Abstract
Background
Studies suggest that racial discrimination impacts health via biological dysregulation due to continual adaptation to chronic psychosocial stress. Therefore, quantifying chronicity is critical for operationalising the relevant aetiological exposure and hence maximising internal validity. Using one of the most common discrimination scales in the epidemiological literature, we develop a novel approach for more accurately assessing chronicity and compare it with conventional approaches to determine whether coding influences differential exposure classification and associations with hypertension and depression among African American women.
Methods
Data are from a socioeconomically diverse cross section of 208 mid-life African American women in Northern California (data collection: 2012–2013). Racial discrimination was assessed using the Everyday Discrimination Scale (α=0.95), and was coded using two conventional approaches: (1) ‘situation-based coding’: number of different situations ever experienced; (2) ‘frequency-based coding’: sum of Likert scale responses ranging from never to almost everyday; and (3) a new ‘chronicity-based coding’ approach: sum of responses, weighted to capture annual chronicity (eg, ‘a few times a month’=3×12=36×/year). Outcomes are hypertension and depressive symptomatology (10-item Center for Epidemiologic Studies-Depression Scale).
Findings
Exposure classification differed by coding approach, by up to 41%. There was a positive association between racial discrimination and hypertension prevalence for chronicity coding only (prevalence ratio=1.61, 95% CI 1.03 to 2.49). For depressive symptoms, a dose–response relationship of similar magnitude was observed for all three coding approaches.
Conclusion
Scale coding is an important methodological consideration for valid exposure assessment in epidemiological research. Coding can impact exposure classification and associations with important indicators of African American women’s mental and physical health.
INTRODUCTION
African American women experience higher rates of numerous adverse mental and physical health outcomes compared with other groups1–5 and a growing body of evidence implicates racial discrimination as a driver of these inequities.4,6–11
Racial discrimination, commonly reported by African American women, is hypothesised to impact health through repeated biological adaptation to chronic psychosocial stress.7,12,13 When chronic, psychosocial stress can cause ongoing activation of the body’s stress response processes, resulting in the overcirculation of stress hormones, which, over time, can lead to multisystem dysregulation and increased risk of poor health.14 Quantifying chronicity, there-fore, is critical for operationalising the aetiologically relevant exposure and hence maximising validity among studies examining racial discrimination as a predictor of health.
The Everyday Discrimination Scale (EDS)—one of the most commonly used measures of discrimination in the epidemiological literature6,9—is well suited to measure chronicity. The EDS was developed as a subjective measure to capture self-reported frequency of routine, relatively subtle discriminatory experiences in everyday social situations. First, respondents are asked: ‘In your day-to-day life, how often have any of the following things happened to you?’ Examples include: ‘people treat you with less respect’ and ‘people act as if they’re afraid of you.’ Second, respondents identify the reason for the unfair treatment (eg, gender, race, ethnicity).9 Responses are typically coded on a 6-point Likert scale ranging from ‘never’ to ‘almost everyday.’15–17
While the EDS has been consistently and positively associated with adverse mental health outcomes among African Americans,9,15,18,19 findings for physical health outcomes are less consistent.6,8 For example, racial discrimination is often conceptualised as a risk factor for hypertension (HT).12,20,21 However, studies examining the association between racial discrimination—including studies using the EDS—and blood pressure outcomes show mixed results.16,17,20–27
One potential explanation for mixed findings across studies using the EDS is inconsistency in scale properties and the coding strategy used. These differences produce distinct exposures that may differentially impact health. For example, the two most common approaches to coding the EDS—’situation-based’ and ‘frequency-based’ coding28—differentially weight the survey response options, which may carry implications for assessing the chronicity of discrimination experiences.
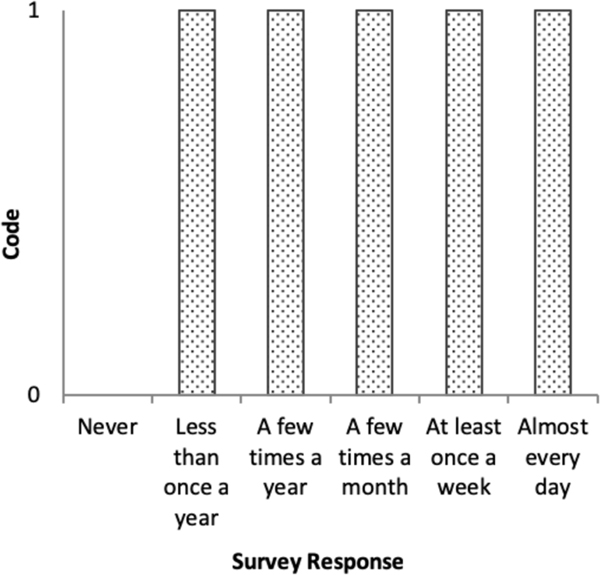
In situation-based coding, each survey item is dichotomised: ‘never’=0 and ‘ever’=1.18,22,25,28 As shown in figure 1, situation-based coding collapses everyone who experienced any discrimination into one category (ie, responses from ‘less than once a year’ through ‘almost everyday’ are combined), obscuring the chronicity of reports. Responses are summed across the items to generate a score ranging from 0 to 10, capturing the number of different situations ever experienced.
Figure 1.
Situation-based coding item weighting structure, Everyday Discrimination Scale.
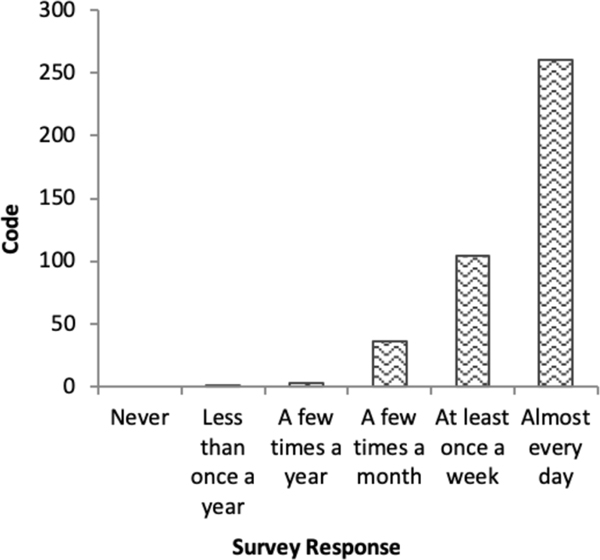
In frequency-based coding,15–17,22–24,28 each response is given a value according to the Likert scale (‘never’=1 to ‘almost everyday’=6). Responses are summed across items to produce a score ranging from 10 to 60. Figure 2 illustrates that although frequency-based coding preserves distinctions between doses of the exposure, it assumes a monotonic change between each response. In reality, each successive response represents increasingly chronic experiences, which the frequency-based coding approach fails to capture.
Figure 2.
Frequency-based coding item weighting structure, Everyday Discrimination Scale.
In summary, the two common approaches to coding the EDS may underestimate the chronicity of everyday racial discrimination experiences. Given that racial discrimination is hypothesized to harm health via repeated physiologic adaptation to chronic psychosocial stress, underestimating chronicity may lead to misclassification of the aetiologically relevant exposure. Such exposure misclassification may threaten internal validity and stall progress toward understanding the potential impact of chronic racial discrimination on racial health inequities. the two common approaches to coding the EDS may underestimate chronicity, resulting in exposure misclassification, which threatens validity and stalls progress towards understanding the potential impact of chronic racial discrimination on racial health inequities.
Study aims
We develop a novel approach to coding the EDS, which weights each response to more accurately reflect the chronicity of EDS experiences (figure 3). We then compare our new coding scheme to the conventional situation and frequency-based approaches to determine whether coding: (A) produces differential expo-sure classification and (B) influences the association of EDS with HT and depressive symptomatology among African American women.
Figure 3.
Chronicity-based coding item weighting structure, Everyday Discrimination Scale.
HT and depression are both stress-related conditions that disproportionately impact African American women, making them salient outcomes to examine in relation to racial discrimination within this population.1,3 Moreover, these are two of the most commonly studied outcomes in the discrimination and health literature, which will allow us to compare our findings to existing work. We hypothesise that the association between the EDS and each study outcome will be differential based on coding approach; and that findings will be most pronounced using chronicity-based coding due to refined exposure assessment in relation to the hypothesised pathway to health (ie, repeated physiologic adaptation to chronic psychosocial stress).
MATERIALS AND METHODS
Study and recruitment
Data are from the African American Women’s Heart and Health Study, an exploratory, cross-sectional study examining associations between social and environmental stressors and mental and physical health among a community sample of 208 African American women in Northern California. We specifically recruited African American women to explore the unique health implications associated with navigating the intersection of multiple marginalised social identities (ie, race and gender) in US society.13
Recruitment and data collection took place from March 2012 to March 2013. Study procedures are described in detail elsewhere.29 Briefly, we used purposive sampling to maximise heterogeneity of sociodemographic factors and risk of experiencing racial discrimination. Women were eligible if they (1) self-identified African American, (2) female sex since birth, (3) aged 30–50, (4) US born, (5) parent(s)/primary caregiver(s) are US-born African American, and (6) could read/write English. Exclusion criteria included: (1) pregnant or lactating, (2) self-reported as physician-diagnosed inflammatory or autoimmune disease.
Missing data
Systolic (SBP) and diastolic blood pressure (DBP) had the highest fraction of missing information (4.8%). We performed multiple imputation of missing values based on socioeconomic, psychosocial, and health status characteristics.30 We excluded one respondent prior to imputation because data were missing for the majority of predictors (n=207). Relative variance increase was <10% for all models and relative efficiency was high (>98%).31
Study measures
Data collection included a computer-assisted self-survey, in-person interview, and physical examination. Resting DBP and SBP was calculated as the average of three consecutive readings using an automated oscillometric monitor.32 HT was defined as: (A) SBP ≥140 mm Hg, and/or (B) DBP ≥90 mm Hg, and/or (C) self-reported current cardiovascular medication usage.33
Depressive symptomatology was assessed using the 10-item Center for Epidemiologic Studies-Depression Scale (CES-D), which captures frequency of self-reported depressive symptoms in the past month (range: 0–30, α=0.83).34–36
Racial discrimination was assessed using the 10-item Everyday Discrimination Scale (EDS).9 Because of the within-group design and focus on racially based discrimination, we used a modified version of the EDS, which asks: ‘In your day-to-day life, how often have the following things happened to you because of your race, ethnicity, or skin color?’ Six response options range from ‘never’ to ‘almost everyday’ (online supplementary table S1).
We used three coding approaches to examine the potential effect of coding on exposure classification and associations with health outcomes (table 1). (1) Situation-based coding—we dichotomised each EDS item to ‘never’=0 and ‘ever’ (collapsing those reporting ‘less than once a year’ or greater into one cate-gory)=1. Items were summed (range: 0–10) to reflect the total number of situations ‘ever’ experienced (α=0.89). (2) Frequency-based coding—we scored responses according to the original Likert scale (range: ‘never’ [1] to ‘almost everyday’ [6]) and summed responses across items (range: 10–60, α=0.94). (3) Chronicity-based coding—we recoded each EDS response to reflect the total number of reported discrimination experiences, standardised on the total number of days per year. ‘Never’ was coded 0. ‘Less than once a year’ was coded as the midpoint between 0 and 1 time per year=0.5×/year. ‘A few’ is generally interpreted as 2–4, so we selected the midpoint=3. Therefore, we coded ‘a few times a year’ as 3×/year and ‘a few times a month’ as 3×12 months=36×/year. We coded ‘at least once a week’ as 2×52 weeks=104×/year and ‘almost everyday’ as 5×52 weeks=260×/year. Recoded items were summed to represent the total number of EDS experiences annually (range: 0–2600, α=0.94). To facilitate comparisons between coding approaches, we collapsed the three EDS measures into tertiles reflecting low, moderate and high exposure (table 1).16
Table 1.
EDS survey response weighting structure, summary and tertile ranges, and description by coding approach, African American Women’s Heart and Health Study, Northern California, 2012–2013 (n=207)
| Situation-based coding | Frequency-based coding | Chronicity-based coding | |
|---|---|---|---|
| EDS response | |||
| Never | 0 | 1 | 0 |
| Less than once a year | 1 | 2 | 0.5 |
| A few times a year | 3 | 3 | |
| A few times a month | 4 | 12 | |
| At least once a week | 5 | 104 | |
| Almost everyday | 6 | 260 | |
| Summary score range | 0–10 | 10–60 | 0–2600 |
| Lower tertile range | 0–7 | 10–22 | 0–24 |
| Middle tertile range | 8–9 | 23–35 | 24.5–448 |
| Upper tertile range | 10 | 36–60 | 482–2600 |
| Description | Number of situations ‘ever’ | Likert scale summary score experienced | Total annual number of EDS experiences |
Owing to the granularity of the chronicity coding, not all possible values of annual EDS experiences are represented.
EDS, Everyday Discrimination Scale.
Covariate selection was outcome specific and guided by directed acyclic graphs (see online supplementary figures S4 and S5).37 Confounders included: age, body mass index (BMI), neuroticism,38,39 education, marital/partnership and employment status. BMI was calculated as weight (kg)/height (m)2. Age was confirmed via driver’s license/state ID. All other covariates were self-reported. To minimise df, we modelled age and neuroticism continuously and dichotomised all other covariates.
Analysis
We generated alpha statistics and performed a polychoric principal component analysis (PCA) to evaluate internal consistency and scale dimensionality under each EDS coding approach. All three coding iterations demonstrated a unidimensional data structure with high internal consistency, indicating recoding did not compromise the integrity of this previously validated scale. We used Goodman-Kruskal’s gamma40 and Cohen’s kappa41 statistics to assess concordance/agreement in EDS exposure classification (low, moderate, high) between the three coding approaches, coded categorically. We also used Pearson’s correlation coefficients to describe the strength of association between the continuous scales.
We fit multivariable modified Poisson regression models with robust SEs to estimate HT prevalence ratios and 95% CIs as a function of each EDS measure.42 The prevalence ratio is a more appropriate and conservative measure of association than the OR when the outcome is not rare (>10%), as was the case with HT in our sample.42 Logistic regression models yielded similar results, although with inflated ORs due to the prevalent outcome (results not shown). Next, we fit multivariable linear regression models to estimate the association between each EDS measure and CES-D. All models used low EDS (bottom tertile) as the reference category. We report models unadjusted and controlling for confounders specific to the exposure–outcome relationship. All analyses were performed using Stata IC V.13.43
RESULTS
Sample
Sample characteristics are displayed in table 2. The mean (SD) SBP was slightly elevated (122 [20]), whereas DBP was in the normal range (80 [12]). Accordingly, 36% of the sample was hypertensive, less than the national prevalence of 46%.1 The mean (SD) CES-D score was approximately 12 (6), slightly above the recommended cut-off of 10 for depression.35
Table 2.
Sample characteristics, African American Women’s Heart and Health Study, Northern California, 2012–2013 (n=207)
| Variable | n (%) or mean (SD) |
|
|---|---|---|
| Poverty status, n (%) | ||
| >100% federal poverty level | 168 | (81) |
| ≤100% federal poverty level | 39 | (19) |
| Educational attainment, n (%) | ||
| >High school diploma | 138 | (67) |
| ≤High school diploma | 69 | (33) |
| Health insurance status, n (%) | ||
| Insured | 152 | (73) |
| Not insured | 55 | (27) |
| Employment status, n (%) | ||
| Employed | 114 | (55) |
| Not employed | 93 | (45) |
| Marital/partnership status, n (%) | ||
| Married/partnered | 61 | (30) |
| Not married/partnered | 146 | (71) |
| Body mass index (BMI), n (%)* | ||
| BMI ≥18.5 and <25 | 28 | (14) |
| BMI <18.5 or ≥25 | 179 | (87) |
| Cardiovascular (CV) medication usage, n (%) | ||
| Not currently taking CV meds | 164 | (79) |
| Currently taking CV meds | 43 | (21) |
| Age (years), mean (SD) | 42 | (5.9) |
| Neuroticism, mean (SD)† | 3 | (0.75) |
| Systolic blood pressure (SBP), mean (SD)‡ | 122 | (20) |
| Diastolic blood pressure (DBP), mean (SD)‡ | 80 | (12) |
| Clinically diagnosable hypertension, n (%)‡ | ||
| Low on SBP and DBP and not taking CV meds | 132 | (64) |
| High on SBP or DBP or taking CV meds | 75 | (36) |
| CES-D score, mean (SD) | 12 | (6.34) |
Seven cases (3.38%) missing.
Four cases (1.93%) missing.
Ten cases (4.83%) missing.
CES-D, Center for Epidemiologic Studies-Depression.
EDS internal consistency and dimensionality by coding approach
Internal consistency was high for all three coding approaches (α=0.89, 0.95 and 0.95 for the situation, frequency and chronicity-based coding approaches, respectively). Our PCA revealed a largely unidimensional data structure for all three approaches. For situation-based coding, the eigenvalue for the first component was 6.35 (63.5% of variance explained). The eigenvalue for the second component was 1.37. All other eigenvalues were <1. For both frequency and chronicity-based coding, the eigenvalue for the first component was 7.57 (75.7% of variance explained). All other eigenvalues were <1.
EDS exposure classification by coding approach
Respondent distribution across low, moderate and high EDS levels was differential by coding approach (online supplementary table S2–S4). Table 3 summarises the number (%) of respondents for whom exposure assessment was discordant (eg, classified as low EDS using one coding approach and moderate or high using another), Pearson’s correlation coefficients, kappa statistics,41 and gamma40 statistics for exposure classification agreement. The frequency and chronicity-based approaches yielded the most concordant exposure classification and highest correlation/agreement, whereas the situation and chronicity-based approaches were most discordant and showed the lowest correlation/agreement.
Table 3.
Discordance n (%), Pearson’s correlation coefficient (r), Goodman-Kruskal’s gamma statistics (γ) and Cohen’s kappa statistics (κ) comparing Everyday Discrimination Scale exposure classification (low, moderate, high) by coding approach, African American Women’s Heart and Health Study, Northern California, 2012–2013 (n=207)
| Coding approaches compared | Discordance n (%) | Pearson’s correlation coefficient (r) | Cohen’s kappa (κ)(P)* | Goodman- Kruskal’s gamma (γ) (ASE)† |
|---|---|---|---|---|
| Frequency versus chronicity | 34 (16) | 0.90 | 0.75 (0.001) | 0.97 (0.009) |
| Situation versus frequency | 64 (31) | 0.76 | 0.54 (0.001) | 0.88 (0.031) |
| Situation versus chronicity | 85 (41) | 0.48 | 0.39 (0.001) | 0.78 (0.044) |
Cohen’s kappa statistics test for agreement in exposure classification (low, moderate, high) between Everyday Discrimination Scale (EDS) measures.41
Goodman-Kruskal’s gamma statistics test for agreement in exposure classification (low, moderate, high) between EDS measures, accounting for ordinal data structure with ties.40 ASE, asymptotic SE.
Associations between EDS and study outcomes by coding approach
Table 4 displays associations between each EDS measure and prevalence of HT. No association was observed using the situation or frequency-based coding approach. In contrast, we found a positive association using chronicity-based coding: moderate (vs low) levels of EDS were associated with a 60% higher estimated prevalence of HT.
Table 4:
Modified Poisson regression with robust error variances for association between Everyday Discrimination Scale and hypertension by coding approach, African American Women’s Heart and Health Study, Northern California, 2012–2013 (n=207)
| Model 1: number of EDS ‘Situations’ ever experienced | Model 2: ‘Frequency’ of EDS experiences (Likert summary score) | Model 3: annual ‘Chronicity’ of EDS experiences | ||||
|---|---|---|---|---|---|---|
| Model 1a: unadjusted | Model 1b: fully adjusted | Model 2a: unadjusted | Model 2b: fully adjusted | Model 3a: unadjusted | Model 3b: fully adjusted | |
| EDS* | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) |
| Moderate | 0.97 (0.61 to 1.56) | 1.00 (0.63 to 1.60) | 1.14 (0.74 to 1.76) | 1.23 (0.81 to 1.88) | 1.52 (0.96 to 2.39) | 1.61 (1.04 to 2.49) |
| High | 1.12 (0.74 to 1.70) | 1.09 (0.72 to 1.65) | 1.01 (0.63 to 1.61) | 1.00 (0.63 to 1.61) | 1.14 (0.69 to 1.90) | 1.10 (0.66 to 1.84) |
| Constant | 0.36 (0.27 to 0.48) | 0.37 (0.20 to 0.68) | 0.35 (0.26 to 0.48) | 0.34 (0.18 to 0.64) | 0.30 (0.21 to 0.44) | 0.31 (0.17 to 0.57) |
Model a: Unadjusted.
Model b: Adjusts for age, body mass index, education (≤high school [HS] diploma), marital/partnership status and employment status.
Referent group=‘low’ EDS.
EDS, Everyday Discrimination Scale; PR, prevalence ratio.
Table 5 displays the results for depressive symptomatology. We observed a consistent dose–response association between EDS and CES-D, irrespective of coding approach.
Table 5.
Linear regression for association between Everyday Discrimination Scale depressive symptomatology (CES-D) by coding approach, African American Women’s Heart and Health Study, Northern California, 2012–2013 (n=207)
| Model 4: number of EDS ‘Situations’ ever experienced | Model 5: ‘Frequency’ of EDS experiences (Likert summary score) | Model 6: annual ‘Chronicity’ of EDS experiences | ||||
|---|---|---|---|---|---|---|
| Model 1a: unadjusted | Model 1b: fully adjusted | Model 2a: unadjusted | Model 2b: fully adjusted | Model 3a: unadjusted | Model 3b: fully adjusted | |
| EDS* | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| Moderate | 2.74 (0.65 to 4.82) | 1.16 (−0.69 to 3.01) | 3.15 (1.14 to 5.17) | 1.75 (−0.05 to 3.54) | 3.47 (1.45 to 5.49) | 2.01 (0.21 to 3.81) |
| High | 3.89 (1.90 to 5.88) | 2.37 (0.63 to 4.11) | 4.75 (2.72 to 6.79) | 3.03 (1.23 to 4.83) | 4.96 (2.93 to 6.98) | 2.85 (1.10 to 4.69) |
| Constant | 9.72 (8.40 to 11.04) | 8.75 (6.88 to 10.61) | 9.13 (7.72 to 10.54) | 8.39 (6.50 to 10.28) | 8.89 (7.45 to 10.32) | 8.34 (6.42 to 10.25) |
| P for trend† | 0.000 | 0.008 | 0.000 | 0.001 | 0.000 | 0.003 |
Model a: Unadjusted.
Model b: Adjusts for age, neuroticism, marital/partnership status, education (≤high school [HS] diploma) and employment status.
Referent group=‘low’ EDS.
P value (two sided) associated with beta coefficient when EDS tertiles are modelled ordinally (vs categorically).
CES-D, Center for Epidemiologic Studies-Depression; EDS, Everyday Discrimination Scale.
DISCUSSION
Accurate exposure assessment is fundamental to validity in epidemiological research and measurement decisions should be based on the hypothesised biological mechanism linking the exposure and outcome.37 We developed a novel approach to coding the EDS for more accurately assessing the chronicity of everyday racial discrimination, a psychosocial stressor that is hypothesised to impact health via repeated stress adaptation. We compared our coding approach with conventional strategies to determine whether risk profiles and associations with health outcomes varied by coding approach. As hypothesised, the three coding schemes produced differential exposure classifications and associations with study outcomes. However, EDS coding was more instrumental for associations with HT than with depressive symptomatology.
Evidence of association between the EDS and HT is mixed;16,17,22–26,44 our findings suggest this may be partially attributed to differences in coding approach, and hence chronicity assessment. One prior cross-sectional analysis showed inconsistent associations between EDS and HT among African Americans when comparing situation vs frequency-based coding strategies.16 The present study corroborates the finding of differential associations based on coding, and extends this work by developing and testing a novel chronicity-based coding approach.
That an association between EDS and HT was observed only using chronicity-based coding could be due to more accurate exposure assessment in relation to the proposed biological path-ways to health. Specifically, chronicity may be underestimated by the frequency-based approach and entirely ignored by the situation-based approach. In both cases, non-differential exposure misclassification may bias results towards the null,37 both here and in other studies seeking to measure chronic exposure to racial disrimination as a social determinant of health. The chronicity coding approach provides a more accurate exposure assessment, potentially reducing misclassification of chronic racial discrimination experiences. Findings may also help identify the mechanisms linking racial discrimination with cardiovascular functioning. Specifically, accurate chronicity measurement was crucial for modelling this association, which is consistent with stress theory and proposed biological pathway (ie, repeated stress adaptation).7–9,12,14
The finding of higher HT prevalence among those reporting moderate, but not high, EDS parallels other work showing an inverse U-shaped relationship between racial discrimination and health among African Americans.5,16,23,44 There are several plausible interpretations for this finding. First, appraisal and coping may differ by chronicity.5,45,46 Those reporting moderate levels of EDS may possess fewer and/or less adaptive racism-specific coping strategies compared with those reporting high levels. Consequently, each encounter may be appraised as more stressful, resulting in exaggerated blood pressure reactivity, which over time may increase HT risk.20 Second, acknowledging and reporting more chronic EDS may be indicative of a problack bias, greater race centrality and/or engagement in system-blame versus self-blame, all of which have been shown to buffer the effects of racial discrimination on health.8,23,44,47 Finally, those reporting high EDS may have a blunted blood pressure response due to a lack of physiological adaptation to chronic psychosocial stress.14 Future research should explore these potential psychosocial and biological modifiers to further explicate the mechanisms through which EDS impacts health.
Unlike with HT, the association between EDS and depressive symptomatology was robust to coding. After adjusting for potential confounders, we saw a dose–response relationship using all three approaches. Findings are consistent with an extensive literature demonstrating a positive association between the EDS and depression, regardless of coding strategy used across studies.15,18,19 Our study demonstrates that capturing chronicity is not critical for modelling the association between everyday racial discrimination and depression, suggesting there are other mechanisms at play that could be explored in future work.
Finally, results provide preliminary evidence of the construct and criterion validity for our new EDS coding scheme. Because frequency-based coding captures EDS with more granularity than situation-based coding, we would expect the chronicity-based coding (the most granular assessment) to be more similar to frequency than situation-based coding both in terms of expo-sure classification and associations with study outcomes. Indeed, we found the highest correlation/agreement between chronicity and frequency-based approaches (convergent validity) and the lowest correlation/agreement between chronicity and situation-based coding approaches (discriminant validity). Relatedly, scale internal consistency and unidimensionality were more robust for the frequency and chronicity coding approaches than for the situation-based approach. This finding provides further evidence that the nature of the exposure may differ when items are dichotomised (situation-based approach) compared with when the gradient in experiences are retained (frequency and chronicity-based approaches). The shape of association between EDS and HT was similar between the frequency and chronicity-based coding approaches, while dissimilar from the situation-based approach (construct validity). While the frequency and chronicity-based coding approaches were highly correlated and showed similar patterns of results, findings of association with HT were most robust using the chronicity-based approach, suggestive of criterion validity based on the proposed pathway to health. Thus, although the frequency and chronicity-based coding are highly correlated, the latter provides a more nuanced exposure assessment, which may explain the more robust findings under this approach.
Next steps for future research include conducting a more formal exploratory and/or confirmatory factor analysis using these three coding approaches, testing these coding schemes on different health outcomes and more diverse study populations, and exploring whether sociodemographic (eg, age, race, gender, socioeconomic status), coping (eg, active vs passive) or other psychosocial factors (eg, social support) modify the scale validity, reliability and health associations differentially by coding approach. Moreover, given previous evidence of differential validity and reliability of the EDS by race and by gender,48,49 future research may also consider comparing psychometric properties of the three coding approaches stratified by these factors, a subanalysis that was not possible in this within-group study of African American women.
This study had several limitations. Data are from a non-representative sample of African American women living in Northern California, and findings are not generalisable to the African American population as a whole. However, recruitment sought to maximise heterogeneity of exposure to discrimination and key covariates such as income and education, and characteristics of our sample are similar to the demographics of the source population, improving external validity.50 The sociodemographic composition of our sample shares similarities with other national data sets; however, there are also important differences (eg, participants in the present study had similar levels of education but lower prevalence of poverty compared with national samples of African Americans).51–53 Previous work suggests that racial discrimination may manifest differently at various socio-economic levels.19,29,54 These findings should therefore be replicated in larger national samples to ensure generalisability and interrogate whether the optimal coding approach differs based on the study sample’s socioeconomic make-up.
While findings cannot be generalised to other gender or racial groups, the purpose of the study was to understand relationships between psychosocial stressors and mental/physical health among African American women, a particularly vulnerable group across numerous health indicators.1–5 Additionally, the within-group study design uniquely facilitates an assessment of racial discrimination—rather than race—as the exposure of interest, a critical step toward understanding the drivers of racial health inequity.55 Future research should explore these coding schemes in relation to health outcomes among African American men.
Cross-sectional data preclude causal inference; HT and depressive symptomatology could influence racial discrimination reporting. However, other studies have shown longitudinal associations between self-reported discrimination and incident HT17 and depressive symptomatology,18 indicating the potential directionality of these associations. Future research should apply these coding schemes to longitudinal data and test associations with disease progression. We also adjusted for neuroticism, a confounder of the association between racial discrimination and depression.8,39 Neuroticism confounded the association between EDS and depressive symptomatology for situation and frequency-based, but not chronicity-based, coding approaches (results not shown). Future research should explore whether reporting bias manifests differently depending on scale coding.
In developing the ‘chronicity’ weighting structure, we made assumptions about the meaning of each response (eg, ‘a few’=3). To assess potential measurement error, we conducted a sensitivity analysis under various assumptions (eg, ‘a few’=2 or 4). Results were largely unchanged, underscoring the robustness of this coding.
Chronicity coding is novel and cannot be directly compared with other studies. However, all three EDS iterations in our sample demonstrated high internal consistency and a unidimensional data structure, similar to other studies using the scale.9,28,48,49 While distribution-based cut-points are sample specific, the tertile ranges shown in table 1 facilitate reproducibility. Finally, although logistical constraints limited the sample to n=208, all models were powered >0.80.
CONCLUSIONS
Different approaches to coding the EDS produce distinct exposures that vary in their associations with important indicators of African American women’s mental and physical health. Coding differences were more influential for associations with HT than with depressive symptomatology, which may help explain a puzzling pattern in the discrimination and health literature: consistent evidence for mental health outcomes, but inconsistent findings for physical, and particularly for cardiovascular, health outcomes.6 Future research using the EDS should explicate hypothesised mechanisms and code the scale accordingly. If the proposed pathway is through the chronic accumulation of discrimination experiences, then the chronicity-based coding approach may be most appropriate. This may be particularly relevant for strengthening internal validity in studies examining the association between racial discrimination and cardiovascular outcomes among African American women.
Supplementary Material
What is already known on this subject
Racial discrimination is believed to harm the health of African Americans through repeated and ongoing adaptation to chronic psychosocial stress. The Everyday Discrimination Scale, one of the most commonly used measures of racial discrimination in the epidemiological literature, has been consistently associated with mental health outcomes among African Americans; whereas associations with physical health outcomes are mixed.
What this study adds
Scale coding is an important methodological consideration that has implications for exposure assessment and validity in epidemiological research. Accurately assessing chronicity may be pivotal for operationalising the aetiologically relevant exposure among studies examining racial discrimination as a social determinant of cardiovascular health.
Acknowledgements
We thank Dr Maureen Lahiff, PhD, for her thoughtful feedback on coding decisions and model selection, Ms Suzanne Dufault, MPH, for her assistance with the principal component analysis, and the many undergraduate, graduate and postdoctoral students working as volunteer research assistants on the African American Women’s Heart and Health Study.
Funding This work was supported by research grants from: University of California, Berkeley (UCB) Hellman Fund, UCB Population Center, UCB Research Bridging Grant, UCB Experimental Social Science Laboratory, Robert Wood Johnson Health and Society Scholars Program (UCB site), UC Center for New Racial Studies, and the UCB Institute for the Study of Societal Issues. EM was partially supported by grant GTDR14301469 from the Susan G Komen Foundation. AA was also partially supported by grant P60MD006902 from the National Institute on Minority Health and Health Disparities. MT was partially supported by grant UL1GM118985 from the National Institute of General Medical Sciences, and by a Ford Foundation Predoctoral Fellowship administered by the National Academies of Sciences, Engineering, and Medicine.
Footnotes
Disclaimer The funders had no involvement in the study design, data collection, analysis and interpretation of the data, writing of the report, nor the decision to submit the paper for publication.
Competing interests None declared.
Patient consent for publication Obtained.
Ethics approval The study was approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Per IRB approval, the data are restricted for transmission to those outside of the primary investigative team. All codes needed to replicate scale recoding and analysis will be provided and can be made publicly available.
► Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/jech-2018–211230).
Correction notice This article has been corrected since it first published. The data collection year range has been corrected in the Methods section.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation 2016;133:e38–60. [DOI] [PubMed] [Google Scholar]
- 2.Statistics NCfH. Health, United States, 2015: with special feature on racial and ethnic health disparities 2016. [PubMed] [Google Scholar]
- 3.Neighbors HW, Caldwell C, Williams DR, et al. Race, ethnicity, and the use of services for mental disorders: results from the National survey of American life. Arch Gen Psychiatry 2007;64:485–94. [DOI] [PubMed] [Google Scholar]
- 4.Geronimus AT, Hicken M, Keene D, et al. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slaughter-Acey JC, Sealy-Jefferson S, Helmkamp L, et al. Racism in the form of micro aggressions and the risk of preterm birth among black women. Ann Epidemiol 2016;26:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paradies Y. A systematic review of empirical research on self-reported racism and health. Int J Epidemiol 2006;35:888–901. [DOI] [PubMed] [Google Scholar]
- 7.Clark R, Anderson NB, Clark VR, et al. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol 1999;54:805–16. [DOI] [PubMed] [Google Scholar]
- 8.Harrell JP, Hall S, Taliaferro J. Physiological responses to racism and discrimination: an assessment of the evidence. Am J Public Health 2003;93:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DR, Jackson JS, et al. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 1997;2:335–51. [DOI] [PubMed] [Google Scholar]
- 10.Williams DR, Mohammed SA. Racism and health I: pathways and scientific evidence. Am Behav Sci 2013;57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health 2008;98(9 Suppl):S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DR, Neighbors H, Racism NH. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis 2001;11:800–16. [PubMed] [Google Scholar]
- 13.Nuru-Jeter A, Dominguez TP, Hammond WP, et al. “It’s the skin you’re in”: African-American women talk about their experiences of racism. an exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J 2009;13:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BS, Stress MBS. Stress, adaptation, and disease. allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 15.Hunte HER, King K, Hicken M, et al. Interpersonal discrimination and depressive symptomatology: examination of several personality-related characteristics as potential confounders in a racial/ethnic heterogeneous adult sample. BMC Public Health 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts CB, Vines AI, Kaufman JS, et al. Cross-sectional association between perceived discrimination and hypertension in African-American men and women: the Pitt County study. Am J Epidemiol 2008;167:624–32. [DOI] [PubMed] [Google Scholar]
- 17.Cozier Y, Palmer JR, Horton NJ, et al. Racial discrimination and the incidence of hypertension in US black women. Ann Epidemiol 2006;16:681–7. [DOI] [PubMed] [Google Scholar]
- 18.Schulz AJ, Gravlee CC, Williams DR, et al. Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: results from a longitudinal analysis. Am J Public Health 2006;96:1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson DL, Neighbors HW, Geronimus AT, et al. Racial discrimination, John Henryism, and depression among African Americans. J Black Psychol 2016;42:221–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolezsar CM, McGrath JJ, Herzig AJM, et al. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol 2014;33:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brondolo E, Love EE, Pencille M, et al. Racism and hypertension: a review of the empirical evidence and implications for clinical practice. Am J Hypertens 2011;24:518–29. [DOI] [PubMed] [Google Scholar]
- 22.Lewis TT, Barnes LL, Bienias JL, et al. Perceived discrimination and blood pressure in older African American and white adults. J Gerontol A Biol Sci Med Sci 2009;64:1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae DH, Nuru-Jeter AM, Adler NE. Implicit racial bias as a moderator of the association between racial discrimination and hypertension: a study of midlife African American men. Psychosom Med 2012;74:961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims M, Diez-Roux AV, Dudley A, et al. Perceived discrimination and hypertension among African Americans in the Jackson heart study. Am J Public Health 2012;102 Suppl 2:S258–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown C, Matthews KA, Bromberger JT, et al. The relation between perceived unfair treatment and blood pressure in a racially/ethnically diverse sample of women. Am J Epidemiol 2006;164:257–62. [DOI] [PubMed] [Google Scholar]
- 26.Moody DLB, Chang Y-F, Pantesco EJ, et al. Everyday discrimination prospectively predicts blood pressure across 10 years in Racially/Ethnically diverse midlife women: study of women’s health across the nation. Ann Behav Med 2018. doi: 10.1093/abm/kay069. [Epub ahead of print: 21 Sep 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MA, Ravenell J, Glynn RJ, et al. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas heart study. Am Heart J 2008;156:1103–9. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N, Smith K, Naishadham D, et al. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61:1576–96. [DOI] [PubMed] [Google Scholar]
- 29.Allen AM, Thomas MD, Michaels EK, et al. Racial discrimination, educational attainment, and biological dysregulation among midlife African American women. Psychoneuroendocrinology 2019;99:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- 31.StataCorp L. Stata multiple-imputation reference manual, 1985. [Google Scholar]
- 32.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Circulation 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 33.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 35.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (center for epidemiologic Studies depression scale). Am J Prev Med 1994;10:77–84. [PubMed] [Google Scholar]
- 36.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (center for epidemiological studies Depression) depression symptoms index. J Aging Health 1993;5:179–93. [DOI] [PubMed] [Google Scholar]
- 37.Savitz DA. Interpreting Epidemiologic Evidence: Strategy for Study Design and Analysis: Oxford. UK: Oxford University Press, 2003. [Google Scholar]
- 38.John OP, Srivastava S. The big five trait taxonomy: history, measurement, and theoretical perspectives. Handbook of personality: theory and research 1999, 1999: 2, 102–38. [Google Scholar]
- 39.Huebner DM, Nemeroff CJ, Davis MC. Do hostility and neuroticism confound associations between perceived discrimination and depressive symptoms? J Soc Clin Psychol 2005;24:723–40. [Google Scholar]
- 40.Goodman LA, Kruskal WH. Measures of association for cross classifications. Journal of the American statistical association 1954;49:732–64. [Google Scholar]
- 41.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 42.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.StataCorp L. Stata data analysis and statistical software. 10, 2007. [Google Scholar]
- 44.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA study of young black and white adults. Am J Public Health 1996;86:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La F. Stress, appraisal, and coping. 1. New York, 1984. [Google Scholar]
- 46.Sapolsky RM. Why zebras don’t get ulcers. New York: WH Freeman, 1994. [Google Scholar]
- 47.LaVeist TA, Sellers R, Neighbors HW. Perceived racism and self and system blame attribution: consequences for longevity. Ethn Dis 2001;11:711–21. [PubMed] [Google Scholar]
- 48.Lewis TT, Yang FM, Jacobs EA, et al. Racial/ethnic differences in responses to the everyday discrimination scale: a differential item functioning analysis. Am J Epidemiol 2012;175:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark R, Coleman AP, Novak JD. Brief report: initial psychometric properties of the everyday discrimination scale in black adolescents. J Adolesc 2004;27:363–8. [DOI] [PubMed] [Google Scholar]
- 50.Bureau U. American community survey. Raleigh-Durham-Chapel Hill, NC Combined Statistical Area, 2013. [Google Scholar]
- 51.Alegria M, Jackson JS, Kessler RC, et al. Collaborative Psychiatric Epidemiology Surveys (CPES), 2001–2003 [United States. Inter-university Consortium for Political and Social Research distributor, 2016. [Google Scholar]
- 52.Williams DR, González HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National survey of American life. Arch Gen Psychiatry 2007;64:305–15. [DOI] [PubMed] [Google Scholar]
- 53.Assari S. High income protects whites but not African Americans against risk of depression. Healthcare 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health 2012;102:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nuru-Jeter AM, Michaels EK, Thomas MD, et al. Relative roles of race versus socioeconomic position in studies of health inequalities: a matter of interpretation. Annu Rev Public Health 2018;39:169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.