Introduction
Bullous impetigo (BI) and pemphigus foliaceus (PF) are 2 distinct cutaneous conditions with substantial clinical overlap. BI is a common, highly contagious cutaneous infection often affecting children, whereas PF is a relatively rare autoimmune blistering condition. Lesions arise from disruption of desmoglein-1, a desmosomal protein required for keratinocyte adhesion, by an epidermolytic toxin or IgG autoantibodies, respectively.1, 2, 3 Given their shared pathophysiology, it is not surprising that these diseases can have similar clinical presentations and have been mistaken for one another in the literature.1,3 Correct diagnosis is critical, as BI is managed with antibiotics to curb rapid spread, and the disease course of PF is meaningfully affected by early systemic immunosuppression. We discuss the diagnosis of a case of BI masquerading as PF and the means of distinguishing the 2 cutaneous conditions.
Report of a case
A 19-year-old woman with an atopic diathesis was admitted with a pruritic, blistering eruption, progressing over 1 week. She was well appearing, and distributed on her chest, back, extremities (upper > lower), and face were dozens of 1- to 2-cm flaccid bullae and well-demarcated superficial erosions with moist red bases (Fig 1). The bullae easily denuded with light palpation. Many erosions had overlying yellow, cornflake-like scale crust, and her mucous membranes were spared. At its onset, the rash was treated topically with triamcinolone ointment for 3 days with little effect and subsequent spread. The differential diagnosis included PF and BI.
Fig 1.
Dozens of 1- to 2-cm flaccid bullae and well-demarcated superficial erosions with moist red bases.
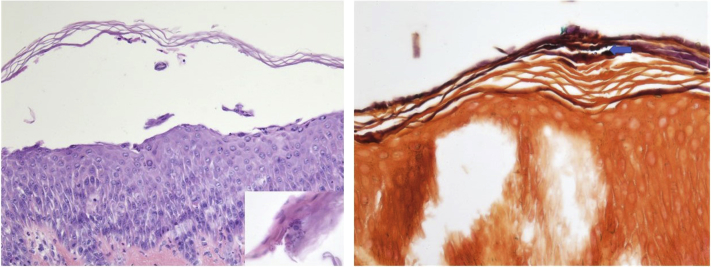
Biopsy of a lesion showed a subcorneal blister with clusters of cocci (Fig 2). Direct immunofluorescence (DIF) of a perilesional biopsy was negative for IgG and C3, and routine laboratory findings were within normal limits. Furthermore, erythrocyte sedimentation rate and C-reactive protein were elevated, whereas IgG enzyme-linked immunosorbent assay (ELISA) for desmoglein-1 and -3 were negative. Importantly, superficial cultures of her erosions grew methicillin-susceptible Staphylococcus aureus. At this point, generalized BI was diagnosed, and the patient was treated with a 14-day course of cephalosporin antibiotics, which successfully improved her rash and associated symptoms.
Fig 2.
Subcorneal blister with clusters of gram-positive bacterial cocci in the stratum corneum (Hematoxylin-eosin stain; original magnification: ×200; Gram stain; original magnification: ×400.)
Discussion
Differentiating BI and PF can occasionally present a significant clinical challenge. In the 18th and 19th centuries, physicians mistakenly christened infantile BI pemphigus neonatorum, highlighting the significant clinical similarity between the two diseases.3 Although rare reports exist of severe BI in the immunocompetent adult population, the infrequency can confound correct diagnosis.2,4 Patients with BI and PF can both present with crusted, scaly superficial erosions with morphologically indistinguishable, easily denuded blisters. Although similarly flaccid, the blisters of PF are typically smaller, and lesions as large as 1 to 2 cm are uncommon.2 Another useful clinical differentiator to consider is rapidity of disease progression. BI tends to generalize quickly, as was the case with our patient, whereas PF is more likely to progress over weeks to months.5 In both conditions, lesions localize to the superficial epidermis, commonly sparing mucous membranes. This phenomenon is the result of desmoglein-1–specific pathology, which allows for desmoglein compensation at mucosal sites, which have much higher levels of desmoglein-3 expression when compared with the nonmucosal epidermis.1,3
Histologically, the blisters of both BI and PF are subcorneal, exhibiting dyskeratotic, acantholytic granular cells in the superficial epidermis, where desmoglein-1 is preferentially expressed.1 In one study, neonatal mice injected with either exfoliative toxin A, the toxin characteristically produced by S aureus in BI, or PF antibodies were found to develop both clinically and histologically indistinguishable blisters.6 The destruction of desmoglein-1, whether by proteolytic toxin or autoantibody, results in loss of intercellular desmosomal junctions in the granular layer of the epidermis.1 Additionally, an inflammatory infiltrate of neutrophils in the dermis is common to both conditions, and Gram stain of BI lesions may show gram-positive cocci in the epidermis. But PF lesions can also become impetiginized, further obfuscating the distinction between the conditions.7
Although histologic architectures of the 2 conditions are similar when visualized with hematoxylin-eosin staining, both the DIF of perilesional biopsies and IgG ELISA for desmoglein-1 and -3 can often aid in distinguishing the 2 diseases. Absent in BI, the characteristic positive DIF of pemphigus foliaceus demonstrates intercellular deposition of IgG and C3, with some cases exhibiting antibody and complement deposition primarily in the superficial epidermis.8 The sensitivity of DIF for any pemphigus disease ranges from 80% to 95%, and the fluorescent staining around keratinocytes is caused by IgG binding of desmoglein-1 on cell surface desmosomes.9 Additionally, ELISA studies of a patient's serum have proven sensitive and specific tests for PF diagnosis, with one study reporting 97.9% sensitivity and 98.9% specificity in detection of desmoglein-1 antibodies in patients with PF.10 In combination, immunofluorescent interrogation has previously aided in the delineation of BI and PF in equivocal cases11 and were equally integral to our patient's diagnosis.
However, rare reports exist of false-positive immunofluorescence findings histologically in bullous impetigo.12 Therefore, detection of the staphylococcal exfoliative toxin of BI has been used for diagnosis.4 In combination with clinical findings and positive superficial wound cultures, the presence of a toxin-producing strain of S aureus can aid in the confirmation of BI. Detection of the exfoliative toxin can be achieved through toxin ELISA, DNA sequence detection via polymerase chain reaction, serologic toxin immunoprecipitation, or radioimmunologic assays.13 Together, these methods allow for the successful diagnosis of BI, preventing potential clinical deterioration in the setting of immunosuppression for PF.
Conclusion
Severe BI, which is uncommon in the adult population, can be mistaken for PF because of significant convergence in clinical presentation and pathophysiology. This mistake is due to the common involvement and destruction of desmoglein-1, resulting in close to identical histologic appearance of subcorneal blisters. Awareness of the significant overlap among BI and PF is necessary, as the treatments for the 2 conditions can be antagonistic, and misdiagnosis can result in significant progression of cutaneous conditions and, in some cases, whole-body involvement.11 As demonstrated through our patient's course, both DIF and ELISA are particularly helpful for both the correct diagnosis and subsequent treatment of adult BI.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Amagai M. The molecular logic of pemphigus and impetigo: the desmoglein story. Vet Dermatol. 2009;20:308–312. doi: 10.1111/j.1365-3164.2009.00831.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoque S., Hextall J., Hay R. Clinicopathological case 3: pemphigus foliaceus; bullous impetigo; subcorneal pustular dermatoses. Clin Exp Dermatol. 2003;28:465–466. doi: 10.1046/j.1365-2230.2003.01292.x. [DOI] [PubMed] [Google Scholar]
- 3.Stanley J.R., Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 4.Cribier B., Piemont Y., Grosshans E. Staphylococcal scalded skin syndrome in adults. A clinical review illustrated with a new case. J Am Acad Dermatol. 1994;30:319–324. doi: 10.1016/s0190-9622(94)70032-x. [DOI] [PubMed] [Google Scholar]
- 5.Bolognia J.J., Jorizzo J.J., Schaffer J.V. Fourth Edition. Elsevier; London: 2017. Dermatology. [Google Scholar]
- 6.Amagai M., Matsuyoshi N., Wang Z.H., Andl C., Stanley J.R. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–1277. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- 7.Buch A.C., Kumar H., Panicker N.H. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364–368. doi: 10.4103/0019-5154.135488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez J., Bystryn J.C. Pemphigus foliaceus associated with absence of intercellular antigens in lower layers of epidermis. Arch Dermatol. 1977;113:1696–1699. [PubMed] [Google Scholar]
- 9.James K.A., Culton D.A., Diaz L.A. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405–412. doi: 10.1016/j.det.2011.03.012. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amagai M., Komai A., Hashimoto T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–357. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 11.Kahn G., Lewis H.M. True childhood pemphigus. Pemphigus foliaceus in an 18-month-old child: immunofluorescence as a diagnostic aid. Am J Dis Child. 1971;121:253–256. [PubMed] [Google Scholar]
- 12.Kouskoukis C.E., Ackerman A.B. What histologic finding distinguishes superficial pemphigus and bullous impetigo? Am J Dermatopathol. 1984;6:179–181. [PubMed] [Google Scholar]
- 13.Mishra A.K., Yadav P., Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150–159. doi: 10.2174/1874285801610010150. [DOI] [PMC free article] [PubMed] [Google Scholar]