Introduction
There is considerable clinical and histologic overlap between frontal fibrosing alopecia (FFA), lichen planopilaris (LPP), fibrosing alopecia with a pattern distribution (FAPD), cicatricial pattern hair loss (CPHL), and lichen planopilaris with a diffuse pattern (LPPDP). Each of these entities may show interface dermatitis involving follicular epithelium and concentric fibrosing around the isthmus and infundibulum, and we favor the term fibrosing alopecia to refer to these entities as a group.1, 2, 3Here we describe 5 patients with focal and diffuse fibrosing alopecia with overlapping features and a slowly progressive course lending further support to the concept that these diseases exist along a spectrum.
Methods
Hair-pull and dermoscopic examination were performed as well as TrichoScan (Tricholog GmbH, Freiburg, Germany), as an objective, noninvasive and observer-independent method4 to quantify the percentages of vellus and terminal hairs. Three patients had biopsies, which were stained with hematoxylin-eosin (H&E) and an elastic tissue stain.5, 6, 7, 8 Complete blood count, free testosterone, syphilis IgG, thyroid function, antinuclear factor, rheumatoid factor, HIV, and hepatitis panels were performed in all patients along with a full physical examination. All diagnoses were made in our hair diseases clinic between August 2017 and December 2018.
Both focal areas of atrichia and diffuse symmetric hair thinning were present in all patients. No exclamation mark hairs, broken hairs, Wickham striae, scalp atrophy, sclerosis, hyperpigmentation, or hypopigmentation were found. Dermoscopy showed decreased hair density, diffuse white dots, and loss of follicular ostia in all patients. Clinical, dermoscopic, and histopathologic features are summarized in Table I, together with our previously published patient with similar but rapidly progressive disease3 for comparison. All laboratory findings were normal or negative. No patient had other evidence or history of systemic lupus erythematosus, discoid lupus erythematosus, or lichen planus. All denied a family history of alopecia or any symptoms such as itching or pain.
Table I.
Clinical, dermoscopic, and histologic details compared with previously published case2
| Case No. | 1 | 2 | 3 | 4 | 5 | Published case |
|---|---|---|---|---|---|---|
| Clinical details | ||||||
| Age (y) | 18 | 32 | 43 | 50 | 28 | 25 |
| Sex | M | F | F | M | M | M |
| Duration (y) | 3 | 5 | 20 | 20 | 2 | 3 |
| Diffused hair loss | Yes | Yes | Yes | Yes | Yes | Yes |
| Symmetric hair loss | Yes | Yes | Yes | Yes | Yes | Yes |
| Hair-pulling test | Negative | Negative | Negative | Negative | Negative | Negative |
| Eyebrow and beard | Sparse | Normal | Sparse | Sparse | Normal | Sparse |
| Axillary and pubic hair | Sparse | Normal | Sparse | Sparse | Normal | Sparse |
| Vellus hair of the body | Disappear | Normal | Disappear | Disappear | Normal | Disappear |
| Lichen planus lesion | - | - | - | - | - | - |
| Dermoscopic and TrichoScan details | ||||||
| Hair density | Decrease | Decrease | Decrease | Decrease | Decrease | Decrease |
| Focal atrichia | + | + | + | + | + | + |
| Exclamation mark hairs | - | - | - | - | - | - |
| Broken hairs | - | - | - | - | - | - |
| Wickham striae | - | - | - | - | - | - |
| Lonely hair sign | + | - | + | + | - | + |
| White spots | + | + | + | + | + | + |
| Loss of follicular ostia | + | + | + | + | + | + |
| Perifollicular keratosis | + | - | + | + | - | + |
| Perifollicular erythema | + | - | Inconspicuous | + | - | - |
| Hair miniaturization | - | + | - | + | - | - |
| Ratio of vellus hairs (%) | 8.7 (crown) | 14.9 (crown) | 2.2 (crown) | 18.1 (crown) | 8.8 (crown) | 5.1 (crown) |
| Histologic details | ||||||
| Lichenoid folliculitis | + | + | + | N/A | N/A | + |
| Concentric fibrosis | + | + | + | N/A | N/A | + |
| Cicatricial | + | + | + | N/A | N/A | + |
| Portion involved | Ist, Inf | Ist, Inf | Ist, Inf | N/A | N/A | Ist, Inf |
Inf, Infundibulum; Ist, isthmus; N/A, not applicable.
Case 1
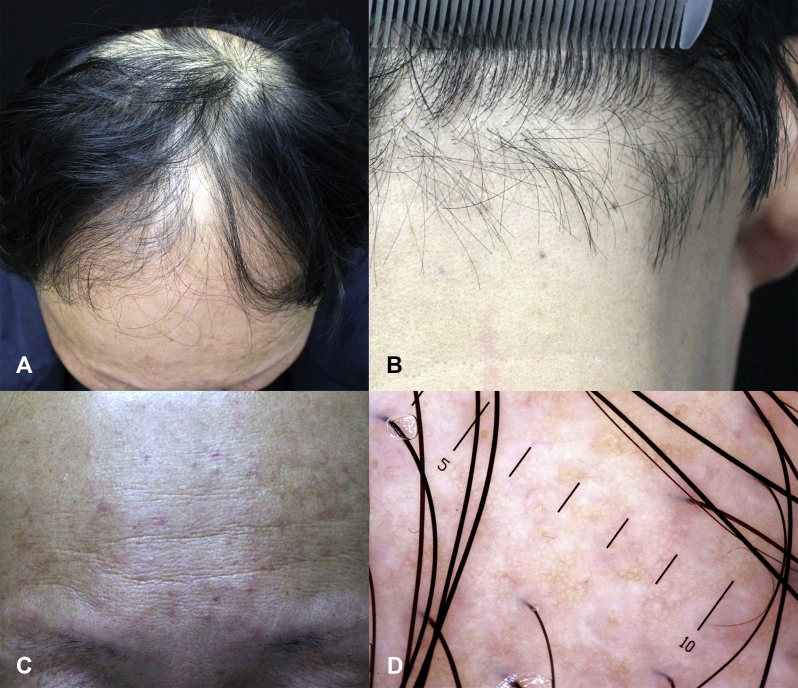
An 18-year-old man presented with a 3-year history of diffuse symmetric hair thinning and hairline recession (Fig 1, A and B). Small patchy areas of atrichia were noted, similar to what Olsen9 described as pencil eraser–sized areas of hair loss. Some areas of focal atrichia merged into larger areas. The “lonely hair sign” characteristic of FFA was evident on the forehead and temporal and occipital scalp. Eyebrow, beard, axillary hair, and pubic hair were all sparse. Vellus hair of the trunk and limbs was absent. Perifollicular erythema and hyperkeratosis were easily noted on the forehead (Fig 1, C) and temporal scalp. Dermoscopic examination found a decrease in hair density and diffuse white dots on the temporal scalp (Fig 1, D), crown, and occiput. TrichoScan found a decrease in hair density on the crown with no significant hair miniaturization. A biopsy from the right temporal scalp found lichenoid folliculitis and concentric fibrosis around both the isthmus and infundibulum, with some follicles collapsed and fibrous tracts retained (Fig 1, E and F). As is common in China, the patient was treated with oral glycyrrhizin capsules, topical tacrolimus (0.1%), and halometasone. After 2 months, the patient stopped all drugs and refused further treatment. One year later, he presented again with no significant progression of disease.
Fig 1.
A and B, Hairline recession, lonely hairs, and diffused small and some larger patchy areas of focal atrichia were observed. C, Perifollicular erythema and hyperkeratosis on the forehead. D, Dermoscopy found hair density decreased and diffused white dots. Some upright regrowing hairs could be seen in the vicinity of white dots on the temporal scalp. E and F, Interface folliculitis and concentric fibrosis around both the isthmus and infundibulum, with some follicles collapsed and fibrous tracts retained. (E and F, H&E stain; original magnifications: E, ×100; F, ×200.)
Case 2
A 32-year-old woman presented with hair thinning for 5 years (Fig 2, A and B). The frontal hairline appeared normal, but thinning was noted in the temporal scalp, and diffuse small patches of alopecia were present in both the crown and temporal scalp merging into larger patches. Neither perifollicular keratosis nor inflammation was noted. Vellus, axillary, and pubic hair were all unaffected. Dermoscopic examination found decreased hair density and many white dots, especially in temporal areas (Fig 2, C). TrichoScan on the crown found mild hair miniaturization (not shown). Biopsy from the frontal scalp and temporal areas found lichenoid inflammation and concentric fibrosis around both infundibular and isthmus (Fig 2, D and E).
Fig 2.
A and B, Frontal hairline is normal, but symmetric hair thinning was present on the crown and temporal scalp. C, Dermoscopy found decreased hair density and white dots; small patches of alopecia were present on the crown and the temporal areas. D and E, Lichenoid inflammation and concentric fibrosis around infundibular and isthmus. (D and E, H&E stain; original magnifications: D, ×100; E, ×200.)
Case 3
A 43-year-old woman presented with advanced diffuse and symmetric hair thinning for more than 20 years (Fig 3, A and B). Frontal recession with many lonely hairs was noted. Diffuse small patches of alopecia were present throughout the scalp, merging into larger ones. Follicular hyperkeratosis and mild inflammation were seen on the crown (Fig 3, C). Eyebrow, beard, axillary hair, and pubic areas were nearly bare. Vellus hair of the trunk and limbs was absent. Dermoscopy found decreased hair density and diffuse white dots both at the crown and the temporal regions (Fig 3, D). TrichoScan found significantly decreased hair density but no obvious hair miniaturization (not shown). Biopsies from the crown (Fig 4, A and B) and the axilla (Fig 4, C) showed lichenoid inflammation and concentric fibrosis around infundibulum and isthmus, with some follicles replaced by connective tissue.
Fig 3.
A and B, Diffuse and symmetric hair thinning with the frontal recession and many lonely hairs. C, Follicular hyperkeratosis and mild inflammation was seen on the crown. D, Dermoscopy found diffuse white dots at both the crown and the temporal scalp and some upright regrowing hairs.
Fig 4.
Biopsies from the crown (A and B) and axilla (C) show the same lichenoid inflammation and concentric fibrosis around infundibular and isthmus, with some follicles replaced by connective tissue. (H&E stain; original magnifications: A and C, ×100; B, ×200.)
Case 4
A 50-year-old man presented with advanced diffuse and symmetric hair thinning for more than 20 years (Fig 5, A and B). Hairline recession with many lonely hairs was noted. Diffuse small patches of alopecia were present with some merging into larger patches. Perifollicular keratosis and perifollicular erythema were seen in the crown and the frontal scalp (Fig 5, C). Eyebrow, beard, axillary hair, and pubic hair were all sparse, and vellus hair of the trunk and limbs was absent. Dermoscopy found a decrease in hair density and diffuse white dots (Fig 5, D). TrichoScan on the crown where the existing hair was thickest showed significantly decreased hair density and mild miniaturization. The patient rejected a biopsy and was lost follow-up 6 months later.
Fig 5.
A and B, Advanced symmetric diffused hair thinning and circumferential hairline recession with lonely hairs and diffuse small alopecia patches merge into larger ones. C, Perifollicular keratosis and perifollicular erythema. Dermoscopy shows decreased hair density and diffuse white dots on the temporal scalp. D, TrichoScan on the crown shows decreased hair density and mild hair miniaturization with the vellus hairs ratio of 18.1%.
Case 5
A 28-year old man presented with gradual hair loss over the last 2 years. Hair thinning was symmetric and diffuse but mild (Fig 6, A). The eyebrows, beard, armpit hair, pubic hair, and vellus hair of the limbs were all normal. TrichoScan on the crown found decreased hair density but without miniaturization (Fig 6, B). After parting the hair, many small patches of hair loss were observed. Dermoscopy found white dots with loss of follicular ostia all over the scalp, and many fine upright regrowing and hypopigmented hairs were seen nearby (Fig 6, C). The patient declined biopsy and rejected drug treatment, and was lost follow-up after the first visit.
Fig 6.
A, Hair thinning was not very apparent at first glance, but small alopecic patches were observed after parting the hair. B, TrichoScan shows decreased hair density but no prominent hair miniaturization. C, Dermoscopy shows sporadic white dots with loss of follicular ostia on the temple and large numbers of fine hairs; upright regrowing hairs and hypopigmented hairs could be seen near white dots.
Discussion
Most patients with scarring hair loss correspond to well-defined entities, but others demonstrate overlap. Entities that share lichenoid folliculitis and concentric fibrosis around the isthmus and infundibulum include FFA, LPP, LPP, LPPDP, FAPD, and CPHL.1, 2, 3 LPP presents as patchy hair loss, FFA involves a band-like area of the hairline, whereas CPHL and FAPD both involve androgen-dependent areas.9 Although each has characteristic features, there exists a wide overlap between these entities and our series of patients suggesting they may belong to a common spectrum of disease.
We previously described a patient with rapidly progressive diffuse fibrosing alopecia and with overlapping clinical and histologic features of FFA, LPP, FAPD, and CPHL, also suggesting that these conditions may exist along a spectrum. We proposed the term fibrosing alopecia to refer to the spectrum of cicatricial entities that share lichenoid folliculitis and fibrosis that mainly involves the upper portions of the follicle.2 The current series of patients presented with slowly progressive alopecia that shared overlapping features. In the 3 younger patients, the hair thinning was more obvious at the hairline and relatively mild on the crown, whereas the 2 older patients had advanced hair thinning at the hairline as well as the crown. This finding may simply represent the time course and natural progression of the disease. Although biopsy specimens were not obtained from 2 patients, the combination of clinical and dermoscopic findings including white dots and irreversible progression suggest that all had progressive fibrosing alopecia.
Recent research shows that FFA can involve the occipital and parietal regions of the scalp as well as the fronto-temporal hairline.10 The lonely hair sign previously described in FFA11 was also noted in each of our patients and not just restricted to the central forehead but also seen in the occipital and temporal regions. Focal atrichia9 was also noted in all of our patients with a tendency to merge into larger patches with absence of emerging hairs.12 In 3 of our patients, hair of the trunk and limbs was absent, and eyebrow, beard, axillary hair, and pubic hair were affected. Two patients had miniaturization in the androgen-dependent region on the crown, but because pattern alopecia is present in roughly half of the population, it may simply represent the background prevalence of androgenetic alopecia.
Perifollicular erythema and hyperkeratosis were noted in cases 1, 3, and 4, whereas in case 2 and 5, there was no obvious inflammation. We speculate that it simply subsided in the chronic phase. Some of our patients were younger than the mean age reported in the literature, suggesting that lymphoid fibrosing alopecia can have early onset.
Our findings support the concept that lymphoid fibrosing alopecia can develop either rapidly or slowly; can involve the forehead, the remainder of the hairline, and androgen-dependent areas; and present with both focal atrichia and larger patches. These findings suggest that previously described disorders may not be unique, but exist along a spectrum. As the number of reported patients is small, these finding should be confirmed by larger studies. The pathogenesis of lymphoid fibrosing alopecia remains speculative but may include hormonal influences, environmental influences, and medication.13, 14, 15 Until discrete pathogenic mechanisms or responses to therapy are defined, there may be little utility in separate classifications.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Sperling L., Cowper S., Knopp E. 2nd ed. Informa Healthcare; New York, NY: 2012. An Atlas of Hair Pathology With Clinical Correlations. [Google Scholar]
- 2.Du X., Li Z., Zhu Q. Rapidly progressive diffuse fibrosing alopecia. JAAD Case Rep. 2019;5(10):883–887. doi: 10.1016/j.jdcr.2019.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starace M., Orlando G., Alessandrini A., Baraldi C., Bruni F., Piraccini B.M. Diffuse variants of scalp lichen planopilaris: clinical, trichoscopic and histopathologic features of 40 patients. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 4.BilgicTemel A., Gulkesen K.H., Dicle O. Automated digital image analysis (TrichoScan) in male patients with androgenetic alopecia; comparison with manual marking of hairs on trichoscopic images. Skin Res Technol. 2018;24(3):515–516. doi: 10.1111/srt.12449. [DOI] [PubMed] [Google Scholar]
- 5.Elston C.A., Kazlouskaya V., Elston D.M. Elastic staining versus fluorescent and polarized microscopy in the diagnosis of alopecia. J Am Acad Dermatol. 2013;69(2):288–293. doi: 10.1016/j.jaad.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Elston D.M., McCollough M.L., Warschaw K.E., Bergfeld W.F. Elastic tissue in scars and alopecia. J Cutan Pathol. 2000;27(3):147–152. doi: 10.1034/j.1600-0560.2000.027003147.x. [DOI] [PubMed] [Google Scholar]
- 7.Fung M.A., Sharon V.R., Ratnarathorn M., Konia T.H., Barr K.L., Mirmirani P. Elastin staining patterns in primary cicatricial alopecia. J Am Acad Dermatol. 2013;69(5):776–782. doi: 10.1016/j.jaad.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Tan T., Guitart J., Gerami P., Yazdan P. Elastic staining in differentiating between follicular streamers and follicular scars in horizontal scalp biopsy sections. Am J Dermatopathol. 2018;40(4):254–258. doi: 10.1097/DAD.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 9.Olsen E.A. Female pattern hair loss and its relationship to permanent/cicatricial alopecia: a new perspective. J Investig Dermatol Symp Proc. 2005;10(3):217–221. doi: 10.1111/j.1087-0024.2005.10109.x. [DOI] [PubMed] [Google Scholar]
- 10.Kanti V., Constantinou A., Reygagne P., Vogt A., Kottner J., Blume-Peytavi U. Frontal fibrosing alopecia: demographic and clinical characteristics of 490 cases. J Eur Acad Dermatol Venereol. 2019;33(10):1976–1983. doi: 10.1111/jdv.15735. [DOI] [PubMed] [Google Scholar]
- 11.Tosti A., Miteva M., Torres F. Lonely hair: a clue to the diagnosis of frontal fibrosing alopecia. Arch Dermatol. 2011;147(10):1240. doi: 10.1001/archdermatol.2011.261. [DOI] [PubMed] [Google Scholar]
- 12.Olsen E.A., Whiting D.A. Focal atrichia: A diagnostic clue in female pattern hair loss. J Am Acad Dermatol. 2019;80(6):1538–1543.e1. doi: 10.1016/j.jaad.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Rezende H.D., Reis Gavazzoni Dias M.F., Trueb R.M. Graft versus host disease presenting as fibrosing alopecia in a pattern distribution: a model for pathophysiological understanding of cicatricial pattern hair loss. Int J Trichology. 2018;10(2):80–83. doi: 10.4103/ijt.ijt_83_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basilio F.M., Brenner F.M., Werner B., Rastelli G.J. Clinical and histological study of permanent alopecia after bone marrow transplantation. An Bras Dermatol. 2015;90(6):814–821. doi: 10.1590/abd1806-4841.20154013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harries M.J., Meyer K., Chaudhry I. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231(2):236–247. doi: 10.1002/path.4233. [DOI] [PubMed] [Google Scholar]