Abstract
1-naphthol (1-NAP) is the main metabolite of pesticide carbaryl and naphthalene, and is also a genotoxic and carcinogenic intermediate in the synthesis of organic compound, dyes, pigment and pharmaceutical industry. In this work, two novel haptens were designed and synthesized for developing a competitive indirect enzyme-linked immunosorbent assay (ciELISA) method for 1-NAP in urine samples. The assay showed a limit of detection of 2.21 ng/mL and working range from 4.02 ng/mL to 31.25 ng/mL for 1-NAP in optimized working buffer. The matrix effect of samples was eliminated via 15-fold dilution of optimized working buffer. Good average recoveries (102.4%-123.4%) with a coefficient of variation from 11.7% to 14.7% was obtained for spiked urine samples. Subsequent instrument verification test showed good correlation between the results of ciELISA and high-performance liquid chromatography. The developed ciELISA is a high-throughput tool to monitor 1-NAP in urine, which can provide technical support for the establishment of biological exposure level for the exposure to carbaryl, naphthalene and other related pollutants.
Keywords: 1-naphthol, hapten design, monoclonal antibody, immunoassay
1. Introduction
1-naphthol (1-NAP, Fig. 1) is the main metabolite of naphthalene or carbaryl. Naphthalene is used extensively as raw material for plasticizer, dyes, surfactants, pesticides, drugs, spices, rubber (Zhao et al., 2019; Zhou et al., 2016), while carbaryl also is the widely product in agriculture of many countries due to its inhibitory effect on acetylcholinesterase (Barr, 2008; Jeon et al., 2013; Zhang et al., 2008). However, as an environmental pollutant, naphthalene and carbaryl are toxic to human health, including mutagenicity, teratogenicity, carcinogenic effects (Preuss et al., 2003; Zhao et al., 2019) and nervous issues (Schreiner, 2003), as well as carbaryl (Beard et al., 2013; Bjorling-Poulsen et al., 2008; Costa et al., 2008; Piel et al., 2018; Weichenthal et al., 2010; Wesseling et al., 2010). Workers, farmers, consumers and ordinary resident might be exposure to naphthalene or carbaryl by smoking (Preuss et al., 2005), aqua environment (Huang et al., 2012), work place (Marczynski et al., 2005), petrol exhaust (Lofgren et al., 1991), lawns and food residues (Meeker et al., 2007). Carbaryl can be metabolized in human body rapidly metabolized and 70–80% is excreted in urine within 24 hour (Berman et al., 2016; Esteve-Romero et al., 2012; Sancho et al., 2003), while 1-NAP is the metabolite which exist in urine as a biomarker (Maroni et al., 2000; Serdar et al., 2008). For naphthalene, it can preferentially accumulate in fat depot of humans and animals due to the high lipophilicity (Viravaidya and Shuler, 2002) and also can be metabolized to 1-NAP and excreted in urine (Ayala et al., 2015; Preuss et al., 2003; Schreiner, 2003). In addition, researches have reported that 1-NAP exhibited genotoxicity, reproductive, and protein toxicity, with a LD50 value of 1700-3300 mg/kg body weight (Huang et al., 2012). Hill et al. (1995) reported that 1-NAP was detected in 86% of subjects in 1000 samples from adults living in USA, with the concentration ranged up to 2500 μg/L. Shafik et al. (1971) measured the concentration of 1-NAP ranging from 0.07 to 1.7 mg/L in the urine of agricultural workers who used carbaryl. These data show that the exposure of naphthalene and carbaryl has become a serious problem. Thus, for assessing exposure of these pollutants, it is necessary to develop rapid, sensitive, low-cost, high-throughput method for monitoring of 1-NAP in urine.
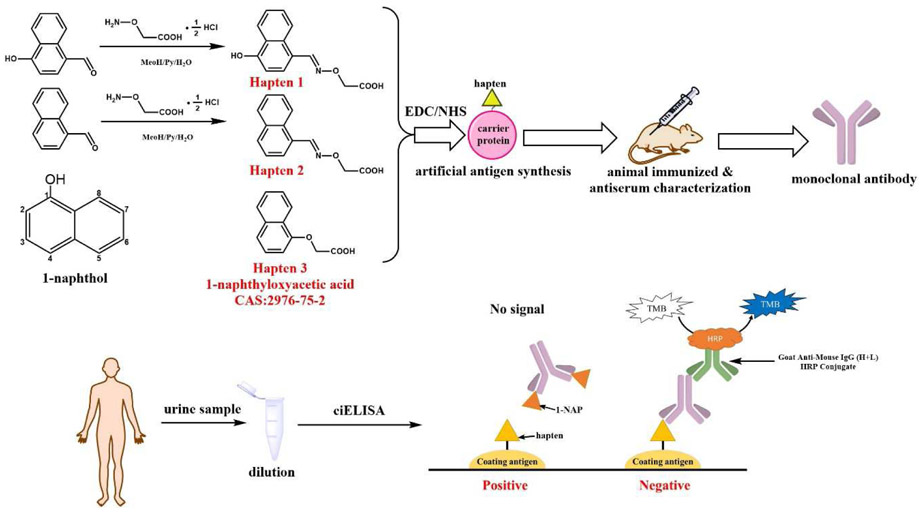
Fig. 1.
The schematic of developed ciELISA for 1-NAP.
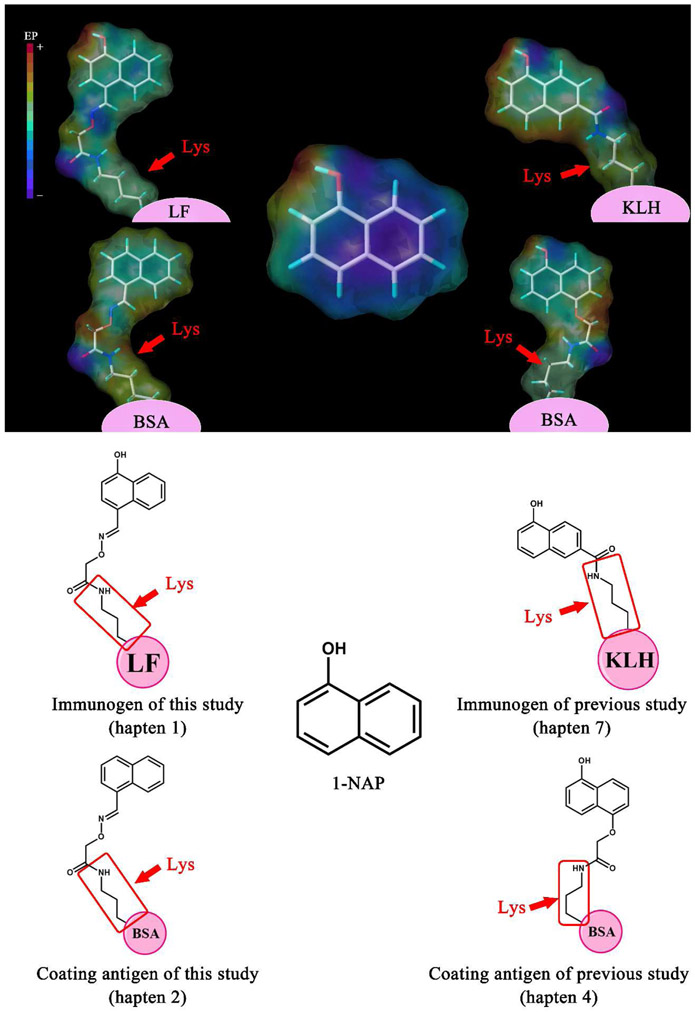
Several methods for 1-NAP detection have been reported, including liquid chromatogram (Sancho et al., 2003; Shealy et al., 1997), gas chromatogram (Bravo et al., 2005; Gaudreau et al., 2016; Petropoulou et al., 2006), capillary electrophoresis (Xu et al., 2016), metal-organic frameworks (MOF) (Qin and Yan, 2018), electrochemistry (Zheng et al., 2015) and immunoassay (Kramer et al., 1994). In comparison with instrumental methods, immunoassay exhibited advantages including rapid, high-throughput, high-sensitive, low-cost, simple and easy-to-use, which is an ideal screening tool for large number of samples before instrumental analysis. However, the production of specific antibodies against 1-NAP was not easy due to its simple structure. In our previous research (Kramer et al., 1994), we synthesized several 1-NAP haptens and the subsequent developed ELISA showed the highest sensitivity (IC50 46.3 ng/mL) by using hapten 7 and hapten 4 (Fig.2). However, the synthesis of the hapten was laborious with multistep and low-yield, and the sensitivity of the developed ELSIA is also not sensitive enough for the monitoring of 1-NAP.
Fig. 2.
Comparison of surface electrostatic potential image of 1-NAP haptens between this study and previous study (Kramer et al., 1994).
In this work, two novel haptens were synthesized via introducing a spacer arm to the No.4 carbon atom of 1-naphthol within one step. The synthesized haptens were further used to prepare artificial antigen for animal immunizing and the development ciELISA for 1-NAP in urine sample. The developed ciELISA was validated by specificity and recovery test which comprised with standard high-performance liquid chromatography (HPLC) method.
2. Materials and Methods
2.1. Animals and Reagents
Complete and incomplete Freund’s adjuvants, keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), polyethylene glycol solution (Hybri-Max™) and 4-hydroxy-1-naphthaldehyde were supplied by Sigma-Aldrich (Shanghai, China). Lactoferrin (LF) from Bovine Milk was purchased from Fujifilm Wako Pure Chemical Co. Ltd. (Japan). The 1-naphthol, 1-naphthaldeyhde, 2-naphthaldeyhde, 1-naphthyloxyacetic acid (hapten 3), 1, 5-dihydroxy naphthalene, 1, 6-dihydroxy naphthalene, 1, 4-dihydroxy naphthalene, 1, 8-dihydroxy naphthalene, 1, 7-dihydroxy naphthalene, 2, 6-dihydroxy naphthalene and phenol were obtained from Energy chemical Co. Ltd. (Shanghai, China). Carbaryl was purchased from Tanmo reference material Technology Ltd. (Beijing, China)Carboxymethoxylamine hemihydrochloride was obtained from Tokyo Chemical Industry (TCI) Co., Ltd. (Shanghai, China). The 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Aladdin Chemical Technology Co., Ltd. (Shanghai, China). HRP-conjugated goat anti-mouse IgG (secondary antibody) and protein G resin were obtained from TransGen Biotech Co. Ltd. (Beijing, China). N, N-dimethylformamide (DMF), pyridine (Py), Tween-20, methanol (MeOH) and ethyl acetate were obtained from Damao Chemical Reagent Co., Ltd. (Tianjin, China). 96-Well polystyrene microplates were purchased from Xiamen Yijiamei Industrial Co.Ltd. (Xiamen, China). RPMI 1640 medium, penicillin-streptomycin solution and pierce™ rapid antibody isotyping kit-mouse were obtained from Thermo Fisher (Shanghai, China). PEG (50%, w/v, average mol wt 1450), HAT and HT media supplement (50×) were purchased from Sigma (Shanghai, China). Fetal Bovine Serum (FBS) was supplied from ExCell Bio. Company (Taicang, China). Bal b/c female mice were supplied by the Guangdong Medical Experimental Animal Centre (license: SCXK (Yue) 2018-0002) and raised at South China Agriculture University Animal Centre (license: SYXK (Yue) 2019-0136). The animal experiment was carried out in a laboratory with a license for experiment animal, which was conformed to the welfare principle (ethical approval number: 2019054, Fig. S1). All other reagents were of analytical reagent grade or higher purity.
2.2. Instruments
The concentration of antibody was detected using a NanoDrop2000c spectrophotometer (Thermo Fisher, Shanghai, China). The ELISA plates were washed in a Wellwasch™ microplate washer (Thermo Fisher, Shanghai, China). Absorbance was measured at a wavelength of 450 nm using a Multiskan MK3 microplate reader (Thermo Fisher, Shanghai, China). HPLC analyses were performed using a Shimadzu LC-20AT system (Kyoto, Japan) equipped with a Shimadzu RF-20A fluorescence detector. Nuclearmagnetic resonance (NMR) spectra were recorded with either a DRX-400 or DRX-600 NMR spectrometer (Bruker, Rheinstetten,Germany).
2.3. Buffers and solutions
The following buffers and solutions were used in this study: (1) serial ionic strength phosphate buffer (PBS) of pH 7.4; (2) serial pH 0.01 M PBS; (3) serial concentration Tween-20 in PBS; (4) 0.1 M pH 9.6 carbonate buffer (CB); (5) PBST (0.01 M PBS with 0.05% Tween-20, pH 7.4); (6) 10% H2SO4 (used as the stop reagent). Cell complete medium: 500 mL RPMI 1640 medium was mixed with 100 mL FBS and 6 mL penicillin-streptomycin solution; HAT medium: 196 mL complete medium was mixed with 4 mL 50× HAT medium; HT medium: 196 mL complete medium was mixed with 4 mL 50× HT medium.
2.4. Synthesis of 1-NAP haptens
4-hydroxy-1-naphthaldehyde was chosen for the synthesis of two haptens (homologous and heterologous) for 1-NAP via introducing carboxymethoxylamine hemihydrochloride as spacer arms to the aldehyde group at within one step reaction. The long spacer arm was conducive to expose the antigenic determinant. The synthetic route of 1-NAP haptens was shown in Fig. 1. The following is a detailed description of the synthetic procedure and characterization of these compounds. The mass and NMR spectrograms are shown in the Supporting Information (Fig. S2 and S3).
Hapten 1, (E)-2-((((4-hydroxynaphthalen-1-yl) methylene) amino) oxy) acetic acid.
The 4-hydroxy-1-naphthaldehyde (1.032 g, 6 mmol) and carboxymethoxylamine hemihydrochloride (3.81 g, 30 mmol) were added into round-bottom flask. Then methanol (12 mL), pyridine (3 mL) and ultrapure water (3 mL) were added. All the mixture was stirred at 50 °C for 6 h. Then mixture was dissolved in 50 mL ethyl acetate and 0.5 M HCl solution was used for extracting pyridine and carboxymethoxylamine hemihydrochloride for three times (50 mL each time). The organic phase was transferred to a round-bottom flask and evaporated at 55 °C. Then 15 mL petroleum ether was added to the round-bottom flask and shook slightly in ultrasound bath. Then the pure product was recrystallized to white solid. After evaporating petroleum ether, the pure product was obtained. ESI-MS analysis (negative): m/z 244 [M - H]−; 1H NMR (600 MHz, DMSO) δ 9.24 (d, J = 8.5 Hz, 1H), 9.15 (s, 1H), 8.79 (d, J = 8.3 Hz, 1H), 8.05 (m, 3H), 7.44 (d, J = 7.9 Hz, 1H), 5.22 (s, 2H).
Hapten 2, (E)-2-(((naphthalen-1-ylmethylene)amino)oxy)acetic acid.
Other than using 1-naphthaldeyhde instead of 4-hydroxy-1-naphthaldehyde, the synthesis method for hapten 2 was the same as that for hapten 1. ESI-MS analysis (negative): m/z 228 [M - H]−; 1H NMR (600 MHz, DMSO) δ 8.95 (m, 1H), 8.64 (dd, J = 16.4, 8.3 Hz, 1H), 8.01 (dd, J = 14.7, 7.9 Hz, 2H), 7.81 (t, J = 7.1 Hz, 1H), 7.60 (m, 3H), 4.75 (s, 2H).
2.5. Simulation of molecular surface electrostatic potential
The energy minimization structure of 3D (three dimensional) of 1-NAP and related compounds and haptens was constructed using Sybyl 8.1 program package (Tripos Inc.) running on a HP xw6600 workstation with an Intel Xeon E5430 2.66 GHz processor. These structures were sketched and then geometry optimized to global low energy conformations using the standard Tripos force field with an 8 Å cutoff for nonbonded interactions in conjunction with Gasteiger-Hückel charges, a 0.005 kcal/(molÅ) termination gradient, and a dielectric constant of 1.00. The surface electrostatic potential of the obtained structures was created by MOLCAD surface program of Sybyl 8.1 using Gasteiger-Hückel charges.
2.6. Synthesis of immunogens and coating antigens
Hapten 1 was conjugated to LF as immunogen and all then haptens (hapten 1, 2 and 3) were conjugated to BSA for coating antigens using the active ester method. Briefly, hapten (0.025 mmol), EDC (0.0375 mmol) and NHS (0.0375 mmol) were dissolved by 0.6 mL of DMF and stirred at for 4 h. Then the mixture was added to 50 mg protein (dissolved in 5 mL CB) and adjusted pH to 9.6 by 3 M NaOH. The solution and stirred overnight. Then the solution was dialyzed by 0.01 M PBS (5 L) for 3 days, and finally stored at −20 °C until use. UV-vis spectral data was used to confirm the structure of the final conjugates (Fig. S4).
2.7. Production of monoclonal antibody
All the female Bal b/c mice were housed and maintained at the South China Agriculture University Animal Center (license: SYXK (Yue) 2019-0136). All the animal experiments were performed incompliance with the protective and administrative laws for laboratory animals of China and conducted with the approval of the Institutional Authority for Laboratory Animal Care (ethical approval number: 2019054), South China Agricultural University, Guangzhou, China.
For the first immunization, each mouse (7-week-old) was intradermally and intramuscularly immunized with 0.1 mL of an emulsion containing 0.5 mL of an immunogen in PBS (1 mg/mL) and 0.5 mL complete Freund’s adjuvant. The following four booster immunizations using the same amount of immunogen emulsified in incomplete Freund’s adjuvant were every three weeks. One week after the fourth booster injection, the serum was collected from the tail tip from each mouse for ciELISA. After serum characterized and coating antigen screening, the mouse that exhibited the best inhibition (%) for 1-NAP was chosen as the donor of spleen cells for hybridoma production. Serum was collected from the tail tip from each mouse prior to the first immunization and used as the negative controls.
Hybridoma preparation was studied in our previous work (Chen et al., 2019). Briefly, the mouse spleen cells and the SP2/0 murine myeloma cells were wash by RPMI 1640 medium for three times. Then the spleen cells and the SP2/0 cells were mixed (5: 1) and fused to hybridomas by 1 mL PEG at 37°C. Then the RPMI 1640 medium was added for stopping fusion. The hybridoma cells were cultured in five 96-well plates with HAT medium and half change to HT medium on the 5th day. The medium of each well was preliminary screened by ciELISA on the 10th day. The well which exhibited highest titer and inhibition was chosen for limit dilution. Briefly, the chosen cell group was sub-cloned five times by limiting dilution (0.6 cell per well) with lower concentration of 1-NAP in each sub-cloning (from 1 to 0.01 μg/mL). The obtained hybridoma was used for ascites preparation. The anti-1-NAP monoclonal antibody (mAb) was separated and purified from ascites by protein G resin. The obtained mAb was stored at −20 °C after measuring the concentration by Nanodrop. The subtype and the affinity of the obtained mAb was IgG1 (Fig. S5) and 6.03×109 L/mol (Fig. S6).
2.8. Optimization of ELISA conditions
To improve the assay performance, the ciELISA was optimized by several parameters, including antibody dilution/coating concentration and working buffer (concentration of Tween 20, ionic strength, and pH). The ciELISA calibration curve was constructed as follows: 1-NAP was serial diluted by artificial urine buffer and were added 50 μL/well as the competitor. Then the calibration curve was generated using Origin pro 7.5 software (OriginLab Corp., Northampton, MA, USA).
2.9. Recovery test
For ciELISA, spiked urine sample (50 μL/well) was diluted by optimized working buffer and added as competitor. High-performance liquid chromatography with fluorescence detection (HPLC-FLD) was used to validate the results of ciELISA. The HPLC system consisted of a Shimadzu LC-20AT system equipped with a Shimadzu RF-20A fluorescence detector. Chromatographic separation was achieved using an XBridge™ C18 chromatographic column (150 mm × 4.6 I.D., 5 μm particle size; Waters Co. Ltd., Ireland). The flow rate was 1.2 mL/min at 35 °C, and the injection volume was 40 μL. The excitation wavelength was 228 nm and measured at 425 nm, with the retention time of 6.45 min (Fig. S7). Spiked urine sample was purified by C18 SPE column. Briefly, C18 SPE column was activated by 9 mL methanol and 9 mL 0.01 M HCl. Then 10 mL urine sample was adjusted to pH 1~2 by HCl and purified by SPE column with the speed of 2~3 mL/min. Analytes was eluted by 10 mL of methanol and filtrated by 0.22 μm membrane.
3. Results and discussion
3.1. Hapten design
As a small organic molecule, hapten design for 1-NAP is the key role in the development of immunoassays. In our previous ELISA study for 1-NAP, highest sensitivity was observed by using hapten 7 (Fig. 2) for immunization (Kramer et al., 1994). However, introducing of a short spacer arm to hapten was not a good strategy for producing high specificity and sensitivity antibodies. The short spacer arm against the recognition between animal immune system and characteristic structure of the hapten because of the steric hindrance of carrier protein (Song et al., 2010). To well understand and compare the influence of hapten design on the specificity of produced antibody from the two studies, molecular surface electrostatic potential simulation was applied. For previous study, Fig. 2 show that the strong negative amide group formed by conjugation was very close to 1-NAP part and therefore became a decisive recognition site of hapten for antibody. Furthermore, the strong negative of amide also influences the charge distribution of 1-NAP part, thereby hapten immune characteristics cannot be maintained very well. Thus, the chance to obtain specific antibody was decreased due to the affection of the amide (Xu et al., 2011). In this work, to avoid the influence of amide, novel immunized hapten was synthesized via introducing a longer spacer arm to 4-hydroxy-1-naphthaldehyd. The longer linker spaces the amide from 1-NAP and therefore reduced the affection of amide, as well as the charge distribution of 1-NAP part. Moreover, the hapten shared a C=N double bond of spacer arm which might provide a rigid support when coupled to carrier protein (Xu et al., 2010), which can make antigenic determinant exposing on the surface of carrier protein as possible. In addition, synthesis route of hapten 1 was easier. In genal, hapten 1 was more reasonable and has potential to produce a higher sensitivity and specificity antibody for 1-NAP.
3.2. Characterization of antisera
Mouse antisera against immunogen were collected and screened for the titer and inhibition in ciELISA against homologous and heterogeneous coating antigens. As shown in Table 1, the ciELISA result exhibited that the highest inhibition was observed by using hapten 2-BSA. Although the lack of hydroxy did not influence the naphthalene obviously, key interaction between antibody and hapten 2 such as hydrogen bond interaction, hydrophilic interaction and electrostatic interaction might be signification reduced. Therefore, the affinity between antibody and the two heterology coating antigen was decreased, while the competitive capacity of 1-NAP to antibody was increased relatively. Furthermore, titer performed by hapten 3-BSA was obviously lower than that of hapten 1-BSA and hapten 1-BSA, suggested that the C=N and oxygen atom was the recognition site for antibody. Previous study also achieved highest sensitivity by using heterologous hapten (hapten 4). Because of the longer linker, the influence of amide was reduced, which leading to the increasing of sensitivity though decreasing the affinity of coating hapten to antibody. That result indicated that the amide was the important recognition site which provide polar interaction, such as hydrogen bond. In general, results from both of the two studies suggested that removing of key polar group from hapten can be a feasible heterologous coating strategy for improving inhibition of 1-NAP.
Table 1.
Characterization of mice antiserum against 1-NAP with homologous and heterologous coating antigen
| Immunogen (Hapten 1-LF) | ||||||
|---|---|---|---|---|---|---|
| Mouse 1 | Mouse 2 | Mouse 3 | ||||
| Coating antigen | Titer | Inhibitionb | Titer | Inhibition | Titer | Inhibition |
| (×103)a | (%) | (×103) | (%) | (×103) | (%) | |
| Hapten 1 - BSA |
16 | 22 | 32 | 44 | 32 | 35 |
| Hapten 2 - BSA |
8 | 57 | 16 | 24 | ·16 | 76 |
| Hapten 3- BSA |
<1 | NDc | 0 | ND | <1 | ND |
Titer is defined as dilution factor of antiserum with the absorbance at 450 nm being situated at about 1.0 ~1.5 at coating concentration of 100 ng/mL.
Percentage inhibition was expressed as follow: inhibition (%) = [1 − (B/B0)] × 100. B0 was mean absorbance of the wells in the absence of competitor; B was mean absorbance of the wells in the presence of certain concentration of competitor.
ND, no detected
According to the antisera screening result in study, mouse 3 exhibited the highest inhibition by using hapten 2-BSA and it was sacrificed for fusion.
3.3. Development of mAb-based ELISA
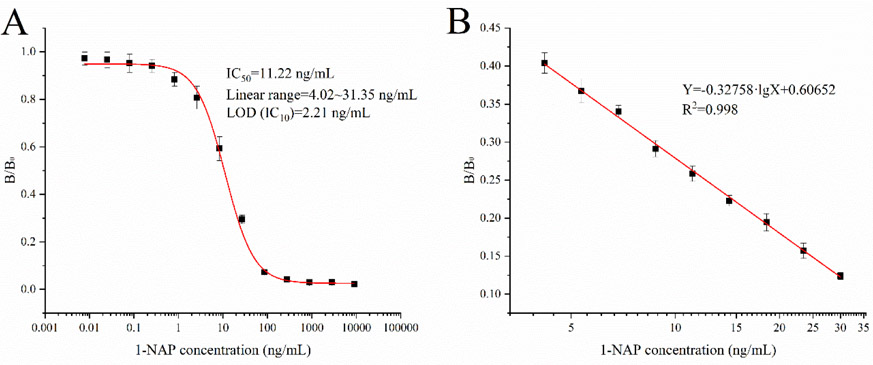
Two cell line (4E2, 4E8) of monoclonal was obtained after hybridoma screening, while cell line 4E8 with best sensitivity was chosen for ascites preparation and purification. The obtained mAb was used for ciELISA development with the optimized conditions. The highest sensitivity was achieved when the coating antigen and antibody concentration were 250 ng/mL and 1000 ng/mL, respectively, and an ionic strength of 0. 1 M without Tween-20 and a pH value of 6.4 were the most suitable for the highest sensitivity (Table S1). The limit of detection (LOD) for 1-NAP was 2.23 ng/mL and the linear range was 3.40~40.0 ng/mL, with the IC50 of 12.47 ng/mL (Fig. 3). The sensitivity of the developed ciELISA was higher than the result of previous study before (Kramer et al., 1994) and indicated that reducing of the polar hydroxyl of 1-NAP hapten is a better strategy to further decrease the competitive of coating antigen.
Fig. 3.
Calibration curve (A) and ciELISA (B) calibration curve for determination of 1-NAP in optimized working buffer (n=6).
3.4. Specificity of the ciELISA
The cross-reactivity (CR) with related compounds was calculated by the optimized condition and summarized in Table 2. Low cross-reactivity (CR) was observed with all the related compounds tested, indicated that the developed mAb showed good specificity for 1-NAP. The highest CR of related compounds was observed for 1, 5-dihydroxy naphthalene while the lowest CR was observed for 1, 8-dihydroxy naphthalene. For explaining the difference between 1-NAP and the related compounds, surface electrostatic potential images of these compounds were also created. In general, there might be serval factors affect the interaction between mAb and the naphthalene compounds in docking process, such as cation-π interaction, π-π stacking, hydrogen bonds, hydrophobic interaction and steric hindrance. Since no significant CR difference between carbaryl (positive charge) and other relate related compounds (negative), indicated that cation-π interaction was not the main factor affect the recognition difference. For carbaryl, steric hindrance resulted by carbamate group might be the main reason that reduced the binding activity from antibody to antigen. For 2-NAP (2-naphthaldeyhde), although its chemical is highly similar to 1-NAP, the CR was significantly lower than 1-NAP, even than some related compounds. Steric hindrance, charge repulsion or hydrophobic interaction at No.2 position might be the main reason that decrease the binging activity, as well as the other 1, n-dihydroxy naphthalene (n=3-8) related compounds. Hence, the position of hydroxyl on naphthalene ring might be the key in the docking process. This speculation will be verified by the computer-assisted simulation by using the homologous modeling in the following work.
Table 2.
Cross reactivity of the obtained mAb with 1-NAP related compounds (n=3)
| Compound | Structure | IC50 (ng/mL) | CR (%)a |
|---|---|---|---|
| 1-naphthol | 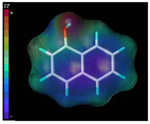 |
12.5 | 100 |
| 2-naphthol | 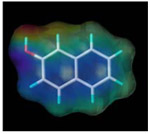 |
1005.0 | 1.2 |
| Carbaryl | 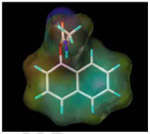 |
300.7 | 4.2 |
| 1, 3-dihydroxy naphthalene | 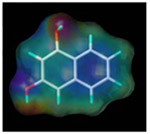 |
178.0 | 6.9 |
| 1, 4-dihydroxy naphthalene | 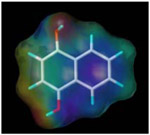 |
200.7 | 6.2 |
| 1, 5-dihydroxy naphthalene | 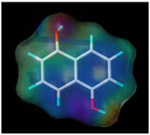 |
87.4 | 14.3 |
| 1, 6-dihydroxy naphthalene | 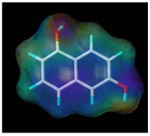 |
502.8 | 2.5 |
| 1, 7-dihydroxy naphthalen | 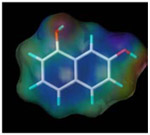 |
881.7 | 1.4 |
| 1, 8-dihydroxy naphthalene | 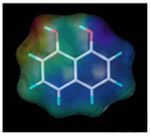 |
4303.4 | 0.3 |
| 2, 6-dihydroxy naphthalene | 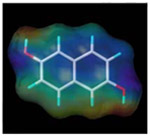 |
2839.7 | 0.4 |
CR, cross-reactivity. The percentage of CR was calculated using the following equation: CR (%) =[IC50 (NAP)/IC50 (cross-reactant)] × 100.
3.5. Recovery test in urine samples
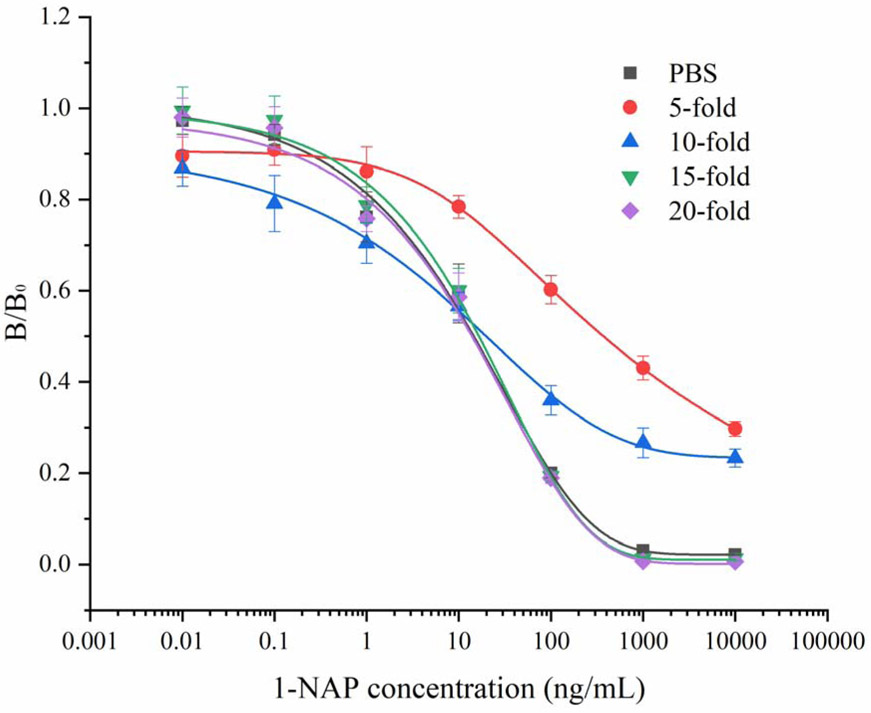
Sample was diluted to four levels (5-fold, 10-fold, 15-fold, 20-fold) by the optimized working buffer for matrix effect test. As shown in Fig. 4, matrix effect had been removed until 15-fold diluted. Three levels of 1-NAP (60, 200 and 600 ng/mL) were spiked in sample for validation of ciELISA. All the spiked samples were analysed by ciELISA and HPLC and the results are summarized in Table 3. The average recoveries of 102.4% to 123.4% were observed using the ciELISA method with a coefficient of variance (CV) ranging from 11.7% to 14.7%. The average recoveries obtained by HPLC method ranged from 90.8% to 123.9% with CVs between 2.2% and 7.5%. The results showed that the accuracy of the ciELISA was comparable to that of the HPLC method. Good correlation and recovery were achieved in this study. However, the sensitivity of the developed ciELSIA was limited by matrix effects and the little narrow linear range. Due to the high dilution, the LOD was increased to 33.15 ng/mL for urine sample. This shortcoming will be dissolved by the following coating antigen improving and standardized producing of ciLEISA kit. The developed ciELISA method could be an ideal tool for screening of urine sample for the monitoring of carbaryl and naphthalene exposure. In addition, immunochromatography and more efficient sample pretreatment (such as immunomagnetic separation) will be developed in the following work for faster detection of 1-NAP.
Fig. 4.
Matrix effect of urine samples on ciELISA performance (n=6).
Table 3.
Recoveries of 1-NAP from spiked urine samples by icELISA and HPLC-FLD (n = 3)a
| Spiked (ng/mL) |
ciELISA |
HPLC-FLD |
||||
|---|---|---|---|---|---|---|
|
mean(ng/mL) (mean±SDb) |
Recovery (%) |
CVc (%) |
mean(ng/mL) (mean±SD) |
Recovery (%) |
CV (%) |
|
| 0d | 1.1±0.2 | NDe | 18.1 | 0.7±0.1 | ND | 14.3 |
| 600 | 741.3±86.9 | 123.4 | 11.7 | 744.7±30.2 | 123.9 | 4.1 |
| 200 | 207.0±30.5 | 102.9 | 14.7 | 184.2±9.0 | 91.5 | 4.9 |
| 60 | 62.6±9.0 | 102.4 | 14.4 | 55.6±1.2 | 90.8 | 2.2 |
For one concentration, three positive samples were spiked and determined by ICA and LC
SD, standard deviation
CV, coefficient of variance, which was obtained from intra-assay.
Blank control
ND, not detected
4. Conclusion
In this work, a monoclonal antibody with improved sensitivity and specificity against 1-naphthol was raised by using novel immunizing hapten and coating hapten. An easy-to-use competitive indirect ELISA was then developed and used to screen the 1-naphthol residue in urine samples to reflect the expose of human being to food, water and environment contaminated by carbaryl and naphthalene. The assay can be finished within about 90 minutes by using a simple pretreatment of dilution, and the limit of detection was 33.15 ng/mL for urine sample. The recoveries of spiked sample ranged from 102.4-123.4%, with a coefficient of variation below 15%. The validation of ciELISA results by standard HPLC-MS/MS suggested that the proposed assay was accuracy and reproducible. It can be used as an ideal tool for the screening of large number of samples with low-cost and high throughput manner.
Supplementary Material
Highlights.
Antibody with good properties for 1-naphthol was obtained based on a novel hapten strategy.
Antibody recognition was analysed by computer-assisted molecular modeling.
An easy-to-used and low-cost immunoassay for 1-naphthol was developed.
Urine samples were analyzed to monitor the expose of pesticide carbaryl and naphthalene.
Acknowledgement
This work was supported by the National Key Research and Development of China (2018YFC1602903; 2018YFC1602904), the Key Project of Guangdong Provincial High School (2019KJDXM002), the Generic Technique Innovation Team Construction of Modern Agriculture of Guangdong Province (2019KJ130), the Open Foundation of Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization, the NIEHS Superfund Research Program (P42ES04699), the NIEHS RIVER Award (R35 ES030443-01), the Graduate Student Overseas Study Program of South China Agricultural University (2019LHPY003) and the China Scholarships Council (201908440392).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ayala DC, Morin D, Buckpitt AR, 2015. Simultaneous Quantification of Multiple Urinary Naphthalene Metabolites by Liquid Chromatography Tandem Mass Spectrometry. Plos One 10, e0121937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, 2008. Biomonitoring of exposure to pesticides. Journal of Chemical Health and Safety 15, 20–29. [Google Scholar]
- Beard JD, Hoppin JA, Richards M, Alayanja MCR, Blair A, Sandler DP, Kamel F, 2013. Pesticide exposure and self-reported incident depression among wives in the Agricultural Health Study. Environ. Res. 126, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman T, Goeen T, Novack L, Beacher L, Grinshpan L, Segev D, Tordjman K, 2016. Urinary concentrations of organophosphate and carbamate pesticides in residents of a vegetarian community. Environ. Int. 96, 34–40. [DOI] [PubMed] [Google Scholar]
- Bjorling-Poulsen M, Andersen HR, Grandjean P, 2008. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health-Glob 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Fernandez C, Smith KD, Gallegos M, Whitehead RD, Weerasekera G, Restrepo P, Bishop AM, Perez JJ, Needham LL, Barr DB, 2005. Quantification of phenolic metabolites of environmental chemicals in human urine using gas chromatography-tandem mass spectrometry and isotope dilution quantification. J. Chromatogr. B 820, 229–236. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Zhou K, Ha WZ, Chen PH, Fu HJ, Shen YD, Sun YM, Xu ZL, 2019. Development of a low-cost, simple, fast and quantitative lateral-flow immunochromatographic assay (ICA) strip for melatonin in health foods. Food Agr. Immunol. 30, 497–509. [Google Scholar]
- Costa LG, Giordano G, Guizzetti M, Vitalone A, 2008. Neurotoxicity of pesticides: a brief review. Front Biosci-Landmrk 13, 1240–1249. [DOI] [PubMed] [Google Scholar]
- Esteve-Romero J, Marco-Peiro S, Rambla-Alegre M, Durgbanshi A, Bose D, Mourya SK, 2012. A micellar liquid chromatographic method for the determination of carbaryl and 1-naphthol in biological samples. J. Liq. Chromatogr. R. T 35, 355–361. [Google Scholar]
- Gaudreau E, Berube R, Bienvenu J, Fleury N, 2016. Stability issues in the determination of 19 urinary (free and conjugated) monohydroxy polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem 408, 4021–4033. [DOI] [PubMed] [Google Scholar]
- Hill RH Jr, Head SL, Baker S, Gregg M, Shealy DB, Bailey SL, Williams CC, Sampson EJ, Needham LL, 1995. Pesticide residues in urine of adults living in the United States: reference range concentrations. Environ. Res 71, 99–108. [DOI] [PubMed] [Google Scholar]
- Huang XF, Zhao GH, Liu MC, Li FT, Qiao JL, Zhao SC, 2012. Highly sensitive electrochemical determination of 1-naphthol based on high-index facet SnO2 modified electrode. Electrochim. Acta 83, 478–484. [Google Scholar]
- Jeon J, Kretschmann A, Escher BI, Hollender J, 2013. Characterization of acetylcholinesterase inhibition and energy allocation in Daphnia magna exposed to carbaryl. Ecotox. Environ. Safe 98, 28–35. [DOI] [PubMed] [Google Scholar]
- Kramer PM, Marco M, Hammock BD, 1994. Development of a selective enzyme-linked immunosorbent assay for 1-naphthol-the major metabolite of carbaryl (1-naphthyl N-methylcarbamate). J. Agr. Food Chem 42, 934–943. [Google Scholar]
- Lofgren L, Persson K, Stromvall AM, Petersson G, 1991. Exposure of commuters to volatile aromatic hydrocarbons from petrol exhaust. The Science of the total environment 108, 225–33. [DOI] [PubMed] [Google Scholar]
- Marczynski B, Preuss R, Mensing T, Angerer J, Seidel A, El Mourabit A, Wilhelm M, Brüning T, 2005. Genotoxic risk assessment in white blood cells of occupationally exposed workers before and after alteration of the polycyclic aromatic hydrocarbon (PAH) profile in the production material: comparison with PAH air and urinary metabolite levels. Int. Arch. Occ. Env. Hea 78, 97–108. [DOI] [PubMed] [Google Scholar]
- Maroni M, Colosio C, Ferioli A, Fait A, 2000. Biological Monitoring of Pesticide Exposure: a review. Introduction. Toxicology 143, 1–118. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R, 2007. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Expo Sci Env Epid 17, 314–320. [DOI] [PubMed] [Google Scholar]
- Petropoulou S, Gikas E, Tsarbopoulos A, Siskos PA, 2006. Gas chromatographic-tandem mass spectrometric method for the quantitation of carbofuran, carbaryl and their main metabolites in applicators' urine. J. Chromatogr. A 1108, 99–110. [DOI] [PubMed] [Google Scholar]
- Piel C, Pouchieu C, Migault L, Beziat B, Boulanger M, Bureau M, Carles C, Gruber A, Lecluse Y, Rondeau V, Schwall X, Tual S, Lebailly P, Baldi I, 2018. Increased risk of central nervous system tumours with carbamate insecticide use in the prospective cohort AGRICAN. Int. J. Epidemiol. [DOI] [PubMed] [Google Scholar]
- Preuss R, Angerer J, Drexler H, 2003. Naphthalene - an environmental and occupational toxicant. Int. Arch. Occ. Env. Hea 76, 556–576. [DOI] [PubMed] [Google Scholar]
- Preuss R, Angerer JR, Drexler H, 2003. Naphthalene-an environmental and occupational toxicant. Int. Arch. Occ. Env. Hea 76, 556–576. [DOI] [PubMed] [Google Scholar]
- Preuss R, Drexler H, Bottcher M, Wilhelm M, Bruning T, Angerer J, 2005. Current external and internal exposure to naphthalene of workers occupationally exposed to polycyclic aromatic hydrocarbons in different industries. Int Arch Occup Environ Health 78, 355–62. [DOI] [PubMed] [Google Scholar]
- Qin SJ, Yan B, 2018. A facile indicator box based on Eu3+ functionalized MOF hybrid for the determination of 1-naphthol, a biomarker for carbaryl in urine. Sensors and Actuators B: Chemical 259, 125–132. [Google Scholar]
- Sancho JV, Cabanes RA, López FJ, Hernández F, 2003. Direct Determination of 1-Naphthol in Human Urine by Coupled-Column Liquid Chromatography with Fluorescence Detection. Chromatographia 58, 565–569. [Google Scholar]
- Schreiner C, 2003. Genetic Toxicity of Naphthalene: A Review. Journal of Toxicology and Environmental Health, Part B 6, 161–183. [DOI] [PubMed] [Google Scholar]
- Serdar B, Waidyanatha S, Zheng Y, Rappaport SM, 2008. Simultaneous determination of urinary 1- and 2-naphthols, 3- and 9-phenanthrols, and 1-pyrenol in coke oven workers. Biomarkers 8, 93–109. [DOI] [PubMed] [Google Scholar]
- Shafik MT, Sullivan HC, Enos HF, 1971. A method for the determination of 1-naphthol in urine. Bull. Environ. Contam. Toxicol. 6, 34-&. [DOI] [PubMed] [Google Scholar]
- Shealy DB, Barr JR, Ashley DL Jr. Patterson DG, Camann DE, Bond AE, 1997. Correlation of environmental carbaryl measurements with serum and urinary 1-naphthol measurements in farmer applicator and his family. Environ. Health Persp 105, 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Wang RM, Wang YQ, Tang YR, Deng AP, 2010. Hapten Design, Modification and Preparation of Artificial Antigens. Chinese J. Anal. Chem 38, 1211–1218. [Google Scholar]
- Viravaidya K, Shuler ML, 2002. Prediction of Naphthalene Bioaccumulation Using an Adipocyte Cell Line Model. Biotechnol. Progr 18, 174–181. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Moase C, Chan P, 2010. A Review of Pesticide Exposure and Cancer Incidence in the Agricultural Health Study Cohort. Environ. Health Persp 118, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling C, van Wendel De Joode B, Keifer M, London L, Mergler D, Stallones L, 2010. Symptoms of psychological distress and suicidal ideation among banana workers with a history of poisoning by organophosphate or n-methyl carbamate pesticides. Occup. Environ. Med 67, 778–784. [DOI] [PubMed] [Google Scholar]
- Xu XD, Li N, Li X, Shi HM, Ma CL, Kang WJ, Cong B, 2016. Capillary electrophoresis chemiluminescence assay of naphthol isomers in human urine and river water using Ni(IV) complex-luminol system. Electrophoresis 37, 2992–3001. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Shen YD, Zheng WX, Beier RC, Xie GM, Dong JX, Yang JY, Wang H, Lei HT, She ZG, Sun YM, 2010. Broad-Specificity Immunoassay for O ,O-Diethyl Organophosphorus Pesticides: Application of Molecular Modeling to Improve Assay Sensitivity and Study Antibody Recognition. Anal. Chem 82, 9314–9321. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Wang H, Shen YD, Nichkova M, Lei HT, Beier RC, Zheng WX, Yang JY, She ZG, Sun YM, 2011. Conformational changes of hapten-protein conjugates resulting in improved broad-specificity and sensitivity of an ELISA for organophosphorus pesticides. The Analyst 136, 2512. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ma GP, Fang GZ, Zhang Y, Wang S, 2008. Development of a capillary electrophoresis-based immunoassay with laser-induced fluorescence for the detection of carbaryl in rice samples. J. Agr. Food Chem 56, 8832–8837. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Li GL, Ning YF, Zhou T, Mei Y, Guo ZZ, Feng YQ, 2019. Rapid magnetic solid-phase extraction based on magnetic graphitized carbon black for the determination of 1-naphthol and 2-naphthol in urine. Microchem. J 147, 67–74. [Google Scholar]
- Zheng XL, Duan S, Liu S, Wei MC, Xia FQ, Tian D, Zhou CL, 2015. Sensitive and simultaneous method for the determination of naphthol isomers by an amino-functionalized, SBA-15-modified carbon paste electrode. Anal Methods-Uk 7, 3063–3071. [Google Scholar]
- Zhou QX, Lei M, Li J, Zhao KF, Liu YL, 2016. Determination of 1-naphthol and 2-naphthol from environmental waters by magnetic solid phase extraction with Fe@MgAl-layered double hydroxides nanoparticles as the adsorbents prior to high performance liquid chromatography. J. Chromatogr. A 1441, 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.