Abstract
Introduction
Elderly people are at increased risk of falls, disability and death due to reduced functional reserve, decline in multiple systems functions, which affects their activities of daily living (ADL) and eventually develop into frailty. The ADL assessment is conducive to early detection to avoid further serious situations. Previous studies on patients’ activities of daily living with chronic kidney disease (CKD) are mainly focused on dialysis patients. Little information is available on non-dialysis patients.
Patients and Methods
A total of 303 elderly patients with CKD stage 3–5 who were admitted to our hospital were selected. ADL evaluation was performed on patients at admission, with Barthel index (BI) as the evaluation tool. They were divided into two groups based on BI (≥60 and <60). Demographic information, lifestyle and clinical profile were collected. The risk factors related to ADL were analyzed by univariate and multivariate models.
Results
The data of 303 patients enrolled in this study were analyzed. The average age of patients was 84.48± 7.14 years and 62.05% were male. There were 88 patients (29.04%) in BI <60 group and 215 patients (70.96%) in the BI ≥60 group. The average age of subjects in the two groups was 87.47 ± 5.85 years and 83.26± 7.28 years, respectively. On univariate analysis, ADL impairment was associated with many factors, such as age, body mass index, blood lipid, heart rate, smoking history, Charlson comorbidity index (CCI), hemoglobin, serum albumin, BNP, eGFR, etc. Multivariate logistic regression showed that age (OR 1.08, 95% CI 1.00–1.17, P=0.0390), Charlson comorbidity index (OR 4.75, 95% CI 1.17–19.30, P=0.0295), and serum albumin (OR 0.80, 95% CI 0.70–0.92, P=0.0012) were the independent risk factors of ADL impairment.
Conclusion
Decline of ADL in CKD patients was independently correlated with age, Charlson comorbidity index and serum albumin. ADL and its influential factors in the elderly CKD patients deserve further attention.
Keywords: chronic kidney disease, activities of daily living, risk factors
Introduction
The global population is aging rapidly and inevitably because of the increase in human life expectancy and the decrease in fertility rate. China, the world’s most populous country, is also experiencing a dramatic increase in its aging population. The number of people aged 60 and over in China is expected to grow from 22.6 million by 2013 to 402 million by 2040.1 Aging is one of the main risk factors for chronic kidney disease (CKD). The incidence of CKD gradually increases with age. According to The Global Burden of Disease, Injuries, and Risk Factors Study (GBD) 2017, the prevalence of CKD was about 9.1% in the world’s population.2 Elderly people are at increased risk of falls, disability and death due to reduced functional reserve, cumulative decline in multiple systems functions of the body, which often affects their activities of daily living and eventually develops into frailty.3 One review suggested that CKD is a risk factor for cognitive impairment and frailty in older adults.4 The incidence of frailty in patients with chronic kidney disease is higher than that in the general elderly population due to changes in protein metabolism, inflammation, oxidative stress, anemia and other reasons.5 It has been pointed out that the incidence of frailty in the healthy elderly is about 11%, while the incidence of frailty in the elderly with CKD is about 6 times (up to 60%) as that in the healthy elderly.6 Frailty patients are characterized by fatigue, weight loss, muscle weakness and decreased functioning, often leading to an increased risk of falls, disability and hospitalization.7
The current assessment methods of frailty, such as frailty index8 and comprehensive geriatric assessment (CGA),9 all include the assessment of physical functions of the elderly, one of which is activities of daily living (ADL). ADL is a convenient way to assess a person’s functional level, referring to the ability of a person to perform his or her daily activities. It ranges from complete independence to heavy dependence, including basic activities of daily living (BADL) and instrumental activities of daily living (IADL), reflecting the overall health of a person.10 The Barthel index (BI) is one of the most commonly used methods to assess a person’s basic self-care ability and the intensity of care required. The total score is 100, and the lower the score, the stronger the dependence on daily life. In studies of patients with stroke, acute heart failure and peripheral artery disease, it was found that low BI score was associated with poor prognosis.11–14 The ADL assessment method is simple, which is conducive to early detection of impaired functional activities, with a view to early intervention to avoid further serious situations such as falls and disability. At present, studies on patients’ abilities of daily living are mainly focused on patients with surgical surgery and cerebrovascular diseases. Related studies on patients with chronic kidney disease are also focused on dialysis patients,15,16 while little information is available on non-dialysis patients.
The aim of this study was to explore the main risk factors related to the decline of activities of daily living in CKD non-dialysis patients, so as to identify and screen frailty population in the early stage, and propose relevant intervention measures to improve the quality of life.
Patients and Methods
Study Design and Subjects
This was a case–control study. We collected 303 patients from the clinical Scientific Research platform. They were admitted to the department of Internal Medicine, Beijing Chaoyang Hospital, Capital Medical University between October 2017 to December 2018. This study was reviewed and approved by the Medical Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. The informed consents were signed after the eligible patients agreed to participate in the study and allowed access to their clinical data. The inclusion criteria for all subjects were as follows: 1) 65 years old and over, 2) Diagnosis of CKD stage 3–5, 3) non-dialysis, whether hemodialysis or peritoneal dialysis. The glomerular filtration rate was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.17 The estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2 was defined as CKD stage 3–5.18
Data Collection and Outcomes
Age, gender, smoking history, body mass index (BMI), heart rate (HR), Left ventricular ejection fraction (LVEF), medical history, psychological components (anxiety, depression), cause of hospital admission, length of stay, and laboratory test were collected and extracted from clinical Scientific Research platform. The patient’s anxiety and depression status was assessed by a neuropsychologist, or they had a prior history of anxiety or depression and were taking antianxiety or antidepressant medications. Since height and weight were recorded at admission, BMI could be calculated by the following formula: BMI = weight (kg)/height (m2). The Charlson comorbidity index (CCI) is a weighted score of the number and severity of various diseases to predict 1-year mortality.19 It should be noted that the selected population in our study were all patients with chronic kidney disease, so the renal disease was not taken into account.
ADL evaluation was performed on patients at admission, with Barthel index (BI) as the evaluation tool. BI consists of 10 items, including feeding, dressing, transferring, grooming, bathing, bowel control, bladder care, toileting, walking, ascending and descending stairs, with a total score ranging from 0 to100.11 Higher BI score indicates lower dependency with ADL. It is reported that BI score ≥60 is classified as basic self-care in life, score<60 indicates functional dependency.20 In our study, the BI cut-off value of patients divided into two groups was 60, which was consistent with previous studies.11–14,21
Statistical Analysis
R 3.3.2 statistical software was used to perform all analyses. For measurement data, the normality of data was tested using the Shapiro test. And homogeneity of variance was tested by Levene test. The variables such as B-type natriuretic peptide (BNP) that presented non-uniformity were transformed via natural logarithm. The variables such as age, BMI, HR, CCI, eGFR and laboratory test with normal distribution or large samples (n>30) were presented as the mean ± standard deviation (SD) and then were assessed by Student’s t-test. Small samples or non-normal distribution data were represented by median (upper quantile–lower quantile), and Wilcox test was adopted. The count data, such as gender, smoking history, LVEF<55%, psychological components were presented as a percentage, then, were compared with the Chi-square test or Fisher’s exact test according to the theoretical frequency. Variables with P<0.2 in the univariate analysis were incorporated into the multivariable models. The multivariable analyses were carried out using the multinomial logistic regression, decision tree and Bayesian model. The odds ratio (OR) was calculated by using logistic regression analysis. The receiver operating characteristic curve and decision curve analysis were conducted for three methods to test the validity of prediction, and the area under the curve (AUC) was established.
Results
Baseline Characteristics
The data of 303 patients enrolled in this study were analyzed. Baseline characteristics of the patients are shown in Table 1. The average age of patients was 84.48± 7.14 years and 62.05% were male. After assessing ADL with Barthel index, subjects were divided into two groups, BI <60 group and BI ≥60 group. There were 88 patients (29.04%) in BI <60 group and 215 patients (70.96%) in the BI ≥60 group. The average age of subjects in the two groups was 87.47 ± 5.85 years and 83.26± 7.28 years, respectively.
Table 1.
Baseline and Univariate Analysis of the Risk Factors of ADL in CKD Stage 3–5 Patients
| Total (n=303) | BI<60 (n =88) | BI≥60 (n = 215) | p value | |
|---|---|---|---|---|
| Age (years) | 84.48±7.14 | 87.47±5.85 | 83.26 ± 7.28 | <0.001 |
| Male, n (%) | 188 (62.05) | 58 (65.91) | 130 (60.47) | 0.450 |
| BMI (kg/m2) | 25.20±3.93 (n=242) | 24.45±3.37 (n=41) | 25.36±4.03 (n=201) | 0.179 |
| HR (beats per min) | 77.21±15.62 (n=284) | 80.41±16.39 (n=85) | 75.85±15.11 (n=199) | 0.024 |
| Smoking, n (%) | 176 (60.07) (n=293) | 59 (68.60) (n=86) | 117 (56.52) (n=207) | 0.073 |
| LVEF<55%, n (%) | 18 (13.85) (n=130) | 4 (12.90) (n=31) | 14 (14.14) (n=99) | 1 f |
| CCI | 1.84±1.56 (n=286) | 2.04±1.73 (n=83) | 1.76±1.49 (n=203) | 0.181 |
| Psychological components (anxiety, depression), n (%) | 13 (4.55) (n=286) | 5 (6.02) (n=83) | 8 (3.94) (n=203) | 0.532 |
| Laboratory Test | ||||
| HbA1C, % | 6.55±1.3 (n=182) | 6.60±1.58 (n=48) | 6.52 ± 1.19 (n=134) | 0.724 |
| Hemoglobin(g/L) | 107.38±21.46 (n=278) | 103.20±20.17 (n=81) | 109.10±21.79 (n=197) | 0.037 |
| Albumin(g/L) | 36.04±4.39 (n=251) | 33.59±4.1 (n=70) | 36.99± 4.13 (n=181) | <0.001 |
| CRP(mg/L) | 3.74±6.81 (n=97) | 4.63±4.8 (n=28) | 3.37± 7.48 (n=69) | 0.412 |
| ln(BNP) (ng/L) | 5.15±1.04 | 5.44±0.96 | 5.01±1.06 | 0.004 |
| TG (mmol/L) | 1.51±0.92 (n=251) | 1.35±0.60 (n=71) | 1.57±1.01(n=180) | 0.084 |
| LDL-C (mmol/L) | 2.25±0.85 (n=251) | 2.39±0.81 (n=71) | 2.19±0.86(n=180) | 0.086 |
| HDL-C (mmol/L) | 1.01±0.32 (n=251) | 0.97±0.30 (n=71) | 1.02±0.33 (n=180) | 0.281 |
| Total bilirubin (μmol/L) | 12.19±10.73 (n=248) | 12.90±13.44 (n=67) | 11.93±9.57(n=181) | 0.528 |
| Direct bilirubin (μmol/L) | 5.09±6.88 (n=248) | 5.93±9.78 (n=67) | 4.78±5.44(n=181) | 0.242 |
| Indirect bilirubin (μmol/L) | 7.10±5.44 (n=248) | 6.97±4.51 (n=67) | 7.15±5.75(n=181) | 0.814 |
| eGFR (mL/min per 1.73 m2) | 40.76±13.65 (n=291) | 38.91±14.66 (n=83) | 41.51±13.23 (n=208) | 0.143 |
| Cause of Admission | ||||
| Respiratory disease, n (%) | 68 (22.44) | 26 (29.55) | 42 (19.53) | 0.058 |
| Cardiovascular disease, n (%) | 41 (13.53) | 10 (11.36) | 31 (14.42) | 0.480 |
| Gastrointestinal disease, n (%) | 16 (5.28) | 6 (6.82) | 10 (4.65) | 0.444 |
| Cerebrovascular disease, n (%) | 85 (28.05) | 20 (22.73) | 65 (30.23) | 0.187 |
| Diabetes, n (%) | 10 (3.30) | 3 (3.41) | 7 (3.26) | 0.946 |
| Cancer, n (%) | 37 (12.21) | 10 (11.36) | 27 (12.56) | 0.773 |
| Others, n (%) | 46 (15.18) | 13 (14.77) | 33 (15.35) | 0.899 |
| Length of stay, days | 8.58±3.08 | 9.32±3.66 | 8.27±2.77 | 0.017 |
| BI scores at discharge | 70.69±26.09 (n=298) | 40.35± 24.14 (n=85) | 82.79±14.38 (n=213) | <0.001 |
| Mortality, n (%) | 6 (1.98) | 5 (5.68) | 1(0.47) | 0.532 |
Note: fFor Fisher exact test.
Abbreviations: BMI, body mass index; HR, heart rate; LVEF, left ventricular ejection fraction; CCI, Charlson comorbidity index; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
The two groups were balanced for gender, LVEF, psychological components, Hemoglobin A1C (HbA1C) level, C-reactive protein (CRP) level, high-density lipoprotein cholesterol (HDL-C) level, total bilirubin level, direct bilirubin level and indirect bilirubin level. The BI <60 group was older, had a higher percentage of males, and had a lower mean BMI and eGFR than the BI ≥60 group. Furthermore, the levels of hemoglobin and serum albumin were lower and the levels of BNP were higher in the BI <60 group than in the BI ≥60 group. The prevalence of smoking history was higher in the BI<60 group than in the BI ≥60 group. The main causes of admission were cerebrovascular disease (28.05%), respiratory disease (22.44%), cardiovascular disease (13.53%). The length of stay was longer in the low BI group. Meanwhile, our study found that the risk of adverse events was significantly higher in the low BI group than in the other group.
Risk Factors of ADL Impairment
Risk factors analysis is shown in Table 2. Age (p<0.001), heart rate (p=0.024), BMI (p=0.179), smoking history (p=0.073), hemoglobin level (p=0.037), serum albumin level (p<0.001), log transformed BNP level (p=0.004), low density lipoprotein cholesterol level (LDL-C) (p=0.086), triglyceride (TG) level (p=0.084), Charlson comorbidity index (p=0.181), eGFR (p=0.143), respiratory disease (p=0.058) and cerebrovascular disease (p=0.187) were found to be associated with low BI score in the univariate analysis (Table 1).
Table 2.
Multivariate Logistic Regression Analysis of the Risk Factors of ADL in CKD Stage 3–5 Patients
| OR | 95% CI | p value | |
|---|---|---|---|
| Age | 1.08 | 1.00, 1.17 | 0.0390 |
| LDL-C | 1.65 | 0.91, 2.98 | 0. 0997 |
| Albumin | 0.80 | 0.70, 0.92 | 0.0012 |
| CCI | 4.75 | 1.17, 19.30 | 0.0295 |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; CCI, Charlson comorbidity index.
Patients with missing data on more of the included variables were excluded from the multivariable analyses. After the collinear analysis, there was no strong collinear between any two factors. Charlson comorbidity index was divided into two groups on 3.5 point calculated by ROC. Then, a total of 12 variables, 132 cases sample entered into the multivariate analysis model. With the step-wise selection and Akaike information criterion (AIC) as the criteria for variables selection, the multivariable logistic regression model with the lowest AIC was finally obtained, including four variables. LDL-C was not associated with ADL impairment (P=0.0997). Age, serum albumin level and Charlson comorbidity index were independently significant predictors of ADL impairment. Among them, age (OR=1.08, 95% CI 1.00–1.17, P=0.0390), Charlson comorbidity index (OR=4.75, 95% CI 1.17–19.30, P=0.0295) were risk factors for ADL impairment, and serum albumin (OR=0.80, 95% CI 0.70,-0.92, P=0.0012) was protective factor for ADL impairment (Table 2).
Predicting ADL Impairment
In order to explore the main risk factors for the decline of those patients’ ADL, three different models, logistic regression, decision tree and naïve Bayes were used to predict ADL impairment. Logistic regression is a regression model in which the dependent variable is the categorical variable and the probability of events is modeled as a linear function of a set of predictive variables. A decision tree is a tree structure in which each internal node represents a test on an attribute, each branch represents a test output, and each leaf represents a category. Naïve Bayes method is to use the prior probability of an object to calculate its posterior probability by using the Bayesian formula.22
Variables like demographics and laboratory test at admission were added into models. To achieve a better performance in discrimination and prediction, severe index is shown in Table 3. Compared with the logistic regression model, naïve Bayes model demonstrated a higher performance in AUC (0.82 vs 0.77), F-score (0.51 vs 0.47), specificity (0.96 vs 0.92) and positive predictive value (PPV) (0.86 vs 0.64), whereas a lower performance in accuracy (0.73 vs 0.77), sensitivity (0.37 vs 0.38) and Negative predictive value (NPV) (0.70 vs 0.79). While decision tree model demonstrated a higher accuracy (0.86 vs 0.77), F-score (0.55 vs 0.47), sensitivity (0.61 vs 0.38), NPV (0.94 vs 0.79), but a lower AUC (0.74 vs 0.77), specificity (0.90 vs 0.92) and PPV (0.50 vs 0.64) comparing with the logistic regression model.
Table 3.
Prediction Performance Evaluation
| Model | AUC (95% CI) | Accuracy | F-score | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| LR | 0.77 (0.66,0.88) | 0.77 (0.68,0.83) | 0.47 | 0.38 | 0.92 | 0.64 | 0.79 |
| DT | 0.74 (0.63,0.84) | 0.86 (0.79,0.92) | 0.55 | 0.61 | 0.90 | 0.50 | 0.94 |
| NB | 0.82 (0.72,0.91) | 0.73 (0.64,0.80) | 0.51 | 0.37 | 0.96 | 0.86 | 0.70 |
Abbreviations: AUC, area under curve; LR, logistic regression; DT, decision tree; NB, naïve Bayes; PPV, positive predictive value; NPV, negative predictive value.
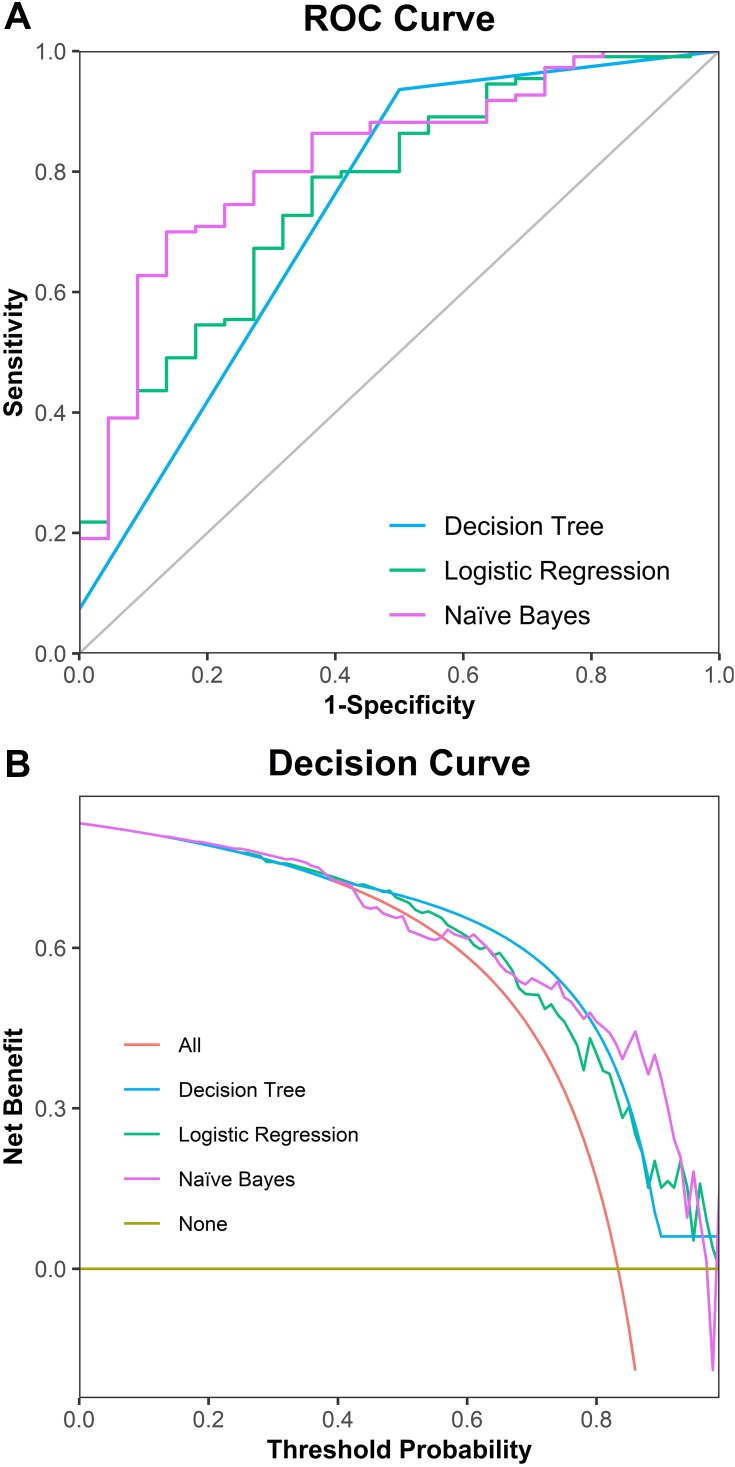
Receiver operating characteristic curve (Figure 1A) demonstrated that naïve Bayes model had the best overall classification performance compared with logistic regression model and decision tree model. Likewise, decision curve analysis (Figure 1B) showed a similar result that the overall net benefit of naïve Bayes model surpassed that of the logistic regression model and decision tree model throughout the threshold ranges.
Figure 1.
Comparison of three models predicting ADL. (A) Comparison of three models using receiver operating characteristic curve; (B) decision curve for prediction of three models.
Discussion
ADL reflects the functional status and self-care ability of the elderly. CKD patients often suffer from a decline in their living ability due to a variety of reasons. Patients with CKD often have a decline in their living ability due to a variety of reasons, which not only significantly reduces their quality of life but also affects the mental health level and social adaptability of the elderly. In addition, it will also bring a heavy burden to the family and society. The study of ADL in elderly CKD patients and its influencing factors can help early detection of risk groups and early intervention to improve the quality of life of the elderly.
In the current practice of nephropathy, the number of patients with non-dialysis CKD (ND-CKD) is increasing. Those patients are characterized by a higher burden of multimorbidity, such as hypertension, diabetes and cardiovascular (CV) disease.23 The high prevalence of both traditional and non-traditional cardiovascular risk factors probably explains the massive burden of cardiovascular disease in this population. De Nicola et al found a linear correlation between cardiovascular risk and LDL levels in patients with non-dialysis CKD.24 Moreover, proteinuria was found to be a risk factor of cardiorenal outcome in these patients. The severity of GFR injury was positively correlated with the level of proteinuria.25 A multicenter prospective study using proteinuria indexed to eGFR showed that filtration-adjusted proteinuria (F-Uprot) could better estimate ESRD risk. In all CKD stages, the risk of ESRD significantly increased with the increase of F-Uprot.26 Another study showed that CKD patients had a relatively high risk of developing ESRD even with long-term care and low proteinuria.25 The aggravation of the burden of CV and renal diseases also leads to the decline of the patients’ quality of life and functional status.
There were a lot of studies about kidney function and ADL. A study of older rehabilitation patients showed that GFR and BI were independent risk indicators for survival.27 A study of people receiving home care for CKD showed a significant correlation between GFR and BI. When GFR decreased, BI scores decreased.28 However, in our study, GFR was associated with low BI in univariate analysis but did not enter the regression analysis model. The effect of GFR on BI was not significant. This may be due to differences in the study population and GFR calculation methods.
Our study used BI scores as a basis for grouping patients. However, BI has some limitations. The score of BI may be influenced by the environment depending on the availability of additional aids. Furthermore, cognitive impairment affects ADL regardless of the patient’s health status. Although BI has some drawbacks, it is still valuable in the study of risk stratification and prognosis. A study by Murcia et al used BI to group patients to investigate whether BI predicted mortality in patients with pneumonia.29 A study of patients with severe limb ischemia also compared patients with high BI and low BI to determine whether BI is useful for risk stratification after bypass surgery.30
It is well known that the dependence of the elderly on the abilities of daily living increases with age.
A study on the factors influencing the daily life of elderly people in China found that older age was a relevant factor for the loss of activities of daily living.31 In a systematic review of 25 studies, age was identified as one of the risk factors for ADL in elderly community residents in several studies.32 Similar results have been found in studies of patients with chronic kidney disease.33 In our study, age showed a strong correlation with ADL in both univariate and multivariate analyses, confirming the effect of age on ADL in patients with chronic kidney disease. Therefore, ADL evaluation should be paid more attention as age increases.
CCI is the most commonly used tool to evaluate comorbidity. It used 604 hospitalized patients as the study object to assign values to patients with different diseases, evaluate their 1-year mortality rate, and verify their ability to predict the risk of death in 685 breast cancer patients.19 CCI, as an independent factor, affects the prognosis and mortality of patients, which has been verified in several studies.34,35 The present study demonstrated that a higher comorbidity index was associated with functional decline in elderly patients with CKD. The elderly are prone to a variety of chronic diseases, and complications have been proven to be one of the risk factors for functional decline, disability, and dependence in multiple studies.36–38 This is consistent with our findings. Therefore, the management of chronic diseases of the elderly should be strengthened.
Our study found that serum albumin levels were an important predictor of ADL declined in elderly CKD patients, which was consistent with previous studies.33,39 Epidemiological studies have shown that there is a certain relationship between serum albumin and ADL.40 A 12-year cohort study of the elderly in Japan found that a decline in activities of daily living was significantly associated with lower serum albumin levels.41 Serum albumin is a common index to evaluate the nutritional status of patients with chronic kidney disease.42 Hypoproteinemia is more common in patients with CKD, which may be due to the impaired gastrointestinal function of CKD patients, resulting in decreased appetite and food intake. Low albumin levels have been associated with poor prognosis in a variety of diseases, including coronary heart disease,43 cancer,44 cerebrovascular diseases,45 CKD and so on. A study on patients with CKD stage 3–4 found that patients with low serum albumin levels had a higher incidence of adverse endpoint events and a worse prognosis.46 Therefore, active nutritional intervention is very necessary for CKD patients to improve their malnutrition and poor prognosis.
Our study found that the risk of adverse events during hospitalization was significantly higher in the low BI group. A recent review of studies indicates that patients with low BI scores in CKD and dialysis populations have a significantly higher risk of death than those with low BI scores.47 BI has an important impact on the prognosis and mortality of patients, which has been verified in postoperative patients and patients with acute heart failure.11,13 A Danish cohort study based on the geriatric database explored the relationship between BI and mortality in elderly patients. The results showed that BI was negatively correlated with the mortality of patients, and the higher the BI score, the lower the mortality of patients.48 However, our study did not further follow up with the patients. Follow-up information should be further increased in later studies.
A recent cross-sectional study in Japan examined the relationship between serum bilirubin levels and ADL in elderly diabetics, and found that serum bilirubin is an important predictor of daily activity disorders in elderly diabetics.49 Some studies have shown that serum bilirubin plays a certain role in predicting the progression of CKD in patients with diabetes.50,51 Liu et al found in the study of non-dialysis patients with chronic kidney disease that serum bilirubin was a protective factor for the prognosis of CKD patients, and patients with high serum bilirubin levels had a better prognosis of kidney.52 We also included this indicator in our study, but there was no significant statistical difference between the two groups in our study. The possible reason is that the patients in our study were older and had more chronic diseases, and the influence of liver function and drugs has not been ruled out.
Several machine-learning algorithms have been used for ADL classification and prediction.53 A study used machine-learning methods, namely logistic regression, support vector machine, and random forest, to predict the state of BI in post-stroke patients.54 Similarly, our study used decision tree, naïve Bayes, logistic regression to predict patients’ ADL. The result demonstrated that naïve Bayes model surpassed that of the other two models in overall performance, although the decision tree model has higher accuracy and sensitivity. Naïve Bayes model has a high potential for predicting ADL impairment.
CKD patients are more prone to functional decline. A prospective study of 1000 elderly people in the community showed that patients with CKD had a threefold decrease in ADL compared with patients without CKD.55 The specific mechanism of CKD leading to the decline of daily living ability is still unclear. There are some possible mechanisms to explain the relationship between the two. First, CKD patients are prone to multiple systemic complications, such as volume and acid–base balance disorders, anemia,56 dyslipidemia, and increased cardiovascular risk, which may lead to fatigue, bone pain, walking difficulties, weakness, and decreased exercise endurance. These complications, by themselves or as part of multimorbidity, complicated the physical status in patients with CKD and may explain the risk of functional decline. Second, CKD patients may also have abnormal metabolism of calcium and phosphorus, parathyroid hormone, vitamin D and other minerals, which may cause CKD-related mineral bone disease (CKD-MBD), promote vascular calcification and osteoporosis, and increase the risk of cardiovascular disease and fracture,57 and thus affect the quality of life and daily activities of patients. Lastly, patients with CKD are often in a state of chronic catabolism, which may lead to muscle atrophy and decreased exercise endurance. In addition, low protein intake, metabolic acidosis, lack of exercise, oxidative stress and inflammation often occur in CKD patients, which affect muscle quality and increase the incidence of sarcopenia in this population, and may also be a factor leading to the decline of ADL.58 Although the association between CKD and functional decline has been widely studied, more studies are needed in the future to further explore the biological mechanism of functional decline in patients with CKD.
There are some limitations to this study that should be taken into consideration. First, this was a single-center study in tertiary care hospital. The potential sources of bias may be due to the monocentric design and the ethnic homogeneity of the sample. Second, follow-up should be added in the later period to clarify the prognosis of the patients. In addition, polypharmacy, cognitive impairment, social factors, physical activity are not included in our study, and these may also be related to patients’ ADL decline.
Conclusion
In conclusion, the present study shows that age, CCI and serum albumin were independently correlated with ADL impairment in CKD patients. Further studies are needed to better understand the relevant biological mechanisms and identify interventions to limit the decline of ADL in CKD patients.
Acknowledgments
We thank the patients and staff who participated in this study. We also thank Chengxiang Du and his team members, Xiao Sun, Qiang Wang, Xingqi Li, Zheyuan Wang, Tianai Mi, of the Hessian Health Statistical Group for their kind assistance in statistical analysis.
Abbreviations
ADL, activities of daily living; CKD, chronic kidney disease; BI, Barthel index; CGA, comprehensive geriatric assessment; BADL, basic activities of daily living; IADL, instrumental activities of daily living; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; BMI, body mass index; HR, heart rate; LVEF, left ventricular ejection fraction; CCI, Charlson comorbidity index; BNP, B-type natriuretic peptide; SD, standard deviation; OR, odds ratio; AUC, area under the curve; HbA1c, Hemoglobin A1c; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; AIC, Akaike information criterion; PPV, positive predictive value; NPV, negative predictive value; CKD-MBD, CKD-related mineral bone disease.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics and Consent Statement
This study was approved by the Medical Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University and informed written consent was obtained from participants prior to data collection.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Chhetri JK, Chan P, Ma L, et al. Prevention of disability in the frail chinese older population. J Frailty Aging. 2019;8(1):2–6. [DOI] [PubMed] [Google Scholar]
- 2.Bikbov B, Purcell CA, Levey AS. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon AC, Bampouras TM, Pendleton N, Mitra S, Dhaygude AP. Diagnostic accuracy of frailty screening methods in advanced chronic kidney disease. Nephron. 2019;141(3):147–155. doi: 10.1159/000494223 [DOI] [PubMed] [Google Scholar]
- 4.Shen Z, Ruan Q, Yu Z, Sun Z. Chronic kidney disease-related physical frailty and cognitive impairment: a systemic review. Geriatr Gerontol Int. 2017;17(4):529–544. doi: 10.1111/ggi.12758 [DOI] [PubMed] [Google Scholar]
- 5.Walker SR, Wagner M, Tangri N. Chronic kidney disease, frailty, and unsuccessful aging: a review. J Ren Nutr. 2014;24(6):364–370. doi: 10.1053/j.jrn.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Nixon AC, Bampouras TM, Pendleton N, Woywodt A, Mitra S, Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11(2):236–245. doi: 10.1093/ckj/sfx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieber CC. Frailty – from concept to clinical practice. Exp Gerontol. 2017;87(Pt B):160–167. doi: 10.1016/j.exger.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Gu D. Predictability of frailty index and its components on mortality in older adults in China. BMC Geriatr. 2016;16:145. doi: 10.1186/s12877-016-0317-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto NA, van Loon IN, Morpey MI, et al. Geriatric assessment in elderly patients with end-stage kidney disease. Nephron. 2019;141(1):41–48. doi: 10.1159/000494222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mlinac ME, Feng MC. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol. 2016;31(6):506–516. doi: 10.1093/arclin/acw049 [DOI] [PubMed] [Google Scholar]
- 11.Uemura Y, Shibata R, Takemoto K, et al. Prognostic impact of the preservation of activities of daily living on post-discharge outcomes in patients with acute heart failure. Circ J. 2018;82(11):2793–2799. doi: 10.1253/circj.CJ-18-0279 [DOI] [PubMed] [Google Scholar]
- 12.Xin X, Chang J, Gao Y, Shi Y. Correlation between the revised brain symmetry index, an EEG feature index, and short-term prognosis in acute ischemic stroke. J Clin Neurophysiol. 2017;34(2):162–167. doi: 10.1097/WNP.0000000000000341 [DOI] [PubMed] [Google Scholar]
- 13.Mii S, Guntani A, Kawakubo E, Shimazoe H. Barthel index and outcome of open bypass for critical limb ischemia. Circ J. 2017;82(1):251–257. doi: 10.1253/circj.CJ-17-0247 [DOI] [PubMed] [Google Scholar]
- 14.Kwon S, Hartzema AG, Duncan PW, Min-Lai S. Disability measures in stroke: relationship among the barthel index, the functional independence measure, and the modified rankin scale. Stroke. 2004;35(4):918–923. doi: 10.1161/01.STR.0000119385.56094.32 [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh NT, Schiller B, Saxena AB, Thomas IC, Kurella Tamura M. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodial Int. 2015;19(4):593–600. doi: 10.1111/hdi.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossola M, Di Stasio E, Antocicco M, et al. Functional impairment is associated with an increased risk of mortality in patients on chronic hemodialysis. BMC Nephrol. 2016;17(1):72. doi: 10.1186/s12882-016-0302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 20.Suarez-Dono J, Cervantes-Perez E, Pena-Seijo M, et al. CRONIGAL: prognostic index for chronic patients after hospital admission. Eur J Intern Med. 2016;36:25–31. doi: 10.1016/j.ejim.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki R, Kikutani T, Yoshida M, Yamashita Y, Hirayama Y. Prognosis-related factors concerning oral and general conditions for homebound older adults in Japan. Geriatr Gerontol Int. 2015;15(8):1001–1006. doi: 10.1111/ggi.12382 [DOI] [PubMed] [Google Scholar]
- 22.Han J, Kamber M, Pei J. Data Mining: Concepts and Techniques. 3rd ed. Amsterdam: Elsevier; 2011. [Google Scholar]
- 23.Bello AK, Levin A, Manns BJ, et al. Effective CKD care in European countries: challenges and opportunities for health policy. Am J Kidney Dis. 2015;65(1):15–25. doi: 10.1053/j.ajkd.2014.07.033 [DOI] [PubMed] [Google Scholar]
- 24.De Nicola L, Provenzano M, Chiodini P, et al. Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: multicenter prospective study in Italy. Nutr Metab Cardiovasc Dis. 2015;25(8):756–762. doi: 10.1016/j.numecd.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 25.De Nicola L, Provenzano M, Chiodini P, et al. Epidemiology of low-proteinuric chronic kidney disease in renal clinics. PLoS One. 2017;12(2):e0172241. doi: 10.1371/journal.pone.0172241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provenzano M, Chiodini P, Minutolo R, et al. Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: multicentre prospective study in nephrology clinics. Nephrol Dial Transplant. 2020;35(1):138–147. doi: 10.1093/ndt/gfy217 [DOI] [PubMed] [Google Scholar]
- 27.Doyle EM, Sloan JM, Goodbrand JA, et al. Association between kidney function, rehabilitation outcome, and survival in older patients discharged from inpatient rehabilitation. Am J Kidney Dis. 2015;66(5):768–774. doi: 10.1053/j.ajkd.2015.04.041 [DOI] [PubMed] [Google Scholar]
- 28.Ozturk GZ, Toprak D. The relationship between glomerular filtration rate, nutrition and activities of daily living in patients with chronic kidney disease receiving homecare. Prog Nutr. 2019;21(1):135–140. [Google Scholar]
- 29.Murcia J, Llorens P, Sanchez-Paya J, et al. Functional status determined by Barthel Index predicts community acquired pneumonia mortality in general population. J Infect. 2010;61(6):458–464. doi: 10.1016/j.jinf.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 30.Koyama A, Sugimoto M, Niimi K, Banno H, Komori K. Association between preoperative frailty and mortality in patients with critical limb ischemia following infrainguinal bypass surgery-usefulness of the Barthel Index. Circ J. 2017;82(1):267–274. doi: 10.1253/circj.CJ-17-0369 [DOI] [PubMed] [Google Scholar]
- 31.Qian JH, Wu K, Luo HQ, Cao PY, Ren XH. Prevalence of loss of activities of daily living and influencing factors in elderly population in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(9):1272–1276. doi: 10.3760/cma.j.issn.0254-6450.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 32.van der Vorst A, Zijlstra GA, Witte N, et al. Limitations in activities of daily living in community-dwelling people aged 75 and over: a systematic literature review of risk and protective factors. PLoS One. 2016;11(10):e0165127. doi: 10.1371/journal.pone.0165127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonner A, Wellard S, Caltabiano M. The impact of fatigue on daily activity in people with chronic kidney disease. J Clin Nurs. 2010;19(21–22):3006–3015. doi: 10.1111/j.1365-2702.2010.03381.x [DOI] [PubMed] [Google Scholar]
- 34.Falsetti L, Viticchi G, Tarquinio N, et al. Charlson comorbidity index as a predictor of in-hospital death in acute ischemic stroke among very old patients: a single-cohort perspective study. Neurol Sci. 2016;37(9):1443–1448. doi: 10.1007/s10072-016-2602-1 [DOI] [PubMed] [Google Scholar]
- 35.Talib S, Sharif F, Manzoor S, Yaqub S, Kashif W. Charlson comorbidity index for prediction of outcome of acute kidney injury in critically Ill patients. Iran J Kidney Dis. 2017;11(2):115–123. [PubMed] [Google Scholar]
- 36.Formiga F, Ferrer A, Perez-Castejon JM, Olmedo C, Pujol R. Risk factors for functional decline in nonagenarians: a one-year follow-up. The NonaSantfeliu study. Gerontology. 2007;53(4):211–217. doi: 10.1159/000100780 [DOI] [PubMed] [Google Scholar]
- 37.Abizanda Soler P, Paterna Mellinas G, Martinez Sanchez E, Lopez Jimenez E. Comorbidity in the elderly: utility and validity of assessment tools. Rev Esp Geriatr Gerontol. 2010;45(4):219–228. doi: 10.1016/j.regg.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 38.Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6(11):e26951. doi: 10.1371/journal.pone.0026951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo S, Kitamura A, Imano H, et al. Serum albumin and high-sensitivity c-reactive protein are independent risk factors of chronic kidney disease in middle-aged japanese individuals: the circulatory risk in communities study. J Atheroscler Thromb. 2016;23(9):1089–1098. doi: 10.5551/jat.33530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schalk BW, Visser M, Penninx BW, Baadenhuijsen H, Bouter LM, Deeg DJ. Change in serum albumin and subsequent decline in functional status in older persons. Aging Clin Exp Res. 2005;17(4):297–305. doi: 10.1007/BF03324614 [DOI] [PubMed] [Google Scholar]
- 41.Okamura T, Hayakawa T, Hozawa A, et al. Lower levels of serum albumin and total cholesterol associated with decline in activities of daily living and excess mortality in a 12-year cohort study of elderly Japanese. J Am Geriatr Soc. 2008;56(3):529–535. doi: 10.1111/j.1532-5415.2007.01549.x [DOI] [PubMed] [Google Scholar]
- 42.Gama-Axelsson T, Heimburger O, Stenvinkel P, Barany P, Lindholm B, Qureshi AR. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol. 2012;7(9):1446–1453. doi: 10.2215/CJN.10251011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chien SC, Chen CY, Leu HB, et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. 2017;241:1–5. doi: 10.1016/j.ijcard.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Ouyang X, Dang Y, Zhang F, Huang Q. Low serum albumin correlates with poor survival in gastric cancer patients. Clin Lab. 2018;64(3):239–245. doi: 10.7754/Clin.Lab.2017.170804 [DOI] [PubMed] [Google Scholar]
- 45.Limaye K, Yang JD, Hinduja A. Role of admission serum albumin levels in patients with intracerebral hemorrhage. Acta Neurol Belg. 2016;116(1):27–30. doi: 10.1007/s13760-015-0504-2 [DOI] [PubMed] [Google Scholar]
- 46.Fan H, Yang J, Liu L, et al. Effect of serum albumin on the prognosis of elderly patients with stage 3–4 chronic kidney disease. Int Urol Nephrol. 2017;49(5):859–865. doi: 10.1007/s11255-017-1542-x [DOI] [PubMed] [Google Scholar]
- 47.Morishita S, Tsubaki A, Shirai N. Physical function was related to mortality in patients with chronic kidney disease and dialysis. Hemodial Int. 2017;21(4):483–489. doi: 10.1111/hdi.12564 [DOI] [PubMed] [Google Scholar]
- 48.Ryg J, Engberg H, Mariadas P, et al. Barthel index at hospital admission is associated with mortality in geriatric patients: a Danish nationwide population-based cohort study. Clin Epidemiol. 2018;10:1789–1800. doi: 10.2147/CLEP.S176035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoguchi T, Fukuhara S, Yamato M, et al. Serum bilirubin level is a strong predictor for disability in activities in daily living (ADL) in Japanese elderly patients with diabetes. Sci Rep. 2019;9(1):7069. doi: 10.1038/s41598-019-43543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uludag K, Oguzhan N, Arikan T, Boz G. Serum bilirubin level and its impact on the progression of chronic kidney disease. Int Urol Nephrol. 2018;50(9):1695–1701. doi: 10.1007/s11255-018-1923-9 [DOI] [PubMed] [Google Scholar]
- 51.Ahn KH, Kim SS, Kim WJ, et al. Low serum bilirubin level predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus. Korean J Intern Med. 2017;32(5):875–882. doi: 10.3904/kjim.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Li M, Song Y, et al. Association of serum bilirubin with renal outcomes in Han Chinese patients with chronic kidney disease. Clin Chim Acta. 2018;480:9–16. doi: 10.1016/j.cca.2018.01.041 [DOI] [PubMed] [Google Scholar]
- 53.Rahman S, Irfan M, Raza M, Moyeezullah Ghori K, Yaqoob S, Awais M. Performance analysis of boosting classifiers in recognizing activities of daily living. Int J Environ Res Public Health. 2020;17:3. doi: 10.3390/ijerph17031082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin WY, Chen CH, Tseng YJ, et al. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int J Med Inform. 2018;111:159–164. doi: 10.1016/j.ijmedinf.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 55.Bowling CB, Sawyer P, Campbell RC, Ahmed A, Allman RM. Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol a Biol Sci Med Sci. 2011;66(6):689–694. doi: 10.1093/gerona/glr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnelle J, Osterweil D, Globe D, Sciarra A, Audhya P, Barlev A. Chronic kidney disease, anemia, and the association between chronic kidney disease-related anemia and activities of daily living in older nursing home residents. J Am Med Dir Assoc. 2009;10(2):120–126. doi: 10.1016/j.jamda.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 57.Tsuruya K, Eriguchi M, Yamada S, Hirakata H, Kitazono T. Cardiorenal syndrome in end-stage kidney disease. Blood Purif. 2015;40(4):337–343. doi: 10.1159/000441583 [DOI] [PubMed] [Google Scholar]
- 58.Lai S, Muscaritoli M, Andreozzi P, et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition. 2019;62:108–114. doi: 10.1016/j.nut.2018.12.005 [DOI] [PubMed] [Google Scholar]