Abstract
The article show the date associated with the work previously reported “Design, theoretical and correlation of the electronic and optical properties of diethynylphenylthiophene as photovoltaic materials”, https://doi.org/10.1016/j.molstruc.2019.127093[1]. The authors reported graphics and tables building from of p-PDT, m-PDT, o-PDT, p-ZnPDT, m -ZnPDT and o-ZnPDT calculations as raw date, with the aim of to show electronic and optical properties, which can be analyzed by the reader. In this context, there exists an important number of renewable energies that are substituting the oil and the charcoal be used in the energetic supply. One of these alternatives is the use of solar cells, which can be use in diverse areas like telecommunications, remote systems of monitoring, lighting systems, water treatment systems, and products of consumption. The employment of the organic photovoltaic technology and photosensitized organic materials are based on the use of molecular organic materials for coverings for ceiling and windows of a house that allow the storage of energy. The OPVs and DSSC present π conjugated systems, giving them a high electronic relocated density, which allows catching the radiations with an energy range of wavelengths between 400 and 800 nm. The systems are derived of diethynylphenylthiophene (LMWOM) coupled to phenyldiamine (PD) as spacer, forming hyper conjugated macrocycles (p-PDT, m-PDT, o-PDT, p-ZnPDT, m -ZnPDT and o-ZnPDT). On the other hand, it is reported process electronic relationship with material sensitized and the bibliographic support of the publication topic.
Keywords: Photosensitized materials, Acceptor- donor structure, Solar applications
Specifications table
| Subject | Organic Chemistry |
| Specific subject area | Science Materials |
| Type of data | Tables and Figures |
| How data were acquired | Spectroscopic characterization (UV- Vis) DFT data (Bond distances and angles of optimized molecules) Structural strategies in photosensitized materials with potential applications in solar cells. |
| Data format | Raw |
| Parameters for data collection | The information is obtained from the raw data derived from Gaussian 09 computing program, which can be analyzed by the reader. The authors reported bond lengths for p-PDT, m-PDT and o-PDT and o-ZnPDT and m-ZnPDT. This allows to interpret the specific effect for atom by presence of zinc(II). Also, the directionally of the dipole moment is shown, the donor - acceptor map in relationship to attacks environmental. The dipole moment allows establishing the planarity of the molecules, and comparing them with similar ones. |
| Description of data collection | Angles and dipolar moment associated to lineal Molecule (LMWOM (1), moment dipolar associated to macrocycles with different spacers a). o-PDT, b). m-PDT and c). p-PDT, optimization of lineal molecule coordinated Lewis acid (angles, structure molecular, HOMO- LUMO description) and Donor- Acceptor capacity for macrocycles studied in relationship with Reactive Species capacity, which can degrade in outdoors conditions, also reported. The readers can calculate the GAP according to the acceptor capacity and if they wish to apply these materials in the photovoltaic cell industry, they can estimate their corrosion or damage by agents such as hydroxyl radicals. Also, the authors show a revision of molecules associated with applications in solar cells, reporting data which the reader can compare the optical and electronic properties, with final results in https://doi.org/10.1016/j.molstruc.2019.127093. Finally, a new synthesized molecule is proposed, for which the data have not been analyzed and is a striking molecule for readers. |
| Data source location | Institution: Universidad Santiago de Cali |
| Data accessibility | The data are found only in this article M. Suarez, C. Caicedo, J. Morales, E. Florez- López, Y. Ávila- Torres, Design, theoretical study and correlation of the electronic and optical properties of diethynylphenylthiophene as photovoltaic materials. Journal of Molecular Structure, 2020, 127093 [1]. |
| Related research article | M. Suarez, C. Caicedo, J. Morales, Flórez- López E, Ávila- Torres Y. Design, theoretical study and correlation of the electronic and optical properties of diethynylphenythiophene as photovoltaic materials, Journal of Molecular Structure 2020, 127093. https://doi.org/10.1016/j.molstruc.2019.127093 |
Value of the Data
-
•
These data are important because the distances and complete angles are reported, which have not been treated in relation to a new molecule derived from diethynylphenylthiophene. Likewise, the authors proposed other molecule derived with benzothiphene (BT), which could have best photovoltaic properties.
-
•
The authors reported theoretical data for precursor molecules of macrocycles, the reader can stablish isomeric effects on the photovoltaic properties and improve the design of new molecules in the field.
-
•
The readers can perform new theoretical calculations matching the macrocycles from diethynylphenyltiophene and benzothiphene (BT) considering o- m and p- phenyldiamine as spacer.
-
•
These molecules can be used as new biomimetic materials to biological macrocycles as porphyrin. This macrocycle allows electronic transport using the metallic ion: iron. The readers can compare the electronic properties with other transition metal in configuration d10, such as: zinc(II).
1. Data
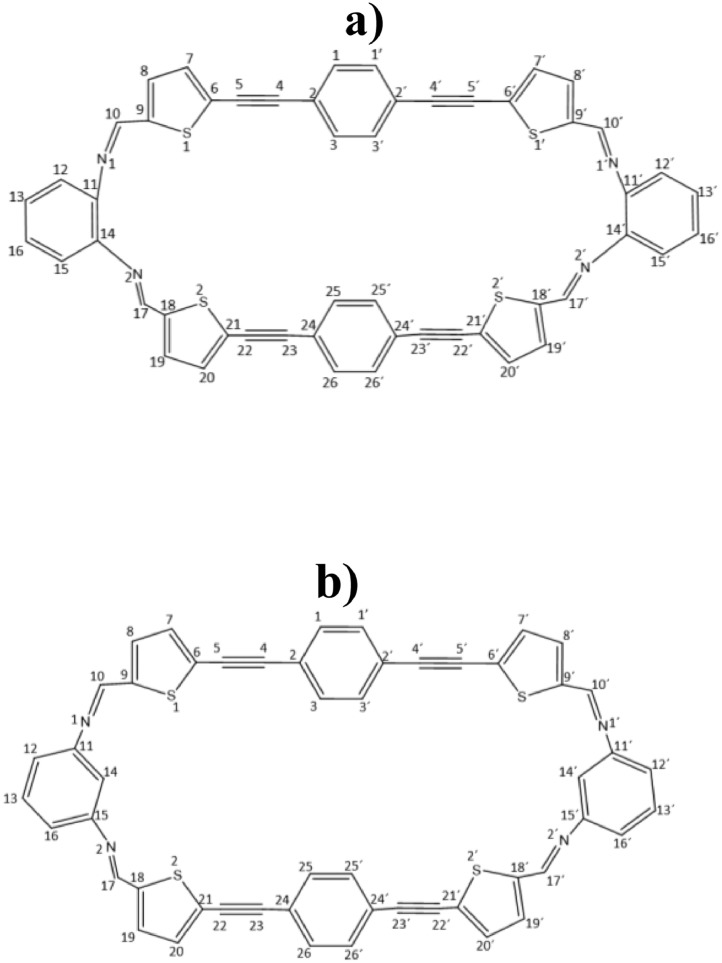
The distances and angles associated to the structure were calculated with the minimum energy in each case, for each optimized spacer and its respective macrocycle, Fig. 1 and 2, Table 1 and 2. The effect of Lewis acid is observed in the Table 3, in where were reported angles and distances associated to molecule optimized with these conditions. In the Fig. 3 is described the electronic process in a sensitized material, by means of which electronic transport occurs in this type of molecules. In the Fig. 4, the vector relationship with dipolar moment is showed for LMWOM (1) and macrocycles, which facilities la visibility on a plane specific, Fig. 5 and 6. The Lewis effect for lineal molecule is observed in the Fig. 7, stabilizing angles, structure molecular and HOMO- LUMO orbitals and its donor and acceptor capacity in sensitized molecules under typical environmental conditions. In the Table 4, the authors show the graphical comparison between molecule reported previously and new molecule synthesized in relation to electronic excitations, with the aim the readers can analyse of date and establish structural correlations. Likewise, in the Fig. 8, the IR spectrum of Synthetized molecule as potential photovoltaic materials derivate of diethynylphenylthiophene and Fig. 9, the mass spectrum m/z for the new molecule derivated of diethynylphenylthiophene, which has been proposed. Finally, in the Table 5 and 6 is reported the evolution in photosensitised materials with similar structural to the molecules synthesized, and the readers realize structural comparisons for to obtain best photovoltaic parameters.
Fig. 6.
Optimization of lineal molecule coordinated Lewis acid, a). Angles, b). Structure molecular, c). HOMO- LUMO description
Fig. 1.
Chemical structure for a). p-Phenylenediamine (p- PD), b). m-Phenylenediamine (m- PD) and c). o-Phenylenediamine (o- PD)
Fig. 2.
Chemical structure for a) o-PDT and b). m-PDT
Table 1.
Bond lengths for p- PD, m- PD and o- PD.
| BOND | p-PD | BOND | m-PD | BOND | o-PD |
|---|---|---|---|---|---|
| N1-C1 | 1.40995 | N1-C1 | 1.40098 | N1-C1 | 1.41077 |
| C1-C2 | 1.40212 | C1-C2 | 1.40516 | C1-C2 | 1.39621 |
| C2-C3 | 1.39240 | C2-C3 | 1.39239 | C2-C3 | 1.39703 |
| C3-C4 | 1.40212 | C3-C4 | 1.39246 | C3-C4 | 1.39289 |
| C4-N2 | 1.40997 | C4-C5 | 1.40510 | C4-C5 | 1.39708 |
| C4-C5 | 1.40211 | C5-N2 | 1.40084 | C5-C6 | 1.39615 |
| C5-C6 | 1.39242 | C5-C6 | 1.40151 | C6-N2 | 1.41086 |
| C6-C1 | 1.40210 | C6-C1 | 1.40141 | C6-C1 | 1.41443 |
| ANGLE | p-PD | ANGLE | m-PD | ANGLE | o-PD |
| N1-C1-C6 | 121.09033 | N1-C1-C6 | 120.46212 | N1-C1-C6 | 117.73855 |
| C2-C1-N1 | 121.08921 | C2-C1-N1 | 120.18014 | C2-C1-N1 | 117.74929 |
| C5-C4-N2 | 121.08974 | C5-C6-N2 | 120.17260 | C5-C6-N2 | 122.91470 |
| C3-C4-N2 | 121.08967 | C5-C4-N2 | 120.47366 | C1-C6-N2 | 122.90703 |
Table 2.
Bond lengths for o-PDT and m-PDT
| BOND | o-PDT | BOND | o-PDT |
|---|---|---|---|
| C1-C2 | 1.41050 | C1′-C2′ | 1.40955 |
| C2-C3 | 1.41198 | C2′-C3′ | 1.41360 |
| C2-C4 | 1.42053 | C2′-C4′ | 1.42036 |
| C4-C5 | 1.21882 | C4′-C5′ | 1.21970 |
| C5-C6 | 1.40409 | C5′-C6′ | 1.40432 |
| C6-C7 | 1.38497 | C6′-C7′ | 1.38814 |
| C6-S1 | 1.75934 | C6′-S1′ | 1.75211 |
| C7-C8 | 1.41023 | C7′-C8′ | 1.41382 |
| C8-C9 | 1.38572 | C8′-C9′ | 1.38233 |
| S1-C9 | 1.74889 | S1′-C9′ | 1.74700 |
| C9-C10 | 1.46061 | C9′-C10′ | 1.45101 |
| C10-N1 | 1.27931 | C10′-N1′ | 1.28328 |
| N1-C11 | 1.39735 | N1′-C11′ | 1.40788 |
| C11-C12 | 1.40467 | C11′-C12′ | 1.40203 |
| C12-C13 | 1.39364 | C12′-C13′ | 1.39185 |
| C13-C16 | 1.39601 | C13′-C16′ | 1.39680 |
| C11-C14 | 1.42227 | C11′-C14′ | 1.42244 |
| C14-C15 | 1.40460 | C14′-C15′ | 1.40797 |
| C15-C16 | 1.39382 | C15′-C16′ | 1.39170 |
| C14-N2 | 1.39592 | C14′-N2′ | 1.40368 |
| N2-C17 | 1.27960 | N2′-C17′ | 1.27947 |
| C17-C18 | 1.46174 | C17′-C18′ | 1.45903 |
| C18-S2 | 1.75088 | C18′-S2′ | 1.75355 |
| C18-C19 | 1.38645 | C18′-C19′ | 1.38542 |
| C19-C20 | 1.40827 | C19′-C20′ | 1.40816 |
| C20-C21 | 1.38631 | C20′-C21′ | 1.38710 |
| S2-C21 | 1.75885 | S2′-C21′ | 1.75909 |
| C21-C22 | 1.40339 | C21′-C22′ | 1.40363 |
| C22-C23 | 1.21935 | C22′-C23′ | 1.21964 |
| C23-C24 | 1.41957 | C23′-C24′ | 1.42004 |
| C24-C25 | 1.41173 | C24′-C25′ | 1.41177 |
| C24-C26 | 1.41240 | C24′-C26′ | 1.41209 |
| ANGLE | o-PDT | ANGLE | o-PDT |
| C6-S1-C9 | 91.64282 | C6′-S1′-C9′ | 91.83895 |
| C4-C5-C6 | 176.76564 | C4′-C5′-C6′ | 170.61957 |
| C9-C10-N1 | 133.41464 | C9′-C10′-N1′ | 123.84971 |
| C10-N1-C11 | 126.44810 | C10′-N1′-C11′ | 117.15231 |
| C14-N2-C17 | 126.71523 | C14′-N2′-C17′ | 125.81983 |
| N2-C17-C18 | 134.24868 | N2′-C17′-C18′ | 132.84811 |
| C18-S2-C21 | 91.68627 | C18′-S2′-C21′ | 91.56327 |
| C22-C23-C24 | 177.23143 | C22′-C23′-C24′ | 173.33124 |
| BOND | m-PDT | BOND | m-PDT |
| C1-C2 | 1.41037 | C1′-C2′ | 1.40985 |
| C2-C3 | 1.41164 | C2′-C3′ | 1.41149 |
| C2-C4 | 1.42061 | C2′-C4′ | 1.42256 |
| C4-C5 | 1.21877 | C4′-C5′ | 1.21864 |
| C5-C6 | 1.40402 | C5′-C6′ | 1.40650 |
| C6-C7 | 1.38601 | C6′-C7′ | 1.38533 |
| C6-S1 | 1.75682 | C6′-S1′ | 1.75705 |
| C7-C8 | 1.40863 | C7′-C8′ | 1.41379 |
| C8-C9 | 1.38577 | C8′-C9′ | 1.38230 |
| S1-C9 | 1.74922 | S1′-C9′ | 1.74531 |
| C9-C10 | 1.46010 | C9′-C10′ | 1.44629 |
| C10-N1 | 1.27938 | C10′-N1′ | 1.28368 |
| N1-C11 | 1.40631 | N1′-C11′ | 1.40182 |
| C11-C12 | 1.40780 | C11′-C12′ | 1.40437 |
| C12-C13 | 1.39011 | C12′-C13′ | 1.39162 |
| C13-C16 | 1.39457 | C13′-C16′ | 1.39392 |
| C11-C14 | 1.40066 | C11′-C14′ | 1.40561 |
| C14-C15 | 1.40539 | C14′-C15′ | 1.40261 |
| C15-C16 | 1.40518 | C15′-C16′ | 1.40446 |
| C15-N2 | 1.40487 | C15′-N2′ | 1.40927 |
| N2-C17 | 1.28431 | N2′-C17′ | 1.28055 |
| C17-C18 | 1.44484 | C17′-C18′ | 1.45943 |
| C18-S2 | 1.74615 | C18′-S2′ | 1.74993 |
| C18-C19 | 1.38320 | C18′-C19′ | 1.38594 |
| C19-C20 | 1.41030 | C19′-C20′ | 1.40873 |
| C20-C21 | 1.38751 | C20′-C21′ | 1.38691 |
| S2-C21 | 1.75824 | S2′-C21′ | 1.75567 |
| C21-C22 | 1.40311 | C21′-C22′ | 1.40336 |
| C22-C23 | 1.21938 | C22′-C23′ | 1.21918 |
| C23-C24 | 1.41902 | C23′-C24′ | 1.41914 |
| C24-C25 | 1.41191 | C24′-C25′ | 1.41218 |
| C24-C26 | 1.41202 | C24′-C26′ | 1.41146 |
| ANGLE | m-PDT | ANGLE | m-PDT |
| C6-S1-C9 | 91.58602 | C6′-S1′-C9′ | 91.25115 |
| C4-C5-C6 | 176.60894 | C4′-C5′-C6′ | 174.54395 |
| C9-C10-N1 | 133.49609 | C9′-C10′-N1′ | 120.67546 |
| C10-N1-C11 | 125.00617 | C10′-N1′-C11′ | 121.79354 |
| C15-N2-C17 | 119.65096 | C15′-N2′-C17′ | 122.90429 |
| N2-C17-C18 | 122.41205 | N2′-C17′-C18′ | 132.54236 |
| C18-S2-C21 | 91.21184 | C18′-S2′-C21′ | 91.60045 |
| C22-C23-C24 | 176.53157 | C22′-C23′-C24′ | 176.52971 |
Table 3.
Bond lengths for o-ZnPDT and m-ZnPDT
| BOND | o-ZnPDT | BOND | o-ZnPDT |
|---|---|---|---|
| C1-C2 | 1.42186 | C1′-C2′ | 1.42085 |
| C2-C3 | 1.42200 | C2′-C3′ | 1.42203 |
| C2-C4 | 1.39809 | C2′-C4′ | 1.40421 |
| C4-C5 | 1.23096 | C4′-C5′ | 1.22642 |
| C5-C6 | 1.37774 | C5′-C6′ | 1.38966 |
| C6-C7 | 1.41401 | C6′-C7′ | 1.39793 |
| C6-S1 | 1.76622 | C6′-S1′ | 1.76099 |
| C7-C8 | 1.38077 | C7′-C8′ | 1.39901 |
| C8-C9 | 1.41980 | C8′-C9′ | 1.39235 |
| S1-C9 | 1.77302 | S1′-C9′ | 1.74363 |
| C9-C10 | 1.39719 | C9′-C10′ | 1.45137 |
| C10-N1 | 1.34876 | C10′-N1′ | 1.28973 |
| N1-C11 | 1.41129 | N1′-C11′ | 1.38923 |
| N1-Zn | 1.94290 | —— | —– |
| C11-C12 | 1.40441 | C11′-C12′ | 1.41240 |
| C12-C13 | 1.38909 | C12′-C13′ | 1.38661 |
| C13-C16 | 1.39950 | C13′-C16′ | 1.39841 |
| C11-C14 | 1.42490 | C11′-C14′ | 1.43019 |
| C14-C15 | 1.40415 | C14′-C15′ | 1.40692 |
| C15-C16 | 1.38940 | C15′-C16′ | 1.39140 |
| C14-N2 | 1.42133 | C14′-N2′ | 1.39140 |
| N2-C17 | 1.32037 | N2′-C17′ | 1.28116 |
| N2-Zn | 1.94290 | ——- | ——- |
| Zn-O1 | 2.04892 | ——- | ——- |
| Zn-O2 | 2.01692 | ——- | ——- |
| C17-C18 | 1.40998 | C17′-C18′ | 1.46033 |
| C18-S2 | 1.76353 | C18′-S2′ | 1.74750 |
| C18-C19 | 1.39872 | C18′-C19′ | 1.38738 |
| C19-C20 | 1.39833 | C19′-C20′ | 1.40658 |
| C20-C21 | 1.39748 | C20′-C21′ | 1.38911 |
| S2-C21 | 1.77176 | S2′-C21′ | 1.75841 |
| C21-C22 | 1.38698 | C21′-C22′ | 1.40041 |
| C22-C23 | 1.22618 | C22′-C23′ | 1.22148 |
| C23-C24 | 1.40883 | C23′-C24′ | 1.41390 |
| C24-C25 | 1.41651 | C24′-C25′ | 1.41519 |
| C24-C26 | 1.41648 | C24′-C26′ | 1.41707 |
| ANGLE | o-ZnPDT | ANGLE | o-ZnPDT |
| C6-S1-C9 | 91.15801 | C6′-S1′-C9′ | 91.21665 |
| C4-C5-C6 | 169.26435 | C4′-C5′-C6′ | 174.68969 |
| C9-C10-N1 | 131.28692 | C9′-C10′-N1′ | 131.44237 |
| C10-N1-C11 | 123.47215 | C10′-N1′-C11′ | 124.63288 |
| N1-Zn-N2 | 89.22684 | ——- | ——- |
| C11-N1-Zn | 107.56032 | ——- | ——- |
| C14-N2-Zn | 107.03021 | ——- | ——- |
| O1-Zn-O2 | 98.13725 | ——- | ——- |
| C14-N2-C17 | 122.32526 | C14′-N2′-C17′ | 126.49626 |
| N2-C17-C18 | 122.32526 | N2′-C17′-C18′ | 117.88437 |
| C18-S2-C21 | 92.11041 | C18′-S2′-C21′ | 91.43543 |
| C22-C23-C24 | 170.30390 | C22′-C23′-C24′ | 174.24680 |
| BOND | m-ZnPDT | BOND | m-ZnPDT |
| C1-C2 | 1.41236 | C1′-C2′ | 1.41337 |
| C2-C3 | 1.41255 | C2′-C3′ | 1.41224 |
| C2-C4 | 1.42046 | C2′-C4′ | 1.41906 |
| C4-C5 | 1.22047 | C4′-C5′ | 1.22193 |
| C5-C6 | 1.39833 | C5′-C6′ | 1.39501 |
| C6-C7 | 1.39135 | C6′-C7′ | 1.37504 |
| C6-S1 | 1.76394 | C6′-S1′ | 1.80502 |
| C7-C8 | 1.40218 | C7′-C8′ | 1.42303 |
| C8-C9 | 1.39395 | C8′-C9′ | 1.37409 |
| S1-C9 | 1.75922 | S1′-C9′ | 1.79440 |
| C9-C10 | 1.41723 | C9′-C10′ | 1.46857 |
| C10-N1 | 1.30903 | C10′-N1′ | 1.27878 |
| N1-C11 | 1.44019 | N1′-C11′ | 1.37785 |
| C11-C12 | 1.39640 | C11′-C12′ | 1.41038 |
| C12-C13 | 1.40451 | C12′-C13′ | 1.38970 |
| C13-C16 | 1.39251 | C13′-C16′ | 1.40912 |
| C11-C14 | 1.40642 | C11′-C14′ | 1.41427 |
| C14-C15 | 1.40185 | C14′-C15′ | 1.42512 |
| C15-C16 | 1.41026 | C15′-C16′ | 1.38499 |
| C15-N2 | 1.38870 | C15′-N2′ | 1.43247 |
| N2-C17 | 1.27802 | N2′-C17′ | 1.31627 |
| C17-C18 | 1.48828 | C17′-C18′ | 1.40972 |
| C18-S2 | 1.78533 | C18′-S2′ | 1.75903 |
| C18-C19 | 1.37287 | C18′-C19′ | 1.39911 |
| C19-C20 | 1.42486 | C19′-C20′ | 1.39690 |
| C20-C21 | 1.37495 | C20′-C21′ | 1.39669 |
| S2-C21 | 1.78017 | S2′-C21′ | 1.75919 |
| C21-C22 | 1.40513 | C21′-C22′ | 1.39778 |
| C22-C23 | 1.22401 | C22′-C23′ | 1.22084 |
| C23-C24 | 1.42425 | C23′-C24′ | 1.41973 |
| C24-C25 | 1.41129 | C24′-C25′ | 1.41229 |
| C24-C26 | 1.41258 | C24′-C26′ | 1.41232 |
| N1-Zn1 | 1.98962 | S1′-Zn2 | 2.40675 |
| Zn1-O1 | 2.01367 | Zn2-O3 | 2.00445 |
| Zn1-O2 | 2.01418 | Zn2-N2′ | 1.99121 |
| ANGLE | m-ZnPDT | ANGLE | m-ZnPDT |
| C6-S1-C9 | 91.50628 | C6′-S1′-C9′ | 92.36433 |
| C4-C5-C6 | 178.39556 | C4′-C5′-C6′ | 176.19523 |
| C9-C10-N1 | 127.20977 | C9′-C10′-N1′ | 133.11961 |
| C10-N1-C11 | 121.37813 | C10′-N1′-C11′ | 125.48684 |
| C15-N2-C17 | 126.38251 | C15′-N2′-C17′ | 122.13497 |
| N2-C17-C18 | 133.58092 | N2′-C17′-C18′ | 126.67908 |
| C18-S2-C21 | 92.97866 | C18′-S2′-C21′ | 91.15672 |
| C22-C23-C24 | 174.32403 | C22′-C23′-C24′ | 176.68674 |
| C10-N1-Zn1 | 176.68674 | S1′-Zn2-N2′ | 132.89784 |
| C11-N1-Zn1 | 91.95766 | S1′-Zn2-O3 | 110.41066 |
| N1-Zn1-O1 | 121.11266 | N2′-Zn2-O3 | 116.31018 |
| N1-Zn1-O2 | 105.11015 | —— | —– |
Fig. 3.
Scheme of electronic traffic through a sensitized material
Fig. 4.
Angles and dipolar moment associated to lineal Molecule (LMWOM (1))
Fig. 5.
Moment dipolar associated to macrocycles with different spacers a). o-PDT, b). m-PDT and c). p-PDT.
Fig. 7.
Donor- Acceptor capacity for macrocycles studied in relationship with Oxygen Reactive Species capacity, which these compounds in can degrade in outdoors conditions.
Table 4.
Graphic comparison between the electronic excitations corresponding to the previously published molecule and the new synthesized molecule not analyzed.
 |
Fig. 8.
IR spectrum of Synthetized molecule as potential photovoltaic materials derivate of diethynylphenylthiophene (BT)
Fig. 9.
Mass spectrum m/z for the new molecule derivated of diethynylphenylthiophene (BT)
Table 5.
 |
 |
Table 6.
 |
(–): It is nor mentioned in the article
Mass spectrum m/z for the new molecule derivated of diethynylphenylthiophene
2. Experimental design and methods
The density functional theory (DFT) approximation as implemented in Gaussian 09, was used for all calculations that were carried out using the B3LYP functional and the 6-31g (2d,p) basis set. Full geometry optimization without symmetry constraints were carried out for all the stationary points. Harmonic frequency analysis allowed us to verify the optimized minima. The local minima were identified when the number of imaginary frequencies is equal to zero. Theoretically, the intensity of the band is expressed in terms of the oscillator strengths (f). Stationary points were modeled in the gas phase (vacuum). The analysis of the changes in electron density for a given electronic transition was based on the electron density difference maps (EDDMs) constructed using the GaussSum suite of programs. The Donor- aceptor capacity is relationshiped to TiO2, •OH, •OOH, and PD spectators. The photo-induced excitations of sunlight occur in the donor material. These excitons disseminate the scope of a donor / acceptor interface, where the transfer of electrons to the acceptor takes place. The Fig. 7 allows to reader understad the donate photogenerated electrons to diatomic oxygen to form the superoxide radical anion that can degrades the structure. The scheme of electronic traffic through a sensitized material is builded for understanding the electronic properties between Donor- Acceptor, which will allow stablish the capacity of the molecule in function of the HOMO- LUMO levels. Finally, the IR and Mass- spectrum were collected in the Spectrophometric Agilent Cary 630 FTIR with Attenuated Total Reflectance (ATR) and GC- MS Perkin Elmer Clarus 600 T- INTEC.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors of the paper thank to Universidad Santiago de Cali, Grants DGI- 63661.
References
- 1.Suarez M., Caicedo C., Morales J., López Florez-, E. Ávila- Torres, Design Y. theoretical study and correlation of the electronic and optical properties of diethynylphenylthiophene as photovoltaic materials. Journal of Molecular Structure. 2020 doi: 10.1016/j.dib.2020.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh C.P., Lu H.P., Chiu C.L., Lee C.W., Chuang S.H., Mai C.L., Yen W.N., Hsu S.J., Diau E.W.G., Yeh C.Y. Synthesis and characterization of porphyrin sensitizers with various electron-donating substituents for highly efficient dye-sensitized solar cells. Journal of Materials Chemistry. 2010;20(6):1127–1134. [Google Scholar]
- 3.Yella A., Lee H.W., Tsao H.N., Yi C., Chandiran A.K., Nazeeruddin M.K., Diau E.W.G., Yeh C.Y., Zakeeruddin S.M., Grätzel M. Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science. 2011;334(6056):629–634. doi: 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- 4.Yella A., Mai C.L., Zakeeruddin S.M., Chang S.N., Hsieh C.H., Yeh C.Y., Grätzel M. Molecular engineering of push–pull porphyrin dyes for highly efficient dye‐sensitized solar cells: The role of benzene spacers. Angewandte Chemie International Edition. 2014;53(11):2973–2977. doi: 10.1002/anie.201309343. [DOI] [PubMed] [Google Scholar]
- 5.Mathew S., Yella A., Gao P., Humphry-Baker R., Curchod B.F., Ashari-Astani N., Tavernelli I., Rothlisberger U., Nazeeruddin M.K., Grätzel M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nature Chemistry. 2014;6(3):242. doi: 10.1038/nchem.1861. [DOI] [PubMed] [Google Scholar]
- 6.Duanglaor P., Thiampanya P., Sudyoadsuk T., Promarak V., Pulpoka B. Synthesis and photophysical properties of donor–acceptor system based bipyridylporphyrins for dye-sensitized solar cells. Journal of energy chemistry. 2015;24(6):779–785. [Google Scholar]
- 7.Prakash K., Manchanda S., Sudhakar V., Sharma N., Sankar M., Krishnamoorthy K. Facile synthesis of β-functionalized “push-pull” Zn (II) porphyrins for DSSC applications. Dyes and Pigments. 2017;147:56–66. [Google Scholar]
- 8.Martín-Gomis L., Parejo, Álvarez C., Fernández-Lázaro J.C., Sastre-Santos F. Á. Dye sensitized solar cells (DSSCs) based on bulky tert-octylphenoxy-carboxyphenyl substituted phthalocyanine without the presence of co-adsorbents. Inorganica Chimica Acta. 2017;468:327–333. [Google Scholar]
- 9.Hara K., Tachibana Y., Ohga Y., Shinpo A., Suga S., Sayama K., Sugihara H., Arakawa H. Dye-sensitized nanocrystalline TiO2 solar cells based on novel coumarin dyes. Solar Energy materials and Solar cells. 2003;77(1):89–103. [Google Scholar]
- 10.Hara K., Kurashige M., Dan-oh Y., Kasada C., Shinpo A., Suga S., Sayama K., Arakawa H. Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New Journal of Chemistry. 2003;27(5):783–785. [Google Scholar]
- 11.Hagberg D.P., Edvinsson T., Marinado T., Boschloo G., Hagfeldt A., Sun L . A novel organic chromophore for dye-sensitized nanostructured solar cells. Chemical Communications. 2006;21:2245–2247. doi: 10.1039/b603002e. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z.S., Cui Y., Dan-oh Y., Kasada C., Shinpo A., Hara K. Thiophene-functionalized coumarin dye for efficient dye-sensitized solar cells: electron lifetime improved by coadsorption of deoxycholic acid. The Journal of Physical Chemistry C. 2007;111(19):7224–7230. [Google Scholar]
- 13.Ito S., Miura H., Uchida S., Takata M., Sumioka K., Liska P., Comte P., Péchy P., Grätzel M. High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chemical Communications. 2008;41:5194–5196. doi: 10.1039/b809093a. [DOI] [PubMed] [Google Scholar]
- 14.Zeng W., Cao Y., Bai Y., Wang Y., Shi Y., Zhang M., Wang F., Pan C., Wang P. Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chemistry of Materials. 2010;22(5):1915–1925. [Google Scholar]
- 15.Bai Y., Zhang J., Zhou D., Wang Y., Zhang M., Wang P. Engineering organic sensitizers for iodine-free dye-sensitized solar cells: red-shifted current response concomitant with attenuated charge recombination. Journal of the American Chemical Society. 2011;133(30):11442–11445. doi: 10.1021/ja203708k. [DOI] [PubMed] [Google Scholar]