Abstract
Purpose
Topical analgesics are an upcoming treatment option for neuropathic pain. In this observational study, we performed a double-blind placebo-controlled response test (DOBRET) in patients with polyneuropathy to determine the personalized analgesic effect of phenytoin 10% cream.
Patients and Methods
In a double-blind fashion, 12 consecutive adult patients with symmetrical painful polyneuropathy and equal pain intensity of ≥4 on the 11-point numerical rating scale (NRS) applied phenytoin10% cream on one painful area and a placebo cream on the corresponding contralateral area. We defined responders as patients who experienced a pain reduction ≥2 NRS points from baseline and ≥1 NRS point difference in pain reduction in favour of phenytoin 10% cream compared with placebo cream within 30 minutes after application. We also evaluated the percentage of pain reduction and frequency of 30% and 50% pain relief from baseline.
Results
Six patients (50%) were responders. Compared with placebo cream, pain reduction was higher in phenytoin 10% cream-applied areas with mean difference in pain reduction of 1.3 (95% CI: 1.1 to 1.8; p<0.001) on the NRS and mean percentage difference in pain reduction of 22% (95% CI: 13% to 32%; p =0.03). All responders had at least 30% pain reduction, and 4 out of 6 had at least 50% pain reduction in the phenytoin 10% cream applied area. All non-responders had less than 30% pain reduction. No side effects were reported.
Conclusion
A DOBRET is easy to perform, quickly identifies an analgesic effect in responders and could be a useful tool to personalize neuropathic pain treatment with topical formulations.
Keywords: neuropathic pain, treatment, topical administration, analgesics, neuropathy
Introduction
Many patients with polyneuropathy experience neuropathic pain, which has a negative influence on the quality of life, daily functioning, work and sleep, and can induce or worsen depression.1–3 Neuropathic pain is often difficult to treat, because the effectiveness of the present-day oral medication is limited by side effects.4,5 New treatment strategies are needed to improve neuropathic pain management with less side effects.
Topical analgesics are an interesting emerging option, because they are meant to influence only the nerve endings in the epidermis without reaching the bloodstream thus systemic side effects may be avoided.6,7 The topical use of analgesics appears to be especially favourable in patients with localized neuropathic pain in areas not larger than a letter-sized piece of paper.8,9 The latest developed topical analgesic is phenytoin, a broad-acting voltage-gated sodium blocker, with favourable qualities such as not entering the bloodstream and having an onset of action within 30 minutes in open and single-blind placebo-controlled response tests in our center.10,11 Limitations of both the open and single-blind placebo-controlled tests are that patient and physician-related factors, expectations and their inter-relationship could influence the outcome of the tests, especially the placebo effect.12–14
To minimize the placebo effect, we continued by performing a double-blind placebo-controlled response test (DOBRET) to compare the analgesic effect of phenytoin 10% cream to placebo cream and evaluate the usefulness of a DOBRET to identify responders. In this paper, we present our experience with the first 12 patients with painful polyneuropathy treated by this paradigm.
The response test is part of daily clinical practice, to ensure that the patient is prescribed an effective topical analgesic and is not carried out with the aim of systematic data collection and analysis. Therefore, according to Dutch law on the conduction of medical research, and in line with European guidelines, no approval from the ethics committee is needed.15,16 This is also in line with the American Medical Association Council on Ethical and Judicial Affairs.17,18
Patients and Methods
Patients
In this observational study from September 2018 to December 2018 with 12 consecutive adult patients with a symmetrical painful polyneuropathy, we report on the analgesic effect of phenytoin 10% cream compared with placebo cream at the Institute for Neuropathic Pain in the Netherlands. Polyneuropathy was defined as the presence of distal sensory or sensorimotor symptoms as well as at least two of the following signs in the distal lower limbs that indicate large nerve fiber involvement: muscle weakness, hypoesthesia for touch, decreased or absent vibration sense, diminished or absent tendon reflexes. Painful polyneuropathy was defined as polyneuropathy with pain in the distally affected areas with two or more of the following typical neuropathic pain characteristics: burning, painful cold, electric shocks, tingling, pins and needles, and itch.
The pain had to be localized in two anatomically symmetrical areas (eg feet) with baseline pain intensity of at least 4 on the 11-point numerical rating scale (NRS, from 0 no pain to 10 worst pain ever), and between both painful areas, there had to be no more than a 1-point difference in baseline NRS. All patient data were treated with confidentiality.
Double-Blind Placebo-Controlled Response Test
The topical formulations were developed and produced by a pharmacist in accordance with GMP standards. The creams consisted of a cetomacrogol base with or without phenytoin as the active ingredient and were delivered in indistinguishable test tubes. The creams had an identical appearance, consistency and odour. The labels of the test tubes (phenytoin 10% and placebo cream) were blinded and renamed with consecutive numbers and as “A” and “B” by an independent person not involved in the execution of the study. Neither the treating physician nor the patient knew which of the tubes contained phenytoin cream or placebo cream.
As part of good clinical practice, patients were informed about the treatment with phenytoin 10% cream and the use of a placebo cream, including the advantages of double-blind testing, the direct unblinding after 30 minutes, and in case a patient was classified as a responder, receiving directly a prescription for phenytoin 10% cream as continued medication. All patients gave informed consent.
The treating physician gave neutral instructions and let the patient apply one of the creams (~0.5 g) to one pain area of one limb and the other cream to a similar pain area on the contralateral limb. To avoid contamination, cream application in each pain area occurred with a different hand. Patients rated the pain intensity in both areas on the NRS prior to and again 30 minutes after cream application, and any side-effects were noted. After this, unblinding followed by the treating physician who removed the stickers from the tubes. Responders were defined as patients who experienced within 30 minutes ≥2 points pain reduction on the NRS from baseline as well as ≥1 point difference in pain reduction on the NRS between the phenytoin 10% cream and the placebo cream applied area in favour of phenytoin 10% cream. We chose a strict responder definition to avoid treating placebo responders.
Data Analysis
Data are summarized as either the mean (standard deviation) or frequency (proportion) for continuous and categorical data, respectively. The primary outcome was the mean difference between placebo and active treatment in NRS scores 30 minutes after cream application. We used a linear mixed-effects (LME) model with a random intercept per subject and a fixed effect for treatment (ie placebo or treatment), adjusted for baseline NRS score (ie analysis of covariance). P-values were based on the likelihood ratio test; 95% confidence intervals (95% CI) around effect estimates were based on the profile likelihood. LME models were fitted with the R library lme4 (version 1.1–21, Bates D, 2019).19 We also determined the number of patients achieving minimum pain relief (MPR) from baseline of at least 30% (moderate benefit: MPR30) and of at least 50% (considerable benefit: MPR50) measured on the NRS. We considered p-values less than 0.05 as statistically significant.
Results
Table 1 shows patient and baseline and pain characteristics of the 12 included patients, of whom six (50%) classified as responders. Most patients (n=10, 83%) had pain in the feet. Baseline NRS was not significantly different between responders and non-responders. Results of the DOBRET are presented in Tables 2 and 3, and Figure 1. The difference in duration of neuropathic pain between responders and non-responders can be explained by three patients in the responder group, who had neuropathic pain for more than 15 years. Without these three patients, average duration of neuropathic pain in the responder group was 3.7 years (SD: 3.3). There appeared no correlation between pain duration and NRS changes in the responder group.
Table 1.
Patient Characteristics
| All Patients (N = 12) |
Responders (N = 6) |
Non-Responders (N = 6) |
|
|---|---|---|---|
| Age in years, mean (SD) | 64.2 (11.0) | 63.3 (14.0) | 65.0 (8.1) |
| Men/women, N | 6/6 | 2/4 | 4/2 |
| Duration of pain in years, mean (SD) | 6.1 (7.0) | 10.3 (7.9) | 1.9 (1.7) |
| Current analgesic co-medication, N (%) | 7 (58) | 4 (67) | 3 (50) |
| Pre-test NRS mean (SD) | 6.1 (1.4) | 6.2 (1.5) | 6.0 (1.4) |
| Use of neuropathic pain medication, N | 6 | 3 | 3 |
| Diagnosis | N | N | N |
| Chronic idiopathic axonal polyneuropathy | 5 | 4 | 1 |
| Chemotherapy induced polyneuropathy | 4 | 2 | 2 |
| Diabetic polyneuropathy | 3 | 0 | 3 |
| Anatomical Areas of Pain | |||
| Both feet | 6 | 3 | 3 |
| Both feet and lower legs | 3 | 2 | 1 |
| Both feet, lower legs and hands | 1 | 0 | 1 |
| Fingertips | 2 | 1 | 1 |
| Pain Characteristics | N (%) | N (%) | N (%) |
| Burning | 10 (83) | 5 (83) | 5 (83) |
| Painful cold | 7 (58) | 4 (67) | 3 (50) |
| Electric shocks | 5 (42) | 2 (33) | 3 (50) |
| Tingling | 12 (100) | 6 (100) | 6 (100) |
| Pins and needles | 6 (50) | 3 (50) | 3 (50) |
| Numbness | 10 (83) | 5 (83) | 5 (83) |
| Itching | 1 (8) | 1 (17) | 6 (100) |
| Hypesthesia to touch | 7 (58) | 4 (67) | 3 (50) |
| Hypesthesia to pinprick | 7 (58) | 2 (33) | 5 (83) |
| Allodynia | 8 (67) | 4 (67) | 4 (67) |
Abbreviations: N: number of patients, SD: standard deviation.
Table 2.
Analgesic Effect of Phenytoin versus Placebo on NRS Score After 30 Minutes
| Population | Phenytoin | Placebo | Mean Difference | ||||
|---|---|---|---|---|---|---|---|
| Post Application NRS Score | Mean NRS Difference | ||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P-value | |
| All patients (N = 12) | 4.3 | 3.5 to 5.1 | 5.2 | 4.4 to 6.0 | 0.9 | 0.4 to 1.4 | 0.003 |
| Responders* (N = 6) | 3.0 | 2.5 to 3.5 | 4.3 | 3.8 to 4.8 | 1.3 | 1.1 to 1.8 | < 0.001 |
| Non-responders (N = 6) | 5.7 | 5.2 to 6.2 | 6.2 | 5.7 to 6.7 | 0.5 | −0.2 to 1.2 | 0.2 |
| % Pain Reduction from Baseline | Mean % Difference | ||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P-value | |
| All patients (N = 12) | 29 | 13 to 45 | 12 | −2 to 26 | 17 | 6 to 28 | 0.006 |
| Responders* (N = 6) | 51 | 40 to 62 | 28 | 15 to 42 | 22 | 13 to 32 | 0.001 |
| Non-responders (N = 6) | 8; | −5 to 20 | −4 | −20 to 11 | 12 | −12 to 36 | 0.3 |
Note: *Responder is defined as ≥2 points pain reduction on the NRS as well as ≥1 point difference between the phenytoin 10% cream and the placebo cream applied area in favour of phenytoin 10% cream.
Abbreviations: NRS, 11-point numerical rating scale, CI, confidence interval; N, number of patients.
Table 3.
Minimal Pain Reduction of 30% and 50%
| Population | Phenytoin | Placebo |
|---|---|---|
| MPR50 N (%) | MPR50 N (%) | |
| All patients (N = 12) | 4 (33) | 1 (8) |
| Responders (N = 6) | 4 (67) | 1 (17) |
| MPR30 N (%) | MPR30 N (%) | |
| All patients (N = 12) | 6 (50) | 3 (25) |
| Responders (N = 6) | 6 (100) | 3 (50) |
Abbreviations: MPR30, minimal pain reduction of ≥30%; MPR50, minimal pain reduction of ≥50%; N, number of patients.
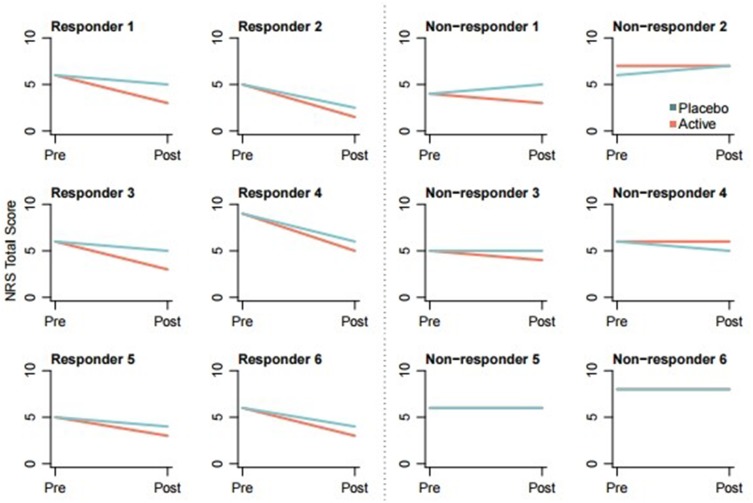
Figure 1.
Individual results of DOBRET.
Abbreviation: NRS, 11-point numerical rating scale in which 0 is no pain and 10 worst pain ever.
Mean NRS difference in pain reduction between the phenytoin 10% cream and placebo cream applied areas in the responder group was 1.3 (95% CI: 1.1 to 1.8; p<0.001), and mean percentage difference in pain reduction was 22% (95% CI: 13% to 32%; p=0.03). All responders had at least 30% pain reduction (MPR30), and 4 out of 6 had at least 50% pain reduction (MPR50) in the phenytoin 10% cream applied area. None of the patients reported local or systemic side effects.
Discussion
This is the first study showing that a DOBRET can identify early responders to the analgesic effect of phenytoin 10% cream within 30 minutes after its application.
The fast onset of topical phenytoin can be explained by its effect on the sensory nerves that reach the epidermis up to the stratum corneum. Molecules smaller than 500 Dalton, such as phenytoin (252 Dalton), can easily penetrate the stratum corneum.20 As a broad spectrum voltage-gated sodium channel blocker, topical phenytoin can inhibit the over-activity of sensory nerves as the source of neuropathic pain. Especially for patients with painful neuropathies (including polyneuropathy) in whom over-active nerve endings mainly reside in the skin, and not in the spinal cord or brain, topical phenytoin could be an optimal candidate to attain analgesia by silencing sodium channels peripherally.
Our hypothesis is that a DOBRET can help to identify those patients with a close fit between the pathogenesis of the pain that resides in the epidermal area where over-active small nerve fibers cross-talk with keratinocytes and immunocompetent cells and the mechanism of action of topical phenytoin.6 In non-responders identified by the response tests, possible explanations for the insufficient analgesic effect might be 1) presence of a significant central sensitization component, 2) absence of an up-regulated or sensitized receptor profile in the epidermis, and/or 3) retraction of the nociceptors from the epidermis.
A reason that some patients also experience pain reduction in the placebo applied area could be that we described to the patient the placebo cream as “a cream that can alleviate pain without knowing how it exactly works”. In future studies, we intend to describe the placebo cream differently “cream without an active compound”. Other limitations of this study are the small number of patients, and due to the exploratory nature, we could not evaluate the long-term efficacy, although patients will be contacted for follow-up. Furthermore, the usefulness of a DOBRET should be validated to predict the long-term effect of patients using phenytoin cream.
In the past, we have prescribed a cream containing an active pharmaceutical ingredient, such as phenytoin, and inquired about its effect at the next visit. Sometimes this resulted in treating patients several weeks to months without adequate analgesia. This timeframe may be reduced to only 30 minutes by performing a DOBRET that could be a useful tool for personalized medicine, as it may identify responders and beforehand reduce unnecessary and ineffective treatments. It will also reduce waiting time for the patient, because it is a quick way to determine which therapy appears to be suitable and it can be performed sequentially with any topical analgesic (eg amitriptyline, clonidine, ketamine, baclofen).
Arguments have been raised that the use of placebos in clinical practice is unethical.21 This may be so, for example, prescribing an antibiotic to satisfy a patient with a viral infection, or even if there was no deficiency prescribing multivitamins that can contain high doses of vitamin B6 and may cause a peripheral neuropathy. However, in our observational study, the placebo cream is used only for the short duration of a DOBRET with fully informed patient consent and the sole purpose to identify the added value of the active topical analgesic. This preserves the integrity of the physician, who can then prescribe only an active topical analgesic. The question could be raised if a placebo cream really is a placebo, when taking into account that success rates with topical placebos are about twice those seen with oral placebo.22 Besides the placebo effect, other still unknown mechanisms could be part of the analgesia with a “placebo” cream, such as creating a layer on the skin, reducing allodynia, or stimulation of A-beta fibers (touch) reducing pain sensation.10
A DOBRET can be regarded as a short n-of-1 trial, which is classified as level 1 evidence according to the Oxford Centre for Evidence-Based Medicine.23 In n-of-1 trials, active medication and placebo are tested in one person, and thus such trials can be seen as a cornerstone of the much advocated personalized medicine approach. In the European Union, including the Netherlands, personalized medicine is increasingly supported as an alternative or a complement to randomized clinical trials.
Conclusion
Phenytoin cream appears to be a safe topical analgesic; no side effects were reported. A double-blind placebo-controlled response test (DOBRET) is easy to conduct and is a promising tool in clinical practice to identify effective topical therapy in patients suffering from painful polyneuropathies (personalized medicine).
Acknowledgments
We thank Tiofarma for providing us phenytoin 10% and placebo creams and J. Mulder for blinding of the cream tubes.
Disclosure
DJK and JMKH are holders of two pending patents: (1) topical phenytoin for use in the treatment of peripheral neuropathic pain (WO2018106107); and (2) topical pharmaceutical composition containing phenytoin and a (co-)analgesic for the treatment of chronic pain (WO2018106108). The authors report no other conflicts of interest in this work.
References
- 1.Poliakov I, Toth C. The impact of pain in patients with polyneuropathy. Eur J Pain. 2011;15(10):1015–1022. doi: 10.1016/j.ejpain.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 2.Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities – a descriptive study. Disabil Rehabil. 2009;31(17):1402–1408. doi: 10.1080/09638280802621382 [DOI] [PubMed] [Google Scholar]
- 3.Erdmann PG, van Genderen FR, Teunissen LL, et al. Pain in patients with chronic idiopathic axonal polyneuropathy. Eur Neurol. 2010;64(1):58–64. doi: 10.1159/000315037 [DOI] [PubMed] [Google Scholar]
- 4.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerup NB, Haroutounian S, Baron R, et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain. 2018;159(11):2339–2346. doi: 10.1097/j.pain.0000000000001340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keppel Hesselink JM, Kopsky DJ, Bhaskar AK. Skin matters! The role of keratinocytes in nociception: a rational argument for the development of topical analgesics. J Pain Res. 2016;10:1–8. doi: 10.2147/JPR.S122765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015;4(1):17–32. doi: 10.1007/s40122-015-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):017–0615. doi: 10.1007/s11916-017-0615-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Management. 2012;2(1):71–77. doi: 10.2217/pmt.11.77 [DOI] [PubMed] [Google Scholar]
- 10.Kopsky DJ, Keppel Hesselink JM. Single-blind placebo-controlled response test with phenytoin 10% cream in neuropathic pain patients. Pharmaceuticals. 2018;11(4):4. doi: 10.3390/ph11040122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopsky DJ, Keppel Hesselink JM. Phenytoin cream for the treatment for neuropathic pain: case series. Pharmaceuticals. 2018;11(2):2. doi: 10.3390/ph11020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191–204. doi: 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- 13.Wampold BE. The therapeutic value of the relationship for placebo effects and other healing practices. Int Rev Neurobiol. 2018;139:191–210. [DOI] [PubMed] [Google Scholar]
- 14.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695. doi: 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Your research: Is it subject to the WMO or not? Central Committee on Research Invovling Human Subjects. Available from https://english.ccmo.nl/investigators/legal-framework-for-medical-scientific-research/your-research-is-it-subject-to-the-wmo-or-not. Accessed March19, 2020.
- 16.REGULATION (EU) No 536/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC.
- 17.Bostick NA, Sade R, Levine MA, Stewart DM Jr. Placebo use in clinical practice: report of the american medical association council on ethical and judicial affairs. J Clin Ethics. 2008;19(1):58–61. [PubMed] [Google Scholar]
- 18.Keppel Hesselink JM, Kopsky DJ, Bhaskar AK. Ethical justification of single-blind and double-blind placebo-controlled response tests in neuropathic pain and N-of-1 treatment paradigm in clinical settings. J Pain Res. 2019;12:345–352. doi: 10.2147/JPR.S180792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. 2015;67(1):48. [Google Scholar]
- 20.Bos JD, Meinardi Marcus MHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x [DOI] [PubMed] [Google Scholar]
- 21.Hrobjartsson A. Clinical placebo interventions are unethical, unnecessary, and unprofessional. J Clin Ethics. 2008;19(1):66–69. [PubMed] [Google Scholar]
- 22.Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Available from http://www.cebm.net/index.aspx?o=5653. Accessed 2 September 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Your research: Is it subject to the WMO or not? Central Committee on Research Invovling Human Subjects. Available from https://english.ccmo.nl/investigators/legal-framework-for-medical-scientific-research/your-research-is-it-subject-to-the-wmo-or-not. Accessed March19, 2020.
- OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Available from http://www.cebm.net/index.aspx?o=5653. Accessed 2 September 2019.