Abstract
Purpose:
(a) To describe the relationship of multimorbidity and physical activity (PA) in cancer survivors and (b) to explore perceived disability and PA in middle-aged and older survivors.
Methods:
The authors analyzed the data from cancer survivors (N = 566), identified using the Pennsylvania Cancer Registry, who responded to a Behavioral Risk Factor Surveillance System-derived questionnaire. They created age groups (e.g., 45–54 years, 55–64 years, 65–74 years, and 75 years and older) and calculated a composite score of eight common comorbidities (e.g., chronic obstructive pulmonary disease, heart disease) to assess multimorbidity. Logistic regression was used to estimate the association of demographic and behavioral/clinical risk factors (e.g., multimorbidity, perceived disability, body mass index) with PA.
Results:
Most respondents were females (62%), older (mean age = 68 years) and represented diverse cancer sites, including breast (n = 132), colorectal (n = 102), gynecologic (n = 106), prostate (n = 111), and lung (n = 80). PA participation was mixed; 44% of survivors reported achieving >150 min of aerobic PA, but half of lung and 37% of gynecologic survivors reported no PA (0 min/week). Higher multimorbidity (odds ratio = 0.82, confidence interval [0.69, 0.98], p < .05), obesity (odds ratio = 0.51, confidence interval [0.30, 0.86], p < .05), and perceived disability (odds ratio = 0.49, confidence interval [0.32, 0.77], p < .001) were negatively associated with PA participation. Strength training was suboptimal across all survivors.
Conclusion:
Most older survivors experienced comorbid conditions, and this was associated with less PA. Survivors who perceived themselves as disabled or who were obese were half as likely as others to participate in PA. This suggests an increasing need to address both physical and psychological limitations in designing PA interventions for real-world needs. Exercise interventions that address the unique needs of older survivors for multimorbidity, obesity, and perceived disability may strengthen opportunities for PA.
Keywords: exercise, geriatric oncology, healthy aging, symptoms
Cancer survivors experience physical deterioration from aging and cancer treatment. Given that 62% of the 15.5 MU.S. survivors are older than 65 years and that number is expected to continue to grow (Bluethmann, Mariotto, & Rowland, 2016), this is a significant clinical challenge for older patients and providers. Physical activity (PA) is recommended as part of survivorship care partly to address common cancer symptoms, including cancer-related pain, fatigue, cognitive impairment, depression, and physical dysfunction (Mustian, Sprod, Janelsins, Peppone, & Mohile, 2012). Similarly, PA is recommended to address common age-related chronic conditions, including heart disease, respiratory issues, bone fragility, and sarcopenia (Garber et al., 2011; Kraus et al., 2015; Law, Clark, & Clark, 2016; Peterson et al., 2009). As up to 80% of all adults older than 65 years live with more than one comorbidity based on Medicare claims and other clinical data (DuGoff, Canudas-Romo, Buttorff, Leff, & Anderson, 2014; Fortin, Bravo, Hudon, Vanasse, & Lapointe, 2005; Salive, 2013), it is not uncommon for older cancer survivors to contend with both multiple cancer symptoms and multiple chronic conditions (“multimorbidity”) simultaneously, resulting in compromised functional capacity and quality of life (Baumann, Pütz, Röhrig, Höffken, & Wedding, 2009). Lifestyle interventions, including PA, have been shown to mitigate both cancer treatment and age-related symptoms (Brown et al., 2011), yet are underutilized. Recent data suggest that healthcare providers are not routinely discussing PA with older cancer survivors as part of survivorship care (Siembida, Kent, Bellizzi, & Smith, 2019). The extent to which multimorbidity impacts PA participation in middle-aged and older cancer survivors’ postcancer diagnosis is not well known, especially across cancer sites. Furthermore, the role of perceived disability due to multimorbidity and its potential impact on PA participation for older survivors has not been fully explored (Brawley, Rejeski, & King, 2003).
The purpose of this paper is (a) to describe the relationship of multimorbidity and PA participation in cancer survivors, considering age and cancer site differences, and (b) to explore the role of perceived disability, especially relative to PA participation in middle-aged and older survivors. A greater understanding of physical limitations associated with multimorbidity and the connection to perceived disability may be useful in developing PA programs for survivors with these unique age-related barriers and be able to address an important gap in the literature.
Methods
Design, Sampling, and Data Collection Procedures
We used data from the Cancer and Pennsylvania Behaviors and Lifestyle Epidemiology (CAPABLE) study. For this cross-sectional study, the Pennsylvania Cancer Registry was used to identify eligible cancer survivors who (a) were at least 20 years of age, (b) had received a breast, lung, colorectal, prostate, or gynecologic (endometrial, ovarian, and cervical) cancer diagnosis between January 1, 2015 and December 31, 2016, and (c) were able to read, speak, and write in English (and respond to mailed surveys). We randomly sampled 2,500 adult cancer survivors from 28 Central Pennsylvania counties served by the Penn State Cancer Institute, 500 from each of the five cancer sites previously specified in our eligibility criteria, who were mailed surveys using selected questions from the Behavioral Risk Factor Surveillance System (BRFSS; Pierannunzi, Hu, & Balluz, 2013). For our adapted survey instrument, we pulled questions from the BRFSS core sections, including health status, health-related quality of life, chronic health conditions and demographics, tobacco use, and exercise (PA). The survey also included questions from the multifactor screener in the 2000 National Health Interview Survey Cancer Control Supplement for dietary questions and the National Health and Nutrition Examination Survey for the weight questions (Wright et al., 2007).
Of the 28 counties surveyed, 18 are categorized as urban/metropolitan and 10 as rural/nonmetropolitan using 2013 Rural/Urban Continuum Codes. To ensure sufficient representation of racial/ethnic minority respondents, we oversampled non-Hispanic Black and Hispanic individuals by a factor of 2. Furthermore, to ensure adequate participation by sex, we sampled equal numbers of male and female patients for lung and colorectal cancers. Participants whose registry data were missing age, race/ethnicity, or zip code were excluded from this study. The overall response rate was approximately 28%, which is similar to response rates to other mailed population-based surveys (Kaplowitz, Hadlock, & Levine, 2004; Moser et al., 2013). Participants who opted in after the initial recruitment letter or returned completed questionnaires provided implied consent to participate in the study. All study procedures and materials were reviewed and approved by Penn State College of Medicine Institutional Review Board. Additional details about the CAPABLE study are described elsewhere (Mama et al., 2018).
Dependent Variables
The main dependent variable of interest was achievement of recommended PA, based on both aerobic and strength training guidelines. According to the American College of Sports Medicine, American Cancer Society, and others, adults should obtain 30 min of moderateto vigorous-intensity exercise, 3–5 times per week (approximately 150 min), and participate in at least two strength training sessions per week to obtain health benefits (Jonas & Phillips, 2012; Kushi et al., 2006; Nelson et al., 2007). To assess self-reported PA participation, participants in our survey were asked, “how many minutes did you take part in PA during the past week?” To align with current PA recommendations, we categorized aerobic PA participation as follows: achieved recommended PA (exercised 150 min or more per week), were insufficiently active (exercised between 1 and 149 min/ week), or were nonexercisers (0 min of exercise). Strength training was assessed by asking, “during the past month, how many times per week did you do physical activities or exercises to strengthen your muscles?” Based on low responses for strength training estimates, we dichotomized strength training reports based on whether the participant reported engaging in any strength training (≥1 time per week) or did not do strength training at all (0 times per week).
Independent Variables—Multimorbidity and Perceived Disability
To compare differences by age, we created age-related subgroups (e.g., 45–54 years, 55–64 years, 65–74 years, and 75 years and older), which was useful in contextualizing PA participation rates across the aging trajectory. For multimorbidity, we calculated a composite score (n/8) based on the same list of common comorbidities (i.e., heart disease, stroke, asthma, chronic obstructive pulmonary disease, arthritis/joint symptoms, depressive disorders, kidney disease, and diabetes) collected in the national BRFSS (Pierannunzi et al., 2013). Perceived disability was assessed by asking participants, “during the past four weeks, were you limited in regular activities you could do as a result of your physical health?”
Statistical Analysis
Statistical analysis was conducted using SAS version 9.4 (SAS, Cary, NC). We used descriptive statistics to characterize the population and to understand differences in these characteristics by cancer site (overall, breast, colorectal, gynecologic, lung, and prostate). We also calculated the prevalence of specific chronic health conditions across cancer sites to understand the extent of multimorbidity burden. Subsequently, a multivariable logistic regression model was created to estimate the overall association of PA participation, controlling for demographic (race/ethnicity, age group, marital status, and educational status) and behavioral/ clinical risk factors (e.g., multimorbidity, perceived disability, body mass index [BMI]). In consideration of age-related subgroups, the youngest age group (45–54 years) was used as the reference group in regression models to compare against older age groups (55–64 years, 65–74 years, and 75 years and older). Logistic regression coefficients were exponentiated and expressed as odds ratios (ORs) for ease of interpretation.
Results
Key Characteristics of Participants
A total of 566 middle-aged and older survivors between the ages of 45–96 years participated in the survey, and 62% were females. Our final sample for the study included middle-aged and older cancer survivors (N = 566) who had received a primary diagnosis of breast, prostate, colorectal, gynecologic, or lung cancer. Participants included breast (n = 139), colorectal (n = 102), prostate (n = 123), gynecologic (n = 122), and lung (n = 80) cancer survivors. We did not observe statistically significant differences in response rate by age or cancer site. The majority of the participants were White (90%) and educated; about 25% had completed some college or vocational school and 34% had completed college (i.e., received a degree from a 4-year college). The mean age of all participants was 68 years (SD = 10.27; range = 45–96 years), but lung cancer survivors were the oldest, with a mean age of 72 years (SD = 9.36; range = 45–94 years) (Table 1).
Table 1.
Characteristics of Participants by Cancer Site
| Variables | Overall (N = 566) |
Breast (n = 139) |
Colorectal (n = 102) |
Gynecologic (n = 122) |
Lung (n = 80) |
Prostate (n = 123) |
p (χ2) |
|---|---|---|---|---|---|---|---|
| Mean age in years (SD); range | 68.16 (10.27); 45–96 | 66.19 (11.72); 45–94 | 69.12 (11.66); 46–96 | 66.59 (9.32); 46–88 | 72.06 (9.36); 45–94 | 68.60 (7.72); 55–93 | |
| 45–54, n (%) | 58 (10.25) | 29 (20.86) | 10 (9.80) | 15 (12.30) | 4 (5.00) | 0 (0.00) | .004 |
| 55–64, n (%) | 149 (26.33) | 35 (25.18) | 26 (25.49) | 34 (27.87) | 14 (17.50) | 40 (32.52) | |
| 65–74, n (%) | 201 (35.51) | 42 (30.22) | 29 (28.43) | 49 (40.16) | 26 (32.50) | 55 (44.72) | |
| 75 and older, n (%) | 158 (27.92) | 33 (23.74) | 37 (36.27) | 24 (19.67) | 36 (45.00) | 28 (22.76) | |
| Gender, n (% female) | 351 (62.01) | 139 (100.00) | 49 (48.04) | 122 (100.00) | 41 (51.25) | 0 (0.00) | .30 |
| Race/ethnicity, n (%) | |||||||
| European American | 511 (90.28) | 124 (89.21) | 90 (88.24) | 109 (89.34) | 76 (95.00) | 112 (91.06) | .31 |
| African American | 21 (3.71) | 5 (3.60) | 7 (6.86) | 2 (1.64 ) | 0 (0.00) | 7 (5.69) | |
| Hispanic | 18 (3.18) | 5 (3.60) | 1 (0.98) | 8 (6.56) | 3 (3.75) | 1 (0.81) | |
| Other | 16 (2.83) | 5 (3.60) | 4 (3.92) | 3 (2.46) | 1 (1.25) | 3 (2.44) | |
| Education, n (%) | |||||||
| High school or less | 43 (7.60) | 4 (2.88) | 11 (10.78) | 8 (6.56) | 12 (15.00) | 8 (6.50) | .004 |
| High school graduate | 189 (33.39) | 44 (31.65 ) | 35 (34.31) | 48 (39.34) | 31 (38.75) | 31 (25.20) | |
| Some college | 140 (24.73) | 33 (23.74) | 26 (25.49) | 31 (25.41) | 19 (23.75) | 31 (25.20) | |
| College or higher | 186 (32.86) | 56 (40.29) | 28 (27.45) | 33 (27.05) | 16 (20.00) | 53 (43.09) | |
| Unknown | 8 (1.41) | 2 (1.44 ) | 2 (1.96) | 2 (1.64) | 2 (2.50) | 0 (0.00) | |
| Marital status, n (%) | |||||||
| Single | 172 (30.39) | 53 (38.13) | 27 (26.47) | 43 (35.25) | 31 (38.75) | 18 (14.63) | <.001 |
| Married/partner | 382 (67.49) | 81 (58.27) | 71 (69.61) | 78 (63.93) | 48 (60.00) | 104 (84.55) | |
| Unknown | 12 (2.12) | 5 (3.60) | 4 (3.92) | 1 (0.82) | 1 (1.25) | 1 (0.81) | |
| Physical activity, min/week (%) | |||||||
| >150 | 257 (45.40) | 68 (48.92) | 43 (42.16) | 46 (37.70) | 25 (31.25) | 69 (56.10) | .004 |
| 1–149 | 70 (12.37) | 17 (12.23) | 14 (13.73) | 12 (9.84) | 7 (8.75) | 13 (10.57) | |
| 0 | 188 (33.22) | 37 (26.62) | 27 (26.47) | 46 (37.70) | 39 (48.75) | 29 (23.58) | |
| Unknown | 51 (9.01) | 17 (12.23) | 18 (17.65) | 18 (14.75) | 9 (11.25 ) | 12 (9.76) | |
| Strength training, n (%) | |||||||
| >1 time per week | 344 (60.78) | 83 (59.71) | 56 (54.90) | 87 (71.31) | 50 (62.5) | 68 (55.28) | .04 |
| 0 times per week | 143 (25.26) | 41 (29.50) | 29 (28.43) | 21 (17.21) | 15 (18.75) | 37 (30.08) | |
| Unknown | 79 (13.96) | 15 (10.79) | 17 (16.67) | 14 (11.48) | 15 (18.75) | 18 (14.63) | |
| Multimorbidity, mean (SD); range | 1.44 (1.3); 0–6 | 1.35 (1.2); 0–6 | 1.19 (1.2); 0–5 | 1.46 (1.3); 0–6 | 2.29 (1.6); 0–6 | 1.19 (1.1); 0–5 | <.001 |
| Perceived disability, n (%) | |||||||
| Yes | 190 (33.57) | 33 (23.74) | 37 (36.27) | 48 (39.34) | 40 (50.00) | 32 (26.02) | .002 |
| No | 353 (62.37) | 101 (72.66) | 59 (57.84) | 70 (57.38) | 36 (45.00) | 87 (70.73) | |
| Unknown | 23 (4.06) | 5 (3.60) | 6 (5.88) | 4 (3.28) | 4 (5.00) | 4 (3.25) | |
| Body mass index, | 30.99 (7.81); | 30.35 (8.00); | 29.10 (5.94); | 33.95 (8.73); | 28.11 (6.33); | 31.42 (7.40); | <.001 |
| mean (SD); range | 13.71–62.32 | 14.26–62.32 | 18.40^19.23 | 15.98–53.37 | 16.31^18.93 | 13.71–56.80 | |
| Normal, n (%) | 117 (20.67) | 35 (25.18) | 23 (22.55) | 20 (16.39) | 22 (27.50) | 17 (13.82) | <.001 |
| Overweight, n (%) | 156 (27.56) | 31 (22.3) | 34 (33.33) | 20 (16.39) | 28 (35.00) | 43 (34.96) | |
| Obese, n (%) | 238 (42.04) | 57 (41.00) | 35 (34.31) | 71 (58.20) | 17 (21.25 ) | 58 (47.15) | |
| Unknown, n (%) | 55 (9.72) | 16 (11.51) | 10 (9.80) | 11 (9.02) | 13 (16.25) | 5 (4.07) | |
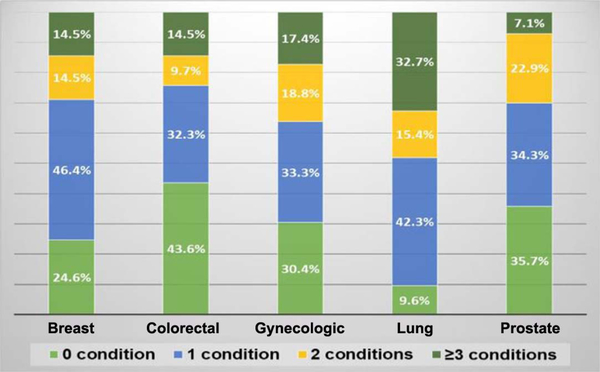
The most prevalent health conditions reported over all survivors were some form of arthritis or joint problems (approximately 56%), depressive disorders (approximately 24%), and heart attack/heart disease (approximately 17%). However, the prevalence of these conditions varied widely by cancer site (Figure 1). A majority of breast (approximately 68%) and gynecologic (approximately 63%) cancer survivors reported arthritis or similar joint conditions. Nearly 60% of lung cancer survivors reported a diagnosis of chronic obstructive pulmonary disease. Lung cancer survivors had the highest mean composite score for multimorbidity of any cancer site at 2.29 (SD = 1.56; range = 0–6), compared with 1.44 (SD = 1.32; range = 0–6) for all survivors (Table 1). The mean multimorbidity composite score for gynecologic cancer survivors was also higher than the overall mean for all survivors; gynecologic survivors reported a mean of 1.46 chronic conditions (SD = 1.28; range = 0–6).
Figure 1 —
Prevalence of multimorbidity in older survivors across cancer sites. Distribution of multimorbidity burden by cancer site. Conditions reported included heart attack/angina, stroke, asthma, chronic obstructive pulmonary disease, arthritis/joint conditions, depressive disorder, kidney disease, and diabetes. See Methods section for additional details.
A high percentage (70%, n = 396) of the survivors were also overweight or obese. The overall mean BMI was 31 (SD = 13.71; range = 13.7–62.3). This was especially noticeable for the gynecologic survivors. These survivors had a mean BMI of 34 (SD = 8.73; range = 15.98–53.3). Breast and prostate cancer survivors also reported excess weight; 63% of breast cancer survivors and 83% of prostate cancer survivors were overweight or obese.
Prevalence of PA and Strength Training Participation by Cancer Site
Achievement of recommended PA was mixed in this sample of survivors. Almost half (45%) reported achieving the recommended 150 min/week of moderate- to vigorous-intensity aerobic exercise. However, only 12% reported being insufficiently active (exercised between 1 and 149 min/week), and 33% reported that they did no aerobic PA at all. Approximately, 37% of gynecologic and 49% of lung cancer survivors reported that they did no aerobic PA. Strength training participation was also very mixed. The majority of survivors reported doing strength training at least one time per week (61%). This was highest among gynecologic (71%) and lung cancer survivors (63%). However, among survivors of other cancer sites (breast, colorectal, and prostate), reported strength training sessions were lower, with about 55–60% indicating that they had done strength training exercises once per week. Overall, strength training prevalence was suboptimal in these survivors, as more than about 25% reported doing no strength training at all.
Association of PA, Multimorbidity, and Perceived Disability
We observed a strong negative association between greater multimorbidity and likelihood of aerobic PA participation (OR = 0.82, confidence interval, CI [0.69, 0.98], p < .05; Table 2). We also observed that those who perceived themselves as disabled were 50% less likely to participate in PA than those who did not perceive themselves as disabled (OR = 0.49, CI [0.32, 0.77], p< .001). There was a linear increase in likelihood of PA participation by educational attainment; participants with a college or graduate school education were nearly three times as likely as less educated participants to participate in PA (OR = 2.87, CI [1.19, 7.12], p < .05). Obese survivors (BMI > 30) were half as likely as nonobese survivors to participate in PA (OR = 0.51, CI [0.30, 0.86], p < .05).
Table 2.
Association of Multimorbidity and Perceived Disability With PA Participationa
| Variables | OR | 95% CI | SE | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 1.00 (referent group) | |||
| Female | 1.17 | [0.57, 2.41] | 0.37 | .67 |
| Age group | ||||
| 45–54 years | 1.00 (referent group) | |||
| 55–64 years | 1.23 | [0.60, 2.53] | 0.37 | .57 |
| 65–74 years | 1.23 | [0.61, 2.43] | 0.35 | .56 |
| 75 years and older | 0.87 | [0.42, 1.79] | 0.37 | .70 |
| Race | ||||
| White | 1.00 (referent group) | |||
| Non-Whiteb | 0.76 | [0.35, 1.68] | 0.40 | .50 |
| Cancer site | ||||
| Breast | 1.00 (referent group) | |||
| Colorectal | 1.18 | [0.56, 2.52] | 0.38 | .67 |
| Gynecologic | 1.04 | [0.57, 1.92] | 0.31 | .89 |
| Lung | 0.57 | [0.26, 1.22] | 0.39 | .15 |
| Prostate | 1.48 | [0.59, 3.74] | 0.47 | .41 |
| Education | ||||
| High school or less | 1.00 (referent group) | |||
| High school graduate | 1.51 | [0.62, 3.77] | 0.46 | .37 |
| Some college/vocational school | 2.23 | [0.92, 5.62] | 0.46 | .08 |
| College/post graduate | 2.87 | [1.19, 7.12] | 0.45 | .02 |
| Marital status | ||||
| Single | 1.00 (referent group) | – | – | – |
| Married/stable union | 1.07 | [0.68, 1.70] | 0.23 | .77 |
| Perceived disability | ||||
| No | 1.00 (referent group) | |||
| Yes | 0.49 | [0.32, 0.77] | 0.23 | .00 |
| Multimorbidity score | 0.82 | [0.69, 0.98] | 0.09 | .03 |
| BMI (kg/m2) | ||||
| <25 | 1.00 (referent group) | |||
| 25–29.9 | 1.23 | [0.69, 2.18] | 0.29 | .48 |
| ≥30 | 0.51 | [0.30, 0.86] | 0.27 | .01 |
Note. PA = physical activity; OR = odds ratio; CI = confidence interval; BMI = body mass index.
PA participation categorized based on meeting PA aerobic guidelines.
Black, Hispanic, or other.
We did not observe any significant associations in PA participation based on gender, race/ethnicity, or marital status. However, PA participation by cancer site varied. For example, lung cancer survivors were 43% less likely than breast cancer survivors to do aerobic PA (OR = 0.57, CI [0.26, 1.22], p = .15). We also did not find significant differences between age-related subgroups.
Discussion
The majority of older cancer survivors had at least one comorbid condition, and this was related to suboptimal PA participation. Greater multimorbidity appeared to further reduce PA participation, especially for gynecologic and lung cancer survivors, whose mean multimorbidity score was higher than that for all other survivors. Gynecologic and lung cancer survivors also reported the highest level of inactivity (especially for aerobic activity) among all cancer sites. Given that the PA guidelines for all survivors also recommend avoiding inactivity in recovery, this is a clinically significant finding. Current literature suggests that advanced age increases risk of multimorbidity in older adults (Fabbri et al., 2015). In this study, multimorbidity was a significant negative correlate for low PA participation, independent of age. This suggests that multimorbidity burden may outweigh chronological age as an impediment to PA.
Perceived disability was also a significant correlate for reduced PA participation. In addition to having the highest multimorbidity scores of all survivors, gynecologic and lung cancer survivors also had the highest reports of perceived disability among survivors of all cancer sites (Table 1), and this was related to lower participation in aerobic activities (Table 2). The majority of breast and gynecologic cancer survivors (>60%) reported joint symptoms related to arthritis or another joint disorder (see Supplemental Table [available online]). This might potentially be a consequence of breast cancer treatment. Aromatase inhibitors are known to produce arthralgias in some breast cancer survivors (Niravath, 2013). Age-related hormonal changes may also influence joint fluidity and function in older women generally (Kulie et al., 2011).
Although we did not categorize obesity as a comorbid condition, we did assess BMI as a covariate and observed that obesity appeared to impede PA participation across survivors of all cancer sites. For gynecologic survivors, for example, 58% had a BMI >30. Most (82%) prostate cancer survivors also reported being overweight or obese. This extreme amount of excess weight (i.e., BMI > 30 or BMI > 35, connoting morbid levels of obesity) could be suggestive of sarcopenic obesity, a newer way to understand the problems created by obesity in older patients (Zamboni, Mazzali, Fantin, Rossi, & Di Francesco, 2008). In sarcopenic obesity, age-related changes in body composition, as well as the increased obesity, may contribute to reduced muscle mass or strength. This combination of obesity and reduced strength can exacerbate disability and increase the risk of comorbidity or death in older adults (Zamboni et al., 2008). For older cancer survivors, age-related sarcopenia may be exacerbated by cancer treatment and increase muscle loss (Fielding et al., 2011; Suskin & Shapiro, 2018); adding obesity to this equation has been found to have important clinical implications for physical function, chemotherapy toxicity, and survival (Colditz & Peterson, 2018; Ligibel et al., 2014). It is conceivable that some survivors had been counseled to do strength training to counteract muscle loss and improve physical function, as many survivors (65%) reported doing some form of strength training at least once per week. However, overall strength training participation was suboptimal and remains an opportunity to fortify the health and function of older survivors. Multiple behavior change strategies may be required to control weight and also promote PA (Shaw, Gennat, Rourke, & Del, 2006) in these survivors.
The high level of multimorbidity and obesity supports recent research stressing that functional age, rather than chronological age, is a superior method of determining physical performance in older adults (Frochen & Mehdizadeh, 2018) and older cancer survivors. Clinical tools like the geriatric assessment can also be important in determining individual benefits and risks of health recommendations following cancer treatment (Wildiers et al., 2014).
While physical limitations are a known challenge for mobility and for doing PA, this study extends the current literature by also considering psychosocial variables, including perceived disability (Gellert et al., 2015), which provides different information about the survivor’s experience. Assessing whether or not a person perceives a set of physical limitations impedes daily activities, provides valuable insight about perceptions, attitudes, and beliefs, which are potentially modifiable constructs in behavioral medicine (Peel, McClure, & Bartlett, 2005).
Physical rehabilitation literature has provided data about barriers and facilitators to PA in people with disabilities. According to the 2008 Barriers to PA for People with Disabilities survey, 40% of educated adults with disabilities do not exercise because they do not believe it will improve their condition, and 50% do not exercise because they do not know how to do so safely with their condition(s) (Rimmer, Wang, & Smith, 2008). These behavioral determinants provide valuable stepping stones to overcoming both real and perceived barriers to PA in older adults (Gellert et al., 2015), and interventions that have targeted these have been demonstrated to be successful in changing PA behaviors and improving health outcomes (Stacey, James, Chapman, Courneya, & Lubans, 2015).
This study had many strengths. We had a large sample of recently diagnosed cancer survivors from a wide age range. We also purposively sampled survivors from multiple cancer sites to better understand the diverse needs of survivors, especially relative to current health habits. The insights about challenges experienced by lung and gynecologic cancer survivors were especially informative. The 28-county source of participants in Central Pennsylvania also represents an underserved region, with limited healthcare access, and so this study fills a gap in understanding the needs of both rural and urban survivors that live in this region. The Pennsylvania Cancer Registry also offered a robust resource from which to identify eligible survivors based on our criteria.
There were also limitations. We used a cross-sectional design for this study, so it is not clear how these one-time estimates relate to longitudinal trends. However, our data offers a valuable basis for future research. We used self-report for PA estimates, which can be biased. However, the PA questions came from a validated questionnaire (BRFSS; Kwon, Hou, & Wang, 2012), and this surveillance strategy has informed the U.S. PA guidelines (U.S. Department of Health and Human Services, 2018). In the future, a mixed PA assessment approach (using objective and self-report instruments) would provide more complete information about PA participation. We also recognize that body composition, rather than BMI, might provide better information about the role of weight, fat distribution, and muscle quality in survivors, but this technique was not feasible with our mailed survey. Body composition would be a useful assessment for future studies (Reinders et al., 2015).
In a time when the focus for cancer survivors is increasingly on the quality of life after diagnosis, a thoughtful consideration of both physical and psychological limitations (Stanton, Rowland, & Ganz, 2015) is required. Additionally, negotiating barriers to PA for older cancer survivors (Bluethmann, Sciamanna, Winkels, Sturgeon, & Schmitz, 2018) to control weight, reduce multimorbidity, and change perceptions of disability will enhance quality of life and quality of years for a growing population of older cancer survivors with complex needs.
Supplementary Material
Acknowledgments
The authors would like to thank the Bureau of Health Statistics and Registries at the Pennsylvania Department of Health for providing data on cancer survivors and to the Community Sciences and Health Outcomes Shared Resource, supported by the Penn State Clinical and Translational Science Institute, for generating the sampling strategy of the study (NIH UL1 TR002014). Dr. S.M. Bluethmann is supported by a Mentored Research Scholar Grant in Applied and Clinical Research, MSRG-18–136-01, from the American Cancer Society. Portions of this study were previously presented at the 2018 annual meetings of the Society of Behavioral Medicine and American College of Sports Medicine. S.M. Bluethmann led the conceptualization, design, analysis, and writing of this manuscript. W. Foo assisted with analysis and data management for the study. S.K. Mama and R.M. Winkels contributed to interpreting data and writing the manuscript. K.H. Schmitz provided senior leadership on study design, concept, data interpretation, and writing of this manuscript. All coauthors consented to submission of this manuscript.
Footnotes
The authors declare that they have no conflicts of interest.
Contributor Information
Shirley M. Bluethmann, Department of Public Health Sciences, College of Medicine, The Pennsylvania State University, Hershey, PA. Penn State Cancer Institute, Hershey, PA.
Wayne Foo, Department of Public Health Sciences, College of Medicine, The Pennsylvania State University, Hershey, PA..
Renate M. Winkels, Department of Public Health Sciences, College of Medicine, The Pennsylvania State University, Hershey, PA. Penn State Cancer Institute, Hershey, PA.
Kathryn H. Schmitz, Department of Public Health Sciences, College of Medicine, The Pennsylvania State University, Hershey, PA. Penn State Cancer Institute, Hershey, PA.
Scherezade K. Mama, Department of Kinesiology, The Pennsylvania State University, State College, PA. Penn State Cancer Institute, Hershey, PA.
References
- Baumann R, Pütz C, Röhrig B, Höffken K, & Wedding U (2009). Health-related quality of life in elderly cancer patients, elderly non-cancer patients and an elderly general population. European Journal of Cancer Care, 18(5), 457–465. doi: 10.1111/j.1365-2354.2008.00975.x [DOI] [PubMed] [Google Scholar]
- Bluethmann SM, Mariotto AB, & Rowland JH (2016). Anticipating the “Silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology, Biomarkers & Prevention, 25(7), 1029–1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluethmann SM, Sciamanna CN, Winkels RM, Sturgeon KM, & Schmitz KH (2018). Healthy living after cancer treatment: Considerations for clinical and community practice. American Journal of Lifestyle Medicine, 12(3), 215–219. doi: 10.1177/1559827618755681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley LR, Rejeski WJ, & King AC (2003). Promoting physical activity for older adults: The challenges for changing behavior. American Journal of Preventive Medicine, 25(3), 172–183. doi: 10.1016/S0749-3797(03)00182-X [DOI] [PubMed] [Google Scholar]
- Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, & Johnson BT (2011). Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiology, Biomarkers & Prevention, 20(1), 123–133. doi: 10.1158/1055-9965.EPI-10-0988 [DOI] [PubMed] [Google Scholar]
- Colditz GA, & Peterson LL (2018). Obesity and cancer: Evidence, impact, and future directions. Clinical Chemistry, 64(1), 154–162. doi: 10.1373/clinchem.2017.277376 [DOI] [PubMed] [Google Scholar]
- DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, & Anderson GF (2014). Multiple chronic conditions and life expectancy: A life table analysis. Medical Care, 52(8), 688–694. doi: 10.1097/MLR.0000000000000166 [DOI] [PubMed] [Google Scholar]
- Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, & Ferrucci L (2015). Aging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical research. Journal of the American Medical Directors Association, 16(8), 640–647. doi: 10.1016/j.jamda.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, … Zamboni M (2011). Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association, 12(4), 249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Bravo G, Hudon C, Vanasse A, & Lapointe L (2005). Prevalence of multimorbidity among adults seen in family practice. Annals of Family Medicine, 3(3), 223–228. doi: 10.1370/afm.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frochen S, & Mehdizadeh S (2018). Functional status and adaptation: Measuring activities of daily living and device use in the national health and aging trends study. Journal of Aging and Health, 30(7), 1136–1155. doi: 10.1177/0898264317707299 [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, … American College of Sports Medicine. (2011). American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine & Science in Sports & Exercise, 43(7), 1334–1359. doi: 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- Gellert P, Witham MD, Crombie IK, Donnan PT, McMurdo ME, & Sniehotta FF (2015). The role of perceived barriers and objectively measured physical activity in adults aged 65–100. Age and Ageing, 44(3), 384–390. doi: 10.1093/ageing/afv001 [DOI] [PubMed] [Google Scholar]
- Jonas S, & Phillips EM (2012). ACSM’s Exercise is Medicine™: A clinician’s guide to exercise prescription. Lippincott Williams & Wilkins. [Google Scholar]
- Kaplowitz MD, Hadlock TD, & Levine R (2004). A comparison of web and mail survey response rates. Public Opinion Quarterly, 68(1), 94–101. doi: 10.1093/poq/nfh006 [DOI] [Google Scholar]
- Kraus WE, Bittner V, Appel L, Blair SN, Church T, Després J, … Whitsel L (2015). The national physical activity plan: A call to action from the American Heart Association: A science advisory from the American Heart Association. Circulation, 131, 1932–1940. doi: 10.1161/CIR.0000000000000203 [DOI] [PubMed] [Google Scholar]
- Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, & Schrager S (2011). Obesity and women’s health: An evidence-based review. Journal of the American Board of Family Medicine, 24(1), 75–85. doi: 10.3122/jabfm.2011.01.100076 [DOI] [PubMed] [Google Scholar]
- Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, Gansler T, … The American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee. (2006). American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA: A Cancer Journal for Clinicians, 56(5), 254–281. doi: 10.3322/canjclin.56.5.254 [DOI] [PubMed] [Google Scholar]
- Kwon S, Hou N, & Wang M (2012). Comparison of physical activity levels between cancer survivors and non-cancer participants in the 2009 BRFSS. Journal of Cancer Survivorship, 6(1), 54–62. doi: 10.1007/s11764-011-0204-8 [DOI] [PubMed] [Google Scholar]
- Law TD, Clark LA, & Clark BC (2016). Resistance exercise to prevent and manage sarcopenia and dynapenia. Annual Review of Gerontology and Geriatrics, 36(1), 205–228. doi: 10.1891/0198-8794.36.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, … Hudis CA (2014). American Society of Clinical Oncology position statement on obesity and cancer. Journal of Clinical Oncology, 32(31), 3568–3574. doi: 10.1200/JCO.2014.58.4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mama SK, Foo W, Winkels R, Wiskemann J, Bluethmann SM, Calo W, … Schmitz KH (2018). Rural-urban differences in meeting physical activity recommendations in cancer survivors in central Pennsylvania: 1549 board# 1 may 31 1. Medicine & Science in Sports & Exercise, 50(5S), 373–374. doi: 10.1249/01.mss.0000536313.40909.f6 [DOI] [Google Scholar]
- Moser RP, Naveed S, Cantor D, Blake KD, Rutten LJF, Ramirez A, … Moser R (2013). Integrative analytic methods using population-level cross-sectional data (HINTS Data Briefs). Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Mustian KM, Sprod LK, Janelsins M, Peppone LJ, & Mohile S (2012). Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: A review. Oncology & Hematology Review, 8(2), 81–88. doi: 10.17925/OHR.2012.08.2.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, … Castaneda-Sceppa C (2007). Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation, 116(9), 1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- Niravath P (2013). Aromatase inhibitor-induced arthralgia: A review. Annals of Oncology, 24(6), 1443–1449. doi: 10.1093/annonc/mdt037 [DOI] [PubMed] [Google Scholar]
- Peel NM, McClure RJ, & Bartlett HP (2005). Behavioral determinants of healthy aging. American Journal of Preventive Medicine, 28(3), 298–304. doi: 10.1016/j.amepre.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Giuliani C, Morey MC, Pieper CF, Evenson KR, Mercer V, … Simonsick EM (2009). Physical activity as a preventative factor for frailty: The health, aging, and body composition study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64(1), 61–68. doi: 10.1093/gerona/gln001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierannunzi C, Hu SS, & Balluz L (2013). A systematic review of publications assessing reliability and validity of the behavioral risk factor surveillance system (BRFSS), 2004–2011. BMC Medical Research Methodology, 13(1), 49. doi: 10.1186/1471-2288-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders I, Murphy RA, Martin KR, Brouwer IA, Visser M, White DK, … Harris TB (2015). Body mass index trajectories in relation to change in lean mass and physical function: The health, aging and body composition study. Journal of the American Geriatrics Society, 63(8), 1615–1621. doi: 10.1111/jgs.13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer JH, Wang E, & Smith D (2008). Barriers associated with exercise and community access for individuals with stroke. Journal of Rehabilitation Research & Development, 45(2), 315–322. doi: 10.1682/JRRD.2007.02.0042 [DOI] [PubMed] [Google Scholar]
- Salive ME (2013). Multimorbidity in older adults. Epidemiologic Reviews, 35(1), 75–83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- Shaw K, Gennat H, Rourke P, & Del M (2006). Exercise for overweight or obesity. Cochrane Database of Systematic Reviews, (4), CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siembida EJ, Kent EE, Bellizzi KM, & Smith AW (2019). Healthcare providers’ discussions of physical activity with older survivors of cancer: Potential missed opportunities for health promotion. Journal of Geriatric Oncology. Advance online publication. pii: S1879–4068(18)30522–8. doi: 10.1016/j.jgo.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Stacey FG, James EL, Chapman K, Courneya KS, & Lubans DR (2015). A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. Journal of Cancer Survivorship, 9(2), 305–338. doi: 10.1007/s11764-014-0413-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Rowland JH, & Ganz PA (2015). Life after diagnosis and treatment of cancer in adulthood: Contributions from psychosocial oncology research. American Psychologist, 70(2), 159. doi: 10.1037/a0037875 [DOI] [PubMed] [Google Scholar]
- Suskin J, & Shapiro CL (2018). Osteoporosis and musculoskeletal complications related to therapy of breast cancer. Gland Surgery, 7(4), 411. doi: 10.21037/gs.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2018). Physical activity guidelines for Americans (2nd ed). Washington, DC: Author; Retrieved from https://health.gov/paguidelines/second-edition/ [Google Scholar]
- Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen MLG, Extermann M, … Hurria A (2014). International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology, 32(24), 2595–2603. doi: 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JD, Borrud LG, McDowell MA, Wang C, Radimer K, & Johnson CL (2007). Nutrition assessment in the National Health and Nutrition Examination Survey 1999–2002. Journal of the American Dietetic Association, 107(5), 822–829. doi: 10.1016/j.jada.2007.02.017 [DOI] [PubMed] [Google Scholar]
- Zamboni M, Mazzali G, Fantin F, Rossi A, & Di Francesco V (2008). Sarcopenic obesity: A new category of obesity in the elderly. Nutrition, Metabolism and Cardiovascular Diseases, 18(5), 388–395. doi: 10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.