Abstract
Background:
An optimal blood pressure (BP) range to mitigate morbidity and mortality on left ventricular assist device (LVAD) support has not been clearly defined.
Methods:
Average Doppler opening pressure, mean arterial pressure (MAP), and/or systolic blood pressure (SBP) were calculated in operative survivors (n=16155) of LVAD support in INTERMACS. BP distributions were used to group patients: low (BP <25th percentile), normal (25-75th percentile), high (75th-95th) and very high (>95th percentile). Associations between BP and adverse events were evaluated using Cox Regression (Hazard ratio (HR), 95% confidence interval).
Results:
The median MAP, Doppler, and SBPs (mmHg) during CFLVAD support were 84 [77, 90], 85 [80, 92], and 99 [90,107] mmHg. BP had a bimodal risk association with survival. At 3 years, survival was 58±1.8% in those with low MAPs (≤75 mmHg) vs. 70±0.9%, 71±1.5%, and 63±3.0% in the those with normal, high, or very high average MAPs. Patients with chronically low MAPs (≤75 mmHg), Dopplers (≤80 mmHg) and SBPs (<90 mmHg) had 35-42% higher adjusted hazards of death than patients with normal or high BPs (p≤0.0001). Patients with MAPs >100 mmHg, Dopplers ≥105 mmHg, and SBPs ≥120 mmHg had 17-20% higher adjusted hazards of death than those with normal pressures (p<0.05). In patients on axial flow LVADs, elevated SBP (HR 1.08 [1.04-1.13] per 10 mmHg increase) but not MAP correlated with increased incident stroke.
Conclusions:
In INTERMACS, BP extremes during LVAD support increase the risk for adverse events, supporting a MAP goal >75 mmHg and <90 mmHg. Hypotension conferred the highest risk for mortality. Excessive BP control should be avoided, and Doppler opening pressure should not be assumed to represent MAP in all patients.
Keywords: LVAD, mortality, blood pressure
Survival in patients supported with durable continuous flow (CF) left ventricular assist devices (LVADs) has improved over time. In the 9th annual STS-INTERMACS (Society of Thoracic Surgeons- Interagency Registry for Mechanically Assisted Circulatory Support) report, nearly 50% of patients implanted after 2015 were alive on support at 5 years, compared with an average survival of 4 years in those implanted prior to 2015.(1) In conjunction with refinements in LVAD candidate selection and device technologies, improvements in outpatient management have also contributed to the survival gains. In addition to optimizing device function through control of afterload, outpatient blood pressure (BP) management in patients on CF-LVAD support has recently received attention in response to HVAD (Medtronic, Minneapolis, MN) clinical trial data demonstrating an association of increased stroke and pump thrombosis incidences in patients with elevated mean arterial pressure.(2-4) In the ENDURANCE supplemental trial, a BP monitoring protocol aiming to achieve a MAP <85 mmHg or a Doppler opening pressure <90 mmHg during HVAD support was associated with improved strokes rates compared with that of prior HVAD studies.(5) Similarly, in the PREVENT (PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management) study, a MAP <90 mmHg along with a tailored surgical implant, anticoagulation, and device management protocol lead to a reduction in HeartMate II (Abbott, Abbott Park, Illinois) pump thrombosis.(6) However, the thresholds for defining maximal BP in these analyses were not derived from the distribution of patient clinical trial BP data; rather they were largely based on expert consensus. Given the differences in pump technology, it also remains unknown if BP goals are similar for axial flow vs. centrifugal flow rotary pumps. Finally, no data exists regarding the lower limit for BP control, and thus the optimal BP range for patients on continuous flow LVAD support has yet to be fully characterized.
Based on prior data, we hypothesize that patients in the highest quartile of BP will have increased frequencies of stroke and pump thrombosis, with associated increased mortality. Using STS-INTERMACS, the primary study aims are as follows: 1) Examine BP trends up to 4 years after device implant in operative survivors of CF-LVAD implant, 2) Examine morbidity and mortality based on average patient Doppler, systolic, and mean arterial blood pressures during CF-LVAD support and by device flow profile (axial flow vs. hydrodynamic centrifugal flow) and 3) examine the utilization of cardiac medications and associated average BP during CF-LVAD support.
Methods:
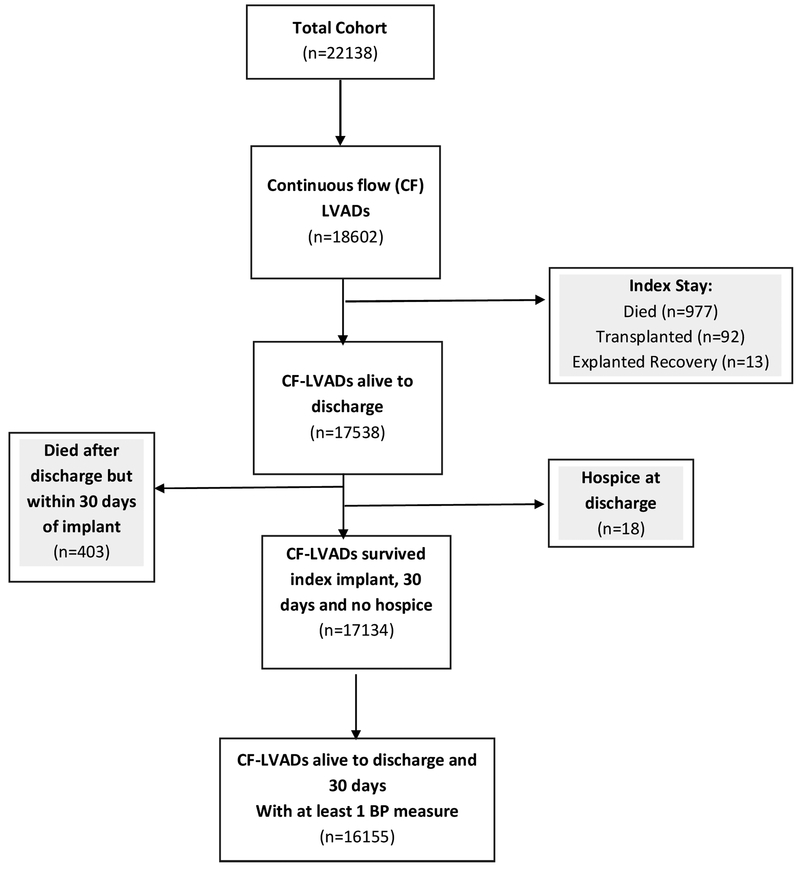
The full INTERMACS cohort consisted of 22138 patients who underwent mechanical circulatory support (MCS) implant between 2006-2015. For the purposes of this analysis, patients receiving total artificial heart support, right ventricular support without an LVAD, patients supported on pulsatile flow LVAD devices, and patients that died during the index implant, within 30 days of LVAD, or those discharged to hospice following index implant were excluded.
Blood pressures were obtained from INTERMACS at 1, 3, and 6 months, and then every 6 months until year 4. In INTERMACS, BP is available as a systolic (SBP), diastolic, and/or Doppler opening pressure. Mean arterial blood pressure (MAP) was calculated from systolic and diastolic BP when available according to the formula (2(DBP) + SBP)/3. Missing data were not imputed and patients had to have at least one BP measure ≥3 months after LVAD implant for study inclusion. Mean patient BPs (MAP, SBP, Doppler individually) were calculated for each patient during LVAD support using measures obtained ≥3 months after LVAD implant. BP measures obtained <3 months from were omitted to avoid confounding from patients with prolonged inpatient stays during index implant. The overall INTERMACS BP distributions by BP measurement modality were used to categorize patients into four BP groups according to MAP, SBP, and/or Doppler: low (<25th percentile), normal blood pressure (25-75th percentile), high (75th-95th) and very high (>95th percentile). The latter category was chosen to fully examine the impact of high BP on LVAD outcome based on previously published clinical trial data.(3, 4) Because it was possible for patients to have BP recorded by more than one measurement modality (MAP, Doppler, SBP) at the same or different follow-up periods, patients could potentially contribute data to the Doppler, SBP, and/or MAP cohorts.
Outcomes of Interest:
The main clinical outcomes of interest included the following:
The distribution of average BP based on SBP, Doppler and MAP during LVAD support.
The association between average BP and mortality overall and by device configuration (axial flow vs. hydrodynamic centrifugal flow).
The association between average BP and INTERMACS-defined morbidities, including right ventricular failure (RVF), incident stroke (any ischemic or hemorrhagic neurologic event), major infection, renal failure, and confirmed pump thrombosis.
The association between average BP during CF-LVAD support and medication use at follow-up.
Statistical Analysis:
SPSS version 24 (Chicago, IL) statistical software was used for analyses. Categorical variables were tallied as frequencies and were compared with Fisher’s exact or Pearson’s X2 tests for >2×2 comparisons. Continuous variables were assessed for normality using histograms and are reported as mean ± standard error or median [25th, 75th], as appropriate, unless otherwise specified. Possible differences between groups were assessed by Student’s t or Mann-Whitney testing, as appropriate. To compare BP changes over time, paired t-testing was used.
Kaplan-Meier survival estimates were calculated at each blood pressure threshold, censoring patients at last follow-up, time of transplant or explant for recovery. For all survival analyses, differences between BP groups were compared with log rank testing and then pairwise comparisons between BP groups were made. For pairwise survival comparisons, the Bonferroni adjustment was applied at an alpha of 0.05.
Finally, a sensitivity analysis was conducted to assess the impact of average BP on mortality based on the number of BP measures (1-2 measures, 3-4 measures, and ≥5 BP measures) that contributed to patient BP average during follow-up.
Mortality hazard ratios based on BP for the whole cohort were calculated with Cox regression modelling. Mortality comparisons were adjusted for known clinical risks. Simultaneous Cox modelling (exit criteria p<0.05) included the following covariates: advanced patient age (age >69 years), sex, prior cardiac surgery, bridge to transplant listed status, INTERMACS Profile 1-2 status, preoperative creatinine and albumin, and concomitant surgery.(1) The occurrence of a major adverse event (stroke, renal failure, infection, confirmed pump thrombosis and/or RV failure) at 30 days as well as device type (axial vs hydrodynamic centrifugal flow) and implant year were also forced into the model. For all analyses except multiple comparisons, a p ≤0.05 was considered significant.
This study, including the manuscript, was approved by the Data Access, Analysis, and Publication Committee of INTERMACS. Patient consent for INTERMACS data collection is obtained at enrolling centers per local Review Board requirements.
Results:
Of 22138 patients in INTERMACS, 16155 CF-LVAD patients survived the index implant and had at least one postoperative BP measure. Figure 1 depicts the reasons for patient exclusion. Table 1 displays the baseline characteristics and demographics of patients comprising the MAP, Doppler, and SBP cohorts. There were no clinically significant differences between the cohorts. The average patient age was 59 years, 25-26% were bridge to transplant intent, and 50-52% were of INTERMACS profile 1-2. Median patient follow-up was just under two years. Within the cohort, at least one BP measure was present in 76% of patients at 1 month, 85% at 3 months, 72% at 6 months, and 51% at 1 year and 25% at 2 years. For most patients, only one modality was used to represent BP at a given time point. For example, at 3 months, only 22% of patients with a recorded SBP also had a concomitant Doppler value recorded in INTERMACS.
Figure 1. Derivation of Final INTERMACS Cohort for Blood Pressure Analysis.
BP= blood pressure. LVAD= left ventricular assist device.
Table 1.
Baseline characteristics and demographics of patients comprising the mean arterial pressure (MAP), Doppler opening pressure, and systolic blood pressure (SBP) cohorts.
| MAP (n=9880) |
Doppler (n=11850) |
SBP (n=11543) |
|
|---|---|---|---|
| Age, years | 59 [50, 67] | 59 [50, 67] | 59 [50, 67] |
| Age >69 years | 1447 (15%) | 1856 (16%) | 1641 (14%) |
| Male, n (%) | 7715 (78%) | 9325 (79%) | 9051 (78%) |
| Ischemic myopathy | 4582 (46%) | 5343 (45%) | 5322 (46%) |
| Profile 1-2, n (%) | 5043 (51%) | 5902 (50%) | 5949 (52%) |
| Prior Sternotomy, n (%) | 3417 (35%) | 3968 (34%) | 3997 (35%) |
| Bridge to Transplant, n (%) | 2450 (25%) | 3036 (26%) | 2989 (26%) |
| Preop. Ventilator, n (%) | 649 (6.6%) | 654 (5.5%) | 763 (6.6%) |
| Preop. ECMO | 228 (2.3%) | 251 (2.1%) | 275 (2.4%) |
| Preop. Creatinine, mg/dL | 1.2±0.51 | 1.2±0.58 | 1.2±0.55 |
| Preop. Albumin, g/dL | 3.7±0.58 | 3.7±0.53 | 3.7±0.56 |
| Axial Flow, n (%) | 8191 (83%) | 9400 (79%) | 9739 (84%) |
| Centrifugal Flow, n (%) | 1589 (17%) | 2450 (21%) | 1804 (16%) |
| Concomitant surgery, n (%) | 4044 (41%) | 4838 (41%) | 4690 (41%) |
| RVAD, n (%) | 271 (2.7%) | 298 (2.5%) | 328 (2.8%) |
| Implant after 2011 | 7136 (72%) | 10150 (86%) | 7663 (66%) |
| Months on Support | 22 [12,38] | 23 [12,40] | 22 [12,36] |
| Frequency of BP measures per patient | |||
| 1 | 35% | 32% | 41% |
| 2 | 24% | 24% | 24% |
| 3 | 17% | 16% | 14% |
| 4 | 10% | 11% | 8% |
| ≥5 | 14% | 17% | 13% |
| AEs at 30 days | |||
| Stroke | 2.1% | 2.3% | 2.0% |
| Pump Thrombosis | 2.0% | 1.9% | 2.0% |
| Major Infection | 18% | 15% | 18% |
| Renal Failure | 4.8% | 4.0% | 4.8% |
| RV Failure | 3.8% | 4.0% | 3.4% |
AE= Adverse event per INTERMACS definition in 30 day survivors. BP= blood pressure. ECMO=extracorporeal membrane oxygenation; Preop. = preoperative. RV= right ventricle. RVAD= right ventricular assist device. Mean ± standard error of the mean shown for continuous variables.
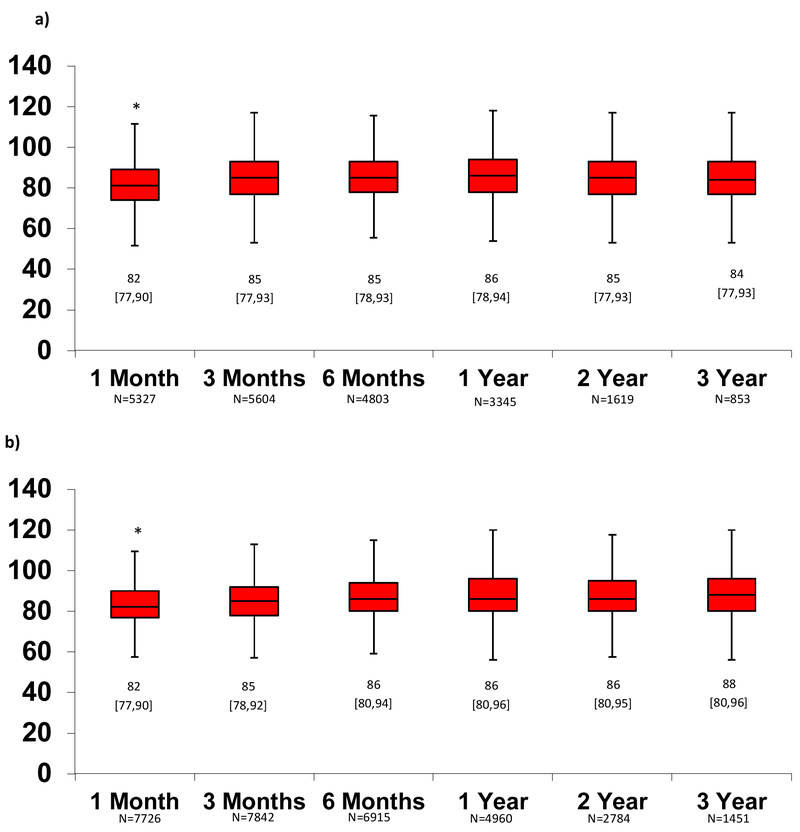
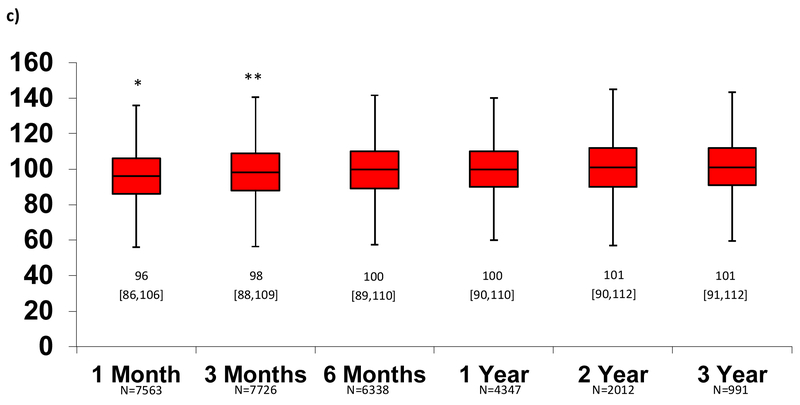
The median [25th, 75th] MAP (figure 2a), Doppler (figure 2b) and SBP (figure 2c) over time for outpatients on MCS support is depicted in Figure 2. Using paired patient data, average MAP, Doppler, and SBP at 1 month were significantly lower than BPs at subsequent follow-up time periods, but differences clinically were small (2-4 mmHg). After 1-3 months, there were no significant differences in BP measures. The average BP during follow-up was not significantly different in patients with 1-2 vs. 3-4 vs. 5+ BP measures. (Online Supplement, table 1)
Figure 2. Change in Blood Pressure Measures Over Time Measured According to Mean Arterial Pressure (MAP), Doppler Opening Pressure, and Systolic Blood Pressure (SBP).
After 1-3 months, there was no change in blood pressure in the MAP (a), Doppler (b) or SBP (c) groups. Median [25th, 75th] shown. *Paired t-test p<0.05 for 1 month vs. all subsequent months. **Paired t-test p<0.05 for 3 months vs. all subsequent months. Otherwise, p>0.05 for all other comparisons.
Overall, the median MAP ≥3 months after CFLVAD support (n=8393 patients) was 85 [78, 91] mmHg, Doppler opening pressure (n=10766) was 86 [80, 93] mmHg, SBP (n=10041) was 100 [90,109] mmHg and diastolic blood pressure (n=9919) was 75 [68,82] mmHg. Using the INTERMACS sample distribution, patients were divided into four groups based on average MAP during CF-LVAD follow-up: Low (≤75 mmHg), normal (76-90 mmHg), high (91-100 mmHg), and very high (>100 mmHg). Doppler groups are as follows: Low (≤80 mmHg), normal (81-95 mmHg), high (96-105 mmHg), and very high (>105 mmHg). Finally, SBP groups include Low (<90 mmHg), normal (91-107 mmHg), high (108-119 mmHg), and very high (≥120 mmHg).
Medication use by Blood Pressure Grouping
Medication use according to MAP category by period of follow-up is shown in table 2 with additional data for Doppler and SBP in Supplemental table 2. The findings below for MAP were clinically similar for the Doppler and SBP cohorts. Within the INTERMACS MAP cohort at 1 year, 42% were on an angiotensin-converting enzyme inhibitor (ACE-I), 12% were on an angiotensin receptor blocker (ARB), 74% were on a β-blocker, 35% were on an aldosterone inhibitor (Aldo-I), 22% were on hydralazine, 66% were on a loop diuretic, and 1.3% were on an intravenous inotrope. Up to two years after LVAD, patients in the low BP group by average MAP, SBP, and Doppler during CF-LVAD support were more likely to be on intravenous inotrope support and an Aldo-I and less likely to be on oral β-blocker therapy than those with higher BPs (p<0.05, table 2 and Supplemental table 2). In contrast, as BPs increased, the frequency of hydralazine and calcium channel blocker use increased (p <0.001). There were no consistent differences in the frequency of ACE-I or diuretic use by BP group after LVAD.
Table 2.
Medication use over time for patients alive on support at each follow-up period based on mean arterial pressure. The Supplemental Table 1 online shows similar results for blood pressure assessed by Doppler and systolic blood pressure.
| Combined Cohort |
MAP ≤75 mmHg |
MAP 76-90 mmHg |
MAP 91-100 mmHg |
MAP >100 mmHg |
p* | |
|---|---|---|---|---|---|---|
| 1 month | ||||||
| ACE-I (n=7671) | 32% | 32.6% | 31.6% | 32.0% | 34.5% | 0.62 |
| ARB (n=7667) | 6.3% | 4.6% | 6.5% | 7.4% | 7.3% | 0.013 |
| β -blocker (n=7678) | 51.6% | 46.7% | 52.0% | 54.7% | 56.7% | <0.001 |
| Digoxin (n=6689) | 22.0% | 21.3% | 22.5% | 22.8% | 16.9% | 0.075 |
| Calcium CB (n=6674) | 7.9% | 6.5% | 7.8% | 9.4% | 9.8% | 0.033 |
| Hydralazine (n=6677) | 18.8% | 15.5% | 18.2% | 23.0% | 25.1% | <0.001 |
| Loop Diuretic (n=7674) | 70.2% | 68.1% | 70.3% | 71.6% | 71.8% | 0.18 |
| Aldo-I (n=7674) | 32% | 34.5% | 32.7% | 29.2% | 31.5% | 0.029 |
| Inotrope (n=3874) | 9.8% | 17.6% | 8.4% | 6.0% | 6.3% | <0.001 |
| 6mo | ||||||
| ACE-I (n=7922) | 42.1% | 44.0% | 41.4% | 42.9% | 40.7% | 0.30 |
| ARB (n=7901) | 10.2% | 9.0% | 10.0% | 10.4% | 14.6% | 0.005 |
| β-blocker (n=7920) | 68.8% | 67.6% | 68.3% | 70.9% | 70.6% | 0.15 |
| Digoxin (n=6084) | 21.0% | 22.1% | 21.3% | 20.6% | 15.2% | 0.044 |
| Calcium CB (n=6069) | 8.6% | 12.6% | 11.3% | 15.3% | 18.2% | <0.001 |
| Hydralazine (n=6079) | 19.8% | 14.3% | 18.9% | 25.1% | 30.1% | <0.001 |
| Loop Diuretic (n=7910) | 66.8% | 66.8% | 66.6% | 66.5% | 68.7% | 0.83 |
| Aldo-I (n=7911) | 35.7% | 38.6% | 36.2% | 32.3% | 32.1% | 0.001 |
| Inotrope (n=4456) | 2.0% | 4.7% | 1.7% | 1.3% | 0.0% | <0.001 |
| 1 Year | ||||||
| ACE-I (n=5749) | 41.5% | 43.0% | 40.8% | 42.6% | 40.7% | 0.57 |
| ARB (n=5736) | 12.2% | 10.5% | 11.8% | 13.2% | 16.5% | 0.017 |
| β-blocker (n=5752) | 73.6% | 71.4% | 73.2% | 75.3% | 78.0% | 0.052 |
| Digoxin (n=4678) | 19.8% | 19.4% | 21.0% | 17.5% | 14.6% | 0.017 |
| Calcium CB (n=4654) | 15.1% | 11.5% | 14.2% | 19.9% | 19.9% | <0.001 |
| Hydralazine (n=4670) | 21.5% | 17.3% | 19.4% | 28.5% | 33.1% | <0.001 |
| Loop Diuretic (n=5743) | 66.4% | 66.5% | 66.2% | 66.7% | 66.9% | 0.99 |
| Aldo-I (n=5745) | 34.9% | 37.3% | 35.5% | 31.7% | 32.2% | 0.034 |
| Inotrope (n=2980) | 1.3% | 2.7% | 1.2% | 0.8% | 0.5% | 0.029 |
| 2 Years | ||||||
| ACE-I (n=3066) | 39.6% | 43.2% | 38.8% | 40.3% | 37.0% | 0.32 |
| ARB (n=3061) | 13.8% | 12.8% | 13.4% | 14.6% | 17.9% | 0.30 |
| β-blocker (n=3071) | 77.9% | 77.0% | 77.1% | 80.5% | 79.5% | 0.35 |
| Digoxin (n=2751) | 19.6% | 21.1% | 21.0% | 16.6% | 11.6% | 0.007 |
| Calcium CB (n=2733) | 17.5% | 13.1% | 15.5% | 25.1% | 23.9% | <0.001 |
| Hydralazine (n=2743) | 24.4% | 18.1% | 22.6% | 32.2% | 33.7% | <0.001 |
| Loop Diuretic (n=3066) | 65.4% | 64.8% | 66.0% | 63.3% | 66.3% | 0.67 |
| Aldo-I (n=3602) | 33.7% | 37.8% | 35.0% | 29.4% | 24.3% | 0.001 |
| Inotrope (1304) | 1.3% | 1.6% | 1.2% | 1.1% | 2.4% | 0.78 |
| 3 Years | ||||||
| ACE-I (n=1608) | 39.3% | 41.9% | 39.7% | 38.3% | 33.3% | 0.50 |
| ARB (n=1607) | 13.4% | 12.1% | 13.1% | 14.1% | 17.1% | 0.62 |
| β-blocker (n=1610) | 79.2% | 80.5% | 77.8% | 80.1% | 87.6% | 0.11 |
| Digoxin (n=1544) | 18.8% | 23.5% | 20.4% | 13.9% | 8.8% | 0.001 |
| Calcium CB (n=1531) | 16.3% | 10.7% | 14.7% | 22.6% | 25.2% | <0.001 |
| Hydralazine (n=1534) | 23.9% | 14.8% | 22.0% | 31.9% | 36.3% | <0.001 |
| Loop Diuretic (n=1597) | 63.6% | 61.6% | 63.5% | 62.3% | 72.4% | 0.25 |
| Aldo-I (n=1607) | 29.9% | 33.0% | 31.5% | 25.9% | 20.0% | 0.025 |
| Inotrope (n=512) | 1.0% | 1.5% | 1.0% | 1.0% | 0.0% | 0.93 |
| 4 Years | ||||||
| ACE-I (n=772) | 35.6% | 37.5% | 37.3% | 30% | 32.7% | 0.39 |
| ARB (n=775) | 14.5% | 15.0% | 14.1% | 16.0% | 12.2% | 0.91 |
| β-blocker (n=775) | 79.5% | 79.0% | 79.6% | 78.7% | 81.6% | 0.98 |
| Digoxin (n=773) | 20.8% | 25.0% | 23.1% | 12.7% | 16.3% | 0.029 |
| Calcium CB (n=764) | 15.7% | 13.0% | 13.1% | 21.3% | 28.6% | 0.006 |
| Hydralazine (n=768) | 23.0% | 9.0% | 22.2% | 27.3% | 40.8% | <0.001 |
| Loop Diuretic (n=767) | 64.7% | 65.4% | 63.1% | 68.0% | 68.8% | 0.66 |
| Aldo-I (n=773) | 31.8% | 40.0% | 32.9% | 26.8% | 22.4% | 0.092 |
| Inotrope (n=140) | 0.7% | 0% | 1.1% | 0% | 0% | 0.90 |
Pearson’s Chi Square p value comparing MAP categories. n represents number of patients with available data for each time point for use or non-use of each medication. ACE-I= angiotensin-converting enzyme inhibitor; ARB= angiotensin receptor antagonist; Aldo-I= aldosterone inhibitor; CB= channel blocker.
Survival and Adverse Events based on Average Blood Pressure during Continuous Flow LVAD Support
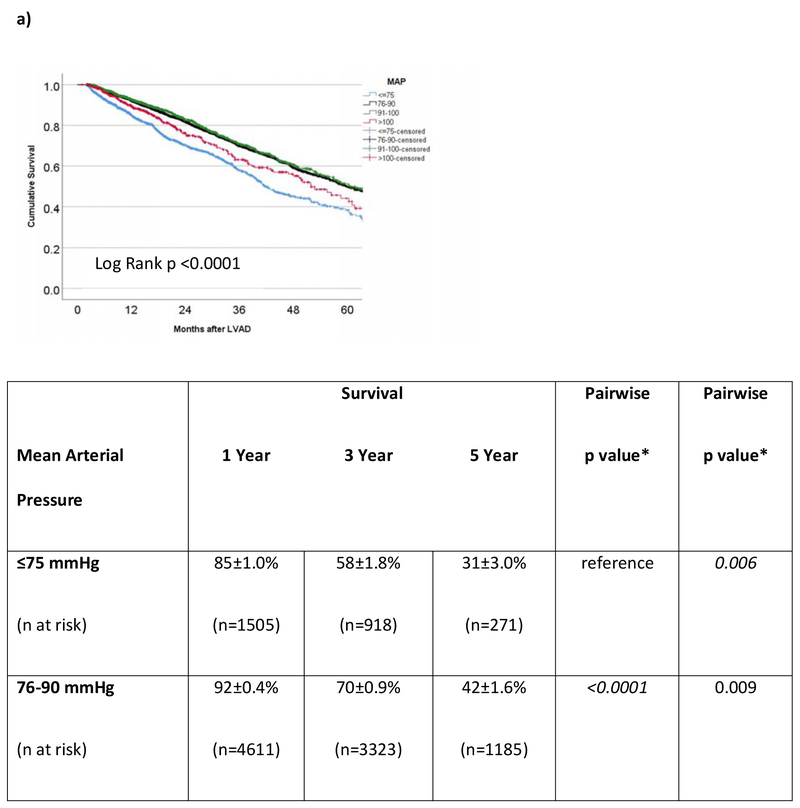
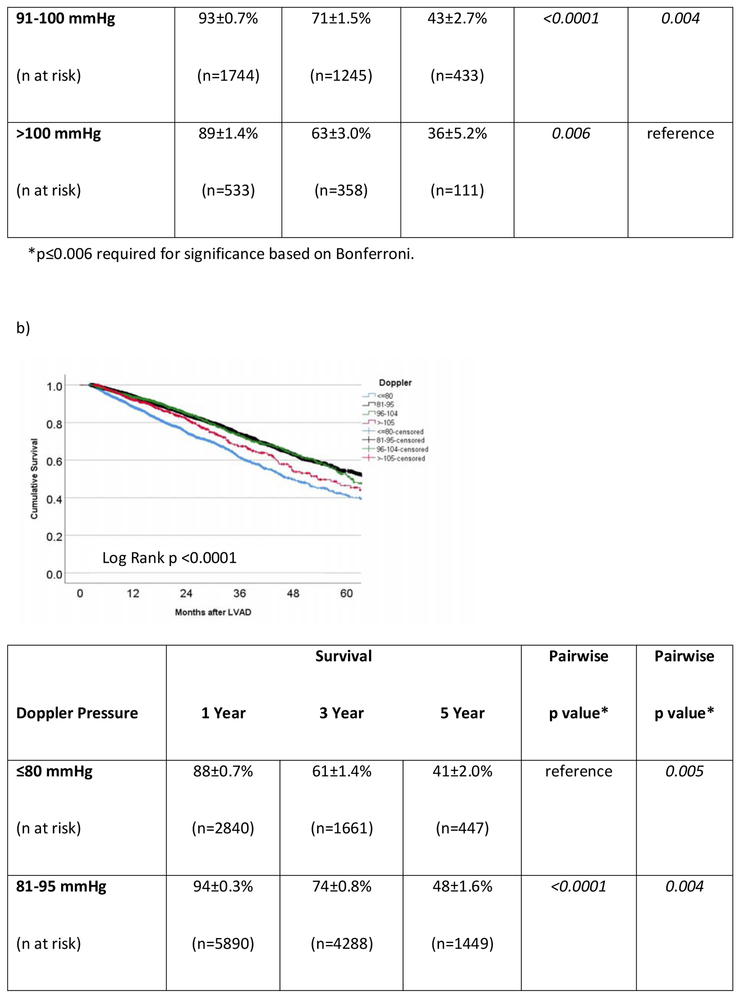
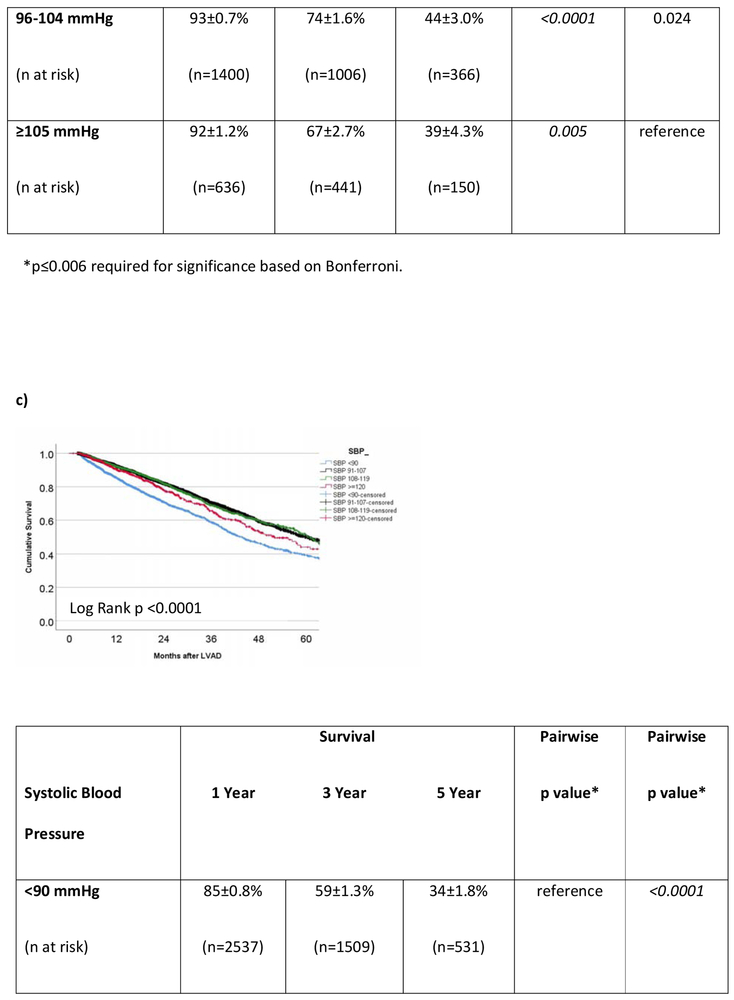
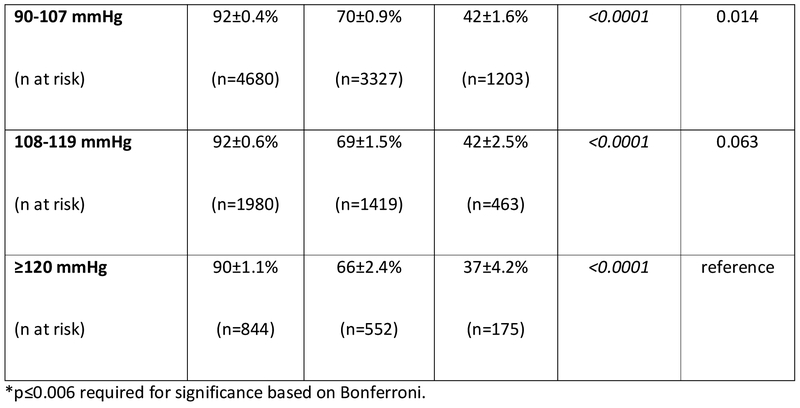
In the cohort of INTERMACS patients that survived to hospital discharge, the 1- and 3-year survivals were 87±0.3% and 64±0.6%, respectively. For the 8393 operative survivors with at least one MAP reading during CF-LVAD support, average MAP was predictive of outcome. However, the correlation was not linear. Survival at 3 years (Figure 3a) was 58±1.8% in those with low MAPs (≤75 mmHg) versus 70±0.9%, 71±1.5%, and 63±3.0% in the those with normal, high, or very high (MAP >100 mmHg) average MAPs. Low and very high MAPs were significantly associated with mortality (Figure 3a). Figure 3b shows the survival of patients (n=10766) based on average Doppler opening pressure during CF-LVAD support. Doppler opening pressure was also associated with a bimodal mortality risk correlation. By Doppler category, patients with low Doppler opening pressures (≤80 mmHg) had inferior survival (61±1.4% at 3 years) compared to patients with normal (74±0.8%, pairwise p<0.0001), high (74±1.6%, pairwise p <0.001), and very high (67±2.7%, pairwise p=0.005) values. In addition, patients with very high Dopplers (≥105 mmHg) also had significantly worse survival when compared to patients who had normal Dopplers (81-95 mmHg, p=0.004) during LVAD support. In the SBP cohort (n=10041), hypotension (SBP <90 mmHg) was again associated with increased mortality when assessed as a continuous variable (unadjusted HR= 0.88 [0.86-0.91] per 10 mmHg increase, p<0.001) or categorically (p<0.0001 for all groups on pairwise comparison, figure 3c). There was suggestion on pairwise SBP comparison that very high average SBPs (≥120 mmHg) may also increase mortality when compared to patients with normal (91-107 mmHg, p=0.014) and high (108-119 mmHg, p=0.063) SBPs, but comparisons were nonsignificant after Bonferroni adjustment.
Figure 3. Survival Based on Average Blood Pressure in INTERMACS LVAD Operative Survivors.
Survival is shown according to average a) mean arterial pressure (MAP), b) Doppler opening pressure, and c) systolic blood pressure (SBP) during continuous flow LVAD support in INTERMACS. LVAD= left ventricular assist device. INTERMACS= Interagency Registry for Mechanically Assisted Circulatory Support
Due to the variability in the number of BP measurements contributing to patient BP average after LVAD implant, a sensitivity analysis was conducted examining outcomes based on BP in those with 1-2, 3-4 and ≥5 BP readings after LVAD implant. There were no clinically significant differences in overall mean Doppler, MAP, or SBP in those with 1-2, 3-4, or ≥5 BP readings during LVAD support (online Supplementary table 3). Patients with fewer BPs by any measurement modality had inferior survival, a finding attributed to prespecified Intermacs BP data capture and follow-up protocols. When survival was assessed by the number of BP values contributing to the BP average, LVAD patients with very low BP by every modality of measure continued to display consistently inferior survivals (Supplemental table 3).
Due to the different patient samples across BP measurement modalities, separate multivariable analyses were conducted to examine the impact of MAP, SBP, and Doppler opening pressure on survival. Controlling for other known risk correlates, low average BPs remained predictive of mortality for all modalities of BP measurement, increasing the hazard of death by 35-39% compared to those with normal or high BPs (table 3 and Supplemental table 4). In addition, patients with very high average MAPs (>100 mmHg), Dopplers (>105 mmHg) and SBPs (≥120 mmHg) had a 17-20% increase in the adjusted hazard of death when compared to patients with correspondingly normal BP values.
Table 3.
Multivariable Analysis of the Impact of Blood Pressure on Mortality. Three separate models were performed, using mean arterial pressure (MAP), Doppler, or systolic blood pressure, along with ten other known correlates of mortality risk.(1)
| Mortality Adjusted HR [95% CI] |
p value | |
|---|---|---|
| Mean Arterial Pressure | ||
| MAP ≤75 mmHg | Reference | -- |
| MAP 76-90 | 0.61 [0.54-0.69] | <0.0001 |
| MAP 91-100 | 0.58 [0.50-0.67] | <0.0001 |
| MAP >100 | 0.77 [0.63-0.94] | 0.012 |
| MAP ≤75 mmHg | 1.31 [1.06-1.60] | 0.012 |
| MAP 76-90 | 0.80 [0.6-0.96] | 0.017 |
| MAP 91-100 | 0.75 [0.61-0.93] | 0.007 |
| MAP >100 | Reference | --- |
| Doppler Opening Pressure | ||
| Doppler ≤80 mmHg | Reference | ---- |
| Doppler 81-95 | 0.63 [0.57-0.70] | <0.0001 |
| Doppler 96-105 | 0.66 [0.57-0.76] | <0.0001 |
| Doppler >105 | 0.77 [0.64-0.93] | 0.006 |
| Doppler ≤80 mmHg | 1.29 [1.08-1.55] | 0.006 |
| Doppler 81-95 | 0.82 [0.68-0.97] | 0.022 |
| Doppler 96-105 | 0.85 [0.70-1.04] | 0.12 |
| Doppler >105 | reference | --- |
| Systolic Blood Pressure | ||
| SBP <90 mmHg | Reference | --- |
| SBP 90-107 | 0.65 [0.59-0.71] | <0.0001 |
| SBP 108-119 | 0.65 [0.58-0.74] | <0.0001 |
| SBP ≥120 | 0.78 [0.66-0.92] | 0.003 |
| SBP <90 mmHg | 1.28 [1.09-1.51] | 0.003 |
| SBP 90-107 | 0.83 [0.71-0.97] | 0.018 |
| SBP 108-119 | 0.84 [0.70-0.99] | 0.039 |
| SBP ≥120 | Reference | --- |
Impact of MAP, Doppler, and SBP on mortality were analyzed controlling for major adverse events (stroke, renal failure, infection, right ventricular failure, confirmed pump thrombosis) at 30 days and the following correlates of risk: age ≥69, sex, prior sternotomy, bridge to transplant intent, INTERMACS profile 1-2, preoperative serum albumin, preoperative creatinine, implant year, concomitant surgery at time of LVAD, centrifugal flow support, and implant year. Models are shown with the highest or lowest BP group as the reference for risk, controlling for the aforementioned covariables. For example, patients with an SBP ≥120 mmHg had an adjusted HR for death that was 17% higher than those with an SBP of 90-107. For those with SBP <90 mmHg, the adjusted HR was 65% higher than those with an SBP of 90-107 mmHg. Hazard ratios for covariables shown in supplemental table 2 online. BTT= bridge to transplant; HR= hazard ratio; MAP= mean arterial pressure, SBP= systolic blood pressure.
Adverse Events based on Blood Pressure Category in Patients on CF-LVAD Support
The frequencies of incident adverse events at 3 years for the entire INTERMACS cohort and each BP sample are shown in table 4. Within INTERMACS, there were 1934 incident strokes during CF-LVAD follow-up. At 3 years, freedom from incident stroke in the entire INTERMACS sample was 82±0.4%. The association of BP overall was inconsistent and differed by method used to represent BP. The mean MAP was 85±0.1 mmHg and 85.6±0.1 mmHg in those with and without an incident stroke, respectively, during support (p=0.13). There was no significant association between incident stroke based on MAP as a continuous variable (HR 1.02 [0.96-1.08] per 10 mmHg increase in MAP, p=0.59) or by MAP category (log rank p=0.22, supplemental figure 1a). Doppler BPs were correlated with stroke such that freedom from stroke was numerically lowest in those patients with low Dopplers (80±1.2%) and very high average Dopplers (79±2.2%) compared with Dopplers of 81-95 mmHg (84±0.6%) and 96-104 mmHg (82±1.3%) (supplemental figure 1b, log rank p=0.016). When analyzed continuously, elevated Doppler opening pressure did not confer a significantly increased stroke risk (HR 0.97 [0.92-1.02] per 10 mmHg increase in Doppler, p=0.26). In contrast, higher average SBP during CF-LVAD support correlated with incident stroke (HR=1.07 [1.03-1.11] per 10 mmHg increase in SBP, p=0.001). When analyzed categorically, patients with high and very high SBPs had numerically the highest stroke frequencies (log rank p=0.001, supplemental figure 1c). Between group comparisons did not meet Bonferroni statistical significance.
Table 4.
Freedom for Incident Adverse Events Categorized by Average Blood Pressure during Continuous Flow LVAD support. Patient average blood pressure was calculated from measures obtained at month 3 and onward.
| Freedom from Incident Event at 3 years | |||||
|---|---|---|---|---|---|
| Stroke | Renal Failure |
Confirmed Pump Thrombosis |
Infection | RV Failure | |
| INTERMACS Cohort | 82±0.4% | 85±0.4% | 95±0.3% | 36±0.6% | 87±0.4% |
| Mean Arterial Pressure Sample (n=8393 patients) | |||||
| Log rank p | 0.22 | <0.0001 | 0.025 | <0.0001 | 0.001 |
| MAP ≤75 (n=1505) | 81±1.5% | 80±1.4% | 94±0.9% | 28±1.7% | 82±1.5% |
| MAP 76-90 (n=4611) | 83±0.7% | 85±0.7% | 96±0.4% | 34±0.9% | 85±0.7% |
| MAP 91-100 (n=1744) | 83±1.1% | 86±1.1% | 96±0.7% | 35±1.6% | 87±1.1% |
| MAP >100 (n=533) | 78±2.5% | 82±1.9% | 94±1.5% | 41±2.8% | 84±2.6% |
| Doppler Opening Pressure Sample (n=10766) | |||||
| Log rank p | 0.016 | <0.001 | 0.031 | <0.0001 | <0.0001 |
| ≤80 mmHg (n=2840) | 80±1.2% | 83±1.1% | 95±0.7% | 35±1.3% | 81±1.2% |
| 81-95 mmHg (n=5890) | 84±0.6% | 88±0.6% | 96±0.4% | 40±0.9% | 84±0.7% |
| 96-104 mmHg (n=1400) | 82±1.3% | 86±1.2% | 96±0.3% | 39±1.7% | 84±1.3% |
| ≥105 mmHg (n=636) | 79±2.2% | 84±1.9% | 93±1.4% | 46±2.6% | 85±2.0% |
| Systolic Blood Pressure sample (n=10041) | |||||
| Log rank p | 0.001 | <0.0001 | 0.17 | <0.0001 | 0.17 |
| <90 mmHg (n=2537) | 84±1.1% | 82±1.0% | 97±0.6% | 31±1.3% | 88±1.0% |
| 90-107 mmHg (n=4680) | 84±0.7% | 86±0.6% | 96±0.4% | 34±0.9% | 86±0.7% |
| 108-119 mmHg (n=1980) | 80±1.2% | 85±1.1% | 95±0.7% | 37±1.4% | 86±1.1% |
| ≥120 mmHg (n=844) | 79±2.0% | 85±1.7% | 96±1.0% | 39±2.3% | 83±2.0% |
Low average BPs by MAP, Doppler and SBP were associated an increased incidence of renal failure and major infection (all p<0.0001, Table 4). Patients in the low MAP and Doppler groups were also more likely to have increased incident RV failure (p<0.001). The correlation between blood pressure and confirmed pump thrombosis was inconsistent and/or of small clinical magnitude.
Survival and Adverse Event Profile According to LVAD Flow Type and Blood Pressure
In patients (n=13227) supported with an axial flow LVAD, survival at 1- and 3-years was 87±0.3% and 64±0.6%, respectively. Blood pressure correlated with mortality during axial flow LVAD support (p<0.0001 for all modes of measure, table 5, supplemental figure 2a-3a). Three-year survival in those with a low MAP (≤75 mmHg) was 57±1.9% compared with survivals of 70-71% in those with normal and high MAPs (all p≤0.005). Overall, patients with a MAP ≤75 mmHg had a 67% increase in the hazard of death during axial flow LVAD support (unadjusted HR=1.67 [1.5-1.9] vs. MAP >75 mmHg). A similar correlation for mortality in those with low pressures was noted based on average Doppler and SBP measurement during axial flow LVAD support (Table 5). When compared to those with normal BPs, a mortality signal was also noted in the 572 patients on axial flow support who had very high Dopplers (≥105 mmHg) and the 478 patients with very high MAPS (>100 mmHg); results of the multiple comparisons should be interpreted with caution.
Table 5.
Estimated survival based on blood pressure group in patients on axial flow LVAD support. Patient average blood pressure was calculated from measures obtained month 3 and onward after LVAD.
| Estimated Survival (%) during Axial Flow LVAD support | |||||
|---|---|---|---|---|---|
| 90 day | 1 Year | 3 Year | p* | P* | |
| MAP Group, mmHg | Log rank p <0.0001 | ||||
| MAP ≤75 (n at risk) | 98.1 ± 0.4% (n=1176) | 84 ± 1.1% (n=1173) | 57 ± 1.9% (n=731) | Reference | 0.005 |
| MAP 76-90 (n at risk) | 99.7 ± 0.01% (n=3896) | 92 ± 0.5% (n=3867) | 70± 1.0% (n=2896) | <0.0001 | 0.004 |
| MAP 91-100 (n at risk) | 99.8 ± 0.1% (n=1496) | 93 ± 0.7% (n=1488) | 71 ± 1.6% (n=1086) | <0.0001 | 0.005 |
| MAP >100 (n at risk) | 99.4 ± 0.4% (n=478) | 89 ± 1.5% (n=470) | 62 ± 3.2% (n=329) | 0.005 | Reference |
| Doppler Group, mmHg | Log rank p <0.0001 | ||||
| Doppler ≤80 (n at risk) | 99.3 ± 0.2% (n=2051) | 88 ± 0.8% (n=2023) | 61± 1.5% (n=1293) | Reference | NS |
| Doppler 81-95 (n at risk) | 99.8± 0.1% (n=4784) | 94± 0.4% (n=4762) | 74± 0.8% (n=3651) | <0.0001 | 0.001 |
| Doppler 96-105 (n at risk) | 99.9± 0.1% (n=1212) | 94± 0.7% (n=1209) | 74± 1.7% (n=899) | <0.0001 | NS |
| Doppler >105 (n at risk) | 99.8 ± 0.2% (n=572) | 92± 1.2% (n=571) | 66± 2.8% (n=399) | NS | Reference |
| SBP Group, mmHg | Log rank p<0.0001 | ||||
| SBP <90 (n at risk) | 98.9 ± 0.2% (n=2150) | 85 ± 0.8% (n=2105) | 58 ± 1.4% (n=1308) | Reference | 0.001 |
| SBP 90-107 (n at risk) | 99.6 ± 0.1% (n=3969) | 92 ± 0.4% (n=3935) | 70 ± 1.0% (n=2897) | <0.0001 | NS |
| SBP 108-119 (n at risk) | 99.9 ± 0.1% (n=1722) | 92 ± 0.7% (n=1717) | 70 ±1.5% (n=1261) | <0.0001 | NS |
| SBP ≥120 (n at risk) | 99.6 ± 0.2% (n=857) | 90 ±1.2% (n=724) | 65 ± 2.5% (n=491) | 0.001 | Reference |
NS= nonsignificant after Bonferroni adjustment (*p threshold p≤0.006)
Survival in patients (n=2928) on hydrodynamic centrifugal flow LVAD support at 1- and 3-years was 88±0.7% and 66±0.6%, respectively. Lower MAP (HR 0.82 [0.72-0.94] per 10 mmHg increase), lower Doppler opening pressure (0.82 [0.72-0.93] per 10 mmHg increase) and lower SBP (HR 0.87 [0.79-0.97] per 10 mmHg increase) correlated with worse survival after hydrodynamic centrifugal flow LVAD implant. Survivals by BP categories are in table 6 (Figure 2b-3b in online supplement). There was no correlation between hypertension and survival in this group of patients.
Table 6.
Estimated survival based on blood pressure group in patients on hydrodynamic centrifugal flow LVAD support. Patient average blood pressure was calculated from measures obtained month 3 and onward after LVAD.
| Estimated Survival (%) during Centrifugal Flow Support | ||||
|---|---|---|---|---|
| 90 day | 1 Year | 3 Year | P | |
| MAP Group, mmHg | p = 0.063 | |||
| MAP ≤75 (n at risk) | 98.2 ± 0.7% (n=329) | 89 ± 1.9% (n=320) | 67 ± 4.7% (n=179) | -- |
| MAP 76-90 (n at risk) | 99.4 ± 0.3% (n=715) | 91 ± 1.2% (n=708) | 69± 3.2% (n=424) | -- |
| MAP 91-100 (n at risk) | 99.6 ± 0.4% (n=248) | 94 ± 1.6% (n=244) | 75 ± 5.9%(n=152) | -- |
| MAP >100 (n at risk) | 100% (n=55) | 93 ± 3.6% (n=52) | 81 ±7.5 (n=27) | -- |
| Doppler Group, mmHg | p=0.038 | |||
| Doppler ≤80 (n at risk) | 99.9 ± 0.1% (n=789) | 88 ± 1.4% (n=773) | 66± 3.7% (n=374) | -- |
| Doppler 81-95 (n at risk) | 99.9 ± 0.1% (n=1106) | 93 ± 0.9% (n=1088) | 69± 3.0% (n=636) | -- |
| Doppler 96-105 (n at risk) | 100% (n=188) | 90 ± 2.5% (n=185) | 73 ± 5.8% (n=104) | -- |
| Doppler >105 (n at risk) | 100% (n=64) | 90 ± 4.1% (n=63) | 84 ± 7.0% (n=399) | -- |
| SBP Group, mmHg | p=0.018 | |||
| SBP <90 (n at risk) | 99.0 ± 0.5% (n=387) | 89 ± 1.8% (n=380) | 66 ± 4.7% (n=203) | |
| SBP 90-107 (n at risk) | 99.7 ± 0.2%(n=711) | 93 ± 1.1% (n=707) | 74 ± 3.1% (n=415) | |
| SBP 108-119 (n at risk) | 99.6 ± 0.4% (n=258) | 91 ± 1.9% (n=254) | 60 ± 6.3% (n=155) | |
| SBP ≥120 (n at risk) | 100% (n=108) | 93 ± 2.7% (n=106) | 79 ± 6.8% (n=61) | |
Association of Blood Pressure and Adverse Events by LVAD Flow Profile
Table 7 shows the overall freedom from incident adverse events at 3 years in patients supported with an axial flow LVAD and by BP grouping. Increased Doppler SBP (HR 1.08 [1.04-1.13] per 10 mmHg increase) was significantly correlative with stroke when analyzed as a continuous variable. Mean arterial pressure (p=0.24) and Doppler opening pressure (p=0.13) were not. On categorial BP comparison, patients with very high Dopplers and very high SBPs had numerically lower freedom from incident stroke when compared to patients with lower average BP values; statistical significance did not reach Bonferroni requirements. Lower BPs measures by all modalities were associated with increased incident infection. The correlation between average BP and RV failure and confirmed pump thrombosis was inconsistent in patients on axial flow LVAD support.
Table 7.
Freedom from Adverse Events Categorized by Average Blood Pressure during Axial Flow LVAD Support. Patient average blood pressure was calculated from measures obtained month 3 and onward after LVAD.
| Freedom from Incident Event at 3 years, Axial Flow Cohort | |||||
|---|---|---|---|---|---|
| Stroke | Renal Failure |
Confirmed Pump Thrombosis |
Infection | RV Failure | |
| All Axial Flow | 83±0.5% | 85±0.4% | 95±0.3% | 37±0.6% | 87±0.5% |
| Mean Arterial Pressure Sample (n=7046 patients) | |||||
| Log rank p | 0.40 | <0.001 | 0.005 | <0.0001 | 0.27 |
| MAP ≤75 mmHg (n=1176) | 84±1.6% | 77±1.4% | 94±1.0% | 29±1.8% | 86±1.5% |
| MAP 76-90 mmHg (n=3896) | 84±0.8% | 84±0.7% | 96±0.4% | 34±1.0% | 86±0.8% |
| MAP 91-100 mmHg (n=1496) | 84±1.2% | 86±1.2% | 96±0.7% | 36±1.7% | 88±1.1% |
| MAP >100 mmHg (n=478) | 79±2.6% | 87±2.0% | 95±1.5% | 42±2.9% | 85±2.6% |
| Doppler Opening Pressure Sample (n=8619) | |||||
| Log rank p | 0.019 | <0.0001 | 0.016 | 0.001 | 0.24 |
| ≤80 mmHg (n=2051) | 82±1.3% | 84±1.2% | 95±0.7% | 36±1.5% | 84±1.3% |
| 81-95 mmHg (n=4783) | 85±0.7% | 88±0.6% | 96±0.4% | 40±0.9% | 86±0.7% |
| 96-104 mmHg (n=1211) | 84±1.4% | 87±1.2% | 97±0.7% | 40±1.8% | 86±1.3% |
| ≥105 mmHg (n=572) | 79±2.3% | 84±2.0% | 94±1.4% | 43±2.7% | 86±2.0% |
| Systolic Blood Pressure sample (n=8577) | |||||
| Log rank p | 0.001 | <0.0001 | 0.63 | <0.0001 | 0.003 |
| <90 mmHg (n=2150) | 84±1.1% | 82±1.1% | 97±0.6% | 31±1.4% | 91±0.9% |
| 90-107 mmHg (n=3969) | 85±0.7% | 86±0.7% | 96±0.4% | 34±1.0% | 89±0.6% |
| 108-119 mmHg (n=1722) | 81±1.3% | 85±1.1% | 96±0.6% | 38±1.5% | 87±1.2% |
| ≥120 mmHg (n=736) | 80±2.1% | 85±1.8% | 96±1.0% | 38±2.4% | 85±2.0% |
INTERMACS= Interagency Registry of Mechanically Assisted Circulatory Support; LVAD= left ventricular assist device; RV= right ventricular
In the hydrodynamic centrifugal flow group, there was no association in operative survivors between stroke and BP when BP was examined as a categorical (table 8) or continuous variable (all p>0.05, data not shown) as MAP, Doppler, or SBP. The mean MAP in those with and without an incident stroke was 82.9±0.7 vs 82.6±0.3 mmHg, respectively. Lower BP was associated with an increased frequency of first infection in the Doppler cohort (HR=0.90 [0.84-0.96] per 10 mmHg increase), development of RV failure (MAP HR=0.87 [0.77-0.98] per 10 mmHg; Doppler HR=0.86 [0.76-0.96] per 10 mmHg) and development of renal failure (MAP HR=0.98 [0.97-0.99] per 10 mmHg; Doppler HR=0.98 [0.96-0.99] per 10 mmHg increase; SBP HR=0.98 [0.96-0.99] per 10 mmHg) (categorical data in table 8). High SBP correlated with an increased frequency of confirmed PT in the hydrodynamic centrifugal flow cohort (SBP HR= 1.03 [1.004-1.05] per 10 mmHg; Doppler and MAP p>0.05). These findings were not confirmed when patients with high Dopplers or high MAPs were examined.
Table 8.
Freedom from Adverse Events by Blood Pressure During Centrifugal Flow LVAD support. Patient average blood pressure was calculated from measures obtained month 3 and onward after LVAD.
| Freedom from Incident Event at 3 years, Centrifugal Flow | |||||
|---|---|---|---|---|---|
| Stroke | Renal Failure |
Confirmed Pump Thrombosis |
Infection | RV Failure | |
| Centrifugal Flow Cohort | 77±1.4% | 83±1.4% | 92±1.2% | 35±1.7% | 73±1.8% |
| Mean Arterial Pressure Sample (n=1347 patients) | |||||
| Log rank p | 0.40 | 0.089 | 0.24 | 0.47 | 0.007 |
| ≤75 mmHg (n=445) | 75±3.3% | 75±5.4% | 95±2.0% | 25±5.3% | 66±5.0% |
| 76-90 mmHg (n=936) | 77±2.3% | 70±3.7% | 90±2.3% | 33±2.9% | 74±3.4% |
| 91-100 mmHg (n=248) | 79±4.0% | 86±3.7% | 90±5.0% | 40±5.0% | 77±4.1% |
| >100 mmHg (n=60) | 75±6.7% | 92±5.7% | 88±6.5% | 28±10% | 66±16% |
| Doppler Sample (n=2147 patients) | |||||
| Log rank p | 0.15 | 0.014 | 0.99 | 0.013 | 0.001 |
| ≤80 mmHg (n=952) | 77±2.4% | 70±4.0% | 92±2.2% | 31±3.2% | 76±2.1% |
| 81-95 mmHg (n=1263) | 79±2.1% | 74±3.1% | 92±1.7% | 38±2.5% | 74±2.9% |
| 96-104 mmHg (n=184) | 74±4.3% | 86±3.5% | 93±2.9% | 34±5.4% | 73±4.4% |
| ≥105 mmHg (n=51) | 83±5.2% | 86±7.9% | 94±4.3% | 44±8.1% | 79±9.2% |
| Systolic Blood Pressure sample (n=1464) | |||||
| Log rank p | 0.34 | <0.0001 | 0.016 | 0.62 | 0.20 |
| <90 mmHg (n=449) | 82±2.6% | 63±5.7% | 96±2.1% | 29±4.1% | 65±6.0% |
| 90-107 mmHg (n=981) | 76±2.5% | 78±3.6% | 93±1.7% | 37±2.7% | 75±3.0% |
| 108-119 mmHg (n=277) | 76±3.4% | 77±4.5% | 80±6.2% | 25±5.0% | 80±3.9% |
| ≥120 mmHg (n=97) | 82±4.1% | 79±7.4% | 98±1.4% | 46±6.4% | 73±8.8% |
INTERMACS= Interagency Registry of Mechanically Assisted Circulatory Support; LVAD= left ventricular assist device; RV= right ventricular
Discussion
Using INTERMACS, several important findings in relation to average BPs during continuous flow LVAD support were noted. First, after 1-3 months of support, BPs changed little. Individuals with higher BPs were more likely to be on supplemental antihypertensive therapies such as calcium channel blockers and hydralazine, while those with lower BPs were more likely to be on inotrope support. Most importantly, BPs correlated with clinically important outcomes. Consistently, individuals with a low average BP during LVAD support had higher mortality and increased frequency of right ventricular failure, incident infection, and renal failure across the different BP measurement modalities and device flow (axial/centrifugal) types. On multivariable analyses, extreme hypertension was also associated with higher mortality, while the correlations between hypertension and neurologic and thrombotic events were incongruous.
The results herein are clinically important as the field accumulates data to devise guidelines for outpatient management of patients on continuous flow LVAD technologies. From this analysis, a lower BP safety limit is suggested based on an increased frequency of incident RV failure and renal failure in those on axial and centrifugal flow devices within the lowest quartile of the MAP, Doppler, and SBP groups. While this analysis is not designed to elucidate cause and effect, it is likely that hypotension is a marker of co-existing illness on device support rather than a cause of RV failure or infection. Hypotheses for the correlation between hypotension and RV and renal failure can be devised based on prior clinical studies.(7, 8) Low pulsatility in the vasculature of CF-LVAD patients compounded by reduced systemic vascular resistance may lead to hypoperfusion in the renal vascular beds and worsening renal function.(7) A reduction in RV contractility further reduces peripheral arterial pulsatility which may lead to a further reduction in renal perfusion. Combined with these data, we feel that overaggressive pharmaceutical management of BP in patients on LVAD support with concomitant severe RV failure should generally be avoided. In addition, these results support avoiding over treatment of BP in stable patients on CF-LVAD support. This may be particularly important to patients on devices with flow response and pulsatility algorithms meant to mimic innate pulse pressures. In this setting, a Doppler opening pressure may only approximate SBP and excessive afterload reduction may be harmful.
A correlation between adverse events and hypertension during CF-LVAD support has been previously published.(4, 5, 9, 10) The largest studies to date have mainly focused on patients on hydrodynamic centrifugal flow LVAD support, demonstrating a correlation between hypertension (MAP≥90 mmHg) and stroke and pump thrombosis (2, 4, 5) The ENDURANCE Supplemental trial was a study of patients randomized to either the HVAD or the HMII for destination therapy support.(5) Within the HVAD group alone, patients were trained on BP monitoring and medications were titrated by practitioners to maintain a MAP ≤85 mmHg or Doppler opening pressure ≤90 mmHg.(5) While there was no difference in incident stroke between HVAD and HMII study groups, HVAD patients in the ENDURANCE Supplemental trial had a 24% lower stroke incidence than those in the original ENDURANCE trial, a finding attributed to improved BP management. The mean MAPs at the time of stroke, however, were not severely elevated in either cohort (HVAD = 80.7 mmHg vs. HMII= 78.1 mmHg).(5) In this INTERMACS analysis, average MAPS in patients on hydrodynamic centrifugal flow support with an incident stroke were only 82±0.5 mmHg. Further, average BP by any modality of measure did not consistently correlate with incident stroke and no findings of increased PT were noted in the centrifugal flow subgroup. While these findings were unanticipated, several factors may explain the discrepant results. First, INTERMACS is a more heterogeneous cohort than that of the patients enrolled into clinical trials. Further, as this was an analysis of outpatients on chronic LVAD support, we purposely excluded individuals who died perioperatively. The early postoperative period, likely for multifactorial reasons, bears the highest hazard for neurologic and PT events after MCS implant.(4, 11) Finally, the average BPs noted herein and in the ENDURANCE Supplemental trial in those with cerebral events suggests that the interplay of BP and stroke during centrifugal flow LVAD support may be more complicated than BP control alone.
In patients on axial flow LVAD support, the correlation between hypertension and adverse events has been less well examined. A cohort study by Nassif et al, composed of 275 patients (244 on axial flow LVAD support) demonstrated a 2.5 fold increased risk of stroke patients with an SBP above the median (SBP >110 mmHg) at the time of hospital discharge.(9) In another study of 99 patients (axial flow = 90; centrifugal flow = 9) on CF-LVAD support, an average Doppler opening pressure ≥90 mmHg during CF-LVAD support was associated with an increased frequency of cumulative adverse events (defined as intracranial hemorrhage, aortic insufficiency, thromboembolism), with the outcome primarily driven by an increased incidence of intracranial hemorrhage.(12)
In the present analysis, we were unable to replicate a consistent correlation between elevated BP and incident stroke and/or confirmed pump thrombosis, even when outcomes were analyzed separately by axial and centrifugal flow designs. Randomized studies with prescripted BP targets in patients on the same CF-LVAD type are needed to determine the attributable risk of hypertension on stroke, renal and RV failure, and such studies need to be conducted separately for each device flow type so that device-specific BP targets can be devised. In the interim, the correlation between stroke and hypertension from prior published trials; the increased adjusted mortality associated with hypertension herein; as well as the known inverse correlation between device flow and afterload support avoidance of hypertension in patients on CF-LVAD support.(2, 3, 5)
Limitations: While INTERMACS is a large database encompassing the two most commonly used LVADs through 2016, the analyses are subject to limitations inherent to registry analyses. Despite the number of patients in INTERMACS, paired BP data was limited. However, the numbers of INTERMACS patients with BP data at 1 year (n=3345-4347) and 2 years (n=1619-2784) still exceeded that of previously published trials examining blood pressure (5,11). In addition, this analysis does not capture outcomes in those patients on centrifugal flow support using rotary pumps with full magnetic levitation of the impeller. INTERMACS is also limited in its ability to precisely time events and BP readings, thus average BP during the entire period of LVAD support were used for outcome assessment. It is possible that adverse events are more likely to occur when BPs are outside the patient average. The INTERMACS registry does not collect BPs at the time of incident stroke nor were we able to determine an inpatient from an outpatient BP measure.
Probably one of the most important limitations of this analysis, and in the management of LVAD patients overall, lies in the challenge of knowing what is measured during BP assessment. Unless an arterial line is present, extrapolation of a Doppler opening pressure beyond systolic pressure is met with uncertainty. Current automated oscillatory BP cuff technologies rely on measuring acoustic waves during cuff deflation that are generated by arterial wall vibrations. Using proprietary manufacturer algorithms, estimates of mean, systolic and diastolic BP are generated. In patients on CF-LVAD support, the dampened arterial pulsatility impairs the accuracy of oscillatory automated BP cuffs.(13) As such, many centers use Doppler opening pressure to assess CF-LVAD patient BP control. However, using this single assessment fails to clearly define if the measure approximates both MAP and SBP (in those with low pulse pressure) or just SBP (in those with higher pulse pressure). Assuming that most patients have low pulse pressure while on the continuous flow technologies studied herein, results from the Doppler and systolic BP cohorts should have been similar. However, this was not consistently noted. The inconsistencies may be due to differences in patient samples or due to the fact that MAP may be the more critical measure during CFLVAD support, reflecting the continuous pressure faced by the end-organs during low pulse-pressure support. Obtaining a MAP in the outpatient setting from automated cuff technologies however, is limited by an inability to measure a precise and accurate diastolic pressure. The addition of pressure sensor technology within the aorta of patients would greatly expand our ability to better management CF-LVAD patients.
In summary, this analysis of patients on continuous flow LVADs in INTERMACS demonstrated little variability in blood pressure values after 1 month of implant. The correlation between hypotension and adverse events during LVAD support would support avoidance of excessive (MAP ≤75 mmHg, Doppler <80 mmHg) pharmacologic BP control. While hypertension was not consistently associated with neurologic and/or thrombotic events, the mortality data herein and findings from prior trials support targeting a MAP goal of >75 mmHg and <90 mmHg. In patients with pulsatility for whom a MAP cannot be accurately measured noninvasively, these results would support targeting a Doppler opening pressure <105 mmHg until device-specific recommendations are gleaned from clinical trial.
Supplementary Material
Acknowledgments
Funding: Data collection for this work was funding in whole or in part with the Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268201100025C.
Footnotes
Disclosures: All investigators work at institutions that receive clinical grant support from Abbott and Medtronic. J Cowger receives compensation from Abbott and Medtronic for speaking engagements and is on the Medtronic Scientific Advisory Committee and the INTERMACS Scientific Committee. P Shah reports grant support from American Heart Association / Enduring Hearts Scientist Development Grant, Merck for unrelated research and consulting for NuPulse CV, Procyrion and Ortho Clinical Diagnostics. S. Pinney receives consulting and speaking fees from Abbott and Medtronic. No other authors have additional disclosures to present.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving Indications, Outcomes, and Scientific Partnerships. Ann Thorac Surg. 2019;107(2):341–53. [DOI] [PubMed] [Google Scholar]
- 2.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34. [DOI] [PubMed] [Google Scholar]
- 3.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376(5):451–60. [DOI] [PubMed] [Google Scholar]
- 4.Teuteberg JJ, Slaughter MS, Rogers JG, McGee EC, Pagani FD, Gordon R, et al. The HVAD Left Ventricular Assist Device: Risk Factors for Neurological Events and Risk Mitigation Strategies. JACC Heart Fail. 2015;3(10):818–28. [DOI] [PubMed] [Google Scholar]
- 5.Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018;6(9):792–802. [DOI] [PubMed] [Google Scholar]
- 6.Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant. 2017;36(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7.Cowger JA, Radjef R. Advanced Heart Failure Therapies and Cardiorenal Syndrome. Advances in Chronic Kidney Disease. 2018;25(5):443–53. [DOI] [PubMed] [Google Scholar]
- 8.Lambert. Perioperative Management of the Right and Left Ventricle. Cardiology Clinics. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Nassif ME, Tibrewala A, Raymer DS, Andruska A, Novak E, Vader JM, et al. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Heart Lung Transplant. 2015;34(4):503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinsino A, Castagna F, Zuver AM, Royzman EA, Nasiri M, Stohr EJ, et al. Prognostic implications of serial outpatient blood pressure measurements in patients with an axial continuous-flow left ventricular assist device. J Heart Lung Transplant. 2019;38(4):396–405. [DOI] [PubMed] [Google Scholar]
- 11.Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, et al. Comprehensive Analysis of Stroke in the Long-Term Cohort of the MOMENTUM 3 Study. Circulation. 2019;139(2):155–68. [DOI] [PubMed] [Google Scholar]
- 12.Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, et al. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circ Heart Fail. 2015;8(3):551–6. [DOI] [PubMed] [Google Scholar]
- 13.Lanier GM, Orlanes K, Hayashi Y, Murphy J, Flannery M, Te-Frey R, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail. 2013;6(5):1005–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.