Abstract
The delivery of noncoding (nc)RNA to target cancer stem cells and metastatic tumors has shown many positive outcomes, resulting in improved and more efficient treatment strategies. The success of therapeutic RNA depends solely on passing cellular barriers to reach the target site, where it binds to the mRNA of the interest. By 2018, 20 clinical trials had been initiated, most focusing on cancer and diabetes, with some progressing to Phase II clinical trials testing the safety and efficacy of small interfering (si)RNA. Many challenges limit RNA interference (RNAi) and miRNA usage in vivo; therefore, various approaches have been developed to promote ncRNA efficiency and stability. In this review, we focus on targeting the tumor microenvironment (TME) via the modification of delivery systems utilizing nanotechnology-based delivery approaches.
Keywords: noncoding RNAs, siRNA, miRNA, nanotechnology, cancer
Introduction
DNA is transcribed to form RNA, which can be translated to form protein. Cellular proteins are the protagonists of cellular function, whereas RNA is a transitional state between genes and proteins [1,2]. Originally, research focused on studying proteins and their role in diseases, whereas, currently, new technologies have shifted the focus from proteins to genes. ncRNAs are a subset of RNAs that lack the potential to participate in protein encoding; rather they work in association with each other to bring about important cellular functions [3]. ncRNA is subdivided into long (lncRNA) and small (sncRNA) ncRNA, which differ in the length of nucleotides (nts) (>200 nts in lncRNA and <200 nts in sncRNA). sncRNAs, such as miRNAs, siRNAs, or PIWI-interacting RNAs (piRNAs), are now receiving increased attention because of their significant role in cancer [4]. The interaction of ncRNA subtypes is essential to enhance their stability; hence, they are key regulators of cellular functions. Disturbances in such functions often lead to cancer, making ncRNAs important mediators of tumor development [5]. The nonfunctional products of alternative splicing, called introns, are a major source of functional ncRNAs [6]. The most studied ncRNA is miRNA, which is a member of the sncRNA family and comprises ~22 nts; in animals, sncRNA has an important role in mediating post-transcriptional gene silencing via regulating the translation of mRNA into protein [7,8]. miRNA has a pivotal role in controlling >60% of protein-coding translation. In cancer cells, miRNA functions in regulating cell differentiation, proliferation, and apoptosis [7,8]. Moreover, the expression level of miRNA differs between normal and cancer cells. Generally, miRNA behaves in a similar way to oncogenes or tumor suppressors, which mediate cell tumorigenesis [9–11]. Genetics and epigenetic alteration can induce a defect in the miRNA processing machinery, resulting in tumor overgrowth [12]. Most of the identified lncRNA is believed to be transcribed via polymerase II, and can be located in the nucleolus or cytosol [13].
siRNA and miRNA have recently been investigated as a promising class of therapy that can be used to treat a range of diseases, including cancer. miRNAs can contribute to changing cell growth and survival rate via targeting functional pathways that are overexpressed at a certain cellular level or in a tissue of interest. Moreover, some miRNAs might result in powerful therapeutic activity when reintroduced inside a specific cell type. Studies have highlighted the potential therapeutic target of miRNA and, therefore, it is likely that many new RNA-based therapeutic technologies will be developed within the next few years [14–16]. siRNA is another promising ncRNA inhibitor that can be utilized to treat several diseases. The major challenge to using siRNA is its limited stability within the body; therefore, different approaches have been developed to deliver it to the target site. Currently, there are 20 ongoing clinical trials using siRNA to treat different diseases, four of which focus on cancer.
In this review, we highlight the therapeutic potential of ncRNA in cancer and address the potential use of ncRNA as a targeted ligand for nanoparticle (NP) formulations.
Mechanism of action of ncRNAs
Despite limited knowledge of the mechanism of lncRNA, it is believed that it is involved in cellular molecular functions. lncRNAs can be divided into three functional groups: transcriptional regulation, post-transcriptional regulation, and ‘others’ [17]. In transcriptional regulation, some lncRNAs are documented to have a function in regulating gene transcription via transcriptional interference. Therefore, lncRNAs have a role in regulating both transcriptional interference and chromatin remodeling. lncRNAs act post-translationally via two major mechanisms, translational control and splicing regulation [17]. In addition, translational control and splicing regulation may function individually or in combination to regulate transcriptional factor synthesis via binding to translational factors or ribosomes or by competing with splicing factors to regulate translation [18,19]. An example of translational lncRNA is small NF90-associated RNA (snaR), which functions by influencing mRNA translation [19]. Zeb2NAT is an example of splicing lncRNA (1.2 kb) that overlaps the 5′-splicing site of the intron, activating the translation of Zeb2 [20]. siRNA and miRNA were recently investigated as a novel class of therapy that can be used to treat a range of diseases, including cancer and infections. siRNA works by competing with endogenous RNA synthesis [21,22]. It acts as a natural antisense inhibitor to enhance mRNA degradation, silencing the targeted protein of interest [23]. Moreover, some lncRNA can interact directly or indirectly with miRNA to enhance mRNA stabilization [24,25]. By contrast, some antisense lncRNA can bind to the mRNA-binding site of miRNA, resulting in mRNA stability [26]. miRNAs are non-protein-coding RNA molecules that complementarily bind to the target mRNA and negatively regulate its function by cleavage and translational repression [27]. Biogenesis of miRNA starts in the nucleus with the action of the RNase III enzyme, Drosha, and DGCR8, which together cleave pri-miRNA to precursor pre-miRNA (~70 nts long). The precursor is then transported to the cytoplasm by Exportin 5, followed by cleavage by Dicer and TRBP, resulting in an double-stranded (ds)RNA duplex containing the mature miRNA, which further binds to the complementary mRNA sequence [28].
Efficient therapeutic applications of ncRNAs
Success of ncRNA as disease treatments
The discovery of functional ncRNA in translation mechanisms has created a revolution in the drug discovery field. Researchers have identified various applications of different types of ncRNA for the efficient chagnosis and treatment of cancer, diabetes, carchovascular diseases, metabolic diseases, renal diseases, and infections. The delivery ncRNA to target cancer stem cells and metastatic tumors has shown many positive clinical outcomes [29]. By 2018, there had been 20 clinical trials with siRNA initiated, mainly focusing on cancer and diabetes, with some progressing to Phase II clinical trials testing the efficacy and safety of siRNA [30,31] (Table 1). There are currently four drugs based on RNAi for treating different cancers in clinical trials [32]. Bevasiranib was the first siRNA drug to enter Phase III clinical trials, and is used to treat eye diseases [33].
Table 1.
New miRNAs, siRNAs, and lncRNAs as putative theraoeutic taraets in cancer
| Study title | Condition | Intervention | Phase | NCT number | |
|---|---|---|---|---|---|
| siRNA | |||||
| Phase Ib/II, multiple center, dose escalation study of DCR-MYC in patients with liver cancer | Hepatocellular carcinoma | Drug: DCR-MYC | Phase I/II | NCT02314052 | |
| Atu027 plus gemcitabine in advanced or metastatic pancreatic cancer (Atu027-I-02)) | Carcinoma, pancreatic ductal | Drug: gemcitabine | Phase I/II | NCT01808638 | |
| A Phase II study of siG12D LODER in combination with chemotherapy in patient diagnosed with locally advanced pancreatic cancer | Pancreatic ductal adenocarcinoma, pancreatic cancer | Drug: siG12D-LODER; drug: gemcitabine+nab-paclitaxel | Phase II | NCT01676259 | |
| Therapeutic target | Condition | Effect | Animal model | Refs | |
| miRNA | |||||
| miR-31 | Lung adenocarcinoma | Induction of lung hyperplasia, adenoma formation, and adenocarcinoma development | Transgenic | [139] | |
| miR-1246 and miR-1290 | Nonsmall cell lung carcinoma | Highly expressed during tumor initiation and cancer progression; process is inhibited by LNAs to these miRNAs | PDX | [140] | |
| miR-34a | Breast cancer | Tumor growth delayed by systemic delivery of tNP-encapsulated miR-34a mimic | Xenograft | [141] | |
| miR-155 | Lymphoma | Tumor growth inhibited by pHLIP- and ANTP-NP-encapsulated anti-miR-155 particles | Transgenic | [142,143] | |
| lncRNA | |||||
| MALAT-1 | Breast cancer | Slower tumor growth comes with significant differentiation into cystic tumors and reduction in metastasis by systemic administration of MALAT-1 ASO inhibitors | Transgenic | [144] | |
| MEG3 | Colorectal cancer | Tumor cell proliferation inhibited by MEG3 overexpression | Xenograft | [145] | |
siRNA therapeutics selectively target gene expression in vivo, resulting in transcriptional repression. The selectivity of this class of ncRNA therapeutics is greater compared with the other ncRNA subtypes because targeting by siRNA involves only the gene of interest, whereas miRNA targets multiple genes. Specifically designed siRNA molecules bind complementarily to mRNA and trigger downstream post-transcriptional silencing of the target genes [34]. Phase I clinical trials have been completed for chemically synthesized siRNA molecules in patients with solid tumors.
Endosome escape mechanism to improve delivery efficiency
The success of RNA therapeutics depends solely on passing through extracellular and intracellular barriers to reach the target site and bind to the mRNA of interest. However, naked RNA inhibitors cannot be introduced directly to biological systems because of limitations, such as high molecular weight negative charge, and, most importantly, their instability. In vivo serum endonuclease is one enzyme that participates in RNAi degradation and facilitates their renal execration [35]. Thus, chemical modification of siRNA, anti-sense oligonucleotides (ASNs), aptamers, and synthetic mRNAs, as well as NPs, can be used to overcome endosomal degradation and promote RNAi efficiency.
To increase RNAi in vivo stability, chemical modifications have been developed that circumvent many of the drawbacks associated with siRNA delivery [36]. Chemical modification can be done by replacing the nonbridging oxygen atom of the phosphate group with a sulfur atom, forming a stable phosphorothioate group. This improves the stability and pharmacokinetic (PK) of RNAi. PK is also improved by increasing RNAi hydrophobicity, which increases the plasma protein affinity and, thus, improves RNAi tissue accumulation. By contrast, introducing the phosphorothioate group into ASNs reduces the miRNA affinity and nucleotide stability [37]. Therefore, the sugar phosphate backbone of RNA can be modified and replaced by introducing polyethyleneimine (PEI) to every second nitrogen atom of ASNs to form a stable amide that enhances RNA stability and affinity [38].
Using NPs is another way to improve RNAi stability and efficiency. NPs can protect RNAi from degradation and selectively deliver it to a tumor site either passively or actively [39]. The ideal characteristics of RNAi delivery systems are that they are nontoxic, biodegradable, and non-immunogenic, and that they can escape endosomal degradation and deliver their RNAi payload safely and intracellularly to the cytoplasm [40]; thus, promoting RNAi silencing [41]. Even though viral vectors are excellent gene delivery systems, safety concerns limit their use in humans. Therefore, nonviral delivery systems have become one of the most viable alternatives.
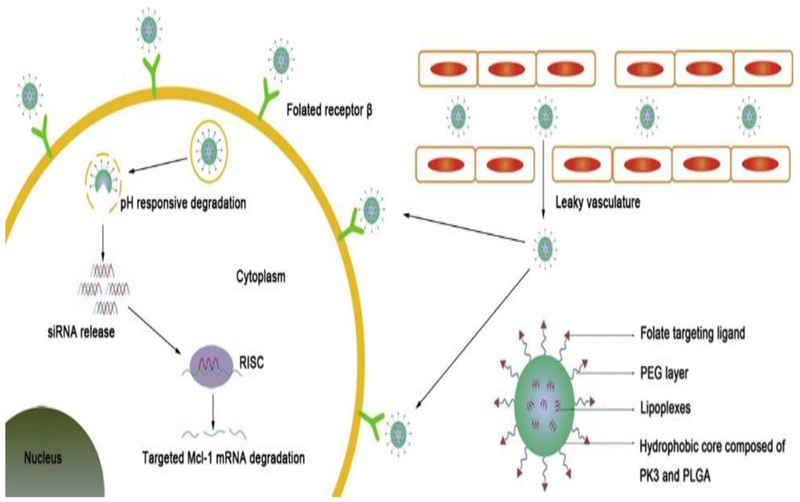
Nonviral delivery systems with targeted polymers, nontargeted lipids, a biodegradable polymer matrix, and PEI have been utilized in Phase I clinical trials [42]. To overcome endosomal degradation, the negative charge on siRNA is widely utilized for complexation with positively charged molecules through electrostatic interactions. One group reported a formulation of mesoporous silica NPs functionalized with PEI combined with cyclodextrin. The authors reported that PEI forms an electrostatic bond with siRNA and cyclodextrin helps to minimize the toxicity associated with PEI [43]. Lipid NPs are safer in terms of their biocompatibility and, hence, reduced toxicity. To improve serum stability and transfection efficacy, noncovalent complexes of lipid and polymers (‘lipoplexes’) are being explored for siRNA delivery. These systems can be PEGylated with long polyethylene glycol (PEG) chains to extend the circulation time of the NPs. Neutral, anionic, and PEGylated phospholipids offer better stability and therapeutic outcome compared with cationic phospholipids used in lipoplexes [44]. To further augment the delivery of liposomal siRNA by evading endosomes and/or lysosomes, polyarginine-based cell-penetrating peptides (CPPs) have been studied; their release is triggered by the acidic TME via an acid-cleavable hydrazine linker [45]. Xiangshi et al. developed a polymeric NP that releases its siRNA payload in acidic TME, maximizing the siRNA silencing efficiency (Figure 1) [46].
Figure 1.
pH-responsive polymeric nanoparticles (NPs) enhanced Mcl-1 small interfering (si)RNA delivery to treat rheumatoid arthritis. These NPs maximized blood circulation and enhanced siRNA release in the acidic environment. In the cytoplasm, siRNA-encapsulated polymeric NPs released siRNA and formed a RNA-induced silencing complex (RISC) with the mRNA, leading to gene silencing. Adapted from [46]. Abbreviations: PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid).
Challenges to using ncRNAs
Clinical approaches for ncRNA targeting and delivery
miRNAs show significant therapeutic potential because they control many targeted genes inside certain signaling pathways or many other targets across multiple independent pathways [47,48]. Therefore, the pleiotropic rule of miRNAs could change cell proliferation based on the cellular context even if the miRNA is directed toward functional pathways that are overexpressed in a certain tissue or cell of interest. Consequently, some miRNAs provide robust therapeutic activity when reintroduced or suppressed inside a certain cell type. In addition, research has highlighted that many miRNAs will be prospective target therapeutic options, revitalizing interest in RNA-based therapeutics [49]. Thus, it is possible that many novel RNA therapeutic agents will be manufactured within the next few years.
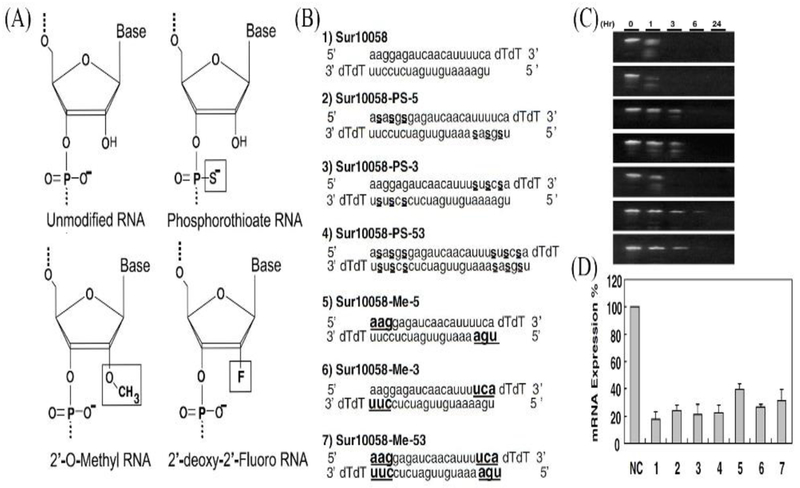
A recent approach to enhancing or suppressing miRNA function includes delivering synthetic oligoribonucleotides (ORNs) that copy the native miRNA duplex in which high miRNA expression is needed to reproduce single-stranded antisense RNA to use the targeted endogenous miRNA for inhibition studies (Figure 2). Utilization of unmodified ORNs faces crucial challenges, such as stability and delivery. To retain RNA stability, changes to the binding affinity of RNA and to protect ORNs from nucleases have been extensively investigated [50,51]. In addition, ORN can be directly conjugated to targeting moieties, such as N-acetyl galactosamine, for delivery to hepatocytes in liver cancer. For instance, Regulus Therapeutics constructed an anti-miR-122 molecule specifically for liver diseases, such as hepatitis C. ORN modifications have mainly involved antisense strategies to sequester mature miRNAs. However, existing chemical modifications have become rate-limiting and require more research. Developing modified duplex RNAs that retain their biological activity is a real challenge and, thus, trials to deliver a single-stranded mature miRNA have been unsuccessful [52] probably because of the inability of duplex RNAs to load into the RNA-induced silencing complex. Thus, continuous efforts have been made to improve the stability and cellular uptake of miRNA to be used to treat various diseases.
Figure 2.
Title. (a) Different structural RNA interference (RNAi) modification (modified moieties are boxed), (b) Name, sequence, and chemical modifications of unmodified and modified Sur10058. Lowercase letters indicate unmodified RNA, underlined bold letters indicate 20-OMe, small underlined s between nucleotides indicates phosphorothioate, (c) Serum stability of small interfering (si)RNAs. Unmodified and modified siRNAs were incubated in 10% human serum at 37°C for 0, 1, 3, 6, or 24 h and aliquots were analyzed on 15% native polyacrylamide gels. (D) Inhibition of survivin mRNA expression in HeLa cells transfected with modified Sur10058 siRNAs (2 nM). Knockdown efficiency was quantitatively measured by real-time PCR analysis. Adapted from [146]. Abbreviations: PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid).
Returning miRNA expression to normal levels and designing therapeutics targeting multiple genes at the same time can be therapeutically viable options in the context of anticancer therapy [53]. Various strategies have been explored to utilize the role of miRNA to develop anticancer therapeutics [54]; for example: (i) miRNA inhibition therapy uses anti-miRNA oligomers, which are 17–22 nts long, single-stranded, chemically modified, competitive inhibitors of miRNA, leading to upregulation of the target mRNA. Examples of this class include antagomers, locked nucleic acid anti-miRs, and miRNA sponges; (ii) miRNA mimetic agents are a class of miRNA replacement therapy that either replace or substitute lost miRNA using synthetic miRNA mimetics; (iii) small-molecule inhibitors of miRNA (SMIRs) work either by inhibition of miRNA biogenesis or by impeding the interaction of miRNA with its target; and (iv) miRNAs can be targeted during transport within the tumor milieu or to distant sites by microvesicles and exosomes. This approach works by blocking crosstalk between the miRNA and the surrounding TME.
Strategies and vehicles for overcoming barriers to RNA delivery
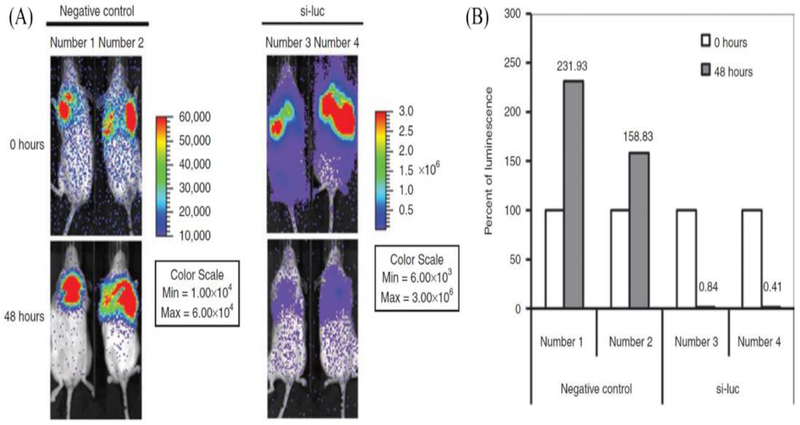
Given that miRNA acts intracellularly, therapeutic agents are needed to increase the cellular uptake of ORNs. ORNs, such as peptide nucleic acids (PNAs), have been invented and optimized to silence miRNAs [55,56]. PNAs can be conjugated chemically to cholesterol moieties not only to enhance their hydrophobicity, but also to maximize the number of CPPs to improve cellular uptake and tissue targeting. The addition of lysine groups to PNAs can promote the endosomal release of PNA once taken up by cells via endocytosis. Despite these advances, ORN doses >5 mg/kg are required to realise therapeutic activities, which could result in adverse effects or toxicity. However, nanotechnology approaches have minimized the problems of target tissue specificity and stability, reducing adverse effects and toxicity profiles, and enabling RNA delivery at <2 mg/kg at therapeutic doses [57]. The most common nanodelivery agents utilized are based on a lipid-based nanodelivery system. These delivery systems are normally modified by PEGylation or through ionization to circumvent unwanted accumulation in plasma serum and overcome nonspecific tissue uptake. For example, cationic lipid complexes were decorated with tumor-targeting markers, such as single-chain fragment variable (scFv) or transferrin [57,58]. Other lipid-based delivery approaches include neutral emulsions, where neutral phospholipids carry the miRNA and, thus, overcome challenges of surface charge when trying to deliver a highly polar (hydrophilic) RNA molecule through the cellular membrane (Figure 3) [59]. This strategy was proven successful for the delivery of let-7 and miR-34a in preclinical lung cancer animal models [59,60].
Figure 3.
Delivering (si-luc) to orthotopic H460-luc lung tumors, (a) I VIS images of luciferase-expressed lung tumors in mice taken at 0 and 48 h after intravenous injection of si-luc (animal numbers 3–4) or negative control (animal numbers 1–2) formulated with neutral lipid emulsion, (b) Quantitative analysis of the relative luminescence data of injected mice [The data are presented as percentage (%) luminescence 48 h post treatment relative to the luminescence at time of injection (100%)]. Adapted from [59].
Numerous advances in delivery technologies that are not lipid-based carriers include polymeric and peptide-based delivery systems. Poly(lactic-co-glycolic acid) (PLGA) NPs are polymeric NPs that can be used to encapsulate both siRNAs and miRNAs [60,61]. PLGA NPs have a significant role in reducing the charge and electrostatic interactions that limit miRNA delivery. Moreover, PLGA NPs can be modified with surface peptides, such as CPPs, to help increase the cellular uptake of miRNA. Furthermore, using different tumor-targeting ligands, such as aptamers and small nucleic acids, also improves PNA tumor specificity and accumulation. As a result, these NP systems are safe and biodegradable, supporting the theory that they could easily be developed into ‘new-generation’ miRNA therapeutics for testing in clinical trials (Table 1).
New strategies for lncRNA delivery
lncRNAs control the translation and modular scaffolds involved in modifying chromatin enzymes to certain genomic loci [62]. lncRNAs produce tight transcriptional control, tissue-specific expression, and dysregulation in many diseases. The first evidence that lncRNAs can be modulated via their characteristic flanking regions and that they might have therapeutic potential came from trials on the H19 gene, which is a lncRNA with oncogenic characteristics that are overexpressed in many cancers. Plasmids expressing diphtheria toxin, which controls the H19 promoter, were injected intratumorally and resulted in tumor reduction in bladder cancer xenograft mouse models [63]. Various Phase I/II clinical studies have begun to investigate the use of tools to modulate misregulated lncRNAs for patients with ovarian, bladder, and some pancreatic cancers. Even though lncRNAs exist in the cytoplasm, they are mostly nuclear [64]. As a result, modifications on ASOs have been performed to target the lncRNA in the nuclear side to trigger RNase H-dependent degradation of the specific target lncRNA [65,66].
Despite these latest developments, the complex structures of lncRNAs preclude ASO from binding to a particular location inside the lncRNA molecule. Although one strategy is to produce many siRNAs targeted to the desired lncRNA, the cost of evaluating off-target effects for each molecule can be prohibitive. Although lncRNAs are regarded as attractive pharmacological and therapeutic targets, the inhibition of lncRNAs in vivo remains challenging. Obstacles to the delivery of molecules into lncRNAs are similar for all RNAi-developed therapeutics. However, one strategy to utilize small molecules that are able to disrupt lncRNA chromatin-modifying complexes is to change the epigenetics of the target cell. Consequently, these delivery strategies, along with other major findings of lncRNA regulatory mechanisms, could result in effective in vivo therapeutic approaches that target lncRNAs.
miRNA as cancer-targeted therapy
Dysregulation of ncRNAs in cancer is involved in managing intrinsic tumorigenic processes [67]. Although the mechanisms of how lncRNAs are associated with cancer pathogenesis remain ambiguous, evidence suggests that lncRNAs control chromatin-modifying proteins, such as PRC2 and coREST [68,69]. In addition, because the hypermethylation of gene promoter sites occurs repeatedly in cancer, lncRNAs might foster tumor-suppressive genes or oncogenes based on how the dysregulation of the respective lncRNA influences the activity of the chromatin-modifying complexes. Oncogenic ncRNAs can prevent growth suppressors. For example, the miR-221/222 family of miRNAs is a key regulator of CDKN1B, which is overexpressed in various tumors. Targeting of CDKN1B by this miRNA family promotes proliferation by enhancing the progress of the cell cycle from G1 to S phase [70]. Similarly, miR-Zip knockdown of miR-221 in a triple-negative breast cancer model (MDA MB-231 cells) inhibited cancer growth, revealing the in vivo pro-proliferative effect of miR-221. In addition, miR-155, which targets another oncogenic miRNA, enhances lymphoma progression in animal models in the absence of Myc [71]. Targeting growth promoters by tumor-suppressive ncRNAs could also be another approach. Although multiple miRNAs are utilized in this process, one example of miRNA mimic-based therapy is miR-34a, which is the first utilized for clinical trials in humans (ClinicalTrials.gov, NCT01829971). In animal models, miR-34a administered systemically shrank lung tumor masses in KrasLSL-G12D p53loxP/loxP mice [60,72]. The therapeutic effectiveness of tumor growth inhibition induced by miR-34a is interesting, given that miR-34a acts as an effective tumor-suppressive agent for many tumor types.
Importantly, ncRNAs regulate the telomeric sites of chromosomes, which continuously shorten at the time of cell division, progressively triggering senescence. Cancer cells avoid this by increasing the expression of telomerase reverse transcriptase (TERT), which adds telomeric repeats to the 3’ end of chromosomes. GAS5 is considered a senescence-promoting ncRNA that promotes the downregulation of CDK6 in gastric, bladder, and pancreatic cancers, among others, and, in many cases, is anticorrelated with tumor volume and clinical stage advancement [73,74]. Suppression of GAS5 is linked to a higher frequency of cells in S phase, elevated amount of CDK6 protein, and altered p21/WAFl levels, minimizing the incidence of cellular senescence [74]. In an animal model of colorectal cancer, GAS5-expressing tumors harbored a substantial reduction in tumor size and tumor burden, indicating targeting GAS5 as a prospective therapy target. In addition, ncRNAs can change the survival response of cells to uncontrolled growth and DNA damage [75]. For instance, miR-221/222 can revoke programmed cell death in multiple tumor types, and the inhibition of the families of these miRNAs elevated apoptosis through activation of SIRT1 and/or downregulation of PUMA [76,77]. Given that tumor size increases, associated capillary growth occurs from cancer cells surrounding the stroma and blood vessels. Therefore, tumor angiogenesis could be a target for therapeutic uses and is why ncRNAs have a role in tumor angiogenesis, also given that various miRNAs modulate angiogenesis in animal models [78–80]. For instance, an miR-7 bioresponsive polymeric NP was delivered to suppress angiogenesis in a human glioblastoma xenograft model [81]. ncRNAs are also basic regulators of the epithelial–mesenchymal transition (EMT). One important ncRNA is miR-21, which promotes the migration and invasion of cells in an array of cancers via phosphatase and tensin homolog (PTEN) and programmed cell death protein 4 (PDCD4) downregulation. miR-21-mediated regulation of PTEN resulted in increased IL-6 signaling, stimulating key characteristics of EMT, including PI3K activation and increased levels of p-STAT3 and NF-κB in HER2-positive breast cancer cells [82]. In another study using breast cancer cells (MDA-MB-231), delivering anti-miR-10b in a NP formulation circumvented lymph node metastasis [83]. Furthermore, numerous targets against metastasis are managed by miR-10b, such as HOXDIO and PTEN, which block miR-10b activity to inhibit the expression of premetastatic factors [84,85]. These results support the use of ncRNAs as novel and innovative antitumor therapeutics.
Nonviral ncRNA delivery systems for cancer therapy
Inorganic materials
ncRNA delivery systems include viral and nonviral vectors. The viral route is not preferred for treating diseases despite having high transfection efficacy because viral vectors are highly immunogenic and carcinogenic and, thus, contraindicated for cancer therapy. Most nonviral vectors are non-immunogenic and provide targeted action at the tumor core. They have been proven to be efficient in delivering ncRNA particles [86,87].
miRNAs have been extensively studied as anticancer drug delivery systems. Within the broad domain of inorganic materials, gold-based nanocarriers have been studied to deliver miRNA to the target site. One group reported the delivery of unmodified miRNA to target cells via cysteamine-functionalized gold NPs (AuNPs). Contrary to using a modified therapeutic intervention that might interfere with the safe and efficient delivery of miRNA, the authors used unmodified miRNA, which could easily be delivered via the proposed system to achieve the transfection of up to 96% of cells in vitro [88]. Another group reported the development of novel spherical nucleic acids (SNAs) comprising a AuNP core surrounded by a shell of uniformly oriented oligonucleotides. Such NPs exhibit high serum stability and cellular uptake, low immunogenicity, and the ability to upregulate target genes, as a result of which they can be loaded in exosomes, leading to efficient gene knockdown [89]. This approach is an example of how the natural cellular machinery can be exploited to enhance the delivery of a highly selective gene targeting platform, with minimal adverse effects and maximum therapeutic efficacy.
lncRNAs have also been studied for their role in regulating NP-mediated toxicity [90]. The malfunctioning of lncRNA regulation is a key indicator in several types of cancer, including prostate cancer. Important lncRNAs, such as PCA3, PCATs, and SChLAP1, are upregulated in prostate cancer, a finding that could help develop tools for the diagnosis and therapy of the disease [91].
Lipid-based nanocarriers
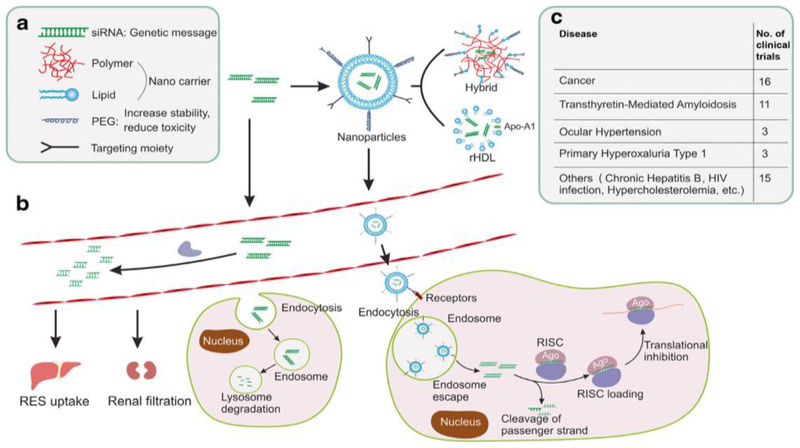
Viral nanocarriers are associated with a lethal immunogenic response; thus, lipid-based nanocarriers (LNPs) are the safest and most non-immunogenic nanocarriers. LNPs comprising lipids and/or phospholipids are biocompatible, are non-immunogenic, and interact well with cell membranes. They are effective for protecting and delivering bioactive molecules, such as chemotherapeutic agents and gene therapies, and can be utilized for imaging purposes (Figure 4) [92]. The most studied classes of lipidic nanocarrier are liposomes, LNPs, and lipid nanoemulsions. Cationic lipids are able to encapsulate multiple bioactive molecules, such as polyanionic RNA molecules and chemotherapeutic agents [86].
Figure 4.
The fate of synthetic small interfering (si)RNA-loaded nanoparticles (NPs) with optimum structural and functional properties, (a) Different components of NPs for RNA interference (RNAi) delivery, (b) Process of payload release within blood stream, (c) Different processes of uptake and clearance of NPs. Adapted, with permission, from [101]. Abbreviations: PEG, polyethylene glycol; RES, reticuloendothelial system; RISC, RNA-induced silencing complex.
A liposome is a LNP comprising a hydrophobic lipid bilayer and a hydrophilic core, in which both hydrophobic and hydrophilic molecules can be incorporated. The hydrophilic core encloses most of the liposome, where hydrophilic macromolecules, such as DNA, protein, and imaging agents, can be incorporated [93]. Moreover, liposomes can be utilized for gene delivery in which cationic liposomes are used to encapsulate the ncRNA for maximum loading efficiency [86]. Different preparation methods, such as Bangham, reverse-phase evaporation, and solvent injection methods, are currently used with high entrapment efficiency (~90%). Cholesterol, which is one liposome component, has a significant role in stability and drug release. Studies showed that the optimum cholesterol percentage in lipid-based nanoparticles is between 17% and 20%, in which maximize drug release was achieved up to 90%, whereas <10% contributed to only a 5% drug release [94]. Therefore, all liposomal components can be manipulated to produce maximum drug encapsulation and minimize toxicity. One of the main disadvantages of conventional liposomal formulations is rapid clearance from the body via liver and spleen [95,96]. Researchers overcame this issue by PEG, which can enhance liposome stability and circulation in the body. Another disadvantage of a liposomal formulation is that some drugs do not have the ability to penetrate the cell membrane; therefore, an active targeting approach can deliver a variety of drugs intracellularly via receptor-mediate endocytosis [97]. DOPC is another liposomal component that is utilized to develop a neutral liposome in which siRNA can be delivered safely, and is currently in a Phase I clinical trial ( NCT01591356). Moreover, pH-sensitive surface-modified liposomes can be utilized to deliver miRNA. Marina Biotech successfully designed a pH-sensitive liposome (‘Smartides’) that is anionic at body pH but turns cationic at lower pH, such as tumor pH [98]. pH-sensitive liposomes successfully delivered miRNA (MRX34) to the TME and restored the tumor suppressors to normal levels, which induced apoptosis in both in vitro and in vivo settings [98].
SLNs remain solid at both room and body temperature [87]. Cationic lipids also act as surfactants to form a primary emulsion. This property is used to protect RNA from environmental enzymes. Their lipophilic core is a challenge for encapsulating RNA molecules. However, they offer an advantage of administering small molecules in combination with ncRNA for synergistic actions [86]. Chen et al. successfully developed a multifunctional NP comprising anionic lipids to deliver vascular endothelial growth factor (VEGF) siRNA combined with doxorubicin (Dox). This technology provided the high entrapment efficiency of both siRNA and Dox, enhancing ovarian cancer growth rate inhibition [99]. SLNs also offer the advantage of using small molecules in combination with ncRNA [86].
Lipid nanoemulsions are another type of NP comprising an oil phase and aqueous phase in thermodynamic equilibrium by using cationic lipids as surfactants. RNA molecules are suspended in the oil phase of the nanoemulsion, which offers protection from degrading enzymes [86]. Brito et al. showed that the activity of a complexed cationic nanoemulsion (CNE) with siRNA improved the stability and delivery of an RNA vaccine. The RNA vaccine-encapsulated nanoemulsions protected RNA against RNAase, elicited an immune response in mice, rabbits, and rats, and showed excellent biocompatibility [100].
Nanostructured lipid carriers (NLCs) are newer lipid nanocarriers that have the advantage of a high loading capacity, longer shelf life, and excellent biocompatibility. They are modified SLNs with a solid lipid core and have a variable solid–liquid state at ambient temperature [101]. NLCs can be utilized as a potent tumor drug delivery systems for encapsulating multiple agents, such as chemotherapeutic agents and gene therapy. Taratula et al. developed multifunctional NLCs that were able to accommodate Dox or paclitaxel, two siRNAs, and hormonal therapy [a modified analog of luteinizing-hormone-releasing hormone (LHRH)] for treating lung cancer [102]. This delivery system successfully enhanced tumor growth inhibition [102].
Polymeric vectors
Polymeric vectors act as an effective way to increase cellular uptake for ncRNA delivery. Factors that deter naked ncRNA from undergoing cellular internalization include the negative charge and enzymatic degradation of nucleic acids. Polymeric vectors counteract these challenges because of their cationic properties and provide protection from degradation [103] Additionally, polymeric vectors act as useful ncRNA carriers because of their simple preparation, typically creating the RNA–polymer complex through hydrophobic interactions with simple mixing (Figure 2) [104]. In vitro, the anionic cell membrane causes the cellular uptake of cationic polymeric vectors [103]. In vivo, however, other negatively charged macromolecules, such as albumen, can interact with the carriers, causing complications [103]. The stability of the particles depends greatly on the balance of cationic and anionic components; therefore, it is important to adjust the composition of the polymeric vectors according to physiological pH conditions [105]. Also, aggregation of the NPs can occur in physiological salt concentrations, creating complications for cellular uptake [103]. Currently, several polymeric and co-polymeric vectors are being researched to study the efficacy of polymeric vectors as a ncRNA delivery system.
PEI has received recent attention because of its high transfection efficiency, despite possible toxicity issues. To address this issue, creating a copolymer of PEI with PEG moieties reduced toxicity as well as increasing nuclease resistance. The advantages of the copolymeric vector stem from the size of the PEG chains used. Chains 0.55 kDa in size were unsuccessful at protecting ncRNA from RNase; however, using PEG chains >2 kDa has sufficiently protected ncRNA from degradation [104].
A second polymeric vector candidate that has possible ncRNA delivery applications is mPEG45-b-PCL100-bPPEEA12. This copolymer complex or micelle NP (MNP) has convenient ncRNA-loading capabilities and has demonstrated a cellular internalization efficiency >90%. This, in conjunction with cytocompatibility at high doses and significant gene knockdown activities, makes MNP a promising contender for future therapeutic options [106].
Cationic polymers are the next most-studied systems for ncRNA delivery. They have potential for delivering ncRNA vectors to targeted sites. A variety of polymers is used to deliver various types of ncRNA, especially miRNA and siRNA, in the treatment of different cancers. Examples include PLGA, cyclodextrin, polylactic acid, chitosan, styrene maleic anhydride, PEI, and dendrimers [87,101]. Polymeric ncRNA vectors, such as the LODER polymer and cyclodextrin-based polymer, have progressed into clinical trials [86]. The advantages of using cationic polymers include [87]: (i) RNA can be encapsulated through different mechanisms, such as conjugation, complexation, adsorption, or entrapment of RNA onto the polymer matrix; (ii) the degradation of polymers can aid the release of the nucleic acid material into the cytosol; (iii) the polymer protects the RNA from being degraded by the external environment; and (vi) they can form spherical NPs, which improves the stability of the formulation. The size range of these nanoparticles is 1–1000 nm. However, one of the main disadvantages of using polymeric NPs is that they are highly non-immunogenic, which can be minimized with structural modifications to the polymer [101].
Targeted delivery of ncRNAs
There are several types of delivery system that alleviate the challenges of using ncRNA as therapy. Further modifying these delivery systems can improve their selectivity towards certain tissues, especially tumors. Increasing selectivity has the advantage of reducing adverse effects, while increasing the efficacy of the drug [107–110]. Using nanocarriers has been proposed as a solution to targeting the aberrant TME because of factors such as hypoxia, acidity, increased interstitial fluid pressure, extravasation via the vascular-endothelial layer, and mononuclear-phagocyte system uptake [111]. Here, we focus on ways to target the TME, both passively and actively, via modification of the delivery systems.
Passive and active targeted delivery
There are generally two types of targeted delivery exhibited by nanocarriers and they are not mutually exclusive. Passive targeted delivery depends greatly on the enhanced permeability and retention (EPR) effect, which occurs in tumors and other inflamed tissue [112,113]. Given the compromised vasculature around these areas, relatively larger sized molecules (especially those 40–200 nm in size) are able to leave the bloodstream, accumulate in these inflamed sites, and be cleared more slowly because of blocked lymphatic drainage [114]. For nanocarriers, such as liposomes, to be fit for this passive targeting, they must maintain their integrity in the blood stream until they reach the targeted site. Strategies to improve this stability have been to add hydrophilic molecules, such as PEG, to the outside surface of the nanocarriers [111]. Passive targeting is a well-known advantage of using nanocarriers as delivery systems and would be especially useful for RNA therapeutics because of their biological instability [115].
The second type of targeted delivery is active targeted delivery, which is the addition of molecules (either directly to a drug or to the surface of a carrier) that exhibit affinity and interact with other molecules in the target environment including on or within cells [3]. This is especially useful when targeting tumors because of their distinct extra and intracellular environment. Research by Li et al. suggests that active targeting is even more important than passive targeting in cancer therapy [113].
There is essentially an endless variety of possible targets, targeting agents, and their combinations to consider when designing a delivery system for RNA. There are a few broad classifications of active targeting entities that are discussed later: small molecules or protein ligands, antibodies, and aptamers.
Types of targeted ligand for the delivery of ncRNAs
Peptides or protein ligands
The optimal targets are those that are overly abundant in a TME, so that, even if a healthy tissue has a target molecule, it will be minimally affected compared with the tumor. Most of the following well-researched peptide and protein ligands fit this description by being overexpressed either on the surface of cancer cells or in the TME.
The discovery of folate as a selective ligand for cancer and activated macrophage cells led to it being one of the first used active targeting agents. Although folate is an essential vitamin, the folate receptor is more highly expressed in cancer cells than in healthy cells. Furthermore, it is easily conjugated to therapeutic molecules while retaining its binding affinity for the folate receptor. It has shown promise for targeting many types of cancer, as well as some autoimmune disorders. Even though imaging studies of folate-conjugated formulations showed significant accumulation in kidney tissue, they were demonstrated to re-enter the bloodstream quickly without significant kidney damage. There are already several drugs using folate targeting being tested in clinical trials, and many new prospects [116].
Hyaluronic acid (HA) is a glycosaminoglycan that is used by cells to form their extracellular matrix, making it a natural and essential biological molecule. It is of interest because some of its receptors, mainly cluster of differentiation (CD44), have demonstrated overexpression in many types of cancer cell [109,117]. Furthermore, HA has multiple functional groups for further molecular conjugation and exhibits receptor-mediated endocytosis. This allows HA-conjugated delivery systems to enter cells and be free to release their contents internally. Yu et ctl. demonstrated that HA-conjugated mesoporous silica NPs containing Dox have higher cytotoxicity than both unconjugated NPs and free Dox against human colon cancer cells [118]. Active targeting of chitosan NPs with HA has also been used to selectively deliver siRNA to silence angiogenic genes in tumor endothelial cells in vivo [119,120]. A similar effect was seen in HA-selenium-PEI NPs containing siRNA for silencing a tumor growth gene, where the targeted NPs exhibited higher toxicity against hepatocellular carcinoma cells in vitro and in vivo. No significant healthy-cell toxicity was seen in mice treated with this formulation [121].
Transferrin (Tf) is a large glycoprotein that functions to bind free iron in the bloodstream. Its receptor, transferrin receptor (TfR), is overexpressed in actively proliferating tumors, making Tf a suitable targeting agent for cancer. The interaction between the two also leads to receptor-mediated endocytosis. It has been successfully used to deliver Dox-loaded liposomes to human hepatocellular carcinoma [122] and ceramides in human ovarian cancer in mouse models [123].
CPPs are a group of uniquely linked amino acids that are known to easily pass through many types of cell membrane. Although CPPs of the TAT class are the most researched, others include LMWP, Penetratin, VP22, MAP, MPG, and pep-1. Most of these retain their penetrating ability while conjugated to other molecules and even while linked to the surface of larger nanocarriers. Even though the mechanism of cell penetration is not fully understood, it is thought to be a mix of direct penetration and endocytosis depending on the size of the CPP-linked complex. Given that the discovery of this group of peptides is relatively recent much research remains to be done on their use [124].
Integrins are transmembrane proteins that help cells adhere to the extracellular matrix and regulate the cell cycle. Some of these receptors are highly expressed on cancer cells and, as such, they are good targets for cancer therapy. One of the best-known peptide ligands is the RGD (Arg-Gly-Asp) tripeptide, which binds to αvβ3 and αvβ5 integrins. Peptides containing this sequence have been linked to the surface of liposomes as active targeting agents, with some success. This targeting strategy has been useful in delivering Dox-containing liposomes to human glioma tumors [125] and paclitaxel-containing liposomes to human lung carcinoma tumors [126] in mouse models.
Antibodies
Antibodies are large macromolecules made solely to bind to a specific molecular pattern with high specificity. This makes them perfect targeting agents, but because of their size, complexity, and the relative difficulty of acquiring large quantities, their use is somewhat limited [110,127–129]. Antibodies are often fragmented, and the targeting region is left intact before being conjugated to nanocarriers [130]. Here, we discuss tumor markers for which antibodies have been made.
Epidermal growth factor receptor (EGFR) is well known for its overexpression, which contributes to tumor proliferation in many cancer types. Monoclonal antibodies, such as cetuximab (Erbitux), are already being used against this receptor clinically. Immunoliposomes have been developed with fragments of cetuximab linked to their surface to successfully deliver a variety of anticancer drugs to several types of tumor in mouse models [131]. Another immunoliposome conjugated with cetuximab was used to deliver survivin and Bcl-2 siRNA to human colorectal adenocarcinoma in mouse models [132].
HER2 (coming from human EGFR) is in the same family as EGFR but is specific to breast cancer tumors. Its overexpression makes it an ideal target in aggressive types of breast cancer by monoclonal antibodies such as trastuzumab (Herceptin). This antibody has been conjugated as part of immunoliposomes to successfully deliver paclitaxel [133] and Dox [134] to HER2-positive breast cancer tumors in mouse models.
Membrane type-1 matrix metalloproteinase (MT1-MMP) is a protein expressed on and around tumor cells that promotes angiogenesis. Its function includes cleaving extracellular matrix components to make way for new vasculature, resulting from its overabundance in the TME. It is a promising target for which antibodies have been recently developed. Hatakeyama et al. showed that anti-MT1-MMP-linked immunoliposomes encapsulating Dox significantly accumulated in tumors and improved the survival rate of tumor-bearing mice [135].
Vascular cell adhesion molecule 1 (VCAM-1) is a marker present in the vascular endothelium of many types of cancer. This makes it a good target, especially for highly vascular tumors that overexpress it. Anti-VCAM-1 monoclonal mouse antibody has been produced and investigated as a part of an immunoliposome in vivo to show high accumulation in human ovarian cancer and nonsmall cell lung cancer [136].
Aptamers
Aptamers are sequences of DNA or RNA designed to have high affinity for a certain target, similar to antibodies. They have the advantage of low immunogenicity, extended shelf-life, lower production costs, and minimum batch-to-batch variation [137]. Aptamers with higher affinity toward a target protein can be produced using the systematic evolution of ligands by exponential enrichment (SELEX) method, which exposes a library of short (20–50 nt) DNA/RNA to the target protein. The oligonucleotides that bind to the protein are separated by those that do not get amplified through PCR and are mixed back in. This process is done several times until the only remaining oligonucleotides are the aptamers that bind tightly to the protein [137].
Another advantage of using aptamers to deliver ncRNA is the relative ease of synthesizing the chimera, with both components comprising oligonucleotides. If the oligonucleotide is modified in such a way that it will be stable in circulation until it reaches a tumor, it is a viable therapeutic. There have been several studies on such siRNA-chimera against tumor targets, such as PSMA, epCAM, CTLA4, Mucin 1, Nucleolin, Mucin-1, 4-BB and others [137]. As is the pattern, aptamers have also been linked to nanocarriers, such as liposomes. Some of these aptamers target previously discussed overexpressed tumor markers, including EGFR, Her-2, TfR, CD44, among others [137,138].
Concluding remarks
ncRNAs, such as siRNA and miRNA, are a promising class of therapy that can be used to treat a range of diseases, including cancer. Researchers face many in vivo challenges with ncRNA, such as siRNA instability and fast miRNA clearance. Chemical modifications of both siRNA and miRNA could overcome these challenges and improve the overall therapeutic efficiency of ncRNAs. Moreover, introducing ncRNA into NPs is another way that could increase its in vivo circulation and maximize its therapeutic efficiency. Moreover, conjugating NPs to a variety of ligands that target specific overexpressed proteins on the surface of cancer cells is a robust method that enhances cellular uptake via active targeting.
Highlights:
The role of non-coding RNA in treating various cancers is emphasized.
Recent advances in drug delivery systems to enhance ncRNA stability and efficiency are reviewed.
Clinical applications of miRNA and its potential use is discussed.
Acknowledgments
R.A. would like to acknowledge the Kingdom of Saudi Arabian Govt for a scholarship to undertake Graduate Studies at Wayne State University. M.J.K. acknowledges the SURF program of the Department of Pharmaceutical Sciences, Wayne State University. S.S. acknowledges the support of Burroughs’ welcome fund collaborative research travel grant (BWFCRTG). A.K.I. acknowledges the US Department of Defense CDMRP KCRP Idea Development Award # W81XWH1810471 and Wayne State University start-up support for funding the Iyer lab.
Biographies
Author biographies

Rami Alzhrani
Rami Alzhrani is a PhD student in the Department of Pharmaceutical Sciences, Wayne State University (WSA), USA. He also works as a faculty member in the College of Pharmacy, Taif University, Saudi Arabia. He was awarded his MSc by the College of Pharmacy and Pharmaceutical Sciences at the University of Toledo, Ohio, USA. He has published more than ten peer-reviewed articles and book chapters. He received the Summer 2019 Dissertation Award from WSA for outstanding work. His current research focuses on synthesizing universal targeted nanoparticles for treating multiple cancers.

Hashem O. Alsaab
Hashem O. Alsaab is an assistant professor and Department Chair of Pharmaceutics and Pharmaceutical Technology, Taif University, Saudi Arabia. He was awarded a PhD by WSU. He is an experienced research scientist working on innovative projects, has published more than 25 peer-reviewed papers, and has both a US and WO patent. Dr Alsaab has received many awards and achievements from WSU, American Association of Pharmaceutical Sciences (AAPS), University of Toledo, and Saudi Arabian Cultural Mission (SACM) for his outstanding contributions to cancer research. His research is broadly focused on exploring the role of nanomedicine and immunotherapy for diagnosis, imaging, and therapy, with an emphasis on cancer.

Samaresh Sau
Samaresh Sau is a senior research scientist in the Department of Pharmaceutical Sciences, WSA. He was awarded a PhD by the CSIR-Indian Institute of Chemical Technology, India. In his first postdoctoral position at Purdue University/Endocyte, he developed clinically translatable drug formulations for cancer and arthritis. He is now working on nanoparticle, small-molecule and antibody–drug conjugates for cancer immunotherapy with a goal to develop therapeutic and diagnostic agents for clinical translation. He is the author of 40 articles and book chapters, and inventor of two US patents.

Arun Iyer
Arun Iyer is the director of the U-BiND Systems Laboratory and assistant professor of Pharmaceutical Sciences at WSA. Dr Iyer was awarded a PhD by Sojo University, Japan, under Hiroshi Maeda (2016 Nobel Prize in Chemistry Nominee). Dr Iyer is a US DoD Early Career Investigator and recipient of the prestigious CRS T. Nagai Research Achievement Award. He has authored >100 publications in peer-reviewed international journals and books and has wide expertise in biomaterials and nanomedicine for treating conditions such as infections, inflammation, and cancer. His laboratory is funded by agencies such as NIH, DoD, American Cancer Society, and private foundations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content and all legal disclaimers that apply to the journal pertain.
Teaser: Drug delivery systems can be utilized to enhance the stability and efficiency of noncoding (nc)RNA, which has a potential role in cancer therapy. Here, we discuss the ability of ncRNA to target tumor microenvironments via modification of a variety of delivery systems utilizing nanotechnology-based delivery approaches.
References
- 1.Wapinski O and Chang HY (2011) Long noncoding RNAs and human disease. Trends Cell Biol. 21, 354–361 [DOI] [PubMed] [Google Scholar]
- 2.Mercer T et al. (2009) Long non-coding RNAs: insights into functions. Nat. Rev. Genet 10, 155–159 [DOI] [PubMed] [Google Scholar]
- 3.Matsui M and Core DR (2017) Non-coding RNAs as drug targets. Nat. Rev. Drug Discov 16, 167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutschner T and Diederichs S (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 9, 703–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anastasiadou E et al. (2017) Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattick JS and Makunin IV (2006) Non-coding RNA. Hum. Mol. Genet 15, R17–R29 [DOI] [PubMed] [Google Scholar]
- 7.Mendell JT (2005) MicroRNAs: critical regulators of development cellular physiology and malignancy. Cell Cycle 4, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 8.He L and Hannon GJ (2004) Erratum: MicroRNAs: small RNAs with a big role in gene regulation, Nat. Rev. Genet 5, 522–531 [DOI] [PubMed] [Google Scholar]
- 9.Esquela-Kerscher A et al. (2006) Oncomirs: microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 [DOI] [PubMed] [Google Scholar]
- 10.Hammond SM (2007) MicroRNAs as tumor suppressors. Nat. Genet 39, 582–583 [DOI] [PubMed] [Google Scholar]
- 11.Nicoloso M et al. (2009) MicroRNAs - the micro steering wheel of tumour metastases. Nat. Rev. Cancer 9, 293–302 [DOI] [PubMed] [Google Scholar]
- 12.Esteller M (2011) Non-coding RNAs in human disease. Nat. Rev. Genet 12, 861–874 [DOI] [PubMed] [Google Scholar]
- 13.Shi X et al. (2013) Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 339, 159–166 [DOI] [PubMed] [Google Scholar]
- 14.Yang X et al. (2015) MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep 5, 8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganesh S et al. (2013) In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 172, 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X et al. (2015) Cluster of differentiation 44 targeted hyaluronic acid based nanoparticles for MDR1 siRNA delivery to overcome drug resistance in ovarian cancer, Pharm. Res 32 2097–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L et al. (2013) On the classification of long non-coding RNAs, RNA Biol. 10 924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D et al. (2008) Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol. Cell. Biol 28, 3008–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrott AM et al. (2011) The evolution and expression of the snaR family of small non-coding RNAs. Nucleic Acids Res. 39 1485–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltran M et al. (2008) A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial–mesenchymal transition. Genes Dev. 22, 756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesana M et al. (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumazin P et al. (2011) An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147, 370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong C et al. (2011) lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature 470, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmena L et al. (2011) ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay Y et al. (2011) Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faghihi MA et al. (2008) Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of 6-secretase. Nat. Med 14, 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang V and Wu W (2009) MicroRNA-based therapeutics for cancer. BioDrugs 23, 15–23 [DOI] [PubMed] [Google Scholar]
- 28.Heneghan HM et al. (2010) MiRNAs as biomarkers and therapeutic targets in cancer. Curr. Opin. Pharmacol 10, 543–550 [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto N et al. (2017) Non-coding RNAs are promising targets for stem cell-based cancer therapy. Noncoding RNA Res. 2, 283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan G et al. (2015) Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev 87, 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty C et al. (2017) Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids 8, 132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ et al. (2016) Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev 104, 61–77 [DOI] [PubMed] [Google Scholar]
- 33.Lares MR et al. (2010) RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 28, 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozpolat B et al. (2014) Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev 66, 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bumcrot D et al. (2006) RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol 2, 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvam C et al. (2017) Therapeutic potential of chemically modified siRNA: recent trends. Chem. Biol. Drug Des 90, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriitzfeldt J et al. (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 [DOI] [PubMed] [Google Scholar]
- 38.Oh SY et al. (2009) A highly effective and long-lasting inhibition of miRNAs with PNA-based antisense oligonucleotides. Mol. Cells 28, 341–345 [DOI] [PubMed] [Google Scholar]
- 39.Weinstein S and Peer D (2010) RNAi nanomedicines: challenges and opportunities within the immune system. Nanotechnology 21, 232001. [DOI] [PubMed] [Google Scholar]
- 40.Oh YK and Park TG (2009) siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev 61, 850–862 [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee S et al. (1997) Endocytosis. Physiol. Rev 77, 759–803 [DOI] [PubMed] [Google Scholar]
- 42.Zuckerman JE and Davis ME (2015) Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov 14, 843–856 [DOI] [PubMed] [Google Scholar]
- 43.Shen J et al. (2014) Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics 4, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ewe A et al. (2017) Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA deliver in vivo. Nanomedicine 13, 209–218 [DOI] [PubMed] [Google Scholar]
- 45.Xiang B et al. (2017) Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomedicine 12, 2385–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X et al. (2019) Delivery of siRNA using folate receptor-targeted pH-sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomedicine 20, 102017. [DOI] [PubMed] [Google Scholar]
- 47.Esquela-Kerscher A et al. (2006) Oncomirs: microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 [DOI] [PubMed] [Google Scholar]
- 48.Adams BD et al. (2014) Aberrant regulation and function of microRNAs in cancer. Curr. Biol 24, R762–R776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeliadt N (2014) Big pharma shows signs of renewed interest in RNAi drugs. Nat. Med 20, 109. [DOI] [PubMed] [Google Scholar]
- 50.van Rooij E et al. (2012) Developing microRNA therapeutics. Circ. Res 110 496–507 [DOI] [PubMed] [Google Scholar]
- 51.Stenvang J et al. (2012) Inhibition of microRNA function by antimiR oligonucleotides. Silence 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry JC et al. (2010) miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem. Biophys. Res. Commun 403, 120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira DM et al. (2013) Delivering the promise of miRNA cancer therapeutics. Drug Discov. Today 18, 282–289 [DOI] [PubMed] [Google Scholar]
- 54.Shah MY et al. (2016) microRNA therapeutics in cancer - an emerging concept. EBioMedicine 12, 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen PE et al. (1994) Peptide nucleic acid (PNA). A DNA mimic with a peptide backbone. Bioconjug. Chem 5, 3–7 [DOI] [PubMed] [Google Scholar]
- 56.Fabani MM and Gait MJ (2008) miR-122 targeting with LNA/2’-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA 14, 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misso G et al. (2014) Mir-34: a new weapon against cancer? Mol. Ther. Acids 3, e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirollo KF et al. (2008) Tumor-targeting nanocomplex delivery of novel tumor suppressor RB94 chemosensitizes bladder carcinoma cells in vitro and in vivo. Clin. Cancer Res 14, 2190–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trang P et al. (2011) Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther 19, 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasinski AL et al. (2015) A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene 34, 3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodrow KA et al. (2009) Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater 8, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinn JL and Chang HY (2012) Genome regulation by long noncoding RNAs. Annu. Rev. Biochem 81, 145–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smaldone MC and Davies BJ (2010) BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr. Opin. Mol. Ther 12, 607–616 [PubMed] [Google Scholar]
- 64.Fatemi RP et al. (2014) De-repressing LncRNA-targeted genes to upregulate gene expression: focus on small molecule therapeutics. Mol. Ther. Acids 3, e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rettig GR and Behlke MA (2012) Progress toward in vivo use of siRNAs-II. Mol. Ther 20, 483–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurreck J et al. (2002) Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 30, 1911–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsang J et al. (2007) MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol. Cell 26, 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trang P et al. (2010) Regression of murine lung tumors by the let-7 microRNA. Oncogene 29, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banch N and Vassella E (2011) miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol. Cancer 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herranz H and Cohen SM (2010) MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 24, 1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babar IA et al. (2012) Nanoparticle-based therapy in an in vivo mieroRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. U. S. A 109, E1695–E1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasinski AL and Slack FJ (2012) miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 72, 5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z et al. (2013) Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS ONE 8, e73991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo X et al. (2015) GAS5 inhibits gastric cancer cell proliferation partly by modulating CDK6. Oncol. Res. Treat 38, 362–366 [DOI] [PubMed] [Google Scholar]
- 75.Yin D et al. (2014) Long noncoding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med. Oncol 31 253. [DOI] [PubMed] [Google Scholar]
- 76.Yang X et al. (2014) Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE 9, e98833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L et al. (2015) Effects of microRNA-221/222 on cell proliferation and apoptosis in prostate cancer cells. Gene 572, 252–258 [DOI] [PubMed] [Google Scholar]
- 78.Stahlhut C et al. (2012) miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Development 139, 4356–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuehbacher A et al. (2007) Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res 101, 59–68 [DOI] [PubMed] [Google Scholar]
- 80.Cimmino A et al. (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. U. S. A 102, 13944–13949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng F et al. (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Mattos-Arruda L et al. (2015) MicroRNA-21 links epithelial-to-mesenehymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in FIER2-positive breast cancer patients. Oncotarget 6, 37269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yigit MV et al. (2013) Context-dependent differences in miR-10b breast oncogenesis can be targeted for the prevention and arrest of lymph node metastasis. Oncogene 32, 1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma L et al. (2010) Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor mode. Nat. Biotechnol 28, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Z et al. (2012) miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int. J. Oncol 40, 1553–1560 [DOI] [PubMed] [Google Scholar]
- 86.Xue H et al. (2015) Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des 21, 3140–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gascón AR et al. (2013) Non-viral delivery systems in gene therapy In Gene Therapy - Tools and Potential Applications (Martin F, ed.), pp. 3–33, InTechOpen [Google Scholar]
- 88.Ghosh R et al. (2013) A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials 34, 807–816 [DOI] [PubMed] [Google Scholar]
- 89.Alhasan AH et al. (2014) Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent MicroRNA regulation agents. Small 10, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao M et al. (2017) Nrf-2-driven long noncoding RNA ODRUL contributes to modulating silver nanoparticle-induced effects on erythroid cells. Bio materials 130, 14–27 [DOI] [PubMed] [Google Scholar]
- 91.Mouraviev V et al. (2016) Clinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancer. Prostate Cancer Pro static Dis. 19, 14–20 [DOI] [PubMed] [Google Scholar]
- 92.Oh YK and Park TG (2009) siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev 61, 850–862 [DOI] [PubMed] [Google Scholar]
- 93.Monteiro N et al. (2014) Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 11, 20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanasty R et al. (2013) Delivery materials for siRNA therapeutics. Nat. Mater 12, 967–977 [DOI] [PubMed] [Google Scholar]
- 95.Gabizon A et al. (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 54, 987–92 [PubMed] [Google Scholar]
- 96.Gabizon A et al. (1991) Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br. J. Cancer 64, 1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen TM and Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev 65, 36–48 [DOI] [PubMed] [Google Scholar]
- 98.Bouchie A (2013) First microRNA mimic enters clinic. Nat. Biotechnol 31, 577–577 [DOI] [PubMed] [Google Scholar]
- 99.Chen Y et al. (2010) Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J. Biol. Chem 285, 22639–22650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brito LA et al. (2014) A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther 22, 2118–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X et al. (2018) RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 37, 107–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen X et al. (2018) RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 37, 107–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gary DJ et al. (2007) Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J. Control. Release 121, 64–73 [DOI] [PubMed] [Google Scholar]
- 104.Oh YK and Park TG (2009) siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev 61, 850–862 [DOI] [PubMed] [Google Scholar]
- 105.Dehousse V et al. (2010) Development of pH-responsive nanocarriers using trimethylchitosans and methacrylic acid copolymer for siRNA delivery. Biomaterials 31, 1839–1849 [DOI] [PubMed] [Google Scholar]
- 106.Sun T et al. (2008) Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials 29, 4348–4355 [DOI] [PubMed] [Google Scholar]
- 107.Sau S et al. (2018) A tumor multicomponent targeting chemoimmune drug delivery system for reprograming the tumor microenvironment and personalized cancer therapy. Drug Discov. Today 23, 1344–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alsaab H et al. (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol 8, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wickens JM et al. (2017) Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 22, 665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhise K et al. (2017) Nanomedicine for cancer diagnosis and therapy: advancement success and structure--activity relationship. Ther. Deliv 8, 1003–1018 [DOI] [PubMed] [Google Scholar]
- 111.Riaz M et al. (2018) Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int. J. Mol. Sci 19, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Torchilin VP (2010) Passive and active drug targeting: drug delivery to tumors as an example. Handb. Exp. Pharmacol 197, 3–53 [DOI] [PubMed] [Google Scholar]
- 113.Li R et al. (2017) Be active or not: the relative contribution of active and passive tumor targeting of nanomaterials. Nanotheranostics 1, 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maeda H et al. (2013) The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev 65, 71–79 [DOI] [PubMed] [Google Scholar]
- 115.Costa DF and Torchilin VP (2018) Micelle-like nanoparticles as siRNA and miRNA carriers for cancer therapy. Biomed. Microdevices 20, 59. [DOI] [PubMed] [Google Scholar]
- 116.Low PS et al. (2008) Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res 41, 120–129 [DOI] [PubMed] [Google Scholar]
- 117.Wang Z et al. (2018) CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomedicine 14, 1441–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu M et al. (2013) Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 5, 178–183 [DOI] [PubMed] [Google Scholar]
- 119.Kim GH et al. (2018) Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Deliv. 25, 1394–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alsaab H et al. (2017) Folate decorated nanomicelles loaded with a potent curcumin analogue for targeting retinoblastoma. Pharmaceutics 9, E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia Y et al. (2018) siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomedicine 13, 1539–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X et al. (2009) Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int. J. Pharm 373, 116–123 [DOI] [PubMed] [Google Scholar]
- 123.Koshkaryev A et al. (2012) Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol. Ther 13, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye, et al. (2016) CPP-assisted intracellular drug delivery, what is next? Int. J. Mol. Sci 17, 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Z, et al. (2012) Cyclic RGD peptide-modified liposomal drug delivery system: enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomedicine 7, 3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meng S et al. (2011) Integrin-targeted paclitaxel nanoliposomes for tumor therapy. Med. Oncol 28 1180–1187 [DOI] [PubMed] [Google Scholar]
- 127.Alsaab H et al. (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol 8, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sau S et al. (2017) Advances in antibody-drug conjugates: a new era of targeted cancer therapy. Drug Discov. Today 22, 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sau S et al. (2018) Multifunctional nanoparticles for cancer immunotherapy: a groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control. Release 274, 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manjappa AS et al. (2011) Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release 150, 2–22 [DOI] [PubMed] [Google Scholar]
- 131.Mamot C et al. (2005) Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 65, 11631–11638 [DOI] [PubMed] [Google Scholar]
- 132.Lee YK et al. (2015) Inhibition of pulmonary” cancer progression by epidermal growth factor receptor-targeted transfection with Bcl-2 and survivin siRNAs. Cancer Gene Ther. 22, 335–343 [DOI] [PubMed] [Google Scholar]
- 133.Eloy JO et al. (2017) Anti-HER2 immunoliposomes for co-delivery of paclitaxel and rapamycin for breast cancer therapy. Eur. J. Pharm. Biopharm 115, 159–167 [DOI] [PubMed] [Google Scholar]
- 134.Park JW et al. (2002) Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin. Cancer Res 8, 1172–1181 [PubMed] [Google Scholar]
- 135.Hatakeyama H et al. (2007) Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int. J. Pharm 342 194–200 [DOI] [PubMed] [Google Scholar]
- 136.Gosk S et al. (2008) VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim. Biophys. Acta Biomembr 1778, 854–863 [DOI] [PubMed] [Google Scholar]
- 137.Sivakumar P et al. (2018) Targeted siRNA delivery using aptamer-siRNA chimeras and aptamer-conjugated nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 11, e1543. [DOI] [PubMed] [Google Scholar]
- 138.Li, et al. (2018) Targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int. J. Nanomedicine 13, 1241–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Edmonds, et al. (2016) MicroRNA-31 initiates lung tumorigenesis and promotes mutant KRAS-driven lung cancer. J. Clin. Invest 126, 349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang W et al. (2016) Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat. Commun 7, 11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adams B et al. (2016) miR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer. Cancer Res. 76, 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Babar I et al. (2012) Nanoparticle-based therapy” in an in vivo mieroRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad Sci. U. S. A 109, E1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao L et al. (2013) pHLIP peptide targets nanogold particles to tumors. Proc. Natl. Acad Sci. U. S. A 110, 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arun G et al. (2016) Differentiation of mammary tumors and reduction in metastasis upon Malatl lncRNA loss. Genes Dev. 30, 34–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin D et al. (2015) Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumor Biol. 36 4851–4859 [DOI] [PubMed] [Google Scholar]
- 146.Choung S et al. (2006) Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun 342, 919–927 [DOI] [PubMed] [Google Scholar]