Abstract
Blood vessels and nerves travel together to supply most tissues in the body. However, there is a knowledge gap in the mechanisms underlying the direct regulation of angiogenesis by nerves. In the current study, we examined the regulation of angiogenesis by sensory nerves in response to inflammation using the cornea, a normally avascular and densely innervated ocular tissue, as a model. We used desiccating stress as an inflammatory stimulus in vivo and found that sub-basal and epithelial nerve densities in the cornea were reduced in dry eye disease (DED). We established a co-culture system of trigeminal ganglion sensory neurons and vascular endothelial cells (VEC) and found that neurons isolated from mice with DED directly promoted VEC proliferation and tube formation compared with normal controls. In addition, these neurons expressed and secreted higher levels of substance P (SP), a proinflammatory neuropeptide. SP potently promoted VEC activation in vitro and blockade of SP signaling with spantide I, an antagonist of SP receptor Neurokinin-1, significantly reduced corneal neovascularization in vivo. Spantide I and siRNA knockdown of SP abolished the promotion of VEC activation by DED neurons in vitro. Taken together, our data suggested that sensory neurons directly promote angiogenesis via SP signaling in response to inflammation in the cornea.
Keywords: ocular surface inflammation, angiogenesis, corneal neovascularization, dry eye disease, corneal nerves, substance P
INTRODUCTION
Blood vessels and nerves are branched structures that typically travel together to supply most tissues in the body. There is mounting evidence that vascular and nervous systems cross-talk and influence each other in development, injury, and repair, with implications in neurodegenerative diseases such as Alzheimer disease and diabetic neuropathy, as well as skin wound healing, and rheumatoid- and osteo-arthritis. (1–6)
The cornea is the outermost and transparent layer of the eye. The normal cornea is avascular and densely innervated, rendering it a unique model to interrogate neuro-vascular interaction. Infection and inflammation of the ocular surface can induce corneal neovascularization (NV) and nerve inflammation/degeneration. (7–10) Corneal epithelium, stromal keratocytes, limbal stem cells, and infiltrating leukocytes have all been implicated in corneal angiogenesis. There is, however, limited evidence on whether corneal nerves play a direct role in corneal NV.
The cornea is innervated by the ophthalmic branch of the trigeminal nerve (cranial nerve V1). Nerve fibers originate from the trigeminal ganglion, enter the cornea from the limbus, transverse in stroma, and then form subbasal nerve plexus that supplies the overlying epithelium. (11–15) In addition to their sensory function, corneal nerves modulate the blink reflex, tear production, and stem cell niche maintenance. (13, 16) They also play critical roles in corneal epithelial wound healing and immune privilege. (17, 18) In the aggregate, these studies support the notion that corneal nerves are not only sensors but major regulators of ocular surface homeostasis.
Spontaneous corneal NV after trigeminal denervation has been reported in mice. (19) This phenomenon is also observed clinically in a patient developing corneal NV after trigeminal nerve block for neuralgia (20). This circumstantial evidence indicates that neuronal support is needed to maintain corneal avascularity. But the direct interaction between blood vessels and nerves in the cornea, especially during inflammation, has not been studied. Dry eye disease (DED) is the most common ocular surface inflammatory disease with an overall prevalence of 5.28% in US population. (21) Both human and animal studies have shown structural and functional alterations of corneal nerves in DED. (22–24) Moreover, response to DED treatment appears to depend on corneal nerve density. (25) Although there is no spontaneous corneal NV in DED, vascular response after injury is heightened. (26) Whether DED-related alterations in corneal innervation play a role in such heightened vascular response have not been explored.
In the current study, we established a co-culture system of sensory neurons derived from the trigeminal ganglion and vascular endothelial cells to directly study their interaction. We induced ocular surface inflammation with desiccating stress using a DED mouse model, and investigated the role of substance P, a neuropeptide secreted by sensory neurons, in promoting angiogenesis after inflammation in vitro and in vivo.
MATERIALS AND METHODS
Animals
Eight-week old male BALB/c and female C57BL/6 mice were obtained from Charles River Laboratories, Wilmington, MA, USA. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Schepens Eye Research Institute of Massachusetts Eye and Ear and were in compliance with the guidelines of the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmology and Vision. Mice were anesthetized for surgical procedures using intraperitoneal injections of 120 mg/kg Ketamine and 20 mg/kg Xylazine. Post-operative pain management and care were provided.
Dry eye disease (DED) induction in vivo
DED was induced in mice as previously described. (27, 28) In brief, female C57BL/6 mice were subjected to desiccating stress by placing them in a controlled-environment chamber with the continuous regulation of relative humidity below 20%, airflow of 15 L/min and a constant temperature of 21 to 23°C for 7 consecutive days. These mice develop DED characterized by increased corneal fluorescein staining and reduced tear secretion within 7 days. Untreated age- and sex-matched mice maintained in the standard vivarium were used as normal controls. Tissues were isolated from mice with and without DED and processed as described below.
Intrastromal suture-induced corneal neovascularization (NV) in vivo
A circular trephine, 1.5 mm in diameter, was used to mark the central corneas of male BALB/c mice. Three intrastromal figure-eight suture knots were placed into the central cornea using 11–0 nylon sutures (AB-0550S, MANI, Tochigi, Japan) as previously described. (29) After suture placement, topical application of 5 μl of 20 μM Spantide I (diluted in phosphate-buffered saline, PBS) was given three times daily and continued for 14 days, same volume of PBS served as the control (N=10 per group). Corneal NV was clinically evaluated and photographed daily. The area covered by blood vessels of the total cornea was analyzed using ImageJ in a masked analysis. The sutures were removed on Day 14 and the corneas were harvested for immunohistochemistry.
Vascular endothelial cell (VEC) culture
Mouse VEC (MILE SVEN1 cell line) was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in complete media DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Flower Branch, GA, USA) and 100U/ml penicillin (MilliporeSigma, Burlington, MA, USA) at 37°C and in 5% CO2.
Primary culture of trigeminal ganglion (TG) neurons
TG neurons were isolated and cultured as described previously. (30) In brief, mice with DED or naïve controls were euthanized (N=5 per group), followed by transcardial perfusion with cold PBS. TG were collected and subjected to enzymatic digestion with papain (Worthington, Lakewood, NJ, USA) and collagenase type II /dispase (Thermo Fisher Scientific). Digested tissues were then subjected to Percoll (VWR, Radnor, PA, USA) gradient and TG neurons were collected from the bottom layer of the gradient by centrifugation at 1300 g for 10 min. The neurons were then washed twice with Neurobasal A medium supplemented with 2% B27 supplement (Thermo Fisher Scientific) and 1% penicillin-streptomycin. Neurons were counted and plated on poly-d-lysine- and laminin-coated coverslips in a 24-well plate at a density of 3,000 neurons per coverslip. Neuronal cultures were maintained with complete neuronal medium, consisting of Neurobasal A medium supplemented with 2% B27 supplement, 1% penicillin-streptomycin, l-glutamine (500 μM), nerve growth factor (NGF; 50 ng/ml), glial-cell-derived neurotrophic factor (GDNF; 50 ng/ml), and mitotic inhibitor fluorodeoxyuridine (60 μM) for 3 days (growth factors were from Thermo Fisher Scientific, and other supplements were from Sigma) and then prepared for co-culture or collection of conditioned media on day 4.
Immunostaining with neuron marker β-tubulin III and glial cell marker GFAP was used to detect the purity of TG neuron culture. TG neurons were fixed in 4% paraformaldehyde (MilliporeSigma, Burlington, MA, USA) at day 4 after conditioned media collection and blocked with 2% bovine serum albumin. TG neurons were incubated overnight at 4°C with Alexa Fluor 488 conjugated anti-beta III Tubulin (MilliporeSigma, Burlington, MA, USA) and Cy3 conjugated anti-GFAP antibody (Abcam, Cambridge, MA, USA) diluted in PBS with 1% BSA. The tissues were then washed five times in PBS and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) with DAPI. Images were obtained using confocal microscopy (TCSSP5; Leica, Wetzlar, Germany). The number of TG neurons was defined as the number of β-tubulin III-positive, relatively large round cells. The number of TG neurons was directly counted in 3 randomly chosen and separate 40× fields, and averaged.
siRNA transfection of TG neurons
Tac1 (substance P precursor) and negative control (non-targeting) siRNAs were from Dharmacon (Lafayette, CO, USA). Tac1 siRNAs were Accell SMARTpool of the following 4 siRNAs, #1 5′-CGAUGAUCUAAAUUAUUGG-3′, #2 5′-GCGCUAUGAGGAAUGAUUA-3′, #3 5′-CCAAUAAGCCUUGUAAUUC-3′ and #4 5′-CUUUCAUGCUGAUAAUGUA-3′. Negative control siRNA (catalogue number D-001930-02-05) has been designed and modified by Dharmacon to have minimal targeting of genes in mouse cells. TG neurons isolated from DED mice were cultured in complete neuronal growth medium as described above for 3 days. On day 4, neurons were treated with 1 μM Tac1 or control siRNAs and cultured for 3 more days. On day 7, the culture media containing siRNA were removed and fresh DMEM supplemented with 1% FBS and 100U/ml penicillin were placed in neuron culture. After 24 hours of culture, supernatants were collected and centrifuged at 1300g for 10 minutes to remove cell debris. The neurons were harvested for PCR analysis.
Co-culture of TG neurons and VEC
TG neurons were cultured on coverslips with complete neuronal medium for 3 days. VECs were seeded in a 12-well plate, cultured in complete media for 24h, and then starved in DMEM supplemented with 1% FBS overnight. The coverslips with TG neurons were washed twice with 1% FBS DMEM and then were placed into Transwell inserts (3.0 mm pore size; Corning, Corning, NY, USA), and the inserts were then put in the 12-well plate where the VECs were already cultured in the bottom well. The co-culture system was incubated in 2ml DMEM supplemented with 1% FBS for 24 hours (Various media were tested to ensure the vitality of both VEC and TG neurons and DMEM supplemented with 1% FBS was the most optimal). After 24 hours, the Transwell inserts were removed and VECs were washed twice with PBS and subjected to the assays described below.
Collection of conditioned media from TG neuron culture
TG neurons isolated from mice with and without DED were cultured in complete neuronal growth medium as described above for 3 days. On day 4, the neurons were washed twice with and then cultured in DMEM supplemented with 1% FBS and 100U/ml penicillin. After 24 hours of culture, the conditioned media (supernatant) were collected and centrifuged at 1300g for 10 minutes to remove cell debris.
VEC proliferation assay
VECs (5 × 104 cells / well in a 12-well plate) were co-cultured with TG neurons or cultured in the TG conditioned media for 24 hours. To determine the role of SP in VEC growth, 2μM spantide I (Tocris Bioscience, Minneapolis, MN, USA) was added to the conditioned media. The cell numbers of VEC were counted directly using a hemacytometer under a 20× objective phase contrast microscope field. Each sample was counted in four separate fields and the mean cell number was used for analysis. The assay was repeated twice. The effect of substance P on VEC growth was evaluated with BrdU Cell Proliferation Kit (MilliporeSigma, Cat. No 2752, Burlington, MA, USA).VEC (5 × 103 cells / well) were seeded in a 96-well plate. Serum-starved VECs were cultured in the presence or absence of 1μM Substance P (Tocris Bioscience) with DMEM supplemented with 1% FBS for 24 hours. Proliferating cells were labeled with BrdU during the last 22 hours of the culture period. BrdU incorporation into the cells was quantified by the absorbance value at 450 nm. The assay was repeated three times.
VEC migration assay
A linear scratch was made using a 10-μl micropipette tip in a confluent monolayer of VECs on 12-well plate. VECs were co-cultured with TG neurons, or cultured in conditioned media with and without 2μM spantide I, or cultured in the presence or absence of 1μM Substance P with DMEM supplemented with 1% FBS for 24 hours. Images were captured at 0 h and 24 h under 5x objective phase contrast inverted microscope field. The areas between leading edges of migration were measured using ImageJ software. Cell migration was expressed as the percentage of healed area. The assay was repeated three times.
VEC tube formation assay
Tube formation assay was performed as previously described. (31) In brief, 65 μl growth factor-reduced Matrigel (Corning, Cat. No 354230; NY, USA) was placed into a 96-well plate and allowed to polymerize for 1.5h at 37°C. VEC (3.0×104 cells/well) were re-suspended in DMEM with 10% FBS and added onto the layer of Matrigel. VEC/Matrigel were incubated for 4 hours at 37°C and photographed (x100 magnification). The total number of tube junctions and length of the tube-like structure were calculated by using Angiogenesis Analyze plug-ins of ImageJ software. The assay was repeated twice.
Corneal whole mount and immunostaining
The entire cornea with limbus was excised under the operating microscope from both control and mice with DED (N=10 per group). Excised corneas were fixed in 4% paraformaldehyde and washed in PBS, incubated in 20 mmol/L pre-warmed EDTA (MilliporeSigma, Burlington, MA, USA) for 30 minutes at 37°C, and incubated with a 0.05% solution of hyaluronidase (MilliporeSigma) in EDTA-PBS for 24 hours at 37°C. The corneas were then washed in PBS and blocked with a 0.3% solution of Triton X-100 in PBS plus 2% BSA for 2 hours at room temperature. After blocking, the corneas were incubated overnight at 4°C with Alexa Fluor 488 conjugated anti-beta III Tubulin diluted in PBS with 1% BSA. The corneas were then washed five times in PBS. Four radial cuts were made to mount the cornea flat on a slide with the epithelium side up using Vectashield. Corneal whole mounts were examined using confocal microscopy. Innervation in a region (200x microscope fields) was calculated as the percentage of area positive for β-tubulin III staining using ImageJ software. To quantitate the number of epithelial nerve terminals, images were analyzed by threshold-based automatic particle counting function of the ImageJ software.
Whole-mount immunostaining was also used to determine the extent of corneal neovascularization. Corneas were excised 14 days after suture placement and fixed in 4% paraformaldehyde. They were washed and incubated in EDTA for 30 minutes, then blocked with 1% BSA for 2 hours at temperature. The corneas were stained with purified rat anti-mouse CD31 (BD Biosciences, Cat. No 550274) overnight, followed by secondary antibody. Four radial cuts were made to mount the cornea flat on a slide with the epithelium side up using Vectashield. Corneal whole mounts were examined using TCSSP5 confocal microscopy.
Real-time PCR
Whole trigeminal ganglia were isolated from mice. TG tissues or cultured TG neurons were placed in TRIzol® Reagent (Thermo Fisher Scientific). Total RNA was isolated with an RNeasy® Micro kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s recommendations and reverse transcribed using a SuperScript™ III kit (Thermo Fisher Scientific). Real-time PCR was performed using TaqMan® Universal PCR Master Mix and predesigned primers for substance P (Mm01166996_m1), CGRP (Mm00801463_m1), alpha-MSH (Mm00435874_m1) and GAPDH (Mm99999915_g1) (Thermo Fisher Scientific) in a LightCycler® 480 II System (Roche Applied Science). The GAPDH gene was used as an endogenous control for each reaction. The results of quantitative PCR were analyzed by the comparative CT method in which the target change = 2−ΔΔCT. The results were normalized by the CT value of GAPDH, and the mean CT of relative mRNA level in the naïve group was used as the reference.
Enzyme-linked immunosorbent assay (ELISA) analysis
The expression of substance P in TG tissue and cultured neurons, and its secretion in the conditioned media of neuronal culture were evaluated with the mouse substance P ELISA kit (Cayman Chemical, Cat. No 583751; Ann Arbor, MI, USA) according to the manufacturer’s protocol. Protein concentrations were measured using BCA Protein Assay (Thermo Fisher Scientific). Substance P concentrations were normalized to total protein concentrations.
Statistical analyses
All quantitative data were presented as means ± SEM from at least two independent experiments. Student’s t-test was used to analyze differences between two samples. Two-way ANOVA was used to assess differences between groups and Bonferroni posttest was used to assess differences within groups. Analyses were performed with Prism (GraphPad, La Jolla, CA, USA). Two-sided of P values less than 0.05 were considered statistically significant.
RESULTS
Corneal innervation, trigeminal ganglion neuron culture, and co-culture with VEC
To determine mouse corneal innervation, whole mount immunostaining of beta tubulin III (nerve marker) was performed. The large thick stromal nerves entered the cornea from the limbus and bifurcated as they traveled centripetally (Fig 1A). The nerves further divided into thinner fibers and formed extensive sub-basal nerve plexus just beneath the basal epithelial cells in a swirling pattern at the corneal apex (Fig 1B) and in a radial pattern peripherally (Fig. 1C). These fibers then traveled perpendicular to the surface of the cornea and ended as free nerve terminals in the epithelium (Fig. 1D).
Figure 1.
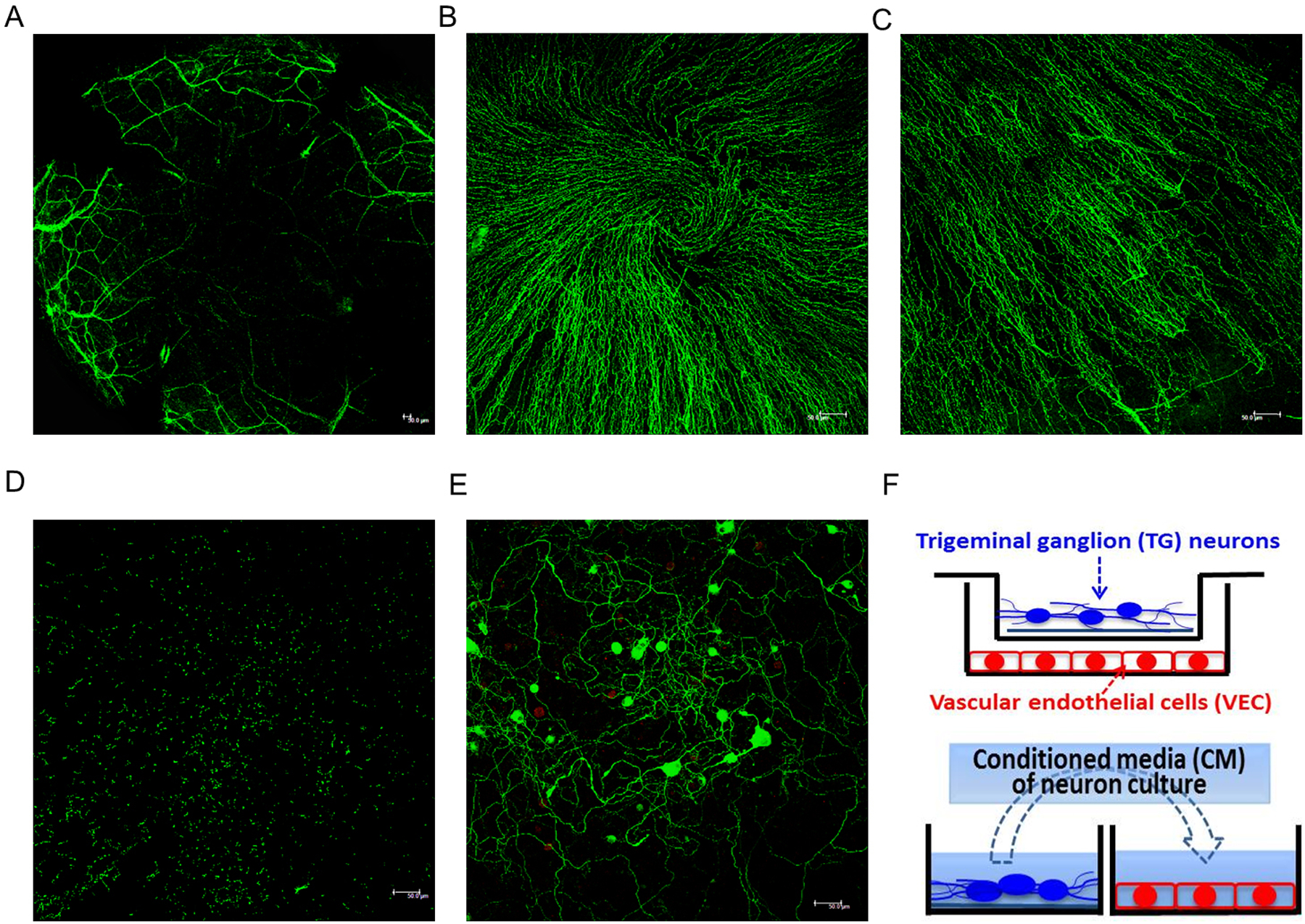
Corneal innervation of naïve C57BL/6 mice and co-culture system of TG neurons and VEC. (A-D) Representative image of corneal whole mount with β-tubulin III immunostaining demonstrates the stromal nerves (A, ×50 magnification), subbasal nerve plexus in the center (B) and periphery (C), and epithelial nerve terminals (D); B-D, x200 magnification. (E) Neuron cultures were maintained with complete neuronal medium for 3 days, and stained for neurons (β-tubulin III; green) and glial cells (GFAP; red), ×200 magnification. Scale bar 50 μm. (F) Schematic of co-culture system with neurons on coverslip in the transwell and VEC in the bottom well of the plate. Conditioned media (CM) were collected between day 3 and day 4 of neuron culture and then applied to VEC culture.
To characterize sensory neurons innervating the cornea in vitro, we harvested TG neurons from C57BL/6 mice and neuron viability and purity can be maintained up to seven days. Figure 1 E shows tubulin III-labeled neurons with large round cell body (20–40 μm in diameter) and extensive network of axons on day 3 of neuron culture. Very few GFAP-labeled glial cells were present, confirming the purity of neuron culture. To interrogate the direct interaction between sensory neurons and blood vessels, we established an in vitro co-culture system of TG neurons and vascular endothelial cells (VEC) using a transwell system. As shown in the schematic in Figure 1F, TG neurons were cultured on a cover slip and placed in the top chamber of the transwell and VEC were cultured in the bottom well. Alternatively, conditioned media of cultured neurons were collected between day 3 and day 4 of culture (24 hours total) and applied to VEC culture. In both systems, VEC activities including proliferation, migration, and tube formation were then assessed.
Alternation of corneal innervation after ocular surface inflammation
Dry eye disease (DED) is the most common ocular surface inflammatory disease. Figure 2 examined the effect of desiccating stress on corneal innervation. Compared to corneal nerves in normal age-matched control mice, these in mice with DED demonstrated similar stromal branches. In the central cornea, there was significant reduction in sub-basal nerve plexus density in DED group (DED vs naïve: 20.1% ± 2.0% vs 28.3% ± 3.1%, p=0.039 Fig. 2B) compared with normal controls; while nerve terminals in the epithelium were similar (Fig. 2C). In the peripheral cornea, both sub-basal nerve plexus density (DED vs naïve: 12.6% ± 0.7% vs 15.3% ± 0.8%, p=0.041, Fig. 2D) and epithelial terminals (DED vs naïve: 535 ± 66 vs 861± 76, p=0.011, Fig. 2E) were reduced in DED group. There was no difference in the number of neurons isolated from each trigeminal ganglion between the normal and DED groups (Data not shown). Cultured TG neuron density and morphology were also comparable between normal and DED groups (Fig. 2F, density of cultured TG neurons was 21 ± 2 per 400x field in DED and 21 ± 3 in control groups, p=0.949), confirming that observations in the co-culture system were not due to a difference in the number of neurons cultured.
Figure 2.
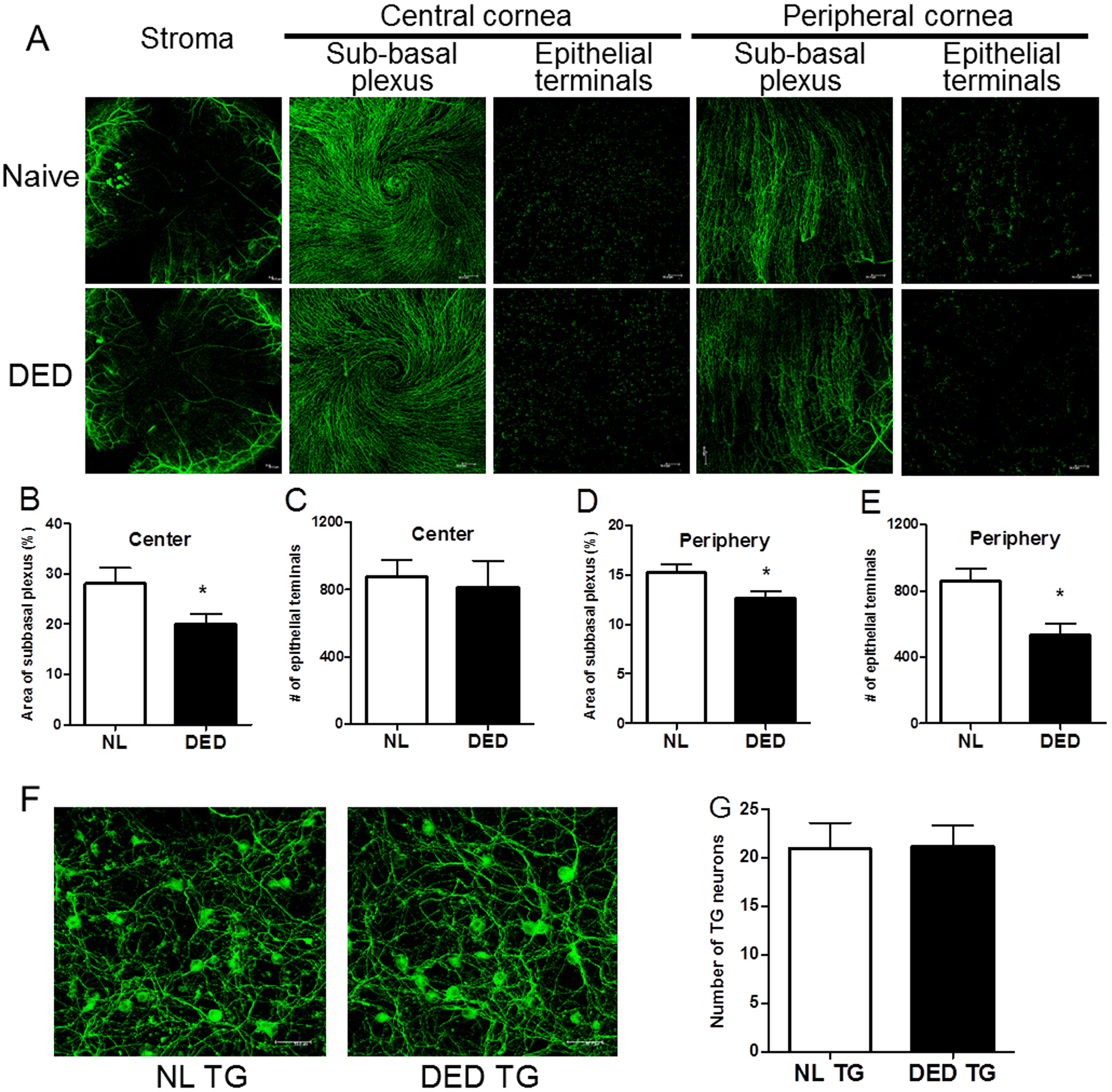
Morphological changes in corneal innervation after desiccating stress. (A) Representative images of corneal whole mount with β-tubulin III immunostaining showed stromal nerves, subbasal plexus and epithelial terminals in the central and peripheral cornea in naïve and mice with DED (n=10 per group). Stromal nerve: x50 magnification. Subbasal plexus and epithelial terminals: x200 magnification. (B-E) Density of subbasal plexus was shown as area occupied by nerve fibers (%) per microscope field (B &D). Density of epithelial terminals was shown as number of terminals per microscope field (C&E). (F) Representative images of cultured TG neurons (β-tubulin III; green) isolated from naïve (n=5) and mice with DED (n=5), x400 magnification. (G) Number of TG neurons counted per microscope field was shown. Error bars represent means ± SEM. *p<0.05.
TG neurons directly promote vascular endothelial cell proliferation and tube formation after inflammation
To determine whether corneal nerves regulate angiogenesis in response to inflammation, we compared VEC activities when co-cultured with TG neurons (or their conditioned media) isolated from normal versus mice with DED. The presence of TG neurons derived from mice with DED significantly promoted VEC proliferation (27% increase, p=0.02, Fig. 3A) and had less impact on migration (6% increase, p=0.68, Fig. 3B–C), compared with neurons derived from control mice. Similarly, the conditioned media of DED TG neuron culture promoted VEC proliferation (15% increase, p=0.027, Fig. 3D), less so migration (9% increase, p=0.14, Fig.3E-F). Furthermore, conditioned media from DED TG neuron culture greatly promoted VEC tube formation (Fig. 3I) with more than 3- and 4-fold increases in the number of junctions (p=0.026, Fig. 3G) and length of tubes formed (p=0.03, Fig. 3H), respectively. These results indicate that in response to inflammation, TG neurons directly promote VEC activation.
Figure 3.
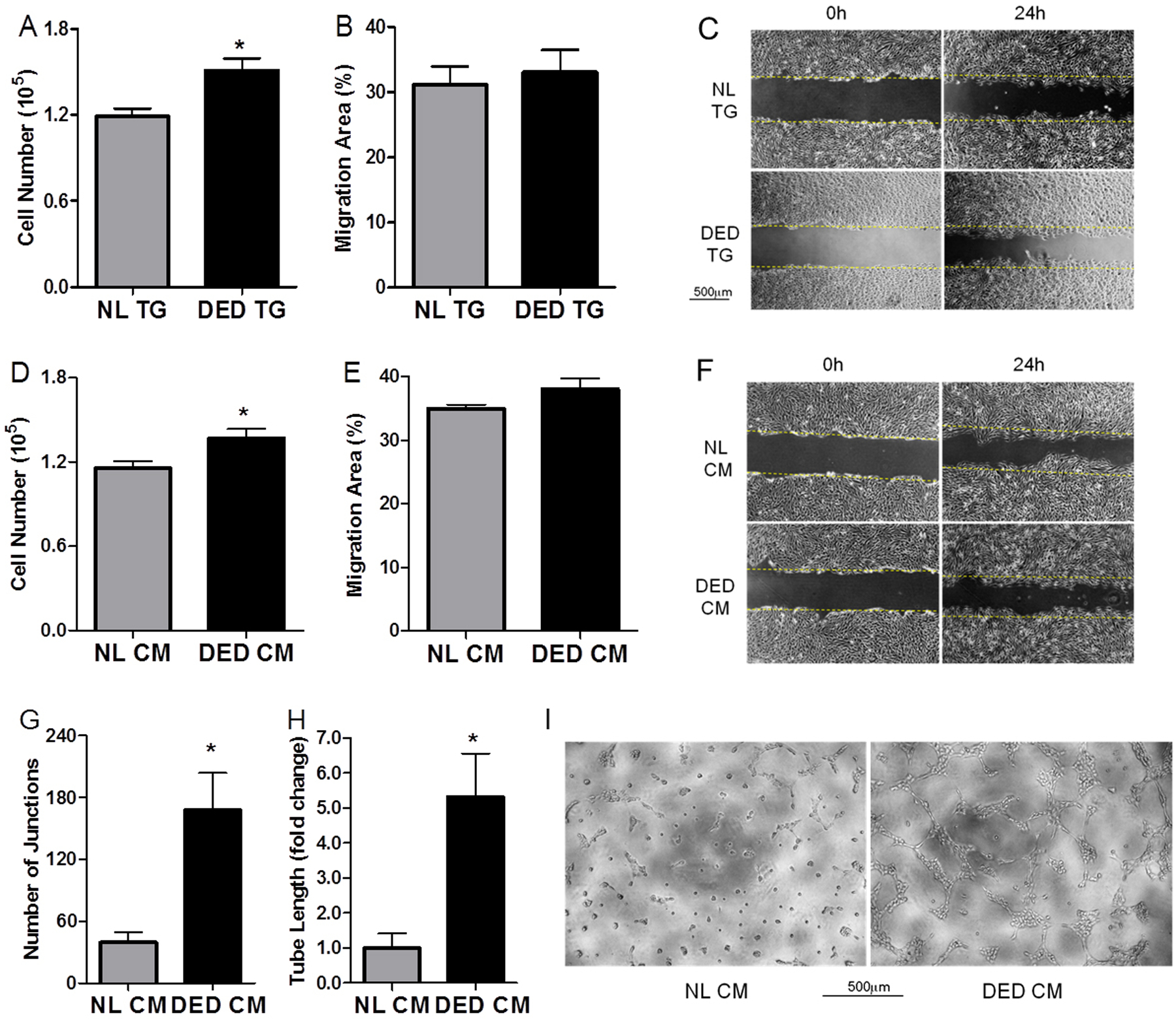
TG neurons promote VEC proliferation and tube formation after desiccating stress. (A) VEC proliferation was evaluated after 24-hour co-culture with naïve or DED TG neurons (n=4). Migration of VEC co-cultured with naïve or DED TG neurons was quantified (B) and photographed at 0h and 24h (C), n=7. (D-H) Conditioned media were applied to VEC culture. VEC proliferation (D, n=8) and migration (E&F, n=4) were determined. Tube formation on Matigel was photographed after 4 hours of culture (I) and quantified as the number of junctions (G) and length of tubes formed (H), n=4. Scale bars, 500μm. *p<0.05.
TG neurons express and secrete higher levels of substance P after inflammation
To investigate the mechanism(s) underlying the promotion of VEC activities by TG neurons after inflammation, we first examined the expression of classic angiogenic factors by TG tissues. Using the Proteome Angiogenesis Array, we found several pro- and anti-angiogenic factors altered in DED but none of them demonstrated marked changes (Supplemental data). Therefore, we turned our attention to neuropeptides known to be secreted by corneal nerves. We evaluated the mRNA expression of three classic neuropeptides, substance P (SP), calcitonin gene-related peptide (CGRP), and alpha-melanocyte stimulating hormone (MSH), in TG tissue lysates. Whereas CGRP and alpha-MSH levels were similar between naïve and DED groups (Fig. 4C & D), SP mRNA expression was significantly increased in DED group (p=0.034, Fig. 4E). An increase in SP protein level in the TG tissue lysates was also observed (p=0.045, Fig. 4F). Similarly, the cultured TG neurons derived from mice with DED expressed significantly higher levels of SP mRNA, compared with those from control mice (p=0.005, Fig. 4G). Most importantly, the concentration of SP in the conditioned media of neuron culture was two-folder higher in the DED group than that in the control group (p=0.045, Fig. 4H). These results suggest that in response to inflammation, TG neurons express and secrete higher levels of SP, which may mediate the promotion of angiogenesis by sensory nerves.
Figure 4.
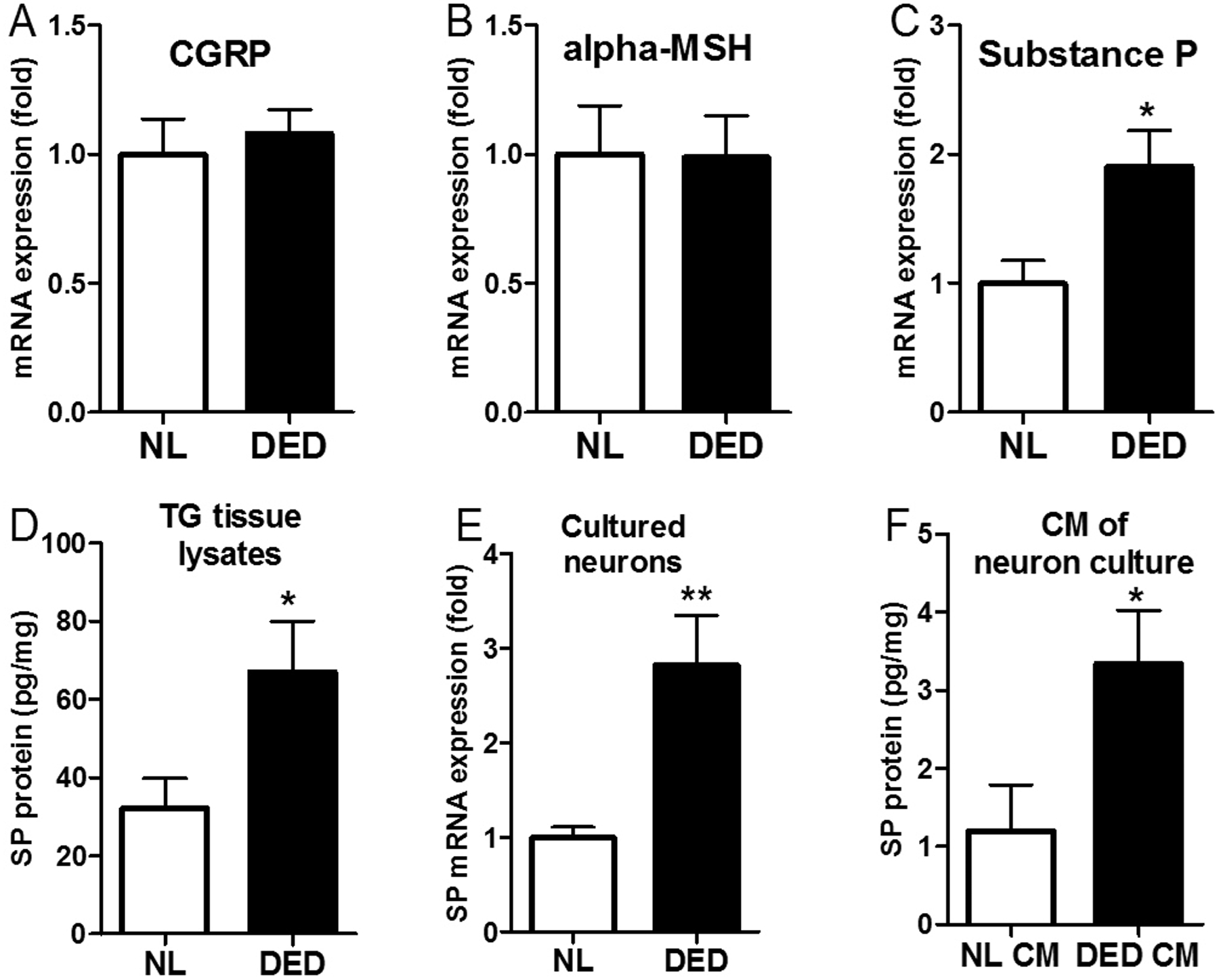
Expression and secretion of substance P is significantly increased in TG neurons after desiccating stress. Expression of CGRP (A), alpha-MSH(B) and substance P (C) mRNA in TG tissue from naïve and DED mice, n=5. (D) SP protein expression in TG tissue was measure with ELISA, n=5. (E) SP mRNA expression in cultured TG neurons isolated from naïve and DED mice, n=8. (F) Protein level of SP secreted by cultured TG neurons in the conditioned media, n=5. *p<0.05, **p<0.01.
SP is potently pro-angiogenic in vitro and in vivo
To examine the angiogenic property of SP, we added SP to VEC culture for 24 hours and observed that exogenous SP potently promoted VEC proliferation (1 μM SP, p<0.0001, Fig. 5A) in a dose-dependent fashion (data not shown) and had little impact on migration (Fig. 5B & C). Furthermore, SP markedly promoted VEC tube formation (Fig. 5F) with more than 5-fold increases in the number of junctions (p<0.005, Fig. 5D) and length of tubes formed (p<0.005, Fig. 5E). The robust promotion of VEC proliferation and tube formation, and minimal effect on migration by SP were similar to the effects of DED TG neurons on VEC in co-culture (Fig. 3). To investigate the role of SP signaling in promoting angiogenesis at the ocular surface in vivo, we placed intrastromal sutures in mouse corneas to induce neovascularization (NV) and applied spantide I, a highly selective antagonist to SP receptor neurokinin 1 (NK1R), topically three times daily. As shown in Fig. 5G, PBS-treated control corneas developed exuberant corneal NV while Spantide I-treated corneas had significantly less NV (85.4%±8.2 in PBS vs. 66.4%±16.0 in spantide I, P=0.03, Fig. 5H). Immunostaining of CD31 (Fig. 5I) confirmed these findings.
Figure 5.
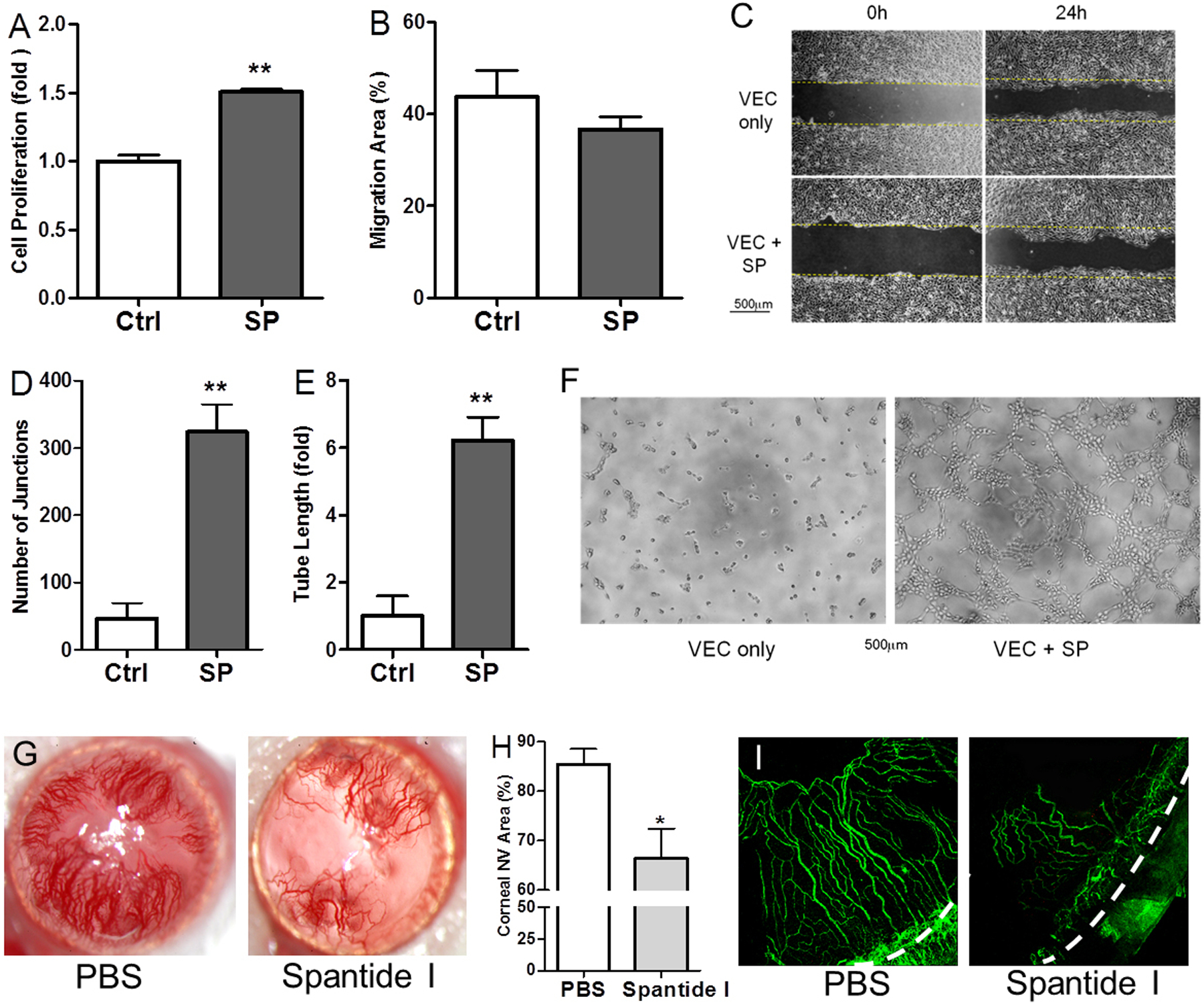
SP promotes angiogenesis in vitro and in vivo. (A) VEC proliferation was determined after 24-hour culture with SP, n=6. (B-C) Migration of VEC when cultured with SP was quantified (B) and photographed at 0h and 24h (C), n=5. (D-F) Tube structure formation in the matrigel cultured with SP was photographed at 4h (F) and quantified as the number of junctions (D) and length of tube formed (E), n=4. Scale bars, 500μm. (G) Representative images of corneal NV with slit lamp photography. (H) Quantification of corneal NV area (%) of slit lamp photography at 14 days after vehicle and spantide I treatment (n=10 per group). (I) Immunohistochemistry with CD31 to highlight corneal NV, x100 magnification, dashed line indicates limbus. *p<0.05, **p<0.01.
SP mediates the promotion of angiogenesis by sensory neurons after inflammation
Although spantide I significantly reduced corneal NV in vivo, the target cell/tissue was non-specific as topical treatment affects the entire ocular surface. To determine the specificity of sensory nerve-derived SP in promoting angiogenesis during ocular surface inflammation, we added spantide I in the neuron-VEC co-culture system. As shown in Figure 6A, while spantide I had minimal effect on the proliferation of VEC co-cultured with normal TG neurons; the DED TG neuron-promoted VEC proliferation was significantly reduced by spantide I (p<0.05, Fig. 6A). VEC migration was not significantly affected by spantide I (Fig. 6B). Similar results were observed with conditioned media of neuron culture, where spantide I reduced VEC proliferation in DED group, but not in control group (Fig. 6C); and had minimal impact on VEC migration (Fig. 6D). The promotion of VEC tube formation by the conditioned media of DED TG neuronal culture was also abrogated by spantide I (Fig. 6G) with a 2-fold decrease in the number of junctions (p<0.01, Fig. 6E) and a 6-fold decrease in length of tube formed (p<0.001, Fig. 6F).
Figure 6.
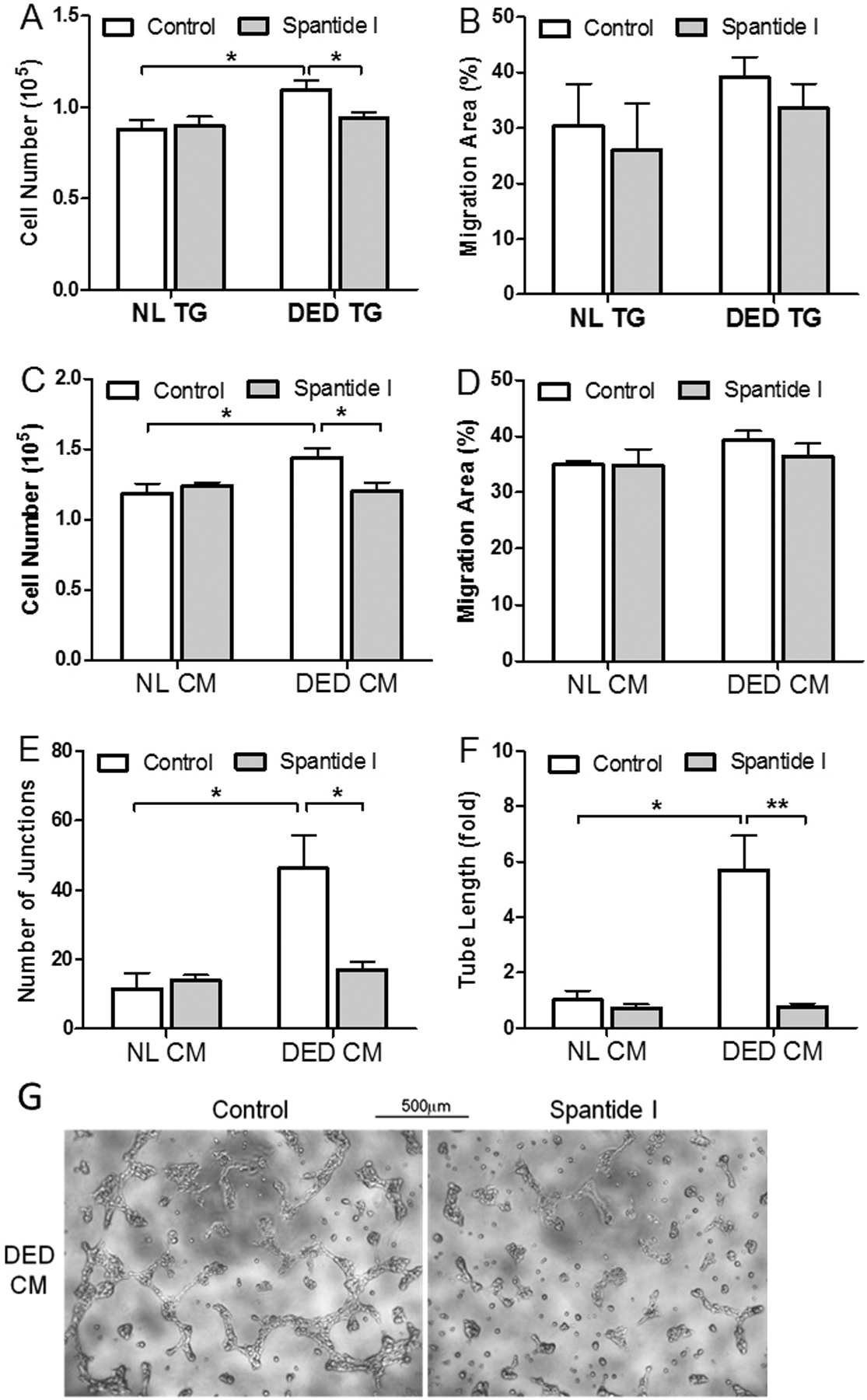
Spantide I abrogates the promotion of VEC proliferation and tube formation by inflamed TG neurons. (A) DED neuron-promoted VEC proliferation was reduced by 2μM spantide I (n=8). (B) VEC migration was minimally affected by spantide I in neuron co-culture (n=6). (C) Conditioned media (CM) derived from DED neuron culture promoted VEC proliferation, which was reduced by Spantide I (n=5). (D) VEC migration was minimally affected by spantide I when cultured in the CM of TG neurons (n=4). (E-G) CM derived from DED neuron culture promoted tube formation, which was reduced by spantide I. Scale bars, 500μm. *p<0.05 **p<0.01 by two-way ANOVA to assess differences between groups and by Bonferroni posttests to assess differences within groups.
To confirm the findings using spantide I, we transfected TG neurons isolated from mice with DED with either control non-targeting or Tac1 (SP precursor gene) siRNA (Fig. 7). The mRNA expression of Tac1 was significantly reduced by Tac1 siRNA, compared with the non-target control (87% decrease, Fig. 7A). Consistent with results using Spantide I, knockdown of Tac1 signaling resulted in significant reduction in VEC proliferation (Fig. 7B&C) and tube formation (Fig. 7D–F). Collectively, these data suggested that sensory nerve-derived SP plays a key role in the promotion of VEC activities during ocular surface inflammation.
Figure 7.
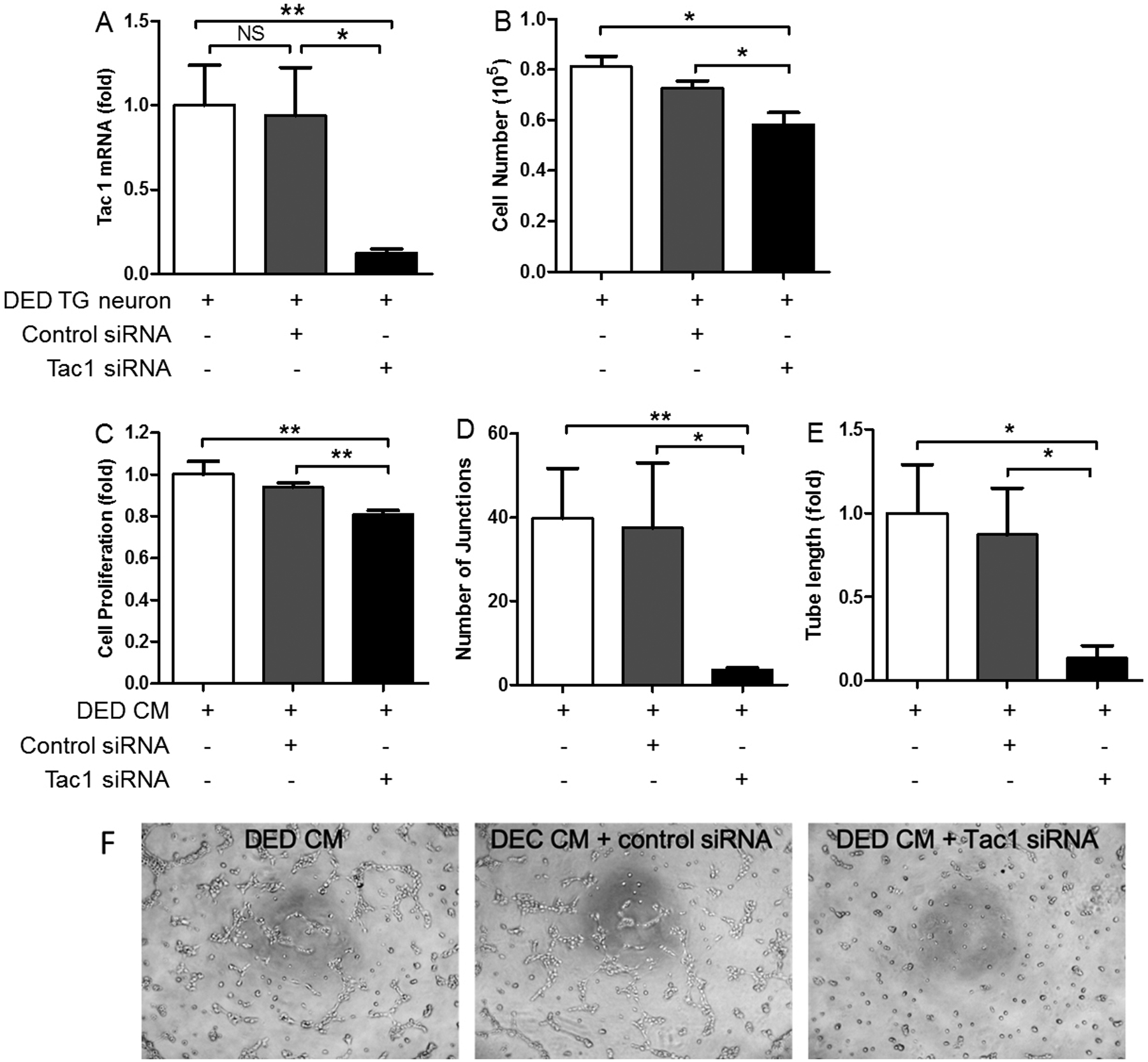
siRNA knockdown of Tac1 abrogates the promotion of VEC proliferation and tube formation by inflamed TG neurons. (A) TG neurons derived from mice with DED were transfected with control non-target or Tac1 siRNA. Tac1 mRNA expression was reduced by 87% (n=4). (B) VEC proliferation was reduced when co-cultured with TG neurons transfected with Tac1 siRNA, compared with control siRNA (n=4). VEC proliferation (C, n=8) and tube formation (D, E, F n=5) were reduced when VEC were cultured in the conditioned media derived from DED TG neurons transfected with Tac1 siRNA, compared with those transfected with control siRNA. *p<0.05, **p<0.01.
DISCUSSION
In the current study, we explored the regulation of angiogenesis by sensory neurons in response to ocular surface inflammation. To examine direct neuro-vascular interaction, we established a co-culture system of TG neurons and VEC and found that neurons isolated from animals exposed to desiccating stress significantly promote VEC proliferation and tube formation, compared with those from control animals. Among the neuropeptides explored, substance P levels are markedly upregulated after desiccating stress both in tissue and in culture. We demonstrated that substance P is potently pro-angiogenic at the ocular surface in vitro and in vivo. More importantly, blockade of substance P signaling, via pharmacological and siRNA manipulation, abolishes the promotion of VEC activities by inflamed sensory neurons in co-culture. In aggregate, the current study shows sensory neurons directly promote angiogenesis in response to ocular surface inflammation and such promotion is mediated via substance P signaling.
Blood vessel and nerves share considerable similarities anatomically and often share common cellular/molecular signaling pathways. (1, 2) There is mounting evidence supporting the notion that the nervous system is critical in modulating angiogenesis. It has been reported that nerves can guide blood vessel patterning by branching, expanding, and pruning in response to local requirements in the brain.(32) Basu et al. reported that ablation of peripheral dopaminergic nerves markedly increased angiogenesis, microvessel density, permeability, and growth of malignant tumors in mice. (33) Similar interactions have been noted at the ocular surface and Ferrari et al. observed that complete denervation of trigeminal nerve results in spontaneous corneal neovascularization. (19) Matsuyama et al. showed that nerve growth factor (NGF), via promoting perivascular innervation, inhibits corneal NV.(34)
These lines of evidence, however, are indirect and circumstantial, as many cellular and molecular components are at play in vivo. Therefore we developed an in vitro co-culture system of TG neurons and VEC, which enables us to explore their direct interaction without the influence of other cells/tissues. To our knowledge, the co-culture system is the first of its kind. The neurons can be maintained in culture with robust viability and purity for up to one week. More importantly, their secretion profile appears to sustain in culture, as shown by the similar pattern of substance P expression/secretion observed in tissue lysates, neuronal lysates, and conditioned media after desiccating stress. Using the co-culture system, we find that in response to desiccating stress, the sensory neurons of the trigeminal ganglion directly promote VEC proliferation and tube formation, while having minimal effects on their migration. This evidence supports the notion of neuro-regulation of angiogenesis, particularly during inflammation.
The normal cornea is the most densely innervated tissue in the human body, and yet is devoid of blood vessels. Corneal neovascularization and nerves can be easily visualized clinically and histopathologically, rendering it an ideal system to study neuro-vascular interaction. Our collective understanding of corneal nerve functions has expanded: in addition to sensory function, they modulate the blink reflex, tear production, and stem cell niche maintenance. (13, 16) In particular, corneal nerves play critical trophic roles in epithelial wound healing. (17, 18) Recently a correlation between subbasal nerve density and corneal endothelial cell loss has been noted, suggesting a potential link between corneal innervation and corneal endothelial cell homeostasis. (35–37) Lastly, there is emerging evidence that corneal nerves contribute to the maintenance of the immune privilege of the cornea. (38, 39) In the aggregate, these studies suggest that corneal nerves are not only sensors but major regulators of ocular surface homeostasis. Consistent with this notion, our study demonstrates that corneal nerves can directly regulate angiogenesis, expanding the list of nerve function at the ocular surface.
To determine the neuro-regulation of angiogenesis during inflammation, we employed a well-established mouse model of dry eye disease (DED). Inflammation plays a key role in the pathogenesis of DED and represents a major driving force in the sensitization, damage and regeneration of the peripheral sensory neurons. (40–43). There is rarely spontaneous corneal NV in response to DED experimentally or clinically. Corneal avascularity is not a simple and passive lack of blood vessels; on the contrary, it is a highly and tightly regulated process, mediated by a plethora of anti-angiogenic factors to actively suppress and regress NV (7–10). This intrinsically high threshold to develop NV is likely the reason for the lack of spontaneous NV during DED. In response to injury (corneal incision and suture placement), however, the corneas of mice with DED had 57% and 140% more heme- and lymph-angiogenesis, respectively, compared to controls. (26) Whether DED-related alterations in corneal innervation play a role in such heightened vascular response have not been explored. Our lab has previously established a DED model utilizing a controlled-environment chamber and reported that desiccating stress significantly increased corneal fluorescein staining, reduced tear production, and resulted in heighted immune response. (28, 44) Consistent with recent reports (23, 45), we found that the sub-basal nerve density is reduced in both central and peripheral cornea, and the number of intraepithelial nerve terminals is decreased in the peripheral cornea after DED induction.
In addition to structural changes, we explored the changes in the secretory profile of TG neurons after desiccating stress. We first turned our attention to classic angiogenesis-related factors using the Proteome Angiogenesis Array, which evaluates the expression of 53 classic angiogenesis-related proteins (supplemental data). We found that TG express both pro-angiogenic factors such as NOV, IGFBP-3, FGF-2, and anti-angiogenic proteins such as PEDF. In response to desiccating stress, no markedly altered target(s) in TG neurons were identified from the Array. It is worth noting that the VEGF was expressed at very low levels and unchanged during DED. Since sensory nerves could also regulate the corneal homeostasis via neurotrophins, neuropeptides and neurotransmitters, we compared the expression of three nerve-secreted peptides between normal and DED TG neurons. While the levels of CGRP and alpha-MSH are unchanged, the levels of substance P are significantly elevated in the trigeminal ganglion isolated from mice with DED, consistent with a recent report. (46) On the other hand, in a study of 19 patients levels of substance P in tears are comparable in subjects with and without DED; while other nerve-derived factors such NGF, CGRP and neuropeptide Y are altered. (47) Interestingly He et al. observed a decrease in Tac1 (substance P precursor) gene expression in the trigeminal ganglion both acutely and chronically after mechanical injuries to the cornea. (48) The discrepancies are likely due to differences in experimental subjects (mice versus humans) and stimulation (DED versus mechanical injury). The high substance P levels in DED group are maintained in neuron culture including the levels of secreted substance P in the conditioned media.
Substance P (SP), an 11- amino acid peptide originating from Tachykinin Precursor 1 (TAC1) gene, belongs to the tachykinin neuropeptide family and is widely distributed in the peripheral and central nervous systems. (49–53) The biological actions of SP are mainly mediated through neurokinin receptor 1 (NK1R). SP is present in normal tears and expressed by corneal epithelium, stromal keratocytes, and up to one third of corneal nerves, and mediates acute neurogenic inflammation. (49–53) Beyond the eye, SP, immobilized on hydrogels, has been shown to promote angiogenesis in myocardial infarction, suggesting its role in neuro-vascular connection during tissue regeneration. (54) In addition, SP has been shown to play a role in neovascularization of the intervertebral discs and discogenic pain. (55) We first determined the effects of exogenous SP on angiogenesis in vitro. Consistent with previous reports in non-ocular tissues (56–58), we found that substance P potently promotes VEC proliferation and tube formation. Interestingly substance P has minimal effects on VEC migration. This divergence between proliferation/tube formation and migration is similar to what is observed in the co-culture with TG neurons derived from mice with DED. This is different from previous reports that substance P could induce the migration of vascular endothelial cells. (59, 60) This may be due to the different cell lines and migratory assays used. Next, we determined the role of substance P signaling in angiogenesis in vivo. We used an intrastromal suture model and applied NK1R antagonist topically for 14 days. Blockade of substance P-NK1R signaling significantly reduces corneal NV. This is consistent with previous reports using Lanepitant, a different NK1R antagonist, in burn and suture NV models (61), as well using spantide I in herpetic stromal keratitis. (62) The in vivo findings using NK1R antagonists have a major drawback: substance P and its receptor NK1R are expressed by many cells at the ocular surface and the observed results are not specific to corneal nerves. We then turned back to the co-culture system of TG neurons and VEC and added spantide I. Indeed, the promotion of VEC proliferation and tube formation by inflamed TG neurons are abolished by spantide I. To confirm these findings, we transfected TG neurons with Tac1 siRNA with high knockdown efficiency. Similar to the results with spantide I, VEC activities in co-culture are reduced when inflamed TG neurons lack substance P signaling. These data prove the critical role of sensory nerve-derived SP in promoting corneal angiogenesis.
In summary, this study provides direct evidence on the promotion of angiogenesis by sensory nerves during inflammation. In addition, we demonstrate that in response to desiccating stress, trigeminal ganglion sensory nerves express and secrete higher levels of substance P, which is potently pro-angiogenic and promotes vascular endothelial cell proliferation and tube formation. Using pharmacological and siRNA manipulation, we show that substance P-NK1R signaling is critical in the promotion of angiogenesis by inflamed nerves.
Supplementary Material
Acknowledgements:
The authors wish to thank Dr. Patricia A. D’Amore, Schepens Eye Research Institute of Massachusetts Eye and Ear, Harvard Medical School, for her scientific and technical support. We also wish to thank Professor Yan Wang, Tianjin Eye Hospital, for her mentorship of LL. The current research is supported by NIH 5K12EY016335 (PI, RD; scholar JY), the New England Corneal Transplant Fund (JY), Harvard Medical School Shore Scholarship (JY), NIH P30 EY003790 (core), and the Chinese Scholarship Council (LL). The authors declare no conflict of interests.
ABBREVIATIONS:
- DED
dry eye disease
- TG
trigeminal ganglion
- VEC
vascular endothelial cell line
- SP
substance P
- NK1R
neurokinin-1 receptor
- NV
neovascularization
- CM
conditioned media
- VEGF
vascular endothelial growth factor
- DMEM
dulbecco’s modified eagle medium
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- GFAP
glial fibrillary acidic protein
- DAPI
4’,6-Diamidino-2-Phenylindole
- CGRP
calcitonin gene-related peptide
- alpha-MSH
alpha-Melanocyte-stimulating hormone
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- BrdU
bromodeoxyuridine
- NOV
nephroblastoma overexpressed
- IGFBP-3
insulin-like growth factor-binding protein 3
- FGF-2
basic fibroblast growth factor
- CXCL12
stromal cell-derived factor 1
- MMP-9
matrix metallopeptidase 9
- PEDF
pigment epithelium-derived factor
- NGF
nerve growth factor
- VEGFR3
epithelial vascular endothelial growth factor receptor 3
- TAC1
tachykinin precursor 1
REFERENCES
- 1.Hogg PJ, and McLachlan EM (2002) Blood vessels and nerves: together or not? Lancet 360, 1714. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P (2003) Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 4, 710–720 [DOI] [PubMed] [Google Scholar]
- 3.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, and Lloyd AC (2015) Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 162, 1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Creemers LB, Cross AK, and Le Maitre CL (2015) Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther 17, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward NL, Hatala DA, Wolfram JA, Knutsen DA, and Loyd CM (2011) Cutaneous manipulation of vascular growth factors leads to alterations in immunocytes, blood vessels and nerves: Evidence for a cutaneous neurovascular unit. J Dermatol Sci 61, 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu XH, Terenghi G, Kangesu T, Navsaria HA, Springall DR, Leigh IM, Green CJ, and Polak JM (1995) Regeneration pattern of blood vessels and nerves in cultured keratinocyte grafts assessed by confocal laser scanning microscopy. Br J Dermatol 132, 376–383 [DOI] [PubMed] [Google Scholar]
- 7.Zhong W, Montana M, Santosa SM, Isjwara ID, Huang YH, Han KY, O’Neil C, Wang A, Cortina MS, de la Cruz J, Zhou Q, Rosenblatt MI, Chang JH, and Azar DT (2018) Angiogenesis and lymphangiogenesis in corneal transplantation-A review. Surv Ophthalmol 63, 453–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cursiefen C, Colin J, Dana R, Diaz-Llopis M, Faraj LA, Garcia-Delpech S, Geerling G, Price FW, Remeijer L, Rouse BT, Seitz B, Udaondo P, Meller D, and Dua H (2012) Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol 96, 3–9 [DOI] [PubMed] [Google Scholar]
- 9.Azar DT (2006) Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 104, 264–302 [PMC free article] [PubMed] [Google Scholar]
- 10.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, and Chang JH (2010) Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res 29, 208–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller LJ, Marfurt CF, Kruse F, and Tervo TM (2003) Corneal nerves: structure, contents and function. Exp Eye Res 76, 521–542 [DOI] [PubMed] [Google Scholar]
- 12.Patel DV, and McGhee CN (2009) In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol 93, 853–860 [DOI] [PubMed] [Google Scholar]
- 13.Shaheen BS, Bakir M, and Jain S (2014) Corneal nerves in health and disease. Surv Ophthalmol 59, 263–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruzat A, Qazi Y, and Hamrah P (2017) In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf 15, 15–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, Aragona P, Geerling G, Messmer EM, and Benitez-Del-Castillo J (2019) Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol 97, 137–145 [DOI] [PubMed] [Google Scholar]
- 16.Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, and Dana R (2012) Dependence of corneal stem/progenitor cells on ocular surface innervation. Investigative ophthalmology & visual science 53, 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beuerman RW, and Schimmelpfennig B (1980) Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol 69, 196–201 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Hirschfeld J, Lopez-Briones LG, and Belmonte C (1994) Neurotrophic influences on corneal epithelial cells. Exp Eye Res 59, 597–605 [DOI] [PubMed] [Google Scholar]
- 19.Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, and Dana R (2013) Nerves and neovessels inhibit each other in the cornea. Investigative ophthalmology & visual science 54, 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodama-Takahashi A, Sugioka K, Sato T, Nishida K, Aomatsu K, Fukuda M, and Shimomura Y (2018) Neurotrophic Keratopathy after Trigeminal Nerve Block for Treatment of Postherpetic Neuralgia. Case Rep Ophthalmol Med 2018, 6815407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dana R, Bradley JL, Guerin A, Pivneva I, Stillman IO, Evans AM, and Schaumberg DA (2019) Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am J Ophthalmol [DOI] [PubMed] [Google Scholar]
- 22.Labbe A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, and Sun X (2013) Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci 54, 5144–5150 [DOI] [PubMed] [Google Scholar]
- 23.Simsek C, Kojima T, Dogru M, and Tsubota K (2018) Alterations of Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. Invest Ophthalmol Vis Sci 59, 1986–1995 [DOI] [PubMed] [Google Scholar]
- 24.Simsek C, Kojima T, Nagata T, Dogru M, and Tsubota K (2019) Changes in Murine Subbasal Corneal Nerves After Scopolamine-Induced Dry Eye Stress Exposure. Invest Ophthalmol Vis Sci 60, 615–623 [DOI] [PubMed] [Google Scholar]
- 25.Kheirkhah A, Dohlman TH, Amparo F, Arnoldner MA, Jamali A, Hamrah P, and Dana R (2015) Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology 122, 662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho YK, Archer B, and Ambati BK (2014) Dry Eye Predisposes to Corneal Neovascularization and Lymphangiogenesis After Corneal Injury in a Murine Model. Cornea [DOI] [PubMed] [Google Scholar]
- 27.Barabino S, Shen L, Chen L, Rashid S, Rolando M, and Dana MR (2005) The controlled-environment chamber: a new mouse model of dry eye. Investigative ophthalmology & visual science 46, 2766–2771 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, Saban DR, Kodati S, Stern ME, and Dana R (2013) Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci 54, 2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inomata T, Mashaghi A, Di Zazzo A, Lee SM, Chiang H, and Dana R (2017) Kinetics of Angiogenic Responses in Corneal Transplantation. Cornea 36, 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malin SA, Davis BM, and Molliver DC (2007) Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2, 152–160 [DOI] [PubMed] [Google Scholar]
- 31.Francescone RA 3rd, Faibish M, and Shao R (2011) A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JA, Choi KS, Kim SY, and Kim KW (2003) Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun 311, 247–253 [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Sarkar C, Chakroborty D, Nagy J, Mitra RB, Dasgupta PS, and Mukhopadhyay D (2004) Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res 64, 5551–5555 [DOI] [PubMed] [Google Scholar]
- 34.Matsuyama A, Takatori S, Sone Y, Ochi E, Goda M, Zamami Y, Hashikawa-Hobara N, Kitamura Y, and Kawasaki H (2017) Effect of Nerve Growth Factor on Innervation of Perivascular Nerves in Neovasculatures of Mouse Cornea. Biol Pharm Bull 40, 396–401 [DOI] [PubMed] [Google Scholar]
- 35.Muller RT, Pourmirzaie R, Pavan-Langston D, Cavalcanti BM, Aggarwal S, Colon C, Jamali A, Cruzat A, and Hamrah P (2015) In Vivo Confocal Microscopy Demonstrates Bilateral Loss of Endothelial Cells in Unilateral Herpes Simplex Keratitis. Invest Ophthalmol Vis Sci 56, 4899–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kheirkhah A, Satitpitakul V, Hamrah P, and Dana R (2017) Patients With Dry Eye Disease and Low Subbasal Nerve Density Are at High Risk for Accelerated Corneal Endothelial Cell Loss. Cornea 36, 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal S, Cavalcanti BM, Regali L, Cruzat A, Trinidad M, Williams C, Jurkunas UV, and Hamrah P (2018) In Vivo Confocal Microscopy Shows Alterations in Nerve Density and Dendritiform Cell Density in Fuchs’ Endothelial Corneal Dystrophy. Am J Ophthalmol 196, 136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, and Niederkorn JY (2015) Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant 15, 1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neelam S, Mellon J, Wilkerson A, and Niederkorn JY (2018) Induction of Contrasuppressor Cells and Loss of Immune Privilege Produced by Corneal Nerve Ablation. Invest Ophthalmol Vis Sci 59, 4738–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pflugfelder SC (2004) Antiinflammatory therapy for dry eye. Am J Ophthalmol 137, 337–342 [DOI] [PubMed] [Google Scholar]
- 41.Basbaum AI, Bautista DM, Scherrer G, and Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139, 267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benowitz LI, and Popovich PG (2011) Inflammation and axon regeneration. Curr Opin Neurol 24, 577–583 [DOI] [PubMed] [Google Scholar]
- 43.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, and Wolffsohn JS (2017) TFOS DEWS II pain and sensation report. Ocul Surf 15, 404–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodati S, Chauhan SK, Chen Y, Dohlman TH, Karimian P, Saban D, and Dana R (2014) CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci 55, 5871–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stepp MA, Tadvalkar G, Hakh R, and Pal-Ghosh S (2017) Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 65, 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu M, Lee SM, Lee H, Amouzegar A, Nakao T, Chen Y, and Dana R (2019) Neurokinin-1 Receptor Antagonism Ameliorates Dry Eye Disease by Inhibiting Antigen-Presenting Cell Maturation and Th17 Cell Activation. Am J Pathol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, and Bonini S (2011) Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol 129, 981–986 [DOI] [PubMed] [Google Scholar]
- 48.He J, Pham TL, Kakazu AH, and Bazan HEP (2019) Remodeling of Substance P Sensory Nerves and Transient Receptor Potential Melastatin 8 (TRPM8) Cold Receptors After Corneal Experimental Surgery. Invest Ophthalmol Vis Sci 60, 2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suvas S (2017) Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol 199, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mistrova E, Kruzliak P, and Chottova Dvorakova M (2016) Role of substance P in the cardiovascular system. Neuropeptides 58, 41–51 [DOI] [PubMed] [Google Scholar]
- 51.Munoz M, and Covenas R (2013) Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 48, 1–9 [DOI] [PubMed] [Google Scholar]
- 52.Munoz M, and Covenas R (2014) Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 46, 1727–1750 [DOI] [PubMed] [Google Scholar]
- 53.Mehta D, and Granstein RD (2019) Immunoregulatory Effects of Neuropeptides on Endothelial Cells: Relevance to Dermatological Disorders. Dermatology 235, 175–186 [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Song M, Kim J, and Park Y (2018) Comparison of Angiogenic Activities of Three Neuropeptides, Substance P, Secretoneurin, and Neuropeptide Y Using Myocardial Infarction. Tissue Eng Regen Med 15, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He M, Pang J, Sun H, Zheng G, Lin Y, and Ge W (2020) Overexpression of TIMP3 inhibits discogenic pain by suppressing angiogenesis and the expression of substance P in nucleus pulposus. Mol Med Rep 21, 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, and Maggi CA (1990) Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res 40, 264–278 [DOI] [PubMed] [Google Scholar]
- 57.Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnett NW, Armstrong CA, Caughman SW, and Ansel JC (1999) VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol 162, 1656–1661 [PubMed] [Google Scholar]
- 58.Greeno EW, Mantyh P, Vercellotti GM, and Moldow CF (1993) Functional neurokinin 1 receptors for substance P are expressed by human vascular endothelium. J Exp Med 177, 1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziche M, Morbidelli L, Geppetti P, Maggi CA, and Dolara P (1991) Substance P induces migration of capillary endothelial cells: a novel NK-1 selective receptor mediated activity. Life Sci 48, PL7–11 [DOI] [PubMed] [Google Scholar]
- 60.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, and Ledda F (1994) Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94, 2036–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bignami F, Giacomini C, Lorusso A, Aramini A, Rama P, and Ferrari G (2014) NK1 receptor antagonists as a new treatment for corneal neovascularization. Invest Ophthalmol Vis Sci 55, 6783–6794 [DOI] [PubMed] [Google Scholar]
- 62.Twardy BS, Channappanavar R, and Suvas S (2011) Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Invest Ophthalmol Vis Sci 52, 8604–8613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


