Highlights
-
•
CI-AKI is associated with prognosis in AMI patients irrespective of its definitions.
-
•
CI-AKIC (Scr elevation ≥ 25% in the first 72 h) had the highest prevalence (18.77%)
-
•
CI-AKIA (Scr elevation ≥ 50%/ ≥0.3 mg/dL in the first 72 h) had the highest PAR.
Keywords: Acute myocardial infarction, Contrast-induced acute kidney injury, Long-term mortality, Population attributable risk
Abstract
Background
Few studies have demonstrated the association between contrast-induced acute kidney injury (CI-AKI) and long-term mortality and explored which definition of CI-AKI accounts for most long-term deaths among patients with acute myocardial infarction (AMI). Therefore, we aimed to evaluate this association and compared the population attributable risks (PARs) of three CI-AKI definitions.
Methods
We analyzed 1300 consecutive AMI patients undergoing angiography in Guangdong Provincial People‘s Hospital. The endpoint was all-cause mortality. CI-AKI was evaluated according to three definitions: (1) CI-AKIA, with a serum creatinine elevation ≥ 50% or ≥ 0.3 mg/dL from baseline in the first 72 h after procedure; (2) CI-AKIB, ≥ 0.5 mg/dL in 72 h; (3) CI-AKIC: ≥ 25% in 72 h; multivariable Cox analysis was conducted to evaluate the association between CI-AKI and long-term mortality. PARs of CI-AKI under different definitions were calculated with their odds ratios and prevalence among our cohort.
Results
During the median follow-up period of 7.0 (5.5; 8.7) years, CI-AKI was significantly associated with poorer outcome regardless of the definition (adjusted hazard ratios: 1.417–2.711). Among the three definitions of CI-AKI, the prevalence was the highest for CI-AKIC (18.77%), and PAR was the highest for CI-AKIA (11.62%, 95% CI: 4.99–19.71), followed by CI-AKIB (9.20%, 95% CI: 4.22–16.00) and CI-AKIC (7.26%, 95% CI: 0.21–15.62).
Conclusions
Our results suggested that CI-AKI is associated with long-term mortality in patients with AMI irrespective of its definitions. Cardiologists and studies regarding long-term prognosis should pay more attention to the presence of CI-AKI, especially CI-AKIA with the highest PAR.
1. Introduction
Contrast-induced acute kidney injury (CI-AKI) is a common adverse complication in patients with acute myocardial infarction (AMI) undergoing coronary angiography (CAG) or percutaneous coronary intervention (PCI), and may cause prolonged hospitalization, a higher incidence of in-hospital events, and increased mortality [1], [2], [3], [4]. However, the challenge is that few studies have demonstrated the association between CI-AKI and long-term mortality in patients with AMI. Some studies suggested that CI-AKI was an independent predictor of worse long-term prognosis among AMI patients [5], [6], [7], while other studies failed to verify this association [8]. One of the reasons for these conflicting results may be the different definitions of CI-AKI, which may also confuse physicians when they are identifying patients at risk [9], [10], [11].
The population-attributable risk (PAR) represents the proportion of cases in a population that would not have occurred in the absence of a risk factor [12]. To the best of our knowledge, no studies have quantified the contributions of different definitions of CI-AKI to long-term mortality in patients with AMI.
Therefore, we conducted this study to evaluate the association between CI-AKI and long-term mortality in patients with AMI and to compare the PARs of three different CI-AKI definitions.
2. Method
2.1. Study population
In this study, 1300 consecutive patients with AMI undergoing coronary angiography (CAG) or percutaneous coronary intervention (PCI) in Guangdong Provincial People’s Hospital were included between January 2010 and December 2013. The inclusion and exclusion criteria were mentioned previously elsewhere [13]. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Guangdong Provincial People s Hospital. All the patients recruited in the study signed written informed consent.
2.2. Protocol
In accordance with standard clinical guidelines, standard guide catheters, guidewires, balloon catheters, and stents were used through the femoral or radial approach [14]. Noninvasive treatment was based on guidelines from the American Heart Association/American College of Cardiology Foundation. Serum creatinine concentrations were measured for all included patients and at 1, 2, and 3 days after contrast exposure.
2.3. Endpoint and definitions
The endpoint of this study was long-term all-cause mortality. All eligible patients included were followed up through office visits or telephone interviews 1 month, 6 months and every 1 year after registration until April 2019. CI-AKI was evaluated according to three definitions: (1) CI-AKIA, with a serum creatinine elevation ≥ 50% or ≥ 0.3 mg/dL from baseline in the first 72 h after procedure; (2) CI-AKIB, ≥ 0.5 mg/dL in 72 h; (3) CI-AKIC: ≥ 25% in 72 h. The definitions of chronic kidney disease (CKD), anemia and hypotension were the same as those in previous studies [15], [16].
2.4. Statistical analysis
We applied the Chi-square test or Fisher’s exact test for categorical variables expressed as counts (percentages). Continuous variables were presented as the mean ± SD or median ± IQR, and compared using the t-test or Wilcoxon rank-sum test (in the two cohorts, with and without CI-AKI observations). Kaplan-Meier analysis was used to count the cumulative mortality, and the log-rank test was used to assess differences between curves. The association between long-term all-cause mortality and CI-AKI was explored by fitting a multivariable Cox regression model adjusting for other risk factors (e.g., age, heart rate, heart function, renal function, and medication). The adjusted risk factors were selected through univariable Cox regression or based on previous studies and clinical importance [17], [18]. Three multivariate Cox proportional hazard regression models were applied for three different definitions of CI-AKI, respectively. PAR was calculated using the equation PAR = P (HR-1)/[1 + P (HR-1)], where P is the prevalence of CI-AKI under different definitions in our database. The standard error of PAR was calculated using the delta method [19]. A two-sided probability value < 0.05 was considered significant. All data analyses were conducted with R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patient characteristics
A total of 1300 patients were included in the analysis. Table 1 details the demographic, clinical and procedural characteristics of the included patients. Suppl. details the baseline characteristics of patients with or without CI-AKI. In general, the mean age was 61.61 ± 12.15 years, and only 16% of the total population was female. A total of 220 patients (16.92%) were older than 75 years. Chronic heart failure (CHF) was present in 588 (45.23%) patients, while CKD was identified in 263 (20.23%) patients.
Table 1.
Baseline characteristics.
| Variables | Total (n = 1300) | CI-AKIA (n = 163) | non-CI-AKIA (n = 1137) | p value | CI-AKIB (n = 77) | non-CI-AKIB (n = 1223) | p value | CI-AKIC (n = 244) | non-CI-AKIC (n = 1056) | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 61.61 ± 12.15 | 68.77 ± 12.15 | 60.59 ± 11.80 | <0.001 | 70.78 ± 11.18 | 61.04 ± 11.98 | <0.001 | 65.31 ± 13.33 | 60.76 ± 11.70 | <0.001 |
| Age > 75, n (%) | 220 (16.92) | 61 (37.42) | 159 (13.98) | <0.001 | 36 (46.75) | 184 (15.04) | <0.001 | 71 (29.10) | 149 (14.11) | <0.001 |
| Female sex, n (%) | 208 (16.00) | 40 (24.54) | 168 (14.78) | 0.001 | 19 (24.68) | 189 (15.45) | 0.032 | 62 (25.41) | 146 (13.83) | <0.001 |
| Weight, kg | 64.92 ± 10.84 | 62.48 ± 11.1 | 65.27 ± 10.77 | 0.003 | 61.33 ± 10.76 | 65.15 ± 10.81 | 0.003 | 63.16 ± 11.43 | 65.32 ± 10.67 | 0.008 |
| SBP, mmHg | 121.92 ± 20.25 | 122.78 ± 26.12 | 121.8 ± 19.28 | 0.644 | 122.17 ± 28.99 | 121.9 ± 19.59 | 0.937 | 122.94 ± 23.87 | 121.68 ± 19.32 | 0.445 |
| DBP, mmHg | 73.56 ± 11.97 | 73.8 ± 13.9 | 73.53 ± 11.67 | 0.812 | 73.16 ± 15.4 | 73.59 ± 11.73 | 0.809 | 74.35 ± 13.5 | 73.38 ± 11.58 | 0.302 |
| HR, bpm | 77.50 ± 15.29 | 81.92 ± 18.11 | 76.87 ± 14.75 | <0.001 | 82.84 ± 19.27 | 77.16 ± 14.95 | 0.013 | 80.01 ± 16.86 | 76.92 ± 14.85 | 0.009 |
| CHF, n (%) | 588 (45.23) | 113 (69.33) | 475 (41.78) | <0.001 | 61 (79.22) | 527 (43.09) | <0.001 | 139 (56.97) | 449 (42.52) | <0.001 |
| CKD, n (%) | 263 (20.23) | 73 (44.79) | 190 (16.71) | <0.001 | 45 (58.44) | 218 (17.83) | <0.001 | 59 (24.18) | 204 (19.32) | 0.088 |
| Hypotension, n (%) | 74 (5.69) | 26 (15.95) | 48 (4.22) | <0.001 | 20 (25.97) | 54 (4.42) | <0.001 | 30 (12.30) | 44 (4.17) | <0.001 |
| LVEF, % | 53.81 ± 10.94 | 49.06 ± 12.32 | 54.5 ± 10.56 | <0.001 | 48.03 ± 13.11 | 54.17 ± 10.7 | <0.001 | 51.02 ± 11.73 | 54.46 ± 10.65 | <0.001 |
| LVEF < 40%, n (%) | 134 (10.31) | 36 (22.09) | 98 (8.62) | <0.001 | 20 (25.97) | 114 (9.32) | <0.001 | 41 (16.80) | 93 (8.81) | <0.001 |
| Hypertension, n (%) | 646 (49.69) | 112 (68.71) | 534 (46.97) | <0.001 | 55 (71.43) | 591 (48.32) | <0.001 | 142 (58.20) | 504 (47.73) | 0.003 |
| Hyperlipidemia, n (%) | 200 (15.38) | 19 (11.66) | 181 (15.92) | 0.158 | 11 (14.29) | 189 (15.45) | 0.783 | 36 (14.75) | 164 (15.53) | 0.762 |
| Hypoproteinemia, n (%) | 606 (46.62) | 75 (46.01) | 531 (46.7) | 0.018 | 40 (51.95) | 566 (46.28) | <0.001 | 103 (42.21) | 503 (47.63) | 0.183 |
| Anemia, n (%) | 431 (33.15) | 76 (46.63) | 355 (31.22) | <0.001 | 36 (46.75) | 395 (32.30) | 0.008 | 89 (36.48) | 342 (32.39) | 0.235 |
| Diabetes, n (%) | 267 (20.54) | 42 (25.77) | 225 (19.79) | 0.077 | 19 (24.68) | 248 (20.28) | 0.354 | 50 (20.49) | 217 (20.55) | 0.984 |
| LDL-C, mmol/L | 2.98 ± 1.05 | 3.09 ± 1.07 | 2.97 ± 1.05 | 0.260 | 2.86 ± 1.18 | 2.99 ± 1.04 | 0.459 | 3.22 ± 1.15 | 2.93 ± 1.02 | 0.003 |
| HDL-C, mmol/L | 0.96 ± 0.41 | 0.97 ± 0.27 | 0.96 ± 0.42 | 0.630 | 0.98 ± 0.31 | 0.96 ± 0.41 | 0.640 | 1.03 ± 0.31 | 0.95 ± 0.42 | 0.006 |
| HS-CRP, mg/L | 31.55 ± 44.29 | 54.52 ± 53.53 | 28.19 ± 41.77 | <0.001 | 66.08 ± 55.06 | 29.32 ± 42.59 | <0.001 | 47.4 ± 54.91 | 27.8 ± 40.53 | <0.001 |
| Lpa, mg/dL | 32.69 ± 36.2 | 31.83 ± 32.65 | 32.81 ± 36.68 | 0.750 | 30.55 ± 30.73 | 32.81 ± 36.5 | 0.585 | 30.77 ± 35.33 | 33.13 ± 36.4 | 0.391 |
| SCR, μmol/L | 95.49 ± 38.01 | 119.03 ± 60.8 | 92.12 ± 32.16 | <0.001 | 140 ± 69.57 | 92.69 ± 33.20 | <0.001 | 93.43 ± 47.04 | 95.97 ± 35.61 | 0.429 |
| eGFR, mL/min/1.73 mm2 | 80.22 ± 26.68 | 67.43 ± 36.36 | 82.05 ± 24.47 | <0.001 | 54.85 ± 27.72 | 81.82 ± 25.8 | <0.001 | 85.78 ± 36.92 | 78.94 ± 23.53 | 0.006 |
| BUN, mg/dL | 5.44 ± 3.05 | 7.13 ± 4.12 | 5.2 ± 2.78 | <0.001 | 8.65 ± 4.94 | 5.24 ± 2.77 | <0.001 | 5.75 ± 3.24 | 5.37 ± 3.00 | 0.096 |
| Hemoglobin, g/L | 132.47 ± 17.19 | 126.1 ± 20.82 | 133.28 ± 16.51 | <0.001 | 123.94 ± 23.14 | 132.94 ± 16.69 | 0.008 | 128.92 ± 20.51 | 133.17 ± 16.37 | 0.014 |
| HbA1c, % | 6.56 ± 1.50 | 6.64 ± 1.33 | 6.55 ± 1.52 | 0.497 | 6.57 ± 1.14 | 6.56 ± 1.52 | 0.936 | 6.57 ± 1.39 | 6.56 ± 1.52 | 0.892 |
| Serum albumin, g/L | 33.22 ± 4.64 | 31.4 ± 4.57 | 33.44 ± 4.6 | <0.001 | 29.48 ± 4.61 | 33.41 ± 4.56 | <0.001 | 32.39 ± 4.23 | 33.38 ± 4.69 | 0.011 |
| ACEI/ARB, n (%) | 1157 (89.00) | 131 (80.37) | 1026 (90.24) | <0.001 | 57 (74.03) | 1100 (89.94) | <0.001 | 210 (86.07) | 947 (89.68) | 0.142 |
| Beta blocker, n (%) | 1064 (81.85) | 108 (66.26) | 956 (84.08) | <0.001 | 42 (54.55) | 1022 (83.57) | <0.001 | 185 (75.82) | 879 (83.24) | 0.018 |
| Statin, n (%) | 1284 (98.77) | 157 (96.32) | 1127 (99.12) | 0.002 | 42 (54.55) | 794 (64.92) | 0.596 | 142 (58.20) | 694 (65.72) | 0.054 |
| PCI, n (%) | 836 (64.31) | 91 (55.83) | 745 (65.52) | 0.826 | 73 (94.81) | 1211 (99.02) | 0.001 | 237 (97.13) | 1047 (99.15) | 0.018 |
| Diuretics, n (%) | 404 (31.08) | 88 (53.99) | 316 (27.79) | <0.001 | 44 (57.14) | 360 (29.44) | <0.001 | 111 (45.49) | 293 (27.75) | <0.001 |
| Metformin, n (%) | 17 (1.31) | 2 (1.23) | 15 (1.32) | 0.705 | 0 (0.00) | 17 (1.39) | 1.000 | 3 (1.23) | 14 (1.33) | 0.745 |
| CV, mL | 132.74 ± 53.03 | 137.06 ± 56.1 | 132.13 ± 52.57 | 0.292 | 138.31 ± 49.05 | 132.39 ± 53.27 | 0.310 | 133.16 ± 56.06 | 132.65 ± 52.33 | 0.898 |
| Peri-procedure IABP, n (%) | 108 (8.31) | 52 (31.90) | 56 (4.93) | <0.001 | 36 (46.75) | 72 (5.89) | <0.001 | 50 (20.49) | 58 (5.49) | <0.001 |
Abbreviations: CI-AKI: contrast-induced acute kidney injury; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; CHF: chronic heart failure; CKD: chronic kidney disease; LVEF: left ventricular ejection fraction; LDL-C: low density lipoprotein-C; HDL-C: high density lipoprotein-C; HS-CRP: high sensitive C-reactive protein; SCR: serum creatinine; Lpa: lipoprotein a; eGFR: estimate glomerular filtration rate; BUN: blood urea nitrogen; ACEI: angiotensin-converting enzymes inhibitors; ARB: angiotensin-receptor blockers; PCI: percutaneous coronary intervention; CV: contrast volume; IABP: intra-aortic balloon pump.
Irrespective of the definition used, patients complicated with CI-AKI following CAG were older, more often female, with lower weight, worse baseline heart and renal function, and higher incidence of hypertension or hypotension; moreover, they had higher levels of high sensitive C-reactive protein (HS-CRP) and lower levels of hemoglobin (Table 1).
3.2. CI-AKI and in-hospital events
In-hospital events are detailed in Suppl. After the procedure, 27 patients underwent hemodialysis, among whom 22 (28.6%) were complicated with CI-AKIB, 24 (14.7%) with CI-AKIA and 21 (8.6%) with CI-AKIC. Regardless of the definitions, patients with CI-AKI tended to have hemodialysis after contrast exposure (p < 0.001). Moreover, patients with CI-AKI were more likely to develop acute heart failure (AHF) and arrythmia during hospitalization.
3.3. Long-term outcomes
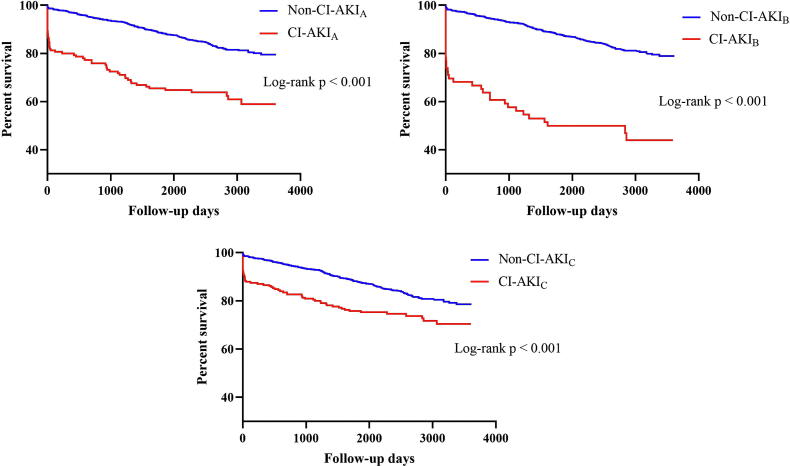
The median follow-up period was 7.0 (5.5; 8.7) years. During the follow-up period, 244 all-cause deaths occurred. The long-term mortality rate was significantly higher in patients with CI-AKIB (51.9%), followed by CI-AKIA (37.4%) and CI-AKIC (26.2%) (p < 0.001). Kaplan-Meier curves revealed that patients with CI-AKI demonstrated poorer long-term prognosis than those without CI-AKI (Fig. 1). In addition, after adjusting for age, gender, heart rate, heart and renal function, hypertension, hypotension, diabetes mellitus, anemia, HS-CRP and medications, CI-AKIA was associated with a 2.049 fold higher risk of long-term death (95% CI: 1.419–2.958), while CI-AKIB was associated with a 2.711 fold higher risk (95% CI: 1.743–4.217) and CI-AKIC was associated with a 1.417 fold higher risk (95% CI: 1.011–1.986) (Table 2).
Fig. 1.
Association between various definitions of contrast-induced acute kidney injury and long-term mortality in patients with acute myocardial infarction undergoing coronary angiography.
Table 2.
Univariable and multivariable analysis of risk factors for long-term mortality.
| Age | UNIVARIABLE ANALYSIS |
MULTIVARIABLE ANALYSIS |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI-AKIA |
CI-AKIB |
CI-AKIC |
||||||||||||||
| HR |
95%Cl |
p-value |
HR |
95%Cl |
p-value |
HR |
95%Cl |
p-value |
HR |
95%Cl |
p-value |
|||||
| 1.378 | 1.010 | 1.881 | 0.043 | 0.992 | 0.978 | 1.006 | 0.251 | 0.992 | 0.978 | 1.006 | 0.265 | 0.994 | 0.980 | 1.008 | 0.414 | |
| Female vs Male | 1.480 | 1.085 | 2.020 | 0.013 | 1.344 | 0.926 | 1.953 | 0.120 | 1.338 | 0.922 | 1.943 | 0.125 | 1.317 | 0.908 | 1.910 | 0.147 |
| Weight | 0.991 | 0.979 | 1.003 | 0.154 | ||||||||||||
| Heart rate | 1.020 | 1.013 | 1.027 | <0.001 | 1.016 | 1.009 | 1.024 | <0.001 | 1.016 | 1.008 | 1.024 | <0.001 | 1.017 | 1.009 | 1.025 | <0.001 |
| CHF | 1.612 | 1.252 | 2.076 | <0.001 | 1.026 | 0.752 | 1.400 | 0.873 | 1.053 | 0.774 | 1.433 | 0.742 | 1.074 | 0.790 | 1.460 | 0.649 |
| CKD | 2.228 | 1.704 | 2.914 | <0.001 | 1.370 | 0.963 | 1.949 | 0.080 | 1.330 | 0.933 | 1.895 | 0.115 | 1.509 | 1.068 | 2.134 | 0.020 |
| Hypertension | 1.344 | 1.044 | 1.731 | 0.022 | 1.039 | 0.767 | 1.409 | 0.804 | 1.053 | 0.776 | 1.428 | 0.742 | 1.061 | 0.784 | 1.436 | 0.700 |
| Hypotension | 2.602 | 1.703 | 3.975 | <0.001 | 1.916 | 1.126 | 3.260 | 0.016 | 1.958 | 1.156 | 3.315 | 0.012 | 1.896 | 1.114 | 3.226 | 0.018 |
| Smoking | 0.825 | 0.642 | 1.062 | 0.135 | ||||||||||||
| Hypoproteinemia | 0.895 | 0.659 | 1.214 | 0.476 | ||||||||||||
| Anemia | 1.574 | 1.219 | 2.033 | <0.001 | 1.369 | 0.999 | 1.877 | 0.051 | 1.378 | 1.006 | 1.888 | 0.046 | 1.409 | 1.029 | 1.929 | 0.033 |
| HS-CRP, mg/L | 1.005 | 1.003 | 1.008 | <0.001 | 1.002 | 0.999 | 1.005 | 0.253 | 1.001 | 0.998 | 1.004 | 0.386 | 1.002 | 0.999 | 1.005 | 0.222 |
| ACEI/ARB | 0.487 | 0.350 | 0.677 | <0.001 | 0.671 | 0.442 | 1.017 | 0.060 | 0.675 | 0.446 | 1.022 | 0.063 | 0.659 | 0.435 | 0.999 | 0.049 |
| Beta-blocker | 0.490 | 0.371 | 0.646 | <0.001 | 0.625 | 0.444 | 0.880 | 0.007 | 0.644 | 0.457 | 0.906 | 0.011 | 0.613 | 0.436 | 0.860 | 0.005 |
| Diuretic | 1.593 | 1.230 | 2.062 | <0.001 | ||||||||||||
| IABP | 3.247 | 2.328 | 4.530 | <0.001 | ||||||||||||
| Diabetes | 1.621 | 1.226 | 2.141 | <0.001 | 1.455 | 1.046 | 2.024 | 0.026 | 1.479 | 1.063 | 2.058 | 0.020 | 1.488 | 1.072 | 2.066 | 0.018 |
| CI-AKIA | 3.098 | 2.318 | 4.140 | <0.001 | 2.049 | 1.419 | 2.958 | <0.001 | ||||||||
| CI-AKIB | 5.145 | 3.664 | 7.225 | <0.001 | 2.711 | 1.743 | 4.217 | <0.001 | ||||||||
| CI-AKIC | 1.831 | 1.376 | 2.435 | <0.001 | 1.417 | 1.011 | 1.986 | 0.043 | ||||||||
Abbreviations: CI-AKI: contrast-induced acute kidney injury; HR: hazard ratio; CHF: chronic heart failure; CKD: chronic kidney disease; HS-CRP: high sensitive C-reactive protein; ACEI: angiotensin-converting enzymes inhibitors; ARB: angiotensin-receptor blockers; IABP: intra-aortic balloon pump.
In addition, in a small fraction of our enrolled patients who underwent hemodialysis after the procedure, a higher mortality rate was observed compared with that of those who did not undergo post-procedure hemodialysis (66.67% vs 18.39%, p < 0.001). Kaplan-Meier curves also revealed the similar results (Suppl. Fig. 1).
3.4. Pars of three CI-AKI definitions
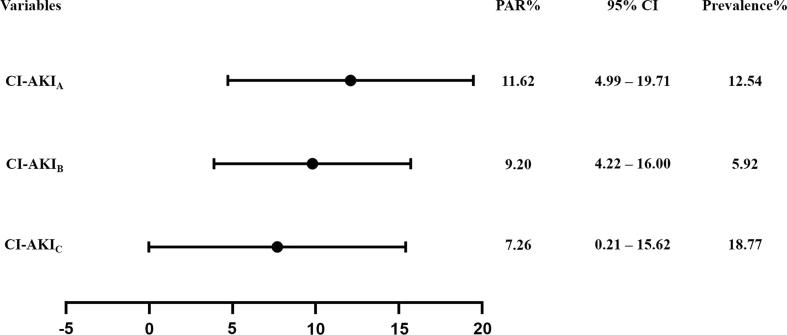
Among the three definitions of CI-AKI, the prevalence was highest for CI-AKIC (18.77%), followed by CI-AKIA (12.54%) and CI-AKIB (5.92%). For the PARs, it was the highest for CI-AKIA (PAR:11.62, 95% CI: 4.99–19.71), followed by CI-AKIB (PAR:9.20, 95% CI: 4.22–16.00), and it was the lowest for CI-AKIC (PAR:7.26, 95% CI: 0.21–15.62) (Fig. 2).
Fig. 2.
Population attributable risk of three different definitions of contrast-induced acute kidney injury.
4. Discussion
Our study evaluated the association between CI-AKI and long-term prognosis in AMI patients, and was the first to compare the PARs for long-term mortality among three different definitions of CI-AKI. In this study, we found that patients with CI-AKI had a higher mortality rate than those without CI-AKI. After adjusting for sociodemographic and cardiorenal risk factors including age, gender, heart rate, CHF, CKD, history of hypertension, history of DM, hypotension, anemia, HS-CRP, and pharmacological therapy, CI-AKI may lead to a 1.417–2.711 fold higher mortality rate depending on the definitions used. Moreover, the highest PAR was found in CI-AKIA, followed by CI-AKIB, and CI-AKIC.
4.1. Findings and comparison with related studies
Our results suggested that CI-AKI was associated with worse long-term outcomes regardless of the definition (adjusted hazard ratios: 1.417–2.711), which was similar to previous results. Centola, M et.al compared two different definitions of CI-AKI in 406 patients with ST-segment elevation myocardial infarction (STEMI). During the median follow-up period of 12 ± 4 months, a significant association was detected between mortality and CI-AKI in both definitions [20]. Our results further verified the previous findings. Indeed, CI-AKI is a marker rather than a mediator of an increased risk of worse long-term outcomes [21]. First, patients who developed CI-AKI following cardiac catheterization tended to have their renal function decline after this acute event [22]. Chalikias G et al. found that AMI patients with acute kidney injury (AKI) during their hospitalization demonstrated a higher rate of deteriorating kidney function (9.5%) than those without AKI (5.1%) during the median follow-up period of 5.6 years [23]. In our cohort, patients with CI-AKI were more likely to have hemodialysis after the procedure and worse baseline renal function, which is similar to previously reported observations [20]. Additionally, deterioration in renal function has long been reported as a strong risk factor for adverse outcomes [18], [24]. Second, patients with CI-AKI are prone to hemodynamic instability or comorbidity associated with prognosis [25]. In our study, patients with CI-AKI were more likely to be complicated with CHF and hypotension, and a higher incidence of arrythmia and AHF was also observed in those with CI-AKI. Third, the distant organ effects of AKI, especially the effect leading to cardiac dysfunction, may have a significant impact on prognosis. Potential mechanisms may be endothelial dysfunction, fluid overload, hypercoagulation and myocardial depression activity during ultrafiltration [26].
In our cohort, the incidence of CI-AKI varied from 5.92% to 18.77% depending on the definitions. As reported by previous results, the lack of a long-established definition of CI-AKI resulted in wide variation in the incidence of CI-AKI among patients with AMI [9], [10], [11], [27]. From July 2006 to June 2007, a study conducted by Jabara et al. enrolled 400 consecutive patients, the incidence of CI-AKI were 3.3% (Scr increase ≥ 0.5 mg/dl), 10.2% (Scr increase ≥ 25%), 7.6% (eGFR decrease ≥ 25%), and 10.5% (the composite), respectively [28]. The incidence of CI-AKI in our cohort was higher than that of Jabara‘s, which may be due to the difference in the included patients (AMI patients in our study).
In this study, we found that CI-AKIA (defined as an increase of Scr ≥ 0.3 mg/dL or ≥ 50% from baseline), which had the highest PAR for long-term mortality, seems to be the most valuable predictor of long-term prognosis in patients with AMI. Previous studies showed that “absolute” criteria (i.e., an increase of 1.0 mg/dL in Scr) neglect a high proportion of patients with small increases in Scr and may lead to underestimation of the incidence of CI-AKI. Actually, a small Scr increase in patients with low baseline Scr may also lead to significant renal impairment [29]. On the other hand, the “relative” criteria (i.e., an increase of 10% in Scr) have a lower discriminative power [28], [30], [31]. CI-AKIA was defined as containing both absolute and relative criteria. A PAR of 11.62% for long-term mortality may reflect the appropriate prevalence and hazard ratio of CI-AKIA.
Our results also indicated that prevention of CI-AKIB defined as an increase of Scr ≥ 0.5 mg/dL from baseline may avert 9.20% of long-term mortality in patients with AMI, which was derived from both the prevalence and the magnitude of its association with long-term mortality. Patients with an absolute Scr increase ≥ 0.5 mg/dL were evaluated as high risk, which is broadly consistent among previous studies. Guillon et al found that patients with an increase in Scr ≥ 0.5 mg/dL had higher based complications. After adjusting for related risk factors, this group (6.9%) had a 2.9-fold increase in 6-month mortality [10]. Budano et al reported a similar finding in 755 patients undergoing contrast exposure [32]. However, this criterion may be too strict and lead to a lack of prognostic value.
In this research, CI-AKIC was identified in 18.77% of the subjects, but its HR value was the lowest among all three CI-AKI definitions, which is quite similar to the result of a large sample randomized CI-AKI trial (HORIZON-AMI) [7]. In addition, CI-AKIC had the lowest PAR, which may only explain 7.26% of the long-term mortality. Its lowest ranking among all three CI-AKI definitions was not unexpected. One explanation may be the inclusion of low risk patients and heterogeneity [33], [34], [35]. This seems to suggest that interventions targeting patients with CI-AKIC may cost the most but generate the lowest effect in reducing long-term mortality in patients with AMI.
4.2. Limitations
First, our study was a sub-study of an observational cohort of unselected patients conducted in a single center located in South China; thus, the prevalence of CI-AKI may not be representative. However, our cohort is one of the largest CI-AKI databases regarding AMI patients. In particular, PAR can only be calculated based on observational data. Second, due to the observational design, we could only indicate that preventing CI-AKI may eliminate 5–11% of long-term all-cause death rather than directly prove it. However, our study evaluated the harm caused by CI-AKI from a new dimension (PAR) and first compared the differences in PAR between three various definitions. Third, some patients were discharged within 72 h after the CAG/PCI and did not have their creatinine level measured thereafter, and it is quite difficult to collect details regarding subsequent kidney insults over a follow-up period as long as 7 years. Therefore, we may not be able to report follow-up renal outcomes (normalization or deterioration) or renal insulting events, which are important for worse outcomes. However, the association between CI-AKI and long-term mortality was adjusted by various important prognostic factors from the TIMI and GRACE scores. Finally, the Scr assay in our laboratory was performed by the Jaffe method, which may have been abated by the others.
5. Conclusions
Our results suggested that CI-AKI is associated with long-term prognosis in patients with AMI irrespective of the definition used. Cardiologists as well as future studies exploring long-term prognosis should pay more attention to the presence of CI-AKI, especially CI-AKIA with the highest PAR.
Funding
This work was supported by the Beijing Lisheng Cardiovascular Pilot Foundation [grant no. LHJJ201612127], the “Lixin Yangfan” Optimized Anti-thrombus Research Fund [grant no. BJUHFCSOARF201801-10], the Progress in Science and Technology Project of Guangzhou [grant no. 201904010470], the Access Research Fund [grant no. 2018-CCA-AF-037], and the China Youth Clinical Research Fund [grant no. 2017-CCA-VG-020]. The funding body did not participate in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
CRediT authorship contribution statement
Li Lei: Methodology, Formal analysis, Investigation, Writing - original draft, Data curation, Writing - review & editing. Yan Xue: Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Zhaodong Guo: Methodology, Investigation, Writing - original draft, Writing - review & editing. Bowen Liu: Methodology, Investigation, Writing - original draft, Writing - review & editing. Yibo He: Investigation, Validation, Writing - review & editing. Feier Song: Investigation, Validation, Writing - review & editing. Jin Liu: Methodology, Data curation, Writing - review & editing. Guoli Sun: Investigation, Data curation, Writing - review & editing. Liling Chen: Investigation, Writing - review & editing. Kaihong Chen: Investigation, Resources, Writing - review & editing. Zhidong Huang: Writing - original draft, Investigation, Writing - review & editing. Ming Ying: Writing - original draft, Investigation. Liyao Zhang: Writing - original draft, Investigation. Zhiqi Su: Writing - original draft. Li Pan: Writing - original draft. Shiqun Chen: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Jiyan Chen: Conceptualization, Methodology, Resources, Supervision, Funding acquisition, Writing - review & editing. Yong Liu: Conceptualization, Methodology, Resources, Supervision, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Beijing Lisheng Cardiovascular Pilot Foundation [grant no. LHJJ201612127], the “Lixin Yangfan” Optimized Anti-thrombus Research Fund [grant no. BJUHFCSOARF201801-10], the Progress in Science and Technology Project of Guangzhou [grant no. 201904010470], the Access Research Fund [grant no. 2018-CCA-AF-037], and the China Youth Clinical Research Fund [grant no. 2017-CCA-VG-020].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100522.
Contributor Information
Jiyan Chen, Email: chenjiyandr@126.com.
Yong Liu, Email: liuyong@gdph.org.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Sato A., Aonuma K., Watanabe M. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: From the CINC-J study. Int. J. Cardiol. 2017;227:424–429. doi: 10.1016/j.ijcard.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell A.M., Kline J.A., Jones A.E., Tumlin J.A. Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann. Emerg. Med. 2015;66(267–74) doi: 10.1016/j.annemergmed.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Senoo T., Motohiro M., Kamihata H. Contrast-induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am. J. Cardiol. 2010;105:624–628. doi: 10.1016/j.amjcard.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Weisbord S.D., Chen H., Stone R.A. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J. Am. Soc. Nephrol.: JASN. 2006;17:2871–2877. doi: 10.1681/ASN.2006030301. [DOI] [PubMed] [Google Scholar]
- 5.Sun G., Chen P., Wang K. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology. 2019;70:621–626. doi: 10.1177/0003319718803677. [DOI] [PubMed] [Google Scholar]
- 6.Nakahashi H., Kosuge M., Sakamaki K. Combined impact of chronic kidney disease and contrast-induced nephropathy on long-term outcomes in patients with ST-segment elevation acute myocardial infarction who undergo primary percutaneous coronary intervention. Heart Vessels. 2017;32:22–29. doi: 10.1007/s00380-016-0836-8. [DOI] [PubMed] [Google Scholar]
- 7.Narula A., Mehran R., Weisz G. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur. Heart J. 2014;35:1533–1540. doi: 10.1093/eurheartj/ehu063. [DOI] [PubMed] [Google Scholar]
- 8.Turan B., Erkol A., Gul M., Findikcioglu U., Erden I. Effect of contrast-induced nephropathy on the long-term outcome of patients with non-ST segment elevation myocardial infarction. Cardiorenal Med. 2015;5:116–124. doi: 10.1159/000371900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chousterman B.G., Bouadma L., Moutereau S. Prevention of contrast-induced nephropathy by N-acetylcysteine in critically ill patients: different definitions, different results. J. Crit. Care. 2013;28:701–709. doi: 10.1016/j.jcrc.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Guillon B., Ecarnot F., Marcucci C. Incidence, predictors, and impact on six-month mortality of three different definitions of contrast-induced acute kidney injury after coronary angiography. Am. J. Cardiol. 2018;121:818–824. doi: 10.1016/j.amjcard.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Capodanno D., Ministeri M., Dipasqua F. Risk prediction of contrast-induced nephropathy by ACEF score in patients undergoing coronary catheterization. J. Cardiovasc. Med. (Hagerstown). 2016;17:524–529. doi: 10.2459/JCM.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 12.Spiegelman D., Hertzmark E., Wand H.C. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control: CCC. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Chen J., Tan N. Safe Limits of Contrast Vary With Hydration Volume for Prevention of Contrast-Induced Nephropathy After Coronary Angiography Among Patients With a Relatively Low Risk of Contrast-Induced Nephropathy. Circ Cardiovasc Interv. 2015;8(6) doi: 10.1161/CIRCINTERVENTIONS.114.001859. [DOI] [PubMed] [Google Scholar]
- 14.Wright R.S., Anderson J.L., Adams C.D. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011;57:1920–1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Liu J., Sun G., He Y. Early beta-blockers administration might be associated with a reduced risk of contrast-induced acute kidney injury in patients with acute myocardial infarction. J. Thorac. Dis. 2019;11:1589–1596. doi: 10.21037/jtd.2019.04.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J. Am. Coll. Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 17.Roe M.T., Chen A.Y., Thomas L. Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am. Heart J. 2011;162(875–83) doi: 10.1016/j.ahj.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Shuvy M., Beeri G., Klein E. Accuracy of the Global Registry of Acute Coronary Events (GRACE) Risk Score in Contemporary Treatment of Patients With Acute Coronary Syndrome. Can. J. Cardiol. 2018;34:1613–1617. doi: 10.1016/j.cjca.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Willey J.Z., Moon Y.P., Kahn E. Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the northern Manhattan study. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centola M., Lucreziotti S., Salerno-Uriarte D. A comparison between two different definitions of contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int. J. Cardiol. 2016;210:4–9. doi: 10.1016/j.ijcard.2016.02.086. [DOI] [PubMed] [Google Scholar]
- 21.Mehran R., Dangas G.D., Weisbord S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019;380:2146–2155. doi: 10.1056/NEJMra1805256. [DOI] [PubMed] [Google Scholar]
- 22.James M.T., Ghali W.A., Tonelli M. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 23.Chalikias G., Serif L., Kikas P. Long-term impact of acute kidney injury on prognosis in patients with acute myocardial infarction. Int. J. Cardiol. 2019;283:48–54. doi: 10.1016/j.ijcard.2019.01.070. [DOI] [PubMed] [Google Scholar]
- 24.Watabe H., Sato A., Hoshi T. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int. J. Cardiol. 2014;174:57–63. doi: 10.1016/j.ijcard.2014.03.146. [DOI] [PubMed] [Google Scholar]
- 25.Dangas G., Iakovou I., Nikolsky E. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am. J. Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 26.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol.: JASN. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marenzi G., Lauri G., Assanelli E. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 2004;44:1780–1785. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Jabara R., Gadesam R.R., Pendyala L.K. Impact of the definition utilized on the rate of contrast-induced nephropathy in percutaneous coronary intervention. Am. J. Cardiol. 2009;103:1657–1662. doi: 10.1016/j.amjcard.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Bachorzewska-Gajewska H., Malyszko J., Sitniewska E., Malyszko J.S., Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am. J. Nephrol. 2006;26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 30.Harjai K.J., Raizada A., Shenoy C. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am. J. Cardiol. 2008;101:812–819. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Liu Y., Smyth B. Clinical implications of contrast-induced nephropathy in patients without baseline renal dysfunction undergoing coronary angiography. Heart Lung Circ. 2019;28:866–873. doi: 10.1016/j.hlc.2018.04.291. [DOI] [PubMed] [Google Scholar]
- 32.Budano C., Levis M., D'Amico M. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am. Heart J. 2011;161:963–971. doi: 10.1016/j.ahj.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Bae E.H., Lim S.Y., Cho K.H. GFR and cardiovascular outcomes after acute myocardial infarction: results from the Korea Acute Myocardial Infarction Registry. Am. J. Kidney Diseases: Off. J. Natl. Kidney Found. 2012;59:795–802. doi: 10.1053/j.ajkd.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Amin A.P., Salisbury A.C., McCullough P.A. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch. Intern. Med. 2012;172:246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 35.Crimi G., Leonardi S., Costa F. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. insights from the all-comer PRODIGY trial. Catheter. Cardiovasc. Interv. 2015;86:E19–E27. doi: 10.1002/ccd.25822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.