The coronavirus disease-2019 (COVID-19) pandemic has caused abrupt shifts in US health care delivery.1 To preserve inpatient resources and minimize COVID-19 transmission, many health systems have expanded telemedicine, limited hospital transfers, and established stringent hospitalization criteria for non–COVID-19-related conditions. States have also enacted, on varying timelines, shelter-in-place (SIP) or equivalent orders to further limit COVID-19 spread. The broader impact of these changes on health care utilization and outcomes in high-risk and vulnerable populations, such as those with cirrhosis, are unknown.
To address these gaps, we used national data in the Veterans Health Administration (VHA), a single integrated system of care and the largest single US provider of liver-related care, to (1) investigate the impact of the COVID-19 pandemic on the volume of national cirrhosis hospitalizations, and (2) identify salient changes in hospitalization-level characteristics.
Methods
This was a retrospective cohort study of VHA cirrhosis hospitalizations using the Veterans Outcomes and Costs Associated with Liver Disease cohort.2 , 3 Veterans Outcomes and Costs Associated with Liver Disease includes patients with cirrhosis identified using a validated algorithm from January 1, 2008, to December 31, 2016, in 125 hospitals nationwide.4 We included patients age ≥18 who were hospitalized for any reason between January 1 and April 15 in 2019 or 2020, and excluded those with prior liver transplantation.
We collected hospital-level data, including length of stay (LOS), hospital region, rurality, community or academic hospital, and discharge disposition. International Classification of Diseases, 10th Revision, discharge codes were used to classify reasons for admission. Patient-level data included demographics, prehospitalization comorbidities, baseline model for end-stage liver disease (MELD) score and Child-Turcotte-Pugh class (measures of liver disease severity), and admission laboratory parameters. Admission MELD was categorized as ≤15, 16–24, and ≥25. Etiology of liver disease was ascertained using a VHA validated algorithm.5 State-level data for new COVID-19 daily cases and deaths, and dates of state SIP orders were obtained from publicly available databases.6 The primary outcome was national weekly cirrhosis hospitalizations.
We plotted weekly hospitalization counts for each year through April 15. Using linear regression, we estimated the difference in weekly hospitalizations in 2019 and 2020 before and after February 29 (date of first publicized US COVID-19 death; called pre-COVID and during-COVID periods herein). We then compared the magnitude of this change (ie, difference-in-differences) between years. Linear regression limited to 2020 was then used to investigate effects of SIP orders, and number of incident COVID-19 cases and deaths on cirrhosis hospitalizations, adjusted for linear time trends in pre- and during-COVID periods. SIP was computed as the proportion of hospitalizations in a given week in a state with an active SIP order. To investigate changes in patient- and hospital-level characteristics during the pandemic, we defined three 2020 subgroup eras: pre-COVID (before February 29), early-COVID (February 29 to March 25), and late-COVID (March 26 to April 15). March 26 was chosen because the VHA announced a COVID-19 Response Plan on March 23 that was widely implemented within 3 days.7 Across these periods, we compared summary statistics using χ2 or Kruskal-Wallis testing as indicated.
Results
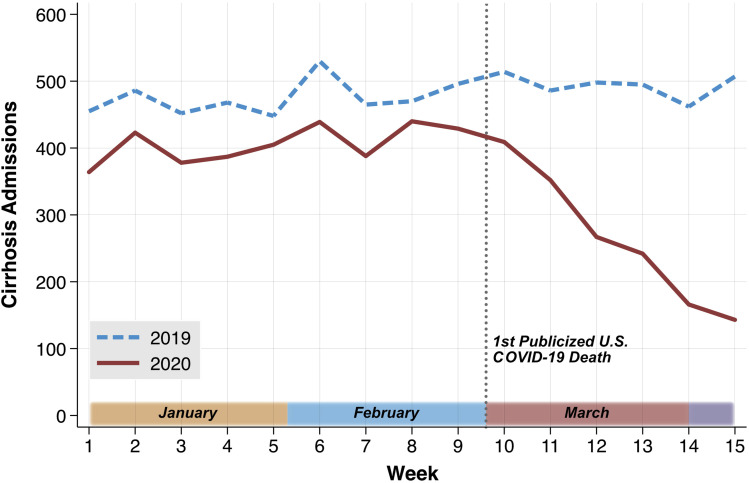
We identified 12,467 hospitalizations from 7216 unique patients in 2019 and 2020. Baseline patient characteristics were similar between years and across equivalent pre- and during-COVID dates (data not shown). In difference-in-differences analysis, weekly cirrhosis hospitalizations were on average 159.47 lower (95% confidence interval −250.03 to −68.90) in the 2020 during-COVID period relative to an expected counterfactual trend (P < .001; Figure 1 ; Supplementary Table 1). When adjusting for a significant linear decrease in weekly hospitalizations in the during-COVID period (−53.51; 95% confidence interval −61.32 to −45.71; P < .001), we did not find independent effects of SIP orders (P = .938), incident COVID-19 cases (P = .682), or incident COVID-19 deaths (P = .875) on weekly hospitalizations.
Figure 1.
National weekly cirrhosis hospitalizations between January 1 and April 15 in 2019 and 2020. Note. Recent reports on April 22, 2020, suggest that the first true COVID-19–related death in the United States occurred in early February 2020; however, before April 22, the first widely publicized death was on February 29, 2020.
We identified significantly higher admission MELD in the late-COVID era, indicating higher degree of liver disease severity (P = .029; Supplementary Table 2). The late-COVID era was also characterized by significantly fewer academic hospital admissions (63.6% vs 68.1% pre-COVID; P = .014), shorter LOS (median 2 vs 3, P < .001), and fewer hospital transfers (7.5% vs 11.1% pre-COVID; P = .046). There were significant differences in post-hospitalization disposition (P = .029), with more patients being discharged to home in the late-COVID era (91.1% vs 88.8% pre-COVID) and fewer to facilities (5.1% vs 9.0% pre-COVID).
Discussion
In this national VHA analysis, we identified a substantial decline in cirrhosis hospitalizations attributable to COVID-19, an effect that has intensified over time. We also identified important changes in hospitalization-level characteristics in the late-COVID era, including significant declines in academic center hospitalizations, decreased LOS, fewer hospital transfers, increased admission MELD, and more frequent discharge to home rather than facilities. These changes likely reflect initiatives to preserve inpatient resources, and guidance encouraging patients to remain home. It is also likely that patients, perhaps because of personal concerns about COVID, are avoiding hospital presentation until symptoms are severe. Importantly, our findings with cirrhosis likely parallel changes in other inpatient resource-intensive conditions, such as congestive heart failure, chronic obstructive pulmonary disease, and myocardial infarction.8
These findings have significant health systems implications. There has been a clear drop-off in hospitalizations for patients who ordinarily would meet acute care criteria. It is unclear how these patients are being managed as outpatients, and given the baseline vulnerability of patients with cirrhosis, it is likely that many do not currently have adequate health care access. Likewise, abbreviated inpatient care may adversely impact outcomes such as short-term mortality, and changes in posthospital disposition away from facilities may increase hospital readmissions and outpatient acuity. Finally, these data may inform anticipatory changes in resource allocation during future pandemics based on expected shifts in utilization.
This study has several limitations. First, there is the possibility of misclassification of exposures and outcomes. Second, we do not yet have access to mortality data this early in the pandemic. Third, our findings in the VHA cohort may not be generalizable to other settings.
In conclusion, national cirrhosis hospitalizations have dramatically declined due to the COVID-19 pandemic. Increased liver disease severity in hospitalized patients raise serious concerns that near-term clinical outcomes may be adversely impacted. Future studies will need to investigate the impact of COVID-19 on cirrhosis-related morbidity and mortality.
Acknowledgments
This study received institutional review board approval from the Corporal Michael J. Crescenz VA Medical Center, Philadelphia. Data management and analyses were performed using Stata 15.1/IC (College Station, TX). This work was supported by resources and facilities available through the Philadelphia Veterans Affairs Healthcare System as well as the central data repositories maintained by the Veterans Affairs Information Resource Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
CRediT Authorship Contributions
Nadim Mahmud, MD MS MPH MSCE (Conceptualization: Lead; Data curation: Equal; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Equal; Validation: Equal; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Equal). Rebecca A. Hubbard, PhD (Investigation: Equal; Methodology: Equal; Validation: Equal). David E. Kaplan, MD, MSc (Conceptualization: Equal; Data curation: Equal; Funding acquisition: Supporting; Methodology: Equal; Validation: Equal; Writing – review & editing: Equal). Marina Serper, MD, MS (Conceptualization: Lead; Data curation: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Equal; Supervision: Lead; Validation: Equal; Writing – review & editing: Equal).
Footnotes
Conflict of interest The authors disclose no conflicts.
Funding Dr Serper is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, award 1K23DK115897-03. This project was supported by a pilot grant from the Leonard Davis Institute of Health Economics, University of Pennsylvania, Philadelphia, Pennsylvania.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.05.005.
Supplementary Material
Supplementary Table 1.
Difference-in-Difference Estimation and Linear Regression Models for National Weekly Cirrhosis Hospitalizations
| Mean national weekly cirrhosis hospitalizations and DiD estimation | |||||
|---|---|---|---|---|---|
| Mean hospitalizations in pre-COVID datesa | Mean hospitalizations in during-COVID datesa | Difference |
|||
| Mean | 95% CI | P value | |||
| 2019 | 472.61 | 494.69 | 22.08 | (−2.47 to 46.63) | .076 |
| 2020 | 408.52 | 271.13 | −137.39 | (−224.56 to −50.22) | <.001 |
| DiD | −159.47 | (−250.03 to −68.90) | .001 | ||
| Linear regression models for national weekly cirrhosis hospitalizations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Base Model |
Base + SIP |
Base + C19 cases |
Base + C19 deaths |
|||||
| Beta | 95% CI | P value | Beta | P value | Beta | P value | Beta | P value | |
| COVID period (≥Feb 29 vs <Feb 29) | 556.15 | (455.75 to 656.55) | <.001 | 544.77 | .003 | 513.54 | .001 | 568.93 | <.001 |
| Calendar week | 6.08 | (2.49 to 14.65) | .147 | 6.11 | .174 | 6.22 | .162 | 6.03 | .176 |
| COVID period x calendar week interaction | −59.59 | (−69.92 to −49.26) | <.001 | −58.53 | .001 | −55.61 | .000 | −60.75 | <.001 |
| Shelter-in-place order | — | — | — | −5.27 | .938 | — | — | — | — |
| Incident COVID-19 cases | — | — | — | — | — | −0.02 | .682 | — | — |
| Incident COVID-19 deaths | — | — | — | — | — | — | — | 0.11 | .875 |
NOTE. All estimates reflect robust standard errors. Dashes indicate that the variable is not part of the model.
CI, confidence interval; C19, COVID-19; DiD, difference in differences.
Note that pre-COVID dates are January 1 to February 28, and during-COVID dates are February 29 (or March 1 for 2019) to April 15
Supplementary Table 2.
Patient and Hospitalization-Level Characteristics in Pre, Early, and Late COVID Erasa in 2020
| Factor | Pre-COVID era (n = 3430) | Early COVID-19 era (n = 1299) | Late COVID-19 era (n = 506) | P value |
|---|---|---|---|---|
| Patient demographics | ||||
| Age, median (IQR) | 61 (56, 65) | 61 (55, 65) | 60 (55, 65) | .16 |
| Male sex, n (%) | 3331 (97.1) | 1268 (97.6) | 489 (96.6) | .48 |
| Race, n (%) | .055 | |||
| White | 2045 (59.6) | 760 (58.5) | 288 (56.9) | |
| Black | 824 (24.0) | 327 (25.2) | 124 (24.5) | |
| Hispanic | 288 (8.4) | 100 (7.7) | 31 (6.1) | |
| Asian | 32 (0.9) | 16 (1.2) | 7 (1.4) | |
| Other | 241 (7.0) | 96 (7.4) | 56 (11.1) | |
| BMI, median (IQR) | 28.5 (24.5, 33.0) | 29.0 (25.3, 33.6) | 28.0 (24.2, 32.5) | .004 |
| Patient comorbidities | ||||
| Etiology of liver disease, n (%) | .41 | |||
| Hepatitis C virus (HCV) | 407 (11.9) | 125 (9.6) | 53 (10.5) | |
| Hepatitis B virus | 92 (2.7) | 37 (2.8) | 19 (3.8) | |
| Alcoholic liver disease (ALD) | 1255 (36.6) | 502 (38.6) | 190 (37.5) | |
| HCV + ALD | 1106 (32.2) | 416 (32.0) | 169 (33.4) | |
| Metabolic-associated fatty liver disease | 422 (12.3) | 162 (12.5) | 50 (9.9) | |
| Other | 148 (4.3) | 57 (4.4) | 25 (4.9) | |
| Diabetes mellitus, n (%) | 1953 (56.9) | 792 (61.0) | 277 (54.7) | |
| Hypertension, n (%) | 3011 (87.8) | 1171 (90.1) | 442 (87.4) | |
| Congestive heart failure, n (%) | 906 (26.4) | 337 (25.9) | 118 (23.3) | |
| Cerebrovascular accident, n (%) | 518 (15.1) | 210 (16.2) | 64 (12.6) | |
| Coronary artery disease, n (%) | 518 (15.1) | 210 (16.2) | 64 (12.6) | |
| Atrial fibrillation, n (%) | 482 (14.1) | 198 (15.2) | 73 (14.4) | |
| Hepatocellular carcinoma, n (%) | 210 (6.1) | 80 (6.2) | 39 (7.7) | |
| Prior decompensated cirrhosis, n (%) | 1193 (34.8) | 464 (35.7) | 189 (37.4) | |
| Baseline CTP, n (%) | .45 | |||
| Class A | 3067 (89.4%) | 1173 (90.3%) | 452 (89.3%) | |
| Class B | 344 (10.0%) | 122 (9.4%) | 49 (9.7%) | |
| Class C | 19 (0.6%) | 4 (0.3%) | 5 (1.0%) | |
| Patient admission labs | ||||
| Sodium, median (IQR) | 137 (135, 140) | 137 (134, 139) | 137 (134, 139) | .21 |
| Creatinine, median (IQR) | 1 (0.8, 1.51) | 1.04 (0.8, 1.6) | 1 (0.8, 1.73) | .27 |
| GFR, median (IQR) | 82.1 (50.7, 109.8) | 80.0 (48.9, 107.1) | 79.5 (42.4, 108.9) | .27 |
| Albumin, median (IQR) | 3.1 (2.6, 3.6) | 3.1 (2.6, 3.6) | 3.2 (2.6, 3.6) | .49 |
| AST, median (IQR) | 32 (21, 53) | 32 (21, 54) | 34 (24, 54) | .12 |
| ALT, median (IQR) | 23 (15.5, 38) | 24 (15, 37) | 25 (17, 40) | .28 |
| Total bilirubin, median (IQR) | 0.9 (0.5, 1.7) | 0.9 (0.6, 1.59) | 0.9 (0.6, 1.7) | .62 |
| INR, median (IQR) | 1.3 (1.16, 1.57) | 1.3 (1.14, 1.55) | 1.35 (1.17, 1.71) | .072 |
| Hemoglobin, median (IQR) | 11.2 (9.2, 13) | 11 (9.2, 13) | 11.15 (9.4, 12.9) | .87 |
| Platelet count, median (IQR) | 134 (85, 194) | 134 (81, 197) | 130 (80, 199) | .80 |
| MELD category, n (%) | .029 | |||
| ≤15 | 207 (59.0) | 87 (58.8) | 25 (48.1) | |
| 16–24 | 118 (33.6) | 49 (33.1) | 16 (30.8) | |
| ≥25 | 26 (7.4) | 12 (8.1) | 11 (21.2) | |
| Hospitalization characteristics | ||||
| US region, n (%) | .66 | |||
| West | 721 (21.2) | 244 (19.1) | 110 (22.0) | |
| Midwest | 647 (19.1) | 256 (20.0) | 89 (17.8) | |
| Northeast | 555 (16.4) | 219 (17.1) | 79 (15.8) | |
| South | 1470 (43.3) | 561 (43.8) | 222 (44.4) | |
| Reason for admission, n (%) | .60 | |||
| Substance abuse | 452 (13.2) | 182 (14.0) | 75 (14.8) | |
| Infection | 881 (25.7) | 352 (27.1) | 126 (24.9) | |
| Gastrointestinal bleed | 148 (4.3) | 60 (4.6) | 21 (4.2) | |
| Hematology/Oncology | 350 (10.2) | 134 (10.3) | 61 (12.1) | |
| Cardiovascular | 677 (19.7) | 238 (18.3) | 82 (16.2) | |
| Psych | 154 (4.5) | 55 (4.2) | 21 (4.2) | |
| FEN/Renal | 351 (10.2) | 136 (10.5) | 66 (13.0) | |
| Other liver-related | 197 (5.7) | 75 (5.8) | 28 (5.5) | |
| Other | 220 (6.4) | 67 (5.2) | 26 (5.1) | |
| Length of stay, median (IQR) | 3 (2, 6) | 3 (1, 6) | 2 (1, 4) | <.001 |
| Hospital transfer, n (%) | 382 (11.1) | 136 (10.5) | 38 (7.5) | .046 |
| Urban/Rural, n (%) | .16 | |||
| Rural | 88 (2.6) | 45 (3.5) | 18 (3.6) | |
| Urban | 3336 (97.4) | 1253 (96.5) | 487 (96.4) | |
| Academic, n (%) | 2337 (68.1) | 836 (64.4) | 322 (63.6) | .014 |
| Disposition, n (%) | .029 | |||
| Home | 3046 (88.8) | 1157 (89.1) | 461 (91.1) | |
| Facility | 307 (9.0) | 107 (8.2) | 26 (5.1) | |
| Other hospital | 59 (1.7) | 28 (2.2) | 13 (2.6) | |
| Hospice | 9 (0.3) | 5 (0.4) | 5 (1.0) | |
| Unknown | 9 (0.3) | 2 (0.2) | 1 (0.2) | |
NOTE. Bold values indicate statistical significance at the alpha = 5% level.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTP, Child-Turcotte-Pugh; FEN, fluid, electrolytes, nutrition; GFR, glomerular filtration rate; INR, international normalized ratio; IQR, interquartile range; MELD, model for end-stage liver disease.
Note that pre-COVID era dates are January 1 to February 28, early COVID era dates are February 29 to March 25, and late COVID era dates are March 26 to April 15.
References
- 1.Murthy S. JAMA. 2020;323:1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 2.Serper M. Hepatology. 2020 doi: 10.1002/hep.31264. [published online ahead of print April 8, 2020] [DOI] [Google Scholar]
- 3.Mahmud N. Hepatology. 2019;69:2150–2163. doi: 10.1002/hep.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg D. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beste L.A. Gastroenterology. 2015;149:1471–1482.e5. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Dong E. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veterans Health Administration . Department of Veterans Affairs; Washington, DC: 2020. Office of Emergency Management. COVID-19 Response Plan. [Google Scholar]
- 8.Tam C.-CF. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]