Abstract
A novel coronavirus, designated as SARS-CoV-2, first emerged in Wuhan City, Hubei Province, China, in late December 2019. The rapidly increasing number of cases has caused worldwide panic. In this review, we describe some currently applied diagnostic approaches, as well as therapeutics and vaccines, to prevent, treat and control further outbreaks of SARS-CoV-2 infection.
Keywords: SARS-CoV-2, Diagnostic approaches, Vaccines, Therapeutics
In late December 2019, a pneumonia of unknown etiology emerged in Wuhan City, Hubei Province, China. Patients presenting with acute respiratory failure were seen in the ER and then admitted to ICU facilities for further treatment [1]. The causative pathogen was later identified as a novel coronavirus named as 2019 novel coronavirus (2019-nCoV) by World Health Organization (WHO), or SARS-CoV-2 by the Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) on February 12, 2020, or human coronavirus 19 (hCoV-19) by a group of virologists in China [2]. The WHO announced coronavirus disease 19 (COVID-19) as the official name of the disease. Respiratory droplet transmission is the main route of transmission, and human-to-human transmission had been occurring among close contacts since the middle of December 2019 [3]. WHO declared COVID-19 as pandemic on March 11, 2020. As of April 22, 2020, a total of 2,475,723 cases had been confirmed globally, with 169,151 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019).
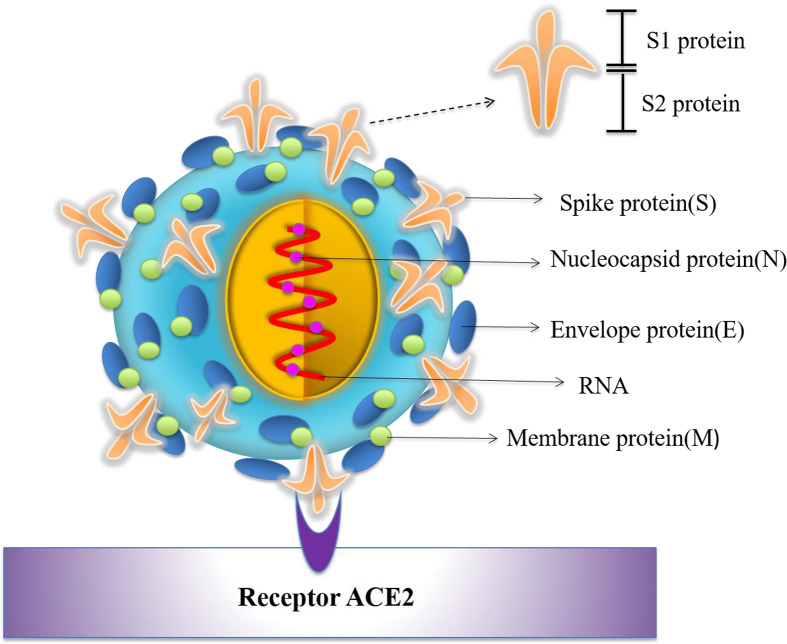
Coronaviruses, which are enveloped non-segmented positive-sense RNA viruses, belong to the family Coronaviridae. They are broadly distributed in humans and other mammals [4]. Previously, six coronavirus species were known to cause human disease, including HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [5]. The first four species caused only mild symptoms, but SARS-CoV and MERS-CoV have caused more than 10 thousand cumulative infections in humans in the past two decades with mortality rates of ∼10% for SARS-CoV and ∼34% for MERS-CoV, respectively [[6], [7], [8]]. SARS-CoV-2 is the seventh number of the family of coronavirus that can infect humans [9]. Although the mortality rate of SARS-CoV-2 is lower than that of MERS-CoV and SARS-CoV, its transmissibility (median R0: 5.7) is much higher than that of either MERS-CoV (R0: <1) or SARS-CoV (R0: 3) [10]. Genome sequencing analysis of clinically isolated samples from patients showed that SARS-CoV-2 shares 88% nucleotide similarity with two bat-derived SARS-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21, but has only around 79% and 50% identity with SARS-CoV and MERS-CoV, respectively [11]. The genome of SARS-CoV-2 and other emerging pathogenic human coronaviruses encodes four major structural proteins, including spike (S), membrane (M), envelope (E) and nucleocapsid (N), as well as sixteen nonstructural proteins (nsp1-16), plus five to eight accessory proteins [12]. Among them, SARS-CoV-2 uses S protein to bind its host cell membrane receptor angiotensin converting enzyme 2 (ACE2) for virus entry (Fig. 1 ) and subsequent pathogenesis [13]. Phylogenetic analysis suggested that bats might be the primary reservoir for SARS-CoV-2 [14], while the intermediate host is still under investigation. The rapid worldwide expansion of SARS-CoV-2 calls for smart POCT diagnostic approaches, safe and effective vaccines, as well as novel therapeutics, to detect, prevent and cure COVID-19 infection. Here, we highlight the safety and effectiveness of vaccines with respect to Dr. Shibo Jiang’s recent appeal that safety be foremost on the minds of vaccine developers as they begin to engage in preclinical and clinical trials to tackle SARS-CoV-2 [15]. Here we briefly summarize current diagnostic approaches and development of vaccines and therapeutics for SARS-CoV-2.
Fig. 1.
Schematic diagram of SARS-CoV-2 structure and ACE2 receptor on the host cell surface.
1. Diagnostic approaches for SARS-CoV-2 detection
Those infected with SARS-CoV-2 may present with such symptoms as fever, dry cough, fatigue, or shortness of breath, with or without nasal congestion, runny nose or other respiratory symptoms [1]. However, patients with mild symptoms may not present any positive signs. Thus, rapid and accurate detection of the causative pathogen is essential in controlling the outbreak among both asymptomatic carriers and individuals showing signs of the disease. However, since SARS-CoV-2 is a newly discovered virus, the diagnostic toolkit is limited.
Up to now, SARS-CoV-2 has been detected from clinical specimens analyzed by electron microscopy, cell culture, real-time reverse-transcription PCR (RT-PCR) and next-generation sequencing [9]. Molecular tests for rapid detection of the causative virus are urgently needed for early identification of infected patients; meanwhile, RT-PCR, which combines the reverse transcription of RNA and amplification of specific cDNA regions, remains the primary means of diagnosing SARS-CoV-2 [16]. Normally, sputum, lower respiratory tract secretions, blood, urine or stool samples are collected form suspected patients. Nasopharyngeal and oropharyngeal swabs are the recommended upper respiratory tract specimen types for SARS-CoV-2 detection [17]. The University of Hong Kong-Shenzhen Hospital enrolled a family of six patients who travelled to Wuhan from Shenzhen between December 29, 2019 and January 4, 2020. Nasopharyngeal or throat swabs were collected. After RNA extraction, an RT-PCR assay was performed to amplify the genes encoding the internal RNA-dependent RNA polymerase (RdRp) and the surface spike (S) protein of SARS-CoV-2. Phylogenetic analysis of the RT-PCR amplicons and two full genomes by next-generation sequencing confirmed that the family was, indeed, infected by SARS-CoV-2 [18]. Prof. Poon’s group developed two 1-step quantitative RT-PCR assays to detect the ORF1b and N genes of SARS-CoV-2. The assays were evaluated using a panel of positive (RNA extracted from cells infected by SARS coronavirus) and negative controls. The detection limits were found to be below 10 copies per reaction, and samples from two SARS-CoV-2 infected patients were positive in the tests [19]. Samples from all collections contained sputum, as well as nose and throat swabs, with or without viral transport medium. RNA extraction and RT-PCR were performed to amplify the RdRp, E and N genes of SARS-CoV-2. The assays were highly sensitive and specific because they did not cross-react with other coronaviruses [20].
Collection of nasopharyngeal or oropharyngeal specimens may cause discomfort and bleeding, especially in patients with thrombocytopenia [18]. This method also requires close contact between healthcare workers and patients, which poses a risk of transmission [17]. Consequently, neither nasopharyngeal nor oropharyngeal specimens are ideal for serial monitoring of viral load. One study used saliva to screen respiratory viruses among hospitalized patients without fever or respiratory symptoms [21]. Prof. Yuen’s group collected saliva from 12 Hong Kong patients with laboratory-confirmed cases of coronavirus infection, and the novel coronavirus could be detected in the saliva specimens of 11 patients (91.7%) by in-house one-step real time RT-qPCR assay targeting the S gene [17], suggesting that saliva could be a practical noninvasive specimen type. Several rapid diagnostic kits for SARS-CoV-2 detection are now commercially available. Among them, one is from the Beijing Genome Institute (BGI). It can detect multiple pathogens using sequencing and microarray technologies, and it has been approved for clinical use [16].
2. Development of vaccines against COVID-19 infection
Vaccine development is the most effective strategy to prevent and eliminate infectious disease. By learning from the vaccine development path of MERS and SARS, several platforms, including DNA, mRNA, recombinant protein, and adenoviral vector, are being investigated. Since S protein and its fragments, such as S1, S2, RBD, and N protein, are prime targets for developing MERS and SARS vaccines, it is expected that similar regions of SARS-CoV-2 could also be considered as critical targets for COVID-19 vaccines [22,23]. Since the genetic sequence of SARS-CoV-2 has been released on 11 January 2020, more than 40 pharmaceutical companies and academic institutions from many countries have engaged in actively developing COVID-19 vaccines, and some candidates have entered efficacy evaluation in animals and clinical trials.
2.1. Nucleic acid vaccines
Several major biotechnology companies have advanced nucleic acid platforms for COVID-19 vaccine development. The Innovation and Value Initiative (IVI), Inovio and the Korea National Institute of Health (KNIH) are collaborating with the Coalition for Epidemic Preparedness Innovations (CEPI) to test the safety and immunogenicity of a DNA vaccine named INO-4800 in Phase 1/2 clinical trial in South Korea [24]. Both Moderna/NIH and CureVac are focusing on mRNA vaccine development, and a safety clinical trial of Moderna’s candidate vaccine mRNA-1273 with a sample size of 45 volunteers was performed in March 2020 [25].
2.2. Subunit vaccines
Subunit vaccines based on recombinant S or S1 protein of SARS-CoV and MERS-CoV have been demonstrated to be efficacious in many studies [[26], [27], [28], [29]]. Clover Biopharmaceuticals is developing a vaccine consisting of a trimerized SARS-CoV-2 S protein using their patented Trimer-Tag technology [30]. The receptor-binding domain (RBD) in SARS-CoV-2 S protein was identified, and it was further demonstrated that SARS-CoV-2 RBD exhibited significantly higher binding affinity to ACE2 receptor compared to binding between SARS-CoV RBD and ACE2 [31], suggesting that the RBD-based SARS-CoV vaccines have the potential to be developed for prevention of SARS-CoV-2 infections. RBD-based vaccines are now under development by several organizations through international collaborations [32]. The pulmonary surfactant-biomimetic nanoparticles used to potentiate heterosubtypic influenza immunity can be used as adjuvant to enhance the immunogenicity of SARS-CoV-2 subunit vaccines [33].
2.3. Inactivated or live-attenuated virus vaccines
Whole inactivated or live-attenuated virus vaccines represent a traditional vaccine strategy. Researchers at the University of Hong Kong have developed a live influenza vaccine that expresses SARS-CoV-2 proteins [34]. Codagenix has developed a “codon deoptimization” technology to attenuate viruses, and the company is exploring COVID-19 vaccine strategies [35].
2.4. Virus vector-based vaccines
Vaccines based on viral vectors offer a high level of protein expression and long-term stability, and induce strong immune responses [36]. Johnson & Johnson is developing an adenovirus-vectored vaccine using AdVac®/PER.C6® vaccine platforms [37]. The first COVID-19 vaccine candidate based on adenovirus-vectored vaccine developed by Chen Wei group entered human clinical testing (NCT04313127) with unprecedented rapidity early on 16 March 2020. Another phase I safety trial of a recombinant adenovirus vaccine candidate (Cansino Biologics Inc., Tianjin, China), Ad5-nCoV, recruited 108 healthy adults in Wuhan, China in March 2020 [38]. Apart from adenovirus vector-based vaccine, two lentivirus vector-based vaccine candidates, COVID-19/aAPC and LV-SMENP-DC have been developed by Shenzhen Geno-Immune Medical Institute. The COVID-19/aAPC vaccine was developed by applying lentivirus modification including the SARS-CoV-2 minigenes and immune modulatory genes, to the artificial antigen presenting cells (aAPCs). The Phase I clinical trial consisting of 100 participants started on February 15, 2020 and the estimated study completion date was December 31, 2024 (NCT04299724). The LV-SMENP DC vaccine was developed by modifying DC with lentivirus vectors expressing SARS-CoV-2 minigene SMENP and immune modulatory genes. The Phase I clinical trial involving 100 patients was conducted on March 24, 2020 and the estimated study completion date was also December 31, 2024 (NCT04276896) (http://clinicaltrials.gov/).
As we all know that adjuvants play a critical role by enhancing immunogenicity of the vaccine candidates and make dose viable in some vaccine platforms. So far, there are at least 10 developers have engaged into developing adjuvanted COVID-19 vaccines. Vaccine developers Dynavax, Seqirus and GlaxoSmithKline have committed to making some liscensed adjuvants including MF59, AS03 and CpG 1018 available for use [36].
No matter which platform we take to develop the COVID-19 vaccines, researchers need to carefully evaluate the effectiveness and safety of the candidate vaccine at each step. In this situation, SARS-CoV-2 –specific animal models seems quite essential. Until now, some different animal models are under developed, including hamsters, ferrets, ACE2-transgenic mice and non-human primates [36].
3. Therapeutic strategies to treat COVID-19
Specific drugs to treat the novel coronavirus will probably take several years to develop and evaluate; however, a range of existing host-directed therapies are under investigation. For example, clinical trials with protease inhibitors (clinical trials.gov: NCT04276688, NCT04255017, and so on) and nucleotide analog remdesivir (clinical trials.gov: NCT04280705, NCT04257656, NCT04252664, and so on) are ongoing in China and the United States [39].
Lopinavir and ritonavir, two licensed HIV protease inhibitors, have been tested in combination for efficacy in 99 COVID-19 patients in China, but the results suggested no difference in the clinical outcome when compared with standard care [40]. Still, one 54-year-old male, the third patient diagnosed with COVID-19 infection in Korea, did show a significant decrease in viral load after lopinavir/ritonavir administration, and no virus titer was observed [41]. The preliminary results of a clinical trial on favipiravir for the treatment of COVID-19 with a total of 80 patients showed that favipiravir had more potent antiviral action than that of lopinavir/ritonavir [42]. Future trials on patients with severe COVID-19 may help to confirm or exclude the efficacy of lopinavir/ritonavir treatment.
Remdesivir, the antiviral agent, was designed for the Ebola virus infection, and it was shown broad-spectrum antiviral activity against several RNA viruses [43]. Remdesivir was shown to be highly effective in the control of SARS-CoV-2 infection in vitro [44]. It was used to treat the first case of COVID-19 infection in the United States, and the patient’s clinical condition improved after only one day [45], indicating the promise of Remdesivir, an anti-SARS-CoV-2 drug. Several clinical trials are ongoing.
It has been demonstrated that SARS-CoV-2 uses the same cell entry receptor ACE2 as SARS-CoV [13]. The use of recombinant ACE2 (rACE2) to neutralize the virus (clinical trials.gov: NCT04287686) is now under clinical investigation [39]. In vitro studies have demonstrated that Vero cells pretreated with chloroquine are refractory to SARS-CoV infection by interruption of the glycosylation process [46]. Chloroquine has been demonstrated to be highly effective in the control of SARS-CoV-2 infection in vitro [44]. Accordingly, chloroquine was first tested in clinical trial by Chinese investigators on more than 100 patients with COVID-19, and it showed a reduction in the duration of symptoms and exacerbation of pneumonia, along with radiological improvement, leading to virus-negative seroconversion [47]. Hydroxychloroquine, a less toxic derivative of chloroquine, was shown to be effective in inhibiting SARS-CoV-2 infection in vitro [48]. However, no confirmed results from a normalized clinical trial cliHydroxychloroquine, along with azithromycin, was tested by French investigators on patients with COVID-19, and it showed that 100% patients with COVID-19 treated with hydroxychloroquine in combination with azithromycin exhibited virological cure on day 6 of the treatment. However, only 57.1% of patients treated with hydroxychloroquine alone have exhibited virologocal cure [49]. However, its use for treatment of COVID-19 outside of the hospital setting or a clinical trial was against by the US FDA due to risk of heart rhythm problems (https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or).
Another interesting strategy is to use convalescent plasma (CP) as treatment, but it should be noted that CP should be collected within two weeks after recovery to ensure a high neutralization antibody titer [50]. It was reported that SARS-CoV-2 isolated from the bronchoalveolar lavage fluid of a severe patient could be neutralized by sera from several other patients [51]. Another preliminary uncontrolled case involved 5 patients with severe COVID-19. After they were administered CP containing neutralizing antibodies (nAb), their clinical status improved [52]. One dose of 200 ml of CP with the nAb titers above 1:640 was transfused to 10 patients with severe COVID-19 as an addition to maximal supportive care and administration of antiviral agents. The clinical symptoms were significantly improved within 3 days, and several parameters were improved compared to pretransfusion, including decreased C-reactive protein and increased lymphocyte counts [53]. Presently, 36 clinical trials are ongoing worldwide (http://clinicaltrials.gov/).
Previous research on MERS-CoV- and SARS-CoV-specific nAbs may provide valuable guidelines for rapid design and development of SARS-CoV-2-specific nAbs. Among the structural proteins of SARS-CoV-2, S fragments, such as S1-NTD, RBD and S2, can be considered as targets for nAb development [12]. Polycloncal human immunoglobulin G (IgG) derived from transgenic cows has been tested successfully for MERS-CoV in animal models [54], and this strategy has been tested for safety in clinical trials (clinical trials.gov: NCT02788188). Because of the high identity of the RBD in SARS-CoV-2 and SARS-CoV, the cross-reactivity of SARS-CoV-specific human monoclonal antibodies was tested on SARS-CoV-2, and it was found that only CR3022 bound potently with SARS-CoV-2 [55], indicating that CR3022 might be a potential therapeutic candidate for treatment of COVID-19 infections. Cocktails consisting of antibodies specific for RBD and other regions in the S protein can be considered to further improve the breadth and efficacy of nAbs against SARS-CoV-2 infection [12].
Studies have also revealed that some coronavirus entry inhibitors have potential to be developed for treatment or prevention of SARS-CoV-2 infection. The peptides derived from the HR2 domain of the spike proteins of SARS-CoV [56], MERS-CoV [6] and SARS-CoV-2 [57,58], have been shown to be effective against the fusion, entry and replication of the corresponding coronavirus. A pan-corovirus fusion inhibitor (EK1) were reported to be highly effective against divergent human coronaviruses, including SARS-CoV, MERS-CoV, HCoV-OC43, HCoV-229E, HCoV-NL-63, and SARS-CoV-2, as well as several bat SARS-related coronaviruses (SARSr-CoVs) [58,59]. A series of lipopeptides derived from EK1, which targeted the HR1 domain, were highly potent in inhibiting entry and infection of divergent human coronaviruses, including SARS-CoV-2. For example, the lipopeptide EK1C4 inhibited SARS-CoV-2 S protein-mediated membrane fusion with IC50 of 1.3 nM [57]. Therefore, these peptides have great potential to be further developed as a therapeutic or prophylactic for treatment or prevention of the current SARS-CoV-2 and MERS-CoV infection and future emerging and reemerging coronavirus infections.
Researchers announced that darunavir, which is a second-generation HIV-1 protease inhibitor, inhibited SARS-CoV-2 infection in vitro and that the inhibition efficiency was 280-fold over that of the untreated group [42]. Another trial (NCT04304053) is looking at the efficacy of a durunavir/cobicistat plus choroquine treatment [60]. Chinese herbal medicines, such as Radix Sophorae and Rhizoma Polygoni Cuspidati, may contain agents against SARS-CoV-2 [42]. The combination of traditional Chinese and Western medicine treatments is also promising. Nonetheless, the efficacy and safety of all these potential candidates in the treatment of COVID-19 need to be confirmed in further preclinical and clinical trials. However, development of safe and effective COVID-19 therapeutics is often hampered by the lack of valid COVID-19 animal models for evaluation their in vivo safety and efficacy [61].
4. Conclusion and perspectives
Here we reviewed recently published information about diagnostic approaches, as well as vaccine and treatment development, for SARS-CoV-2. Quickly identifying a person with SARS-CoV-2 infection is critical to control the continuing spread of the virus. The noninvasive specimen collection strategy has the advantage when collecting clinical specimens. It cannot be overstated that both vaccine development and investigation into potential drugs are subject to further studies to validate safety and efficacy, including, for example, immunization strategies, adjuvant selection, or establishment of animal models. International collaborations, or consortia, will promote COVID-19 and move vaccine development forward. Safety evaluation of candidate vaccines against SARS-CoV-2 is paramount, and this issue is related to the type of vaccines to be selected and immunogens to be designed. Potential therapeutics include lopinavir/ritonavir, remdesivir, chloroquine, hydroxychloroquine, CP and polyclonal/monoclonal antibodies. Again, however, clinical trials are needed for further confirmation of the efficacy and safety of these agents in treating COVID-19.
Declaring of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82041025 and 81630090 to S.J.).
Contributor Information
Lanying Du, Email: ldu@nybc.org.
Shibo Jiang, Email: shibojiang@fudan.edu.cn.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S., Shi Z., Shu Y., Song J., Gao G.F., Tan W. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu L., Liu Q., Zhu Y., Chan K.H., Qin L., Li Y. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Middle East Respiratory Syndrome Coronavirus (MERS-CoV) [(accessed 22 Apr 2020)]; Available online: http://www.who.int/emergencies/mers-cov/en/.
- 9.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579:321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 16.Pang J., Wang M.X., Ang I.Y.H., Tan S.H.X., Lewis R.F., Chen J.I. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To K.K., Tsang O.T., C. Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. pii: ciaa149 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.To K.K.W., Chan K.H., Ho J., Pang P.K.P., Ho D.T.Y., Chang A.C.H. Respiratory virus infection among hospitalized adult patients with or without clinically apparent respiratory infection: a prospective cohort study. Clin Microbiol Infect. 2019;25:1539–1545. doi: 10.1016/j.cmi.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microb Infect. 2020;9:275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inovio Pharmaceuticals . 2020. Inovio collaborating with Beijing advaccine to advance INO-4800 vaccine against new coronavirus in China.http://ir.inovio.com/news-and-media/news/press-release-details/2020/IVI-INOVIO-and-KNIH-to-Partner-with-CEPI-in-Phase-12-Clinical-Trial-of-INOVIOs-COVID-19-DNA-Vaccine-in-South-Korea/default.aspx [Google Scholar]
- 25.Kaiser Permanente Washington Health Research Institute . Kaiser Permanente, Washington Health Research Institute; Seattle: 16 March 2020. Kaiser Permanente launches first coronavirus vaccine trial. [Internet] Retrieved: 23 March 2020. [Google Scholar]
- 26.Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q., Wong G., Lu G., Yan J., Gao G.F. MERS-CoV spike protein: targets for vaccines and therapeutics. Antivir Res. 2016;133:165–177. doi: 10.1016/j.antiviral.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du L., Tai W., Yang Y., Zhao G., Zhu Q., Sun S. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat Commun. 2016;7:13473. doi: 10.1038/ncomms13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clover Biopharmaceuticals . 2020. Clover initiates development of recombinant subunit-trimer vaccine for Wuhan coronavirus (2019-nCoV) [Google Scholar]
- 31.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W.H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020 doi: 10.1007/s40475-020-00201-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Li P., Yu Y., Fu Y., Jiang H., Lu M. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367 doi: 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung E. China coronavirus: Hong Kong researchers have already developed vaccine but need time to test it, expert reveals. South China Morning Post. https://www.scmp.com/news/hong-kong/health-environment/article/3047956/china-coronavirus-hongkong-researchers-have. Accessed 28 Feb 2020.
- 35.Shieber J. Codagenix raises $20 million for a new flu vaccine and other therapies. Tech Crunch. https://techcrunch.com/2020/01/13/codagenix-raises-20-million-for-a-new-flu-vaccine-and-othertherapies/. Accessed 28 Feb 2020.
- 36.Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 37.J&J working on coronavirus vaccine. thepharmaletter; 2020. https://www.thepharmaletter.com/article/j-j-working-on-coronavirusvaccine [Google Scholar]
- 38.IVI, INOVIO, and KNIH to partner with CEPI in phase 1/2 clinical trial of INOVIO’s COVID-19 DNA vaccine in South Korea. 2020. http://ir.inovio.com/news-and-media/news/press-release-details/2020/IVI-INOVIO-and-KNIH-to-Partner-with-CEPI-in-Phase-12-Clinical-Trial-of-INOVIOs-COVID-19-DNA-Vaccine-in-South-Korea/default.aspx [Google Scholar]
- 39.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 43.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luke T., Wu H., Zhao J., Channappanavar R., Coleman C.M., Jiao J.A. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med. 2016;8:326ra21. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 55.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0374-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5:eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrawal S., Goel A.D., Gupta N. Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis. 2020;90(1) doi: 10.4081/monaldi.2020.1289. [DOI] [PubMed] [Google Scholar]
- 61.Yu F., Du L., Ojcius D.M., Pan C., Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microb Infect. 2020;22:74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]