Abstract
Coronavirus disease 2019 (COVID-19) is a novel and highly contagious disease caused by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Older adults and patients with comorbidities and immunosuppressive conditions may experience severe signs and symptoms that can lead to death. This case series assesses the clinical course, imaging features, and outcomes for 12 patients with COVID-19 and a history of kidney transplantation. Patients were evaluated for symptoms, laboratory data, imaging findings, and outcomes from February 2020 to April 2020. Fever, cough, and dyspnea were the most common clinical symptoms, noted in 75% (nine/12), 75% (nine/12), and 41.7% (five/12) of the patients, respectively. Most of the patients had a normal white blood cell count, while 33.3% (four/12) had leukopenia and 8.3% (one/12) had leukocytosis. A combination of consolidation and ground glass opacity was the most predominant (75%) pattern of lung involvement on computed tomography (CT). Eight patients died of severe COVID-19 pneumonia and acute respiratory distress syndrome and four were discharged. All recovered cases had a unilateral peripheral pattern of involvement limited to only one zone on initial chest CT. It seems that CT imaging has an important role in predicting COVID-19 outcomes for solid organ transplant recipients. Future studies with long-term follow up and more cases are needed to elucidate COVID-19 diagnosis, outcome, and management strategies for these patients.
Keywords: Disease attributes, COVID-19, Computed tomography, Immunocompromised, Kidney transplantation
1. Case series
1.1. Background
On December 31, 2019, a novel corona virus was extracted from the respiratory secretions of several patients presenting with lower respiratory tract infection of unknown origin in Wuhan, China [1]. While the mortality rate of coronavirus disease 2019 (COVID-19) is believed to be approximately 5% [2], older adults and those with an underlying chronic disease are specifically at high risk of presenting with severe symptoms, having poorer prognosis, and even developing fatal conditions [3]. Use of immunosuppressive medications such as steroids may also be linked to severe manifestation of the disease, as in patients with previous respiratory viral infections such as H1N1 [4].
Solid organ transplant recipients are on long-term immunosuppressive regimens and are at particular risk of contracting severe respiratory tract infection and possibly with atypical presentation [5]. There are currently limited data on the clinical course, imaging features, and outcomes for COVID-19 in renal transplant recipients. Although some studies noted no other outstanding severe disease among immunosuppressed patients with COVID-19 [6], other studies revealed that immunocompromised patients may have an impaired immune response and high levels of viral load [7].
Here we report clinical and imaging findings for COVID-19 and outcomes in a group of patients with a functioning renal transplant under an immunosuppressive regimen.
1.2. Cases
The study cases consisted of 12 patients with a functioning renal transplant who were admitted to our tertiary hospital with a confirmed diagnosis of COVID-19 between February 25, 2020 and April 12, 2020.
All subjects met the following inclusion criteria: (1) symptoms suggestive of COVID-19 pneumonia (ie, fever, cough, dyspnea, sore throat, myalgia, headache, nausea, or abdominal pain); (2) positive SARS-CoV-2 nasopharyngeal sample (polymerase chain reaction–based test); (3) chest computed tomography (CT) scan suggestive of COVID-19; and (4) lymphocytopenia (absolute lymphocyte count <1000) or dyspnea or O2 saturation <93% or respiratory rate >30/min.
Nine patients were male (75%) and three were female (25%). The mean age (± standard deviation) of the patients was 47.66 ± 1.35 (range 29–66) yr. The most common symptoms were fever, cough, and dyspnea, noted in 75% (nine/12), 75% (nine/12), and 41.7% (five/12), respectively. On admission, all patients were on standard triple immunosuppressive therapy (steroid, calcineurin inhibitor/sirolimus, mycophenolate mofetil/azathioprine). The medical history of the patients was reviewed for any chronic conditions other than chronic renal failure. Table 1 shows detailed clinical characteristics of the cases. Leukopenia was observed in 33.3% (four/12) and leukocytosis in 8.3% (one/12). C-reactive protein was elevated in 83.3% (10/12) and creatine phosphokinase in 55% (five/nine). On admission, mean blood urea nitrogen was 82.9 ± 55.2 mg/dl and creatinine was 2.30 ± 1.09 mg/dl. Graft function on admission in terms of estimated glomerular filtration rate based on the Modification of Diet in Renal Disease equation was 39.9 ± 24.5 cm3/min. Table 2 lists detailed laboratory findings.
Table 1.
Demographic and clinical features and outcomes for the 12 patients.
| Pt | Gender | Age (yr) | Age of KTx (yr) | CMBs | Fever | Cough | HA | Myalgia | Dyspnea | GIS | SO2 (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 29 | 8 | None | + | + | – | – | – | – | 88 | Discharged |
| 2 | Male | 32 | 12 | HTN | + | + | – | + | + | + | 85 | Discharged |
| 3 | Male | 58 | 14 | None | – | – | – | – | – | – | 88 | Death |
| 4 | Male | 38 | 15 | None | – | + | – | + | + | – | 84 | Death |
| 5 | Male | 54 | 18 | Asthma + SA | + | + | – | – | – | – | 90 | Death |
| 6 | Male | 46 | 3 | None | + | + | – | – | + | – | 85 | Discharged |
| 7 | Male | 66 | 4 | None | + | – | – | – | – | – | 85 | Death |
| 8 | Male | 32 | 17 | None | + | + | – | – | + | – | 84 | Death |
| 9 | Male | 64 | 6 | None | + | + | – | – | + | – | 90 | Death |
| 10 | Male | 64 | 3 | None | + | + | – | – | – | – | 84 | Death |
| 11 | Female | 49 | 17 | HTN | – | + | + | – | – | – | 90 | Discharged |
| 12 | Female | 40 | 16 | None | + | – | – | – | – | – | 88 | Death |
KTx = kidney transplantation; CMBs = comorbidities; HTN = hypertension; SA = stable angina; HA = headache; GIS = gastrointestinal symptoms (abdominal pain/diarrhea); SO2 = oxygen saturation on room air.
Table 2.
Baseline laboratory findings.
| Patient |
Summary | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | (mean ± SD) | |
| White blood cell count | 2000 | 5500 | 3800 | 5600 | 12 200 | 8700 | 7200 | 8800 | 4200 | 3100 | 7500 | 2500 | 5925 ± 3050 |
| Neutrophil count | 1040 | 4345 | 2470 | 4984 | 11 590 | 6264 | 5976 | 7920 | 3318 | 2697 | 6000 | 2050 | 4887.83 ± 2934.14 |
| Lymphocyte count | 840 | 1100 | 1140 | 560 | 610 | 2001 | 1152 | 704 | 798 | 279 | 1200 | 375 | 896.58 ± 464.81 |
| Eosinophils (%) | 3 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 2 | 1 | – |
| Hemoglobin (g/dl) | 9.1 | 11.7 | 15.4 | 11 | 9.9 | 12.4 | 9.1 | 11.7 | 13.6 | 11.1 | 15.2 | 8.5 | 11.55 ± 2.28 |
| Platelets (×103) | 131 | 485 | 123 | 148 | 263 | 465 | 160 | 171 | 107 | 54 | 161 | 98 | 197 ± 139 |
| C-reactive protein (mg/l) | 17 | 30 | 5 | 61 | 40 | 66 | 24 | 49 | 3 | 31 | 23 | 30 | 31.58 ± 19.76 |
| Lactate dehydrogenase (U/l) | 305 | 384 | 412 | 110 | 506 | 80 | 326 | 641 | 592 | 931 | 898 | 928 | 509.41 ± 297.36 |
| Creatinine (mg/dl) | 2.99 | 0.92 | 1.53 | 2.55 | 2.96 | 1.77 | 2.50 | 4.60 | 1.23 | 1.50 | 1.51 | 3.60 | 2.3 ± 1.09 |
| Blood urea nitrogen (mg/dl) | 85 | 23 | 33 | 119 | 156 | 60 | 145 | 150 | 30 | 39 | 18 | 137 | 82.91 ± 55.16 |
| Aspartate transaminase (U/l) | 19 | 17 | 25 | 24 | 19 | 26 | 12 | 28 | 37 | 167 | 31 | 12 | 34.75 ± 42.3 |
| Alanine transaminase (U/l) | 6 | 11 | 17 | 8 | 19 | 42 | 14 | 14 | 19 | 96 | 43 | 8 | 24.75 ± 25.52 |
| Alkaline phosphatase (U/l) | 92 | 118 | 203 | 205 | 128 | 106 | 90 | 102 | 148 | 204 | 237 | 134 | 147.25 ± 51.52 |
| Albumin (g/l) | 3.1 | 4 | 3 | 4.5 | 3.1 | 3 | 3.6 | 3.2 | 3.4 | 2.7 | 3.3 | 3.9 | 3.4 ± 0.51 |
| Na (mEq/l) | 138 | 139 | 138 | 137 | 141 | 143 | 141 | 133 | 137 | 133 | 142 | 140 | 138.5 ± 3.2 |
| K (mEq/l) | 4.5 | 3.5 | 4.1 | 3.6 | 3.4 | 5 | 5 | 4.1 | 4 | 4.4 | 3.7 | 3.4 | 4.05 ± 0.57 |
| Prothrombin time (s) | 10.5 | 10.6 | 9.8 | 11.6 | 13.3 | 10 | 11.7 | 11.1 | 9.8 | 14.8 | 9.8 | 9.9 | 11.07 ± 1.58 |
| Partial thromboplastin time (s) | 20 | 20 | 20 | 22 | 27 | 20 | 23 | 69 | 20 | 37 | 26 | 20 | 27.05 ± 14.18 |
| International normalized ratio | 1.01 | 1.06 | 0.9 | 1.13 | 1.3 | 1.1 | 1.14 | 1.07 | 0.94 | 1.4 | 0.9 | 0.95 | 1.07 ± 0.15 |
SD = standard deviation.
On admission, the oral steroid was changed to intravenous steroid administration. The immunosuppressive dose was reduced according to the protocol in our center under consultation with multidisciplinary team comprising a nephrologist, urologist, and infectious disease specialist. Hydroxychloroquine 400 mg stat, Kaletra (lopinavir/ritonavir) 400/100 mg twice daily, and suitable intravenous antibiotics were initiated for all patients. Intravenous immunoglobulin 1–2 g/kg in segregated doses over 5 d was administered in the case of hypoxemia and a creatinine rise with clinical suspicion of kidney transplant rejection.
As a part of our national COVID-19 guidelines, all patients underwent noncontrast chest CT imaging using a low-dose protocol. Two expert radiologists with 9 and 18 yr of experience interpreted the images independently. In the case of disagreement between the readings, the two radiologists reassessed the images in order to reach consensus. The laterality of the disease (unilateral vs bilateral), the distribution (peripheral vs central, anterior vs posterior), and the predominant zonal involvement (upper, middle, lower, or diffuse) were recorded. The predominant pattern of involvement in each lobe was assessed and categorized as ground-glass opacity (GGO) or consolidation. When there was a combination of GGO and consolidation, the allocation was divided between them accordingly. The percentage of lobar involvement was scored using the following system: 0, no involvement; 1, <25%; 2, 26–50%; 3, 51–75%; and 4, >75% involvement [8]. The scores for each lobe were summed to calculate the total lung score (maximum score 20). The final score was multiplied by five to estimate the percentage lung involvement. The presence of other imaging features was also assessed on CT scan images, including interlobular septal thickening, crazy-paving pattern, reverse halo sign, cystic changes, presence of cavitations, lymphadenopathy (defined as lymph node with short axis >10 mm), and pleural and pericardial effusion.
The initial CT scan on admission showed bilateral lung involvement in eight patients and unilateral involvement in four. The lower lobes were frequently involved, as observed in 11/12 of patients (Table 3 ). A combination of consolidation and GGO was the most predominant (75%) pattern, followed by GGO only (25%; Fig. 1 ). In terms of lesion distribution, a combination of peripheral plus central involvement was the most common. The mean lung involvement score was 9.5 ± 5.5 out of 20, which gives a mean estimate of 47.5 ± 27.8% for total lung involvement (Table 4 ).
Table 3.
Frequency of chest computed tomography scan features in the 12 patients.
| Parameter | Patients, n (%) | |
|---|---|---|
| Lung involvement | Bilateral | 8 (66.7) |
| Unilateral | 4 (33.3) | |
| Lobar anatomy | Right upper lobe | 9 (75) |
| Right middle lobe | 10 (83.3) | |
| Right lower lobe | 11 (91.7) | |
| Left upper lobe | 9 (75) | |
| Left lower lobe | 11 (91.7) | |
| Zonal anatomy | Upper | 1 (8.3) |
| Middle | 3 (25) | |
| Lower | 3 (25) | |
| Diffuse | 5 (41.7) | |
| Axial distribution | Peripheral | 4 (33.3) |
| Peripheral + central | 8 (66.7) | |
| Segmental distribution | Posterior | 8 (66.7) |
| Anterior | 2 (16.7) | |
| Diffuse | 2 (16.7) | |
| Computed tomography features | Ground glass opacity | 12 (100) |
| Consolidation | 9 (75) | |
| Interlobular septal thickening | 5 (41.7) | |
| Dilated small vessels in the lesion | 9 (75) | |
| Crazy-paving | 2 (16.7) | |
| Pleural effusion | 2 (16.7) | |
| Pericardial effusion | 1 (8.3) | |
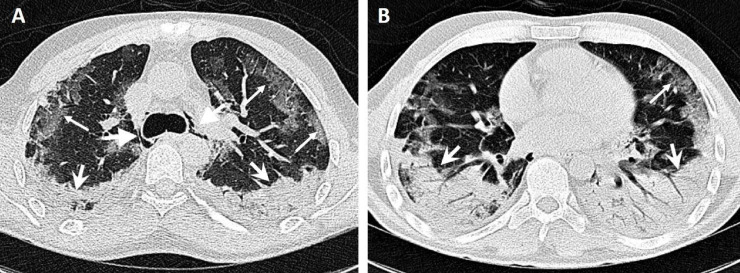
Fig. 1.
A 38-yr-old male patient presented with a dry cough and dyspnea and a history of kidney transplantation 15 yr previously. Computed tomography images show bilateral peripheral ground glass opacity (thin arrows) on the anterior side and an area of bilateral consolidation with air bronchogram (wide arrows) on the posterior side, predominantly in the lower lobes. Incidental pneumomediastinum is noted (thick-headed arrows). The patient died after 22 d in hospital.
Table 4.
Chest computed tomography scores for each lobe and total lungs.
| Lobar anatomy | Ground glass opacity | Consolidation | Total score |
|---|---|---|---|
| Right upper lobe | 1.5 | 0.16 | 1.66 |
| Right middle lobe | 1.66 | 0 | 1.66 |
| Right lower lobe | 1.41 | 0.91 | 2.32 |
| Left upper lobe | 1.41 | 0.08 | 1.49 |
| Left lower lobe | 1.58 | 0.75 | 2.33 |
| Total lungs | 7.58 | 1.9 | 9.46 |
We did not observe cavitation, cystic changes, or lymphadenopathy in any of the study population. Three patients had pleural or pericardial effusion or both. Four patients had a mildly elevated cardiothoracic ratio on chest CT. Pneumomediastinum was observed in two patients who had severe changes on chest CT, but neither was intubated or underwent noninvasive ventilation (NIV) before acquisition of the CT scan.
Follow-up of the patients was performed to the study endpoints, which were death or complete recovery and discharge. Follow-up chest CT was carried out for three patients, while a portable chest X-ray was used for follow-up for the remaining nine. Follow-up imaging revealed progressive radiologic changes such as bilateral areas of air space opacity (Fig. 2 ).
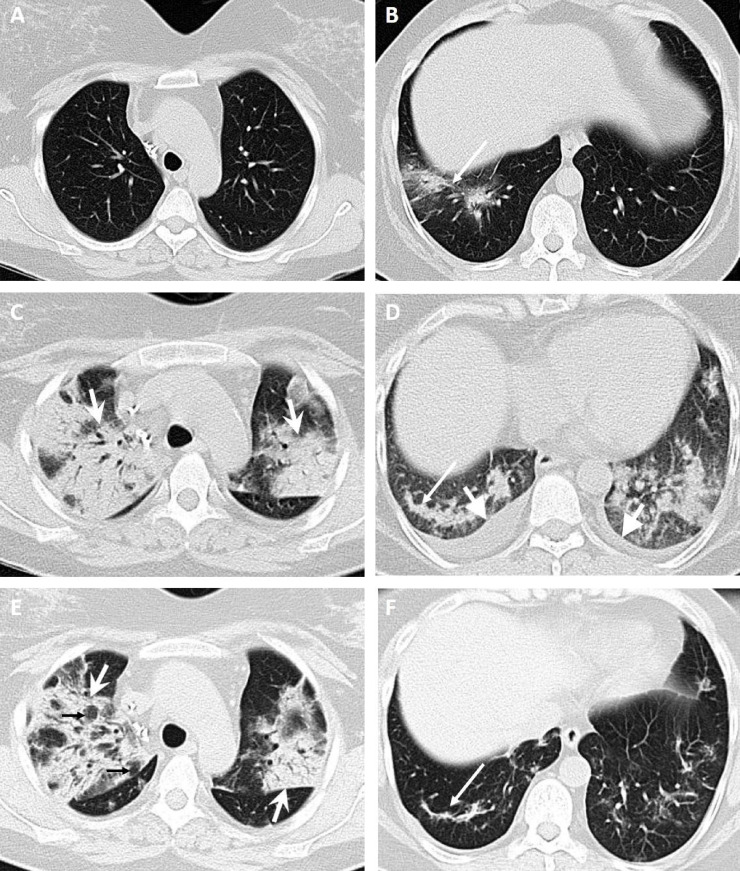
Fig. 2.
A 49-yr-old woman presented with a dry cough, sore throat, headache, and a history of kidney transplantation 17 yr previously. (A,B) Computed tomography (CT) images obtained 3 d after the onset of symptoms show patchy ground-glass opacity in the right inferior lobe with slight central consolidation (long arrows). (C,D) CT images obtained 25 d after the onset of symptoms with secondary superimposed bacterial pneumonia show diffuse bilateral ground-glass opacity and consolidation with air bronchogram (wide arrows) predominantly in the upper lobes and bilateral mild plural effusion (thick-headed arrows). (E,F) CT images obtained 28 d after the onset of symptoms and intravenous antibiotic therapy show evolution of the area of consolidation with vacuolar signs (small black arrows) in the right upper lobe and fibrotic bands (long arrow) in the right lower lobe. The patient was discharged after 37 d.
Of the 12 patients, ten were admitted to an intensive care unit, nine were intubated, eight died of severe COVID-19 pneumonia and acute respiratory distress syndrome (ARDS), and four were discharged after complete recovery. Three patients required neither NIV nor intubation. The median hospital stay was 15 d (interquartile range [IQR] 8.0–21.5), with a longer stay for patients who died (18.0 d, IQR 12.3–21.5) than for those who were discharged (7.0 d, IQR 6.0–28.3), but the difference was not statistically significant.
2. Discussion
Owing to long-term immunosuppressive therapy, kidney transplant recipients are at higher risk of COVID-19 involvement in comparison to immunocompetent individuals. However, a few studies with small sample sizes have reported conflicting results for the characteristics of COVID-19 in these patients. In solid organ transplant recipients, the clinical presentation, imaging findings, laboratory data, and outcomes may differ from those for other adults and vary among patients.
On initial presentation, just one of our 12 cases had gastrointestinal symptoms; the most typical presentation was cough and fever, as for other adults [9], [10]. Guillen et al [11] reported on a patient with a history of third kidney transplant who presented with vomiting and fever as a first symptom and was finally diagnosed with COVID-19 on follow-up.
Laboratory findings revealed that although the majority of COVID-19 patients in the general population have leukopenia and lymphocytopenia (70%) [12], normal white blood cell count was a more frequent finding in our series and leukopenia was detected in one-quarter of our subjects. On admission, lymphocytopenia was detected in 58.33% of our patients, whereas all cases had lymphocytopenia in another study [13]. In contrast to our results, Fishman and Grossi [14] reported that leukopenia and lymphopenia were the prevalent finding in immunocompromised transplant recipients.
According to CT images, the most common pattern of lung involvement was bilateral involvement with a diffuse pattern and a posterior segmental distribution. GGO, a feature highly suggestive of COVID-19, was observed in all cases and consolidation in the majority of cases. A crazy-paving pattern was observed in two patients, which is consistent with late-phase COVID-19. One-third of our cases had unilateral involvement; in other studies, one case had bilateral GGO [15] and one of five cases described by Zhang et al [13] had unilateral involvement.
All the initial CT scan findings were compatible with a normal immune function except for unilateral involvement and consolidation, which were slightly more frequent in our series than in a previous multicenter study of 101 COVID-19 patients [16]. Most patients in the later stages showed subpleural involvement as a less common finding. As the disease progresses, other uncommon findings include pleural/pericardial effusion, lymphadenopathy, cavitation, halo sign, and pneumothorax. The most common imaging features in severely ill patients were bilateral multilobar involvement and subsegmental consolidation [17].
Our study revealed that interlobular septal thickening, multilobar patterns, consolidative lesions, and a high score for lung involvement were more frequent among the patients with poor outcome and complicated cases with ARDS. In addition, all cases with pleural and pericardial effusion or a crazy-paving appearance had poor outcome. Interestingly, two cases had pneumomediastinum as a rare finding on chest CT at the time of presentation, without any history of intubation or other predisposing procedures. Patients who survived had a shorter hospital stay and a unilateral peripheral pattern limited to only one zone of the lung, with none of the other findings mentioned for patients with poor outcome.
In conclusion, CT imaging features may have an important role in predicting COVID-19 outcomes for kidney transplant recipients. However, the majority of the findings are similar to those from other adult studies for the general population.
Conflicts of interest: The authors have nothing to disclose.
Associate Editor: James Catto
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.04.064.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordero E., Aydillo T., Farinas M. Immunosuppressed patients with pandemic influenza A 2009 (H1N1) virus infection. Eur J Clin Microbiol Infect Dis. 2012;31:547–556. doi: 10.1007/s10096-011-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godbole G., Gant V. Respiratory tract infections in the immunocompromised. Curr Opin Pulmon Med. 2013;19:244–250. doi: 10.1097/MCP.0b013e32835f82a9. [DOI] [PubMed] [Google Scholar]
- 6.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. In press. 10.1002/lt.25756. [DOI] [PMC free article] [PubMed]
- 7.Chen Y, Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect Dis. In press. 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed]
- 8.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. In press. 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed]
- 9.Kolifarhood G., Aghaali M., Saadati H.M. Epidemiological and clinical aspects of COVID-19; a narrative review. Arch Acad Emerg Med. 2020;8:e41. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Li X, Cao G, Wu X, Wang Z, Yan T. COVID-19 in a kidney transplant patient. Eur Urol. In press. 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed]
- 11.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transpl. In press. 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed]
- 12.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. In press. 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed]
- 14.Fishman JA, Grossi PA. Novel coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #flatteningthecurve. Am J Transpl. In press. 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed]
- 15.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transpl. In press. 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed]
- 16.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 17.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. In press. 10.2214/AJR.20.23034. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.