Highlights
-
•
Myasthenic crises is a potentially severe complication of COVID-19.
-
•
Hydroxychloroquine can aggravate myasthenia crises.
-
•
IVIg is a potential treatment for both Myasthenic crises and COVID-19.
-
•
IVIg treatment may cause thrombosis in susceptible patients.
Keywords: Viral infection, Myasthenia, COVID-19
Dear Editor,
The neurotropic potential and the neurological complications of coronavirus disease of 2019 (COVID-19) are still being uncovered. Those documented so far include headaches, acute cerebrovascular incidents, anosmia, post-infectious disseminated and brainstem encephalitis, viral meningitis [1] and Guillain-Barré Syndrome [2]. Myasthenia gravis (MG) patients are particularly susceptible to infections causing crises due to the combination of reduced neuromuscular safety factors adversely affected by pyrexia and the effect of acute inflammatory mediators compounded by immunosuppression in many patients [3,4]. Acute respiratory distress (ARDS) seen in COVID-19 coupled with respiratory muscle failure seen in MG crises may result in a dire prognosis. We present a case of a 56-year-old woman who developed MG crisis and concomitant COVID-19.
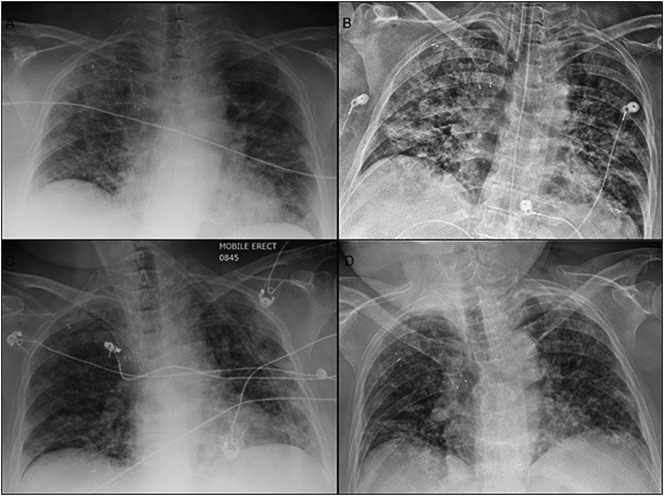
The patient is a 56-year-old woman with a history of acetylcholine receptor antibody (AChR-Ab) positive MG for over five years maintained on pyridostigmine 60 mg four times daily, prednisone 20 mg twice a day and intravenous immunoglobulin (IVIG) infusions (650 mg/kg for two days every two weeks). The patient had refused thymectomy. She also took hydroxychloroquine 200 mg twice daily for mixed connective tissue disease. Her exacerbations typically consisted of weakness of her lower extremities, ptosis and dysphagia but no history of mechanical ventilator support. She presented to the emergency department with dyspnea, fevers, rhinorrhea and diffuse myalgias. She had 70% oxygen saturation, was placed on 15 L of oxygen on non-rebreather but continued to deteriorate requiring mechanical ventilation. Initial chest x-ray showed bi-basilar infiltrates compatible with pneumonia (Fig. 1A). She was started on vancomycin, cefepime, azithromycin and prednisone was increased to 40 mg twice daily. Influenza A and B testing was negative but COVID-19 (confirmed by CDC COVID-19 real-time RT PCR on Roche cyclers) was reported positive on day 2 of admission. Antibiotics were stopped and hydroxychloroquine, 200 mg daily was resumed. On Day 3, neurology recommended restarting pyridostigmine and IVIG 400 mg/kg for five days. Neurological examination prior to IVIG was significant for motor strength of 4/5 in the proximal upper and lower extremities, intact cranial nerves with speech and swallowing function difficult to assess due to intubation. Worsening respiratory status and bilateral pulmonary infiltrates required prone positioning for 16 h on days 6 and 7 (Fig. 1B). Patient showed improvement on ventilator settings after her fifth IVIG dose on day 8 and continued to improve over the course of treatment. Patient was extubated and placed on CPAP on day 13 and 4 L Oxygen by nasal cannula (NC) on day 15 with chest x-ray showing improved bilateral infiltrates (Fig. 1C). However, due to worsening proximal weakness of upper and lower extremities (2/5), she received another course of IVIG 650 mg/kg two days in a row. Repeat COVID-19 testing was positive on Day 19. By day 25, patient was able to stand, walk 4–5 steps and required 3 L oxygen by NC with chest x-ray remaining stable (Fig. 1D). Patient was awaiting placement for subacute rehab facility.
Fig. 1.
Sequential chest radiographs of patient with COVID-19 and myasthenic crises.
Chest radiographs of bilateral pneumonia progression on days 1(A) and 6(B) and improvement on days 15(C) and 25(D).
We present this first known case in the literature of MG crises with simultaneous COVID-19. Recent guidelines for the management of MG during the COVID-19 pandemic suggest individualized therapy, however, MG crises has not been addressed as a potential complication of COVID-19 [4]. While coronavirus infections have not been documented as a cause of MG crisis, viral infections in general have been reported to trigger autoimmunity through augmentation of T cell signaling causing a pro-inflammatory environment due to a hyper-reactive antiviral immune response, epitope spreading and due to the effects of fever on neuromuscular junction function [5]. A shared component between the immunopathogenesis of COVID-19 and MG crises is cytokine dysregulation which promotes the increase of pro-inflammatory cytokines and chemokines that attack organ systems, particularly the lungs which can result in ARDS [5,6].
IVIG uses pooled normal IgG that works through numerous mechanisms, which include: blocking both cytokine production and pathologically activated differentiation of Th1, Th17 and Tfh subsets, overwhelming the neonatal Fc receptor which in turn causes reduction in endogenous and exogenous IgG leading to reduction of AChR antibodies, neutralization of autoantibodies by anti-idiotypic antibodies and inhibition of complement activation [7]. The potential anti-viral and immune-modulating actions of hydroxychloroquine and IVIG against COVID-19 are currently under investigation with mixed findings [8]. Hydroxychloroquine is also reported to worsen MG [3]. In our patient, the combined use of hydroxychloroquine and azithromycin, a macrolide that aggravates MG, may have caused the worsening of MG, requiring additional doses of IVIG [5]. Additionally, a potential complication of IVIG is thrombosis and widespread thrombosis has also been reported in critically ill COVID-19 patients [9,10]. Therefore, careful administration of IVIG is required in MG patients with concomitant COVID-19.
COVID-19 in patients with MG, particularly those who are already immunosuppressed, raises several therapeutic dilemmas, including side effects of MG treatments like IVIG and plasma exchange, as well as side effects of many medications in patients with MG. Ongoing registries of patients with COVID-19 and MG should prove helpful.
Study sponsorship
None.
Disclosures
Dr. Delly reports no disclosures.
Dr. Syed reports no disclosures.
Dr. Lisak participated as a speaker in meetings sponsored by and received consulting fees and/or grant support from: Alexion, Argenx, Ra Pharmaceuticals, Novartis, Mallinckdrot, Catalyst, Teva Pharmaceuticals, Genentech, Chugai, Medimmune, GLG Consulting, Alpha Sites Consulting, Schlesinger Group Consulting, Slingshot Consulting, Health Sources, Adivo Associates, and Smart Analyst. He has also received funding from the NIH and National MS Society.
Dr. Zutshi reports no disclosures.
References
- 1.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94 doi: 10.1212/WNL.0000000000009455. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., Vigano M., Giovannelli G., Pirro F., Montisano D.A., Appollonio I., Ferrarese C. Guillain-Barre syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(4):741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidon A.C., Amato A.A. COVID-19 and neuromuscular disorders. Neurology. 2020;94 doi: 10.1212/WNL.0000000000009566. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.M.G.C.-W.G. International, Jacob S., Muppidi S., Guidon A., Guptill J., Hehir M., Howard J.F., Jr., Illa I., Mantegazza R., Murai H., Utsugisawa K., Vissing J., Wiendl H., Nowak R.J. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J. Neurol. Sci. 2020;412:116803. doi: 10.1016/j.jns.2020.116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilhus N.E., Romi F., Hong Y., Skeie G.O. Myasthenia gravis and infectious disease. J. Neurol. 2018;265(6):1251–1258. doi: 10.1007/s00415-018-8751-9. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.Y., Park K.D., Richman D.P. Treatment of myasthenia gravis based on its immunopathogenesis. J. Clin. Neurol. 2011;7(4):173–183. doi: 10.3988/jcn.2011.7.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020;1591:30589–30594. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marie I., Maurey G., Herve F., Hellot M.F., Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br. J. Dermatol. 2006;155(4):714–721. doi: 10.1111/j.1365-2133.2006.07390.x. [DOI] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;3848:30120–30121. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]