The SARS-CoV-2 virus has infected more than 1.8 million people across 213 countries and killed more than 110 000 [1]. Emerging reports across countries indicate higher COVID-19 mortality among men compared to women, but the underlying reasons remain unclear [2]. The extent to which this disparity is due to biological rather than behavioral or comorbidity sex differences is unknown. The SARS-CoV-2 receptor ACE2 and the entry-associated serine protease TMPRSS2 are expressed in lung and other tissues implicated in the clinical manifestations of COVID-19. However, less is known about the exact cell types expressing ACE2 and TMPRSS2 that serve as cells of entry and pathogenesis for SARS-CoV-2 [3].
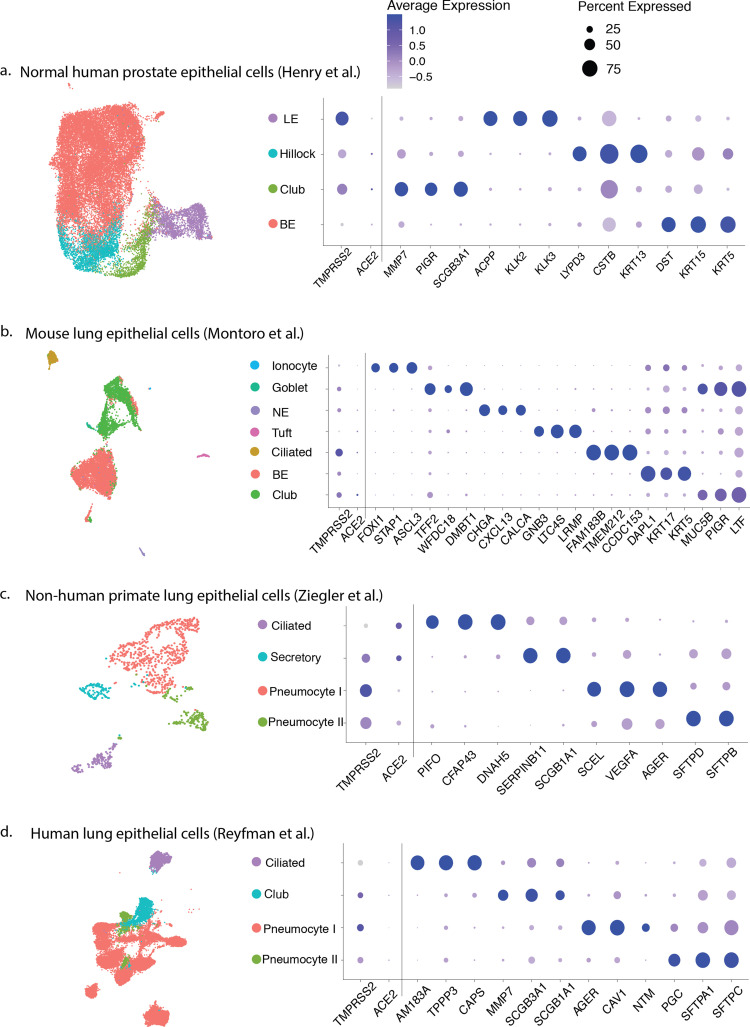
Intriguingly, TMPRSS2, one of the most dysregulated genes in prostate cancer, is highly expressed in human prostate epithelial cells and is androgen-responsive [4]. Given high TMPRSS2 expression in the prostate, we investigated whether TMPRSS2 and ACE2 are co-expressed in human prostate epithelial cells.[5] Using publicly available single-cell RNA sequencing data, we analyzed 24 519 epithelial cells from a normal human prostate data set [5]. In this data set (Supplementary material), 0.32% of all epithelial cells (78 of 24 519) expressed ACE2 and 18.65% expressed TMPRSS2 (4573 of 24 519). Overall, the prostate cell types co-expressing ACE2 and TMPRSS2 were hillock and club cells that were originally identified in lung. We identified 0.61% of club cells and 0.40% of hillock cells that were double-positive (Fig. 1 ; Supplementary Fig. 1).
Fig. 1.
Cell type distribution and top differentially expressed gene marker expression of the four datasets used in the current study. (A) Normal human prostate epithelial cells. (B) Mouse lung epithelial cells. (C) Non-human primate lung epithelial cells. (D) Human lung epithelial cells. Each data set was reclustered and annotated by cell type, with distribution shown in the uniform manifold approximation and projection. For each data set, a dot plot was generated showing the percentage of expression (marker radius) and the average expression level (color gradient) for the most differentially expressed genes in each cell type, as well as ACE2 and TMPRSS2. BE = basal epithelial; LE = luminal epithelial.
We then investigated lung single-cell data sets (one non-human primate, one human, and one mouse) to determine whether lung club cells also co-express ACE2 and TMPRSS2 (Supplementary Table 1). Double-positive cells were found in 16.07% (18 of 112) of non-human primate lung secretory cells in data set 1, 0.33% (7 of 2113) of human lung club cells in data set 2, and 1.86% (48 of 2578) of mouse lung club cells in data set 3 (Supplementary Fig. 1).
To test for sex differences in the expression of these genes, we compared TMPRSS2 and ACE2 expression in lung epithelial cell types [6]. Overall, there was no significant difference in TMPRSS2 expression between males and females in human lung, but higher ACE2 expression in males (log 2 normalized expression level 0.02 vs 0.0065 in females; p < 0.001; Supplementary Fig. 2A). Examining the cell types expressing ACE2 and TMPRSS2, we found that pneumocytes I/II in males compared to females had a higher proportion of cells with expression (Supplementary Fig. 2C). However, we caution against the generalizability of these findings due to the confounding variables that may modulate ACE2 or TMPRSS2 expression such as smoking and age. It is not clear if TMPRSS2 and ACE2 expression is regulated by the same process, but their expression levels are positively correlated in lung cell lines (Supplementary Fig. 3).
In summary, we found a small percentage of prostate hillock and club cells that co-express TMPRSS2 and ACE2. Whether differences in TMPRSS2 and ACE2 expression mediate SARS-CoV-2 pathogenesis and whether androgen signaling can affect COVID-19 disease remain to be studied; sex differences in TMPRSS2 expression alone may not drive the higher burden of SARS-CoV-2 disease among men. Further research into TMPRSS2 expression and its modulation within the lung and other relevant cell types that may impact ACE2 and SARS-CoV-2 pathogenesis is needed.
Conflicts of interest: The authors have nothing to disclose.
Acknowledgments: This work was funded by the Department of Defense through grant W81XWH-17-PCRP-HD (F.W.H., H.S.), National Institutes of Health/National Cancer Institute grants P20 CA233255-01 (F.W.H., H.S) and U19 CA214253 (F.W.H., H.S), and the Prostate Cancer Foundation (F.W.H.).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.04.065.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic. www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Purdie A, Hawkes S, Buse K, et al. Sex, gender and COVID-19: disaggregated data and health disparities. BMJ Global Health blog. https://blogs.bmj.com/bmjgh/2020/03/24/sex-gender-and-covid-19-disaggregated-data-and-health-disparities/.
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. In press. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 4.Tomlins S.A., Rhodes D.R., Perner S. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 5.Henry G.H., Malewska A., Joseph D.B. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Reports. 2018;25:3530–3542. doi: 10.1016/j.celrep.2018.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyfman P.A., Walter J.M., Joshi N. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.