Abstract
While the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) spreads all over the world, the healthcare systems are facing the dramatic challenge of simultaneously fight against the outbreak and life-threating emergencies.
In this biological setting, emergency departments and neurovascular teams are exposed to high risk of infection and should therefore be prepared to deal with neurological emergencies safely.
The purpose of this article is to analyze the current evidence on COVID-19 in the context of acute ischemic stroke and to describe the model of behavior we are putting into action to maintain the stroke pathway both rapid for the patient and safe for the healthcare professionals.
We reserve a specific focus on personal protection equipment, dress code and healthcare professional behavior.
Key Words: Acute stroke therapy, Ischaemic stroke, Stroke, Thrombolysis, Intervention, Stroke teams, Pandemic, Coronavirus
Background
On March 11 2020, the World Health Organization (WHO) defined COVID-19 as a pandemic.1 On April 02, 896,450 confirmed cases in 204 countries were reported.2
Coronaviruses are not-segmented, enveloped, positive-sense, single-strand ribonucleic acid viruses, belonging to the Coronaviridae family.3 Human-to-human transmission occurs primarily via direct contact, through surfaces touch,4 air droplets or airways diffusion.5 , 6 The higher risk of transmission is within approximately 1 or 2 meters from the infected person but the minimum distance is still undetermined.7 Evidences showed that 74% of asymptomatic SARS-CoV-2 positive patients may be infective.8 Although this background makes healthcare professionals’ job difficult and risky, patients with ischemic stroke still need fast intervention.
Since five trials demonstrating the efficacy and safety of mechanical thrombectomy (MT) for the treatment of ischemic strokes due to large vessel occlusion (LVO) have been published,9, 10, 11, 12, 13 the endovascular treatment is now the standard of care for patients presenting within 6 hours from symptoms onset. Also patients who arrives between 6 and 24 hours from symptoms onset can benefit from this treatment if they meet specific neuro-radiological criteria.14 , 15
The whole process of patient's neurological evaluation, radiological examination and medical or interventional treatment should be carried out in the shortest time to reduce brain damage (the so-called “time-is-brain” concept).16 , 17
Relying on our experience in a COVID-19-reference and tertiary stroke center, we analyze each step of the acute ischemic stroke (AIS) pathway in order to identify the phases at the highest risk of infection and to simplify, when possible, the diagnostic process. Consequently, we developed an intra-hospital protocol to balance the management of ischemic stroke patients (including the access to time-dependent procedures) and the risk of infection.
COVID-19 and ischemic stroke: a possible link?
A brief report claims the decreasing by 50% of mechanical thrombectomies in Shanghai since the end of January compared with the same period in 2019.18
Data of our hospital shows no reduction in the number of mechanical thrombectomies performed during the outbreak; conversely, we noted an increase of 88,8% in mechanical thrombectomies when comparing the same period (1th March – 1th April) with 2019.
Although this increase could hide a bias due to a natural improvement of the stroke network performances, it goes against the trend of previous evidences.
However, this trend is not surprising because ischemic stroke was reported as one of the possible complications to COVID-19.19 The disease is associated with transiently raised serum concentrations of inflammatory cytokines 20 that cause endothelial dysfunction and increases the procoagulant activity of the blood, which can contribute to the formation of an occlusive thrombus over a ruptured arterial plaque.21 Another proposed mechanism is cardioembolism from virus-related cardiac injury.22 Cohorts of patients from three Chinese hospitals showed up to 36% of neurological symptoms23 , 24 that included dizziness, headache, encephalopathy, anosmia, dysgeusia, muscle injury (detected by elevated creatine kinase) and stroke.
The impact of Covid-19 on ischemic stroke management: from TIA to LVO
Current acute stroke management guidelines recommend designing and implementing public education programs focused on stroke systems, referring acute stroke patients to emergency care and evaluating risk factors for patients presenting with TIA.14 Since AIS is a time-dependent disease and the treatment effectiveness is mainly related to timely access to the therapy,25 , 26 current guidelines state that emergency therapy should be administrated quickly avoiding unnecessary and potentially time-consuming procedures.14 , 27
Before COVID-19 outbreak, stroke guidelines have been universally accepted by clinicians involved in acute stroke.14 On March 09 2020, the Italian Government imposed national quarantine, restricting movement of population except for necessity, work, and health circumstances, in response to the growing pandemic of COVID-19 in the country.28
Although healthcare system resources have been shifted towards COVID-19 pandemic, both stroke inpatients and outpatients keep requiring high standard of care.
The role of Telemedicine
In the face of Covid-19, the need to reduce the exposure of doctors and patients is vital, as is ensuring the continuity of care for those who need it.
Because hospitals are filling up by infected patients and health workers may be asymptomatic carriers, low risk patients should be discouraged to refer to hospitals whenever possible. To make matters even worse, reports that as many as 100 health care workers at a single institution have to be quarantined at home because of exposure to Covid-19 have raised concern about workforce capacity.29
Telehealth can be a valuable help to solve these problems. It allows patients who have non–Covid-19 issues to receive consultations without the risk of exposure and quarantined doctors to continue to provide care.30
A pivotal strategy for virus spreading control is forward triage that is the categorization of patients before they arrive in the emergency department (ED). However, such a strategy needs to be integrated into the actual stroke system hub-and-spoke model.31 To overcome such a difficult situation, software solutions are being used in some regional healthcare systems, playing a key role in this emergency. Telehealth allows transmission of radiological images and video-assisted neurological examination, preventing unnecessary centralization and supporting spoke decisions (Table 1 ).
Table 1.
Telehealth uses in stroke patients: from TIA to LVO
| Patients | Main purpose |
|---|---|
| TIA |
|
| AIS without LVO |
|
| AIS with LVO |
|
The aims of the coming weeks is encouraging the diffusion of telehealth system in still-lacking centers enrolling more patients in our patient portals.30
Stroke pathway during COVID-19 outbreak: balancing risks for patients and clinicians
Due to COVID-19 outbreak, self-presentation in emergency room (ER) is highly discouraged because of a potential risk of infection; highly suspected for infection or COVID-19 positive (COVID+) patients should be pre-triaged in a separate box.
The approach to patients referred to ER should focus on early recognition of suspected COVID-19 positive cases that require immediate isolation and infection control measures. Current WHO guidelines suggest that once COVID-19 infection is suspected, control measures should be immediately implemented and public health officials notified. However, following current public health official guidelines only a minority of population has already been tested for SARS-CoV-2, mainly due to the reduced availability of these tests.32 , 33 Commonly used tests require several hours for the results and therefore cannot provide useful information for the management of stroke patients in the ER setting. Consequently, to assess the likelihood of infection, risk factors for COVID-19 disease should be taken into consideration. Even if diagnostic criteria are still debated, WHO clinical criteria for pursuing COVID-19 diagnostic evaluation are commonly used. According to WHO,32 COVID-19 should be considered in patients with fever and/or respiratory tract symptoms (eg, cough, dyspnea) who have had any of the following in the prior 14 days:
-
1.
Close contact with a confirmed or suspected case of COVID-19, including through work in health care settings. Close contact includes being within approximately two meters of a patient for a prolonged time or having direct contact with infectious secretions while not wearing personal protective equipment (PPE).
-
2.
Residence in (or travel to) areas where widespread community transmission has been reported.
-
3.
Potential exposure through attendance at events where COVID-19 cases have been reported.
In suspected cases, the epidemiological link to a probable or confirmed case may have occurred within a 14‐day period before the symptoms onset.34
According to WHO's criteria, we developed a risk stratification table to assess infective risk of patients referred to ER (Table 2 ).
Table 2.
Risk stratification, symptoms and characteristics.
| Low risk |
|
| Intermediate risk |
|
| High risk |
|
The WHO has warned that the severe shortage of the global supply of PPE – caused by rising demand, panic buying, and misuse – is putting lives at risk from the novel coronavirus. Then, although clinicians involved in management of AIS patients are at risk of infection and require specific PPE (Table 3 ), they are requested to optimize PPE use and to avoid useless waste.35 Common hygienic habits such as washing hands with soap and water or alcohol-based hand rub before and after all patient interaction, contact with potentially infectious sources, and before putting on and upon removal of PPE, have to be put into practice.
Table 3.
Personal Protective Equipment: level of precaution.
| A) Standard precaution | B) Contact and droplet precaution | C) Airborne precaution |
|---|---|---|
| All patients (regardless of the risk) must wear a disposable surgical mask | ||
|
|
|
Notes: 1. When health care workers put on a disposable particulate respirator, they must always perform the seal check. Note that facial hair (e.g. a beard) may prevent a proper respirator fit.36
ABBREVIATION: FFP2: Filtering Face Piece 2
Neurovascular team should wear opportune disposable equipment (Table 4 ).
Table 4.
Suggested Personal Protective Equipment's classes according to patient's risk and stroke pathway's phase.
| Risk | Phase | ||
|---|---|---|---|
| Triage – Neurological evaluation | Diagnostic imaging | Angiography | |
| Low | A | A | A |
| Intermediate | B | B | C |
| High | B | C | C |
| COVID-19 + | C | C | C |
Diagnostic imaging
According to the AHA / ASA,14 patients with suspected stroke need brain computed tomography (CT), computed-tomography angiography (CTA) and, in selected cases, computed tomography perfusion (CTP).
How to safely do a CT examination
It is highly recommended to have a CT room dedicated to intermediate-to-high risk or COVID+ patients (Table 2). This room should have a dedicated access route separated from the other areas of the radiology unit and must have, on the ground of the entrance, a cloth soaked in sodium hypochlorite. Two radiologic technologists (RT) should be employed for the CT scanning of patients with intermediate-to-high risk or COVID+ patients. One RT (RT1) uses PPE to set up the patient on the CT table while the other RT (RT2) operates on the CT console 37 (Table 4).
CT examination should be performed following a previously described procedure 37: RT1 and the accompanying medical doctor (MD), both equipped with adequate PPE, set up the patient on the CT table and then remove their contaminated PPE and put them into a dedicated garbage inside the room. Then, they leave the CT room. While RT2 operate on the CT scan, RT1 and MD wear a new pair of PPE and, once the examination is over, they transfer the patient from the CT table to the bed.
In patients referred from spoke center for LVO, this step could be avoided and the patient could be directly transferred to the angio suite, if all these conditions are satisfied:
-
2-
patient's arrival time within 6 hours from symptoms onset.
-
2-
radiological imaging acquired within the previous two hours at the spoke center;
-
2-
availability of a CT-capable angiograph to rule out hemorrhage;
This indication comes from the idea that patients who arrive to the hub center (following the drip-and-ship model) within 7.3 hours of time last seen well, the repetition of the brain imaging before angiography could be unnecessary, but it is still debated.38 However, in these epidemiological setting, we follow this more rapid approach.
A special mention is required for acute stroke patients who are confused, aphasic or unable to provide information for a comprehensive COVID-19 screening. Although these patients should be treated as potentially infected, such an approach is hardly possible due to shortage of PPE. In these cases, an extension of the CT examination to the chest should be taken into consideration to exclude signs of interstitial pneumonia.39 If pulmonary CT scan exclude signs of interstitial pneumonia, patients could be identified as low-risk and all the remaining procedure could be performed with class A precautions (Table 2). Conversely, if pulmonary CT scan shows signs of interstitial pneumonia, the patient should be considered as high risk and PPE should be used accordingly (Table 4).
Angiography suite
The angiography suite is the place where mechanical thrombectomy is performed. This procedure should be performed in an adequately ventilated room: natural ventilation with air flow of at least 160 L/s per patient or in negative-pressure rooms with at least 12 air changes per hour and controlled direction of air flow when using mechanical ventilation.40
Since mechanical thrombectomy may require patient intubation (that is classified as an aerosol-producing procedure), intermediate-to-high risk patients need airborne precautions36 , 41 and all healthcare workers must wear proper protection (Tables 4).
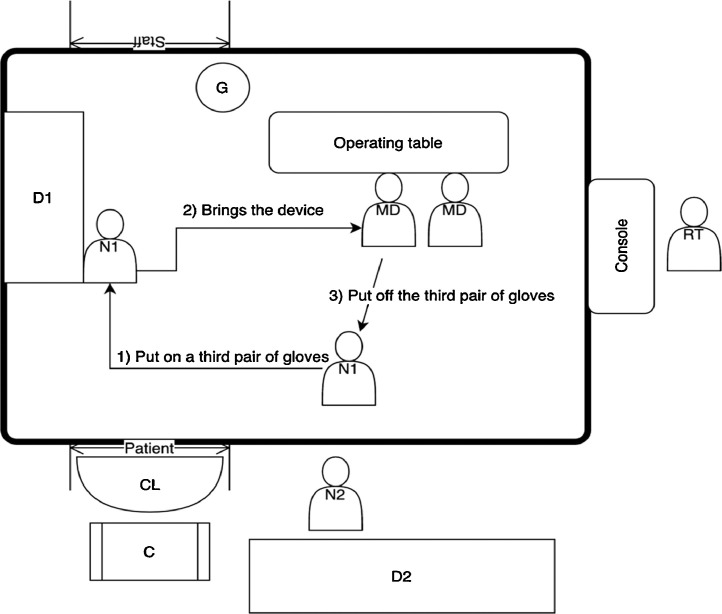
The access to the angio suite should be allowed only through one door (“Patient” in Figure 1 ); in front of this door, on the ground, a cloth soaked in sodium hypochlorite should be placed (CL in Figure 1). Both the ray tube and the detector should be covered with a single-use plastic coverage.
Fig. 1.
Angio Suite – medical devices: storage and claiming.MD: medical doctor; N1: nurse n.1; N2: nurse n.2; D1: device stored within the angio suite cupboard; D2: device stored within the depot; G: contaminated PPE dedicated garbage; CL: cloth soaked with sodium hypochlorite; C: cart located outside the angio suite; RT: radiologist technician; “Patient”: patient's entrance door; “Staff”: staff's exit door.
In many hospitals, not all the medical devices required for mechanical thrombectomy are stored inside the angio suite: devices that are rarely used are kept in depots outside the room. Before patient arrival, the availability of the necessary devices should be checked. However, devices needed to do a mechanical thrombectomy can vary from case to case; therefore, it is not possible to foresee (and prepare) all the devices needed before starting the procedure and a behavioral model is needed to allow quick and safe access to all the necessary tools.
Following the scheme illustrated in Figure 1, we hypothesized a way of working that involves two nurses: N1 and N2. N1 is intended to be inside the angio suite, wearing appropriate PPE. When a device (D1) is needed, N1 wears a third pair of gloves and takes it from the cupboard; after delivering the material to the first operator, the third pair of gloves will be removed. If the requested device is in the depot (D2), N2 put it on a cart (C) placed outside the entrance door and N1 repeats the above-mentioned procedure.
Decontamination
At the end of the intervention, the undressing of the staff must take place inside the angio suite and near an exit door (preferably a different door from the one used for the patient's exit) (“Staff” in Figure 1); near this door, a dedicated garbage (G) must be set up for the collection of contaminated waste.
Acute stroke: in-hospital management
At the end of acute treatment, patient is admitted to a dedicated ward, according to clinical conditions and infective risk. In our hospital, to limit virus diffusion and provide adequate level of care, we have provided separate wards dedicated to COVID+ and non-COVID+ patients both for those who need intensive care and for those who need sub intensive care.
Shortage of Intensive Care Units beds: the unsolved problem of decompressive hemicraniectomy
A particular evaluation should be done for patients with extensive middle cerebral artery (MCA) territory infarct. MCA occlusion can produce a large brain injury and swelling that may increase intracranial pressure causing malignant brain edema and brain herniation.42, 43, 44 Some studies demonstrated that early decompressive hemicraniectomy (DHC) in patients up to 60 years reduces mortality and improves functional outcome 45, 46, 47, 48; however, several surviving patients remain severely dependent 45, 46, 47, 48 and the ideal candidate for DHC and timing of DHC is still a matter of debate.49 After COVID-19 outbreak, due to the shortage of intensive care units beds and the allocation of the intensive care resources,50 DHC should be thoughtfully weighted on a case by case basis.
Conclusion
Having regard to the rapid spreading of the COVID-19, many hospitals will soon have to develop internal protocols to deal with this emergency and to cope with the associated challenging circumstances.
Declaration of Competing Interest
Nothing to declare
References
- 1.Organization WH. Coronavirus Disease 2019 (COVID-19) Situation Report – 512020.
- 2.World Health Organization (WHO). Coronavirus Disease 2019 (COVID-19) Situation Report – 73, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_4 (accessed 3 April 2020) 2020.
- 3.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. 1st ed. Elsevier Inc., 2011. Epub ahead of print 2011. DOI: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed]
- 4.van Doremalen N, Bushmaker T, Morris D, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-12020; 1–3. [DOI] [PMC free article] [PubMed]
- 5.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Horby P.W., Hayden F.G. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel J.D., Rhinehart E., Jackson M. Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35 doi: 10.1016/j.ajic.2007.10.007. Epub ahead of print 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shohei I., Fujikawa A., Jitsu M. Chest CT findings in cases from the cruise ship “diamond princess” with coronavirus disease 2019 (COVID-19) Radiol Epub Ahead Print. 2020 doi: 10.1148/ryct.2020204002. https://doi:10.1148@ryct.2020200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovin T.G., Chamorro A., Cobo E. Thrombectomy within 8 Hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 10.Saver J.L., Goyal M., Bonafe A. Stent-Retriever Thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M., Demchuk A.M., Menon B.K. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 12.Campbell B.C.V., Mitchell P.J., Kleinig T.J. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 13.Berkhemer O.A., Fransen P.S.S., Beumer D. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 14.Powers W.J., Rabinstein A.A., Ackerson T. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/American stroke association. 2018. Epub Ahead Print. 2018 doi: 10.1161/STR.0000000000000158. [DOI] [Google Scholar]
- 15.Turc G., Bhogal P., Fischer U. European stroke organisation (ESO) – European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for Europe (SAFE) Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marler J.R., Tilley B.C., Lu M. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow G.C., Smith E.E., Saver J.L. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J., Rudd A., Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 31 March 2020 doi: 10.1161/STROKEAHA.120.029701. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020 doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 20.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 25 March 2020 doi: 10.1001/jamacardio.2020.0950. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrales-Medina V.F., Musher D.M., Shachkina S. Acute pneumonia and the cardiovascular system. The Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 22.Temporary emergency guidance to us stroke centers during the COVID-19 pandemic. Stroke. 1 April 2020 doi: 10.1161/STROKEAHA.120.030023. Epub ahead of print. [DOI] [Google Scholar]
- 23.Mao L., Wang M., Chen S. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. Preprint Infect Dis (Except HIV/AIDS) 25 February 2020 doi: 10.1101/2020.02.22.20026500. Epub ahead of print. [DOI] [Google Scholar]
- 24.Li Y., Wang M., Zhou Y. Acute cerebrovascular disease following covid-19: a single center, retrospective, observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3550025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez C.R. Editorial: time is brain! J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 1993;3:1–2. doi: 10.1016/S1052-3057(10)80125-9. [DOI] [PubMed] [Google Scholar]
- 26.Hacke W., Kaste M., Bluhmki E. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 27.Toni D., Mangiafico S., Agostoni E. Intravenous thrombolysis and intra-arterial interventions in acute ischemic stroke: Italian Stroke Organisation (ISO)-spread guidelines. Int J Stroke. 2015;10:1119–1129. doi: 10.1111/ijs.12604. [DOI] [PubMed] [Google Scholar]
- 28.Ulteriori disposizioni attuative del decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia di contenimento e gestione dell'emergenza epidemiologica da COVID-19, applicabili sull'intero territorio nazionale. D.P.C.M. 09/03/20202020.
- 29.Hollander J.E., Carr B.G. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMp2003539. NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 30.Mehrotra A, Ray K, Brockmeyer DM, et al. Rapidly converting to “virtual practices”: outpatient care in the era of COVID-19. 2020; 5.
- 31.Elrod J.K., Fortenberry J.L. The hub-and-spoke organization design: an avenue for serving patients well. BMC Health Serv Res. 2017;17:457. doi: 10.1186/s12913-017-2341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Considerations in the investigation of cases and clusters of COVID-19, https://www.who.int/publications-detail/considerations-in-the-investigation-of-cases-and-clusters-of-covid-19 (accessed 21 March 2020) 2020.
- 33.Normativa emergenza coronavirus. dipartimento della protezione civile, http://www.protezionecivile.gov.it/attivita-rischi/rischio-sanitario/emergenze/coronavirus/normativa-emergenza-coronavirus (accessed 22 March 2020) 2020.
- 34.Novel coronavirus (2019-nCoV) situation reports, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 22 March 2020) 2020.
- 35.Organization WH . World Health Organization; 2020. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19): Interim Guidance, 27 February 2020. Technical Documents. [Google Scholar]
- 36.World Health Organization. How to perform a particulate respirator seal check. 2008 [Google Scholar]
- 37.Nakajima K., Kato H., Yamashiro T. COVID-19 pneumonia: infection control protocol inside computed tomography suites. Jpn J Radiol. 2020:20–22. doi: 10.1007/s11604-020-00948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albers G.W., Molina C.A., Selim M.H. Advanced brain imaging in late-arriving drip and ship patients with known large vessel occlusion. Stroke. 2019;50:1940–1943. doi: 10.1161/STROKEAHA.118.020574. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W., Zhong Z., Xie X. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson J., Chartier Y., Pessoa-Silva C., et al. Natural ventilation for infection control in health-care settings., https://apps.who.int/iris/handle/10665/44167 (2009, accessed 17 January 2020) 2020. [PubMed]
- 41.Tran K., Cimon K., Severn M. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035797. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hacke W., Schwab S., Horn M. Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 43.Moulin D.E., Lo R., Chiang J. Prognosis in middle cerebral artery occlusion. Stroke. 1985;16:282–284. doi: 10.1161/01.str.16.2.282. [DOI] [PubMed] [Google Scholar]
- 44.Jeon S.-B., Koh Y., Choi H.A. Critical care for patients with massive ischemic stroke. J Stroke. 2014;16:146–160. doi: 10.5853/jos.2014.16.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmeijer J., Kappelle L.J., Algra A. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 46.Jüttler E., Schwab S., Schmiedek P. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38:2518–2525. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 47.Vahedi K., Vicaut E., Mateo J. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 48.Vahedi K., Hofmeijer J., Juettler E. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 49.Jüttler E., Unterberg A., Woitzik J. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370:1091–1100. doi: 10.1056/NEJMoa1311367. [DOI] [PubMed] [Google Scholar]
- 50.Sorbello M., El-Boghdadly K., Di Giacinto I. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 27 March 2020 doi: 10.1111/anae.15049. Epub ahead of print. [DOI] [PubMed] [Google Scholar]