Abstract
Subclinical ketosis (SCK) and periparturient diseases considerably account for economic and welfare losses in dairy cows. The majority of scientific reports investigating the prevalence of SCK and production diseases are based on empirical studies conducted in Western Europe and North America. The present study surveyed the prevalence of SCK and production-related clinical diseases in early lactating cows in various countries across the world other than those in North America and Western Europe. Twelve countries of South and Central America (Argentina, Brazil, Chile, Colombia, Mexico), Africa (South Africa), Asia (Thailand, China), Eastern Europe (Russia, Ukraine), Australia, and New Zealand were assessed, and data from a total of 8,902 cows kept at 541 commercial dairy farms were obtained. A minimum of five cows per farm were blood sampled and examined once after parturition up to day 21 of lactation. Blood concentration of β-hydroxybutyrate was measured (threshold for SCK: 1.2 mmol/L), and the presence of production-related diseases such as milk fever, retained placenta, mastitis, metritis, displaced abomasum, lameness, and clinical ketosis was recorded. More than 95% of all cows were examined in their second week of lactation. Across all investigated countries, the SCK prevalence was 24.1%, ranging from 8.3% up to 40.1%. The prevalence of production-related diseases detected during the first 21 d of lactation was relatively low (<5%). Calculated odds ratios did not indicate an elevated risk for production diseases in cows with SCK. Despite differences in production systems across countries and variation between individual farms within a region, the present study data on SCK prevalence align with observations in Western European and North American dairy herds. At the very early stage of sampling and clinical examination for detection of SCK, it cannot be excluded that certain production diseases such as displaced abomasum, lameness, and mastitis have developed later.
Keywords: β-hydroxybutyrate, dairy cow, prevalence, production diseases, subclinical ketosis
INTRODUCTION
At the onset of lactation, dairy cows experience a marked metabolic load due to the prevailing negative energy balance, which makes them susceptible toward infectious and metabolic diseases (Gross et al., 2011; Bruckmaier and Gross, 2017). Increased concentrations of circulating ketone bodies, predominantly β-hydroxybutyrate (BHBA), without the presence of clinical signs of ketosis are considered as subclinical ketosis (SCK; Duffield, 2000). The blood BHBA thresholds for SCK diagnosis in literature range between 1.2 and 1.4 mmol/L (LeBlanc, 2010; Rollin et al., 2010; Suthar et al., 2013; Compton et al., 2014; Garro et al., 2014). Although symptoms of clinical ketosis (CK) such as reduced milk production, lethargy, and loss of appetite are commonly observed at greater concentrations of BHBA (>3.0 mmol/L), some cows, however, may be exposed to high BHBA concentrations without showing any clinical signs, whereas others develop ketosis characteristics already at reduced BHBA concentrations (Andersson, 1988; Bruckmaier and Gross, 2017; Zbinden et al., 2017).
Subclinical ketosis affects performance and is obviously related to an increased risk of production-related diseases such as CK, displaced abomasum (DA), retained placenta, and metritis (Duffield et al., 2009; McArt et al., 2012). Concomitantly, production efficiency decreases (e.g., lower milk production, poor fertility, and increased culling rates), which results in economic losses (Walsh et al., 2007; Duffield et al., 2009; McArt et al., 2012, 2015; Rutherford et al., 2016). Serum BHBA concentrations ≥ 1.2 mmol/L in the first week after calving were associated with a several-fold increased risk of subsequently developing DA and metritis, respectively (Duffield et al., 2009). Ospina et al. (2010) found increased odds ratios (OR) of 4.9 for CK, 2.3 for metritis, and 6.9 for DA when plasma BHBA concentrations exceeded a threshold of 1.0 mmol/L during days 3 to 14 postpartum. A negative impact of SCK on milk yield of early lactating cows was reported by several authors (Dohoo and Martin, 1984; Duffield et al., 2009; McArt et al., 2012; Zbinden et al., 2017). Furthermore, cows with BHBA concentrations greater than 1.0 mmol/L in the first week after calving were significantly less likely to be diagnosed pregnant after first insemination (Walsh et al., 2007). The economic impact of SCK is therefore indisputable, although exact figures quantifying the actual costs of SCK are difficult to collect. Based on an earlier investigation on Canadian dairy farms including more than 2,600 cows, McLaren et al. (2006) estimated that a reduction of 1% in SCK incidence would amount to a saving of 584 USD per year. Recently, McArt et al. (2015) calculated the average total costs of hyperketonemia (blood BHBA concentrations ≥ 1.2 mmol/L) to be 289 USD per case diagnosed.
Prevalence of SCK in the existing literature ranges between 10% and 40% in early lactation with greatest values occurring within the first 3 wk of lactation (Dohoo and Martin, 1984; Oetzel, 2004; Duffield et al., 2009). Most of these studies, however, included only a small number of dairy herds and cows (mainly research stations) and were performed in North America and Western Europe (e.g., Rollin et al., 2010; McArt et al., 2012; Suthar et al., 2013; Compton et al., 2014; Garro et al., 2014).
The objective of the present study was to survey the prevalence of SCK and the concomitant occurrence of health disorders in early lactating dairy cows in various countries across the world other than those in North America and Western Europe. Dairy farms in 12 countries of South and Central America (Argentina, Brazil, Chile, Colombia, and Mexico), Africa (South Africa), Asia (Thailand, China), Eastern Europe (Russia, Ukraine), Australia, and New Zealand were investigated. To our knowledge, this is the first large-scale approach to investigate the prevalence of SCK beyond Western Europe and North America.
MATERIALS AND METHODS
Animals and Sampling
All procedures in terms of blood sampling and veterinary examinations followed the animal care and welfare legislation criteria of the involved countries. The study was conducted from June 2011 to September 2013 at different commercial dairy farms in 12 different countries worldwide (Argentina, Australia, Brazil, Chile, China, Colombia, Mexico, New Zealand, Russia, South Africa, Thailand, and Ukraine). Breeds included were dairy breeds (e.g., predominantly Holstein-Friesian, Jersey), local crossbred lines (e.g., Girolondo in Brazil, Simmental × Red Steppe in Russia), and others (e.g., Simmental). Management systems varied from grazing systems to indoor housing of animals throughout the year. In total, 8,902 early lactating dairy cows housed on 541 farms were evaluated. At least five randomly selected cows of a herd in early lactation (days 2 to 21 postpartum [p.p.]) inconspicuous of obvious health disorders and without any previous treatments were clinically examined, and blood was sampled for diagnostic purposes and health monitoring during regular herd health management visits by veterinarians in the field. Each cow was only tested once, and no further restrictions in selection criteria such as parity number, breed, or performance were implied. In all cows, blood samples were taken from the coccygeal vein, and BHBA concentrations were directly determined on-site using a handheld meter (Precision Xceed, Abbott Diabetes Care Inc., Alameda, CA), which was previously validated for the use in cows (Iwersen et al., 2009; Pineda and Cardoso, 2015). Concomitantly, each sampled cow was clinically examined for the presence of metabolic and infectious health disorders (milk fever, retained placenta, DA, lameness, mastitis, metritis, and CK) following standardized diagnostic definitions (Table 1).
Table 1.
Definitions used for disease diagnosis during veterinary examinations of dairy cows in early lactation
| Postpartum disease | Definition | Reference |
|---|---|---|
| Milk fever | Cow requires Ca injections due to hypocalcaemia, based on clinical signs such as muscular weakness or lying cow unable to rise. | Duffield et al. (1999); LeBlanc et al. (2002); DeGaris and Lean (2008) |
| Retained placenta | Failure to pass placenta within 24 h after calving | Duffield et al. (1999); LeBlanc et al. (2002) |
| Mastitis | Visually abnormal milk appearance and/or changes in the appearance of the udder (inflammatory signs, swollen or hard quarters) | Duffield et al. (1999); LeBlanc et al. (2002); Pérez-Cabal et al. (2009) |
| Displaced abomasum | Presence of gas-filled abomasum on left or upper right flank, based on a characteristic “ping” sound at auscultation or percussion, confirmed by surgery | Duffield et al. (1999); LeBlanc et al. (2002 |
| Lameness | Abnormal findings during claw examinations (such as interdigital and digital dermatitis) and/or lameness with a locomotion score1 of ≥3 (scale of 1 to 5) | Duffield et al. (1999); LeBlanc et al. (2002); Thomsen et al. (2008) |
| Metritis | Enlarged uterus and/or purulent, smelly uterine discharge associated with systemic signs (fever2, inappetence/anorexia, inactivity, decreased milk yield) | Duffield et al. (1999); LeBlanc et al. (2002); Sheldon et al. (2006) |
| Clinical ketosis | Decreased milk production, reduced feed intake or inappetence, reduced activity, positive blood, milk or urine ketone test, absence of displaced abomasum or other primary disease, ketone odor in breath or milk | Duffield et al. (1999); LeBlanc et al. (2002) |
1Scale 1 to 5.
2Body temperature > 39.5 °C.
Statistical Analyses
Cows sampled between days 2 and 21 p.p. were categorized “SCK negative” if blood BHBA concentrations were <1.2 mmol/L or “SCK positive” if BHBA concentrations were ≥1.2 mmol/L. The prevalence of SCK was calculated as proportion of animals with BHBA concentrations ≥ 1.2 mmol/L relative to all investigated cows. Prevalence data were estimated at country and average global levels. In addition, overall and country-specific prevalence of postpartum diseases was calculated. The occurrence of postpartum diseases in relation to SCK was evaluated by OR analyses using the statistical package TESTIMATE (version 6.5, idv-Data Analysis & Study Planning, Krailling, Germany) according to the following formula: where p1 indicates the probability of postpartum diseases under SCK conditions and p2 the probability of postpartum diseases under non-SCK conditions.
RESULTS
Data analyses were based on a total of 8,902 dairy cows from 541 different commercial dairy farms. The number of farms investigated per country and their herd size ranged from 2 farms with 83 cows in Thailand to 102 farms housing 2,989 cows in New Zealand. On average, cows of the present data set were in their third lactation (range of parity number: 1 to 14, 26.9% primiparous and 73.1% multiparous dairy cows; Table 2). The majority (95.1%) of the cows was examined and sampled between days 7 and 15 p.p.
Table 2.
Information on animals and farms of the different participating countries in the present study
| Country | Farms (n) | Cows (n) | Percentage of cows | Cattle breed | Housing | Parity number1 | Milk yield (kg/d per cow) | |
|---|---|---|---|---|---|---|---|---|
| Primiparous | Multiparous | |||||||
| Argentina | 27 | 720 | 26.0 | 74.0 | Holstein-Friesian | Pasture and indoors | 2.8 ± 1.8 | 28.5 |
| Australia | 22 | 208 | 15.0 | 85.0 | Holstein-Friesian | Pasture | 3.4 ± 2.0 | 18.4 |
| Brazil | 26 | 159 | 35.5 | 64.5 | 50% Holstein- Friesian, 50% others (Girolondo, Gyr, Brown Swiss) | Pasture and indoors | 2.5 ± 1.7 | 13 to 30 |
| Chile | 17 | 183 | 24.6 | 75.4 | Holstein-Friesian | Pasture and indoors | 2.6 ± 1.3 | 23.0 |
| China | 41 | 404 | 63.1 | 36.9 | Holstein-Friesian | Indoors | 1.3 ± 1.5 | 25 to 35 |
| Colombia | 79 | 791 | 27.1 | 72.9 | Holstein-Friesian, Brahman, Brahman crossbred | Pasture | 2.9 ± 1.8 | 17 to 21 |
| Mexico | 63 | 2,060 | 37.6 | 62.4 | Holstein-Friesian or HF crossbred | Pasture and indoors | 2.3 ± 1.7 | 18 to 22 |
| New Zealand | 102 | 2,989 | 13.7 | 86.3 | Holstein-Friesian, Jersey, HF/Jersey crossbred | Pasture | 4.0 ± 2.4 | 13 to 19 |
| Russia | 77 | 764 | 39.3 | 60.7 | Holstein-Friesian, Simmental, Red Steppe | Indoors | 2.2 ± 1.2 | 13 to 23 |
| South Africa | 54 | 377 | 18.1 | 81.9 | Holstein-Friesian | Pasture | 3.3 ± 1.9 | 20 to 25 |
| Thailand | 2 | 83 | 32.5 | 67.5 | 88% Holstein-Friesian | Indoors | 3.3 ± 2.3 | 12 to 20 |
| Ukraine | 31 | 164 | 21.3 | 78.7 | 50% Holstein-Friesian, 50% others | Indoors | 2.6 ± 1.3 | 13 to 25 |
| Overall | 541 | 8,902 | 26.9 | 73.1 | 3.0 ± 2.1 | |||
1Data are expressed as mean ± SD.
Concentration of Blood BHBA and SCK
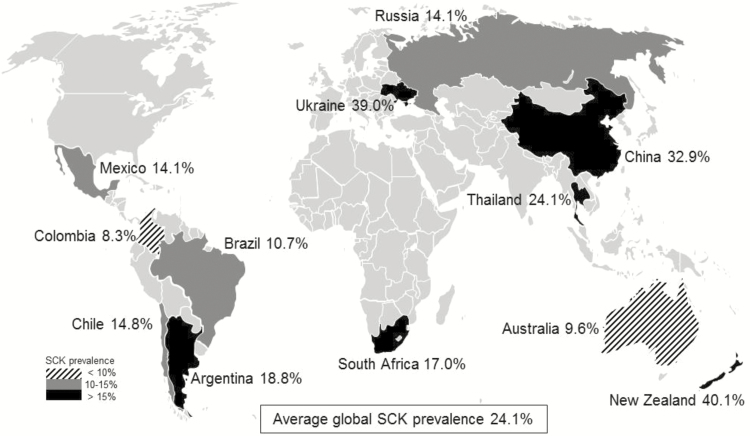
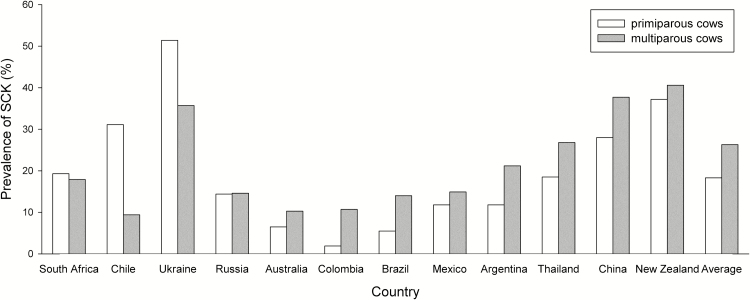
Blood BHBA concentrations observed between days 2 and 21 p.p. were 1.0 ± 0.8 mmol/L, ranging from an average value of 0.7 mmol/L (Australia, Brazil, Colombia, and Russia) to 1.5 mmol/L (Ukraine). In two thirds of the investigated countries, mean BHBA concentrations were less than 1.0 mmol/L (0.7 to 0.9 mmol/L), whereas in one third of the countries (namely, China, New Zealand, Thailand, and Ukraine) mean BHBA concentrations of 1.0 mmol/L and above were observed (Table 3). Blood BHBA concentrations of 3.0 mmol/L and beyond during the first 21 d of lactation were predominately observed in China and Ukraine (Table 3). Overall, SCK (BHBA concentrations ≥ 1.2 mmol/L) was diagnosed in 24.1% of all cows examined, ranging from 8.3% (Colombia) up to 40.1% (New Zealand; Figure 1). In only 2 (Australia and Colombia) out of the 12 investigated countries, SCK prevalence on the participating farms was less than 10%. In four countries (Brazil, Chile, Mexico, Russia), SCK prevalence varied between 10.7% and 14.8%, whereas in the remaining countries (Argentina, China, New Zealand, South Africa, Thailand, Ukraine), SCK prevalence was more than 15% (between 17.0% and 40.1%). More than 80% of all cows diagnosed SCK were multiparous. In contrast, we observed SCK occurring more frequently (P < 0.05) in primiparous cows in three countries (Chile, South Africa, and Ukraine; Figure 2).
Table 3.
Blood BHBA concentrations in early lactating dairy cows (2- to 21-d postpartum) in different countries and proportion of cows with highly elevated BHBA concentrations
| BHBA1 concentration (mmol/L) | Cows with BHBA ≥ 3.0 mmol/L | ||||
|---|---|---|---|---|---|
| Country | n | Mean ± SD | Range | n | % |
| Argentina | 720 | 0.9 ± 0.9 | 0.1 to 6.2 | 36 | 5.0 |
| Australia | 208 | 0.7 ± 0.7 | 0.1 to 7.0 | 4 | 1.9 |
| Brazil | 159 | 0.7 ± 0.6 | 0.1 to 4.8 | 3 | 1.9 |
| Chile | 183 | 0.8 ± 0.7 | 0.0 to 4.6 | 3 | 1.6 |
| China | 404 | 1.2 ± 1.0 | 0.0 to 5.8 | 33 | 8.2 |
| Colombia | 791 | 0.7 ± 0.4 | 0.1 to 4.2 | 4 | 0.5 |
| Mexico | 2,060 | 0.8 ± 0.7 | 0.0 to 6.0 | 53 | 2.6 |
| New Zealand | 2,990 | 1.2 ± 0.7 | 0.0 to 8.0 | 101 | 3.4 |
| Russia | 764 | 0.7 ± 0.7 | 0.1 to 6.4 | 18 | 2.4 |
| South Africa | 376 | 0.9 ± 1.0 | 0.1 to 7.2 | 20 | 5.3 |
| Thailand | 83 | 1.0 ± 0.8 | 0.1 to 5.8 | 2 | 2.4 |
| Ukraine | 164 | 1.5 ± 1.5 | 0.0 to 6.7 | 22 | 13.4 |
| Overall | 8,902 | 1.0 ± 0.8 | 0.0 to 8.0 | 299 | 3.4 |
1BHBA = β-hydroxybutyrate.
Figure 1.
Prevalence of subclinical ketosis (SCK, blood β-hydroxybutyrate concentrations ≥ 1.2 mmol/L) in early lactating dairy cows studied in 12 countries worldwide.
Figure 2.
Occurrence of subclinical ketosis (SCK; blood β-hydroxybutyrate concentrations ≥ 1.2 mmol/L) in primiparous and multiparous cows during early lactation in different countries worldwide.
Prevalence of Concomitant Production Diseases and Their Association With SCK
The overall prevalence of production diseases was 4.3% for milk fever, 4.0% for retained placenta, 3.4% for mastitis, 1.7% for lameness, and 5.3% for metritis (Table 4). Displaced abomasum and CK showed a very low prevalence (0.3% and 0.7%, respectively). During the first 21-d p.p., DA was not seen in 50% (6/12) of the investigated countries and CK was diagnosed in only 7 out of 12 countries. We further investigated the relationship of SCK with the concomitant presence of health disorders in early lactation by performing an OR analysis. However, due to the generally low prevalence of diseases, OR were <1, and an increased risk of disease in association with SCK could not be identified in the present study (data not shown). The greatest OR was calculated for CK as cows diagnosed SCK had a 1.062 greater probability of having subsequently CK.
Table 4.
Prevalence of postpartum diseases between 2- and 21-d postpartum
| Milk fever | Retained placenta | Mastitis | DA1 | Lameness | Metritis | CK2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | % | n | % | n | % | n | % | n | % | n | % | n | % | n |
| Argentina | 0.7 | (5/720) | 2.1 | (15/720) | 5.1 | (37/720) | 1.0 | (7/720) | 2.2 | (16/720) | 15.7 | (113/720) | 4.0 | (29/720) |
| Australia | 10.1 | (21/208) | 7.2 | (15/208) | 3.8 | (8/208) | 0 | (0/208) | 0.5 | (1/207) | 6.3 | (13/208) | 1.9 | (4/208) |
| Brazil | 0 | (0/159) | 8.8 | (14/159) | 3.8 | (6/159) | 0.6 | (1/159) | 1.3 | (2/159) | 0 | (0/159) | 0 | (0/159) |
| Chile | 7.1 | (13/182) | 5.5 | (10/183) | 9.3 | (17/183) | 1.1 | (2/183) | 5.5 | (10/183) | 9.8 | (18/183) | 2.2 | (4/183) |
| China | 1.7 | (7/403) | 4.0 | (16/404) | 2.0 | (8/404) | 2.7 | (14/404) | 1.0 | (4/404) | 3.7 | (15/404) | 1.2 | (5/404) |
| Colombia | 1.5 | (12/791) | 4.3 | (34/791) | 4.6 | (36/791) | 0.1 | (1/791) | 1.0 | (8/791) | 0.9 | (7/791) | 0 | (0/791) |
| Mexico | 0.6 | (13/2,060) | 4.7 | (97/2,060) | 1.4 | (29/2,060) | 0.2 | (4/2,060) | 1.3 | (27/2,060) | 5.7 | (118/2,059) | 0.6 | (12/2,060) |
| New Zealand | 0.7 | (20/2,990) | 0.2 | (7/2,990) | 1.2 | (36/2,990) | 0 | (0/2,990) | 0.1 | (3/2,990) | 0.3 | (10/2,990) | 0.1 | (2/2,989) |
| Russia | 37.4 | (286/764) | 17.0 | (130/764) | 14.9 | (114/764) | 0 | (0/764) | 10.5 | (80/764) | 13.5 | (103/764) | 0.9 | (7/764) |
| South Africa | 0.8 | (3/377) | 1.3 | (5/377) | 0.8 | (3/377) | 0 | (0/377) | 0 | (0/377) | 6.6 | (25/377) | 0 | (0/377) |
| Thailand | 1.2 | (1/83) | 0 | (0/83) | 4.8 | (4/83) | 0 | (0/83) | 1.2 | (1/83) | 19.3 | (16/83) | 0 | (0/83) |
| Ukraine | 0.6 | (1/164) | 7.3 | (12/164) | 3.7 | (6/164) | 0 | (0/164) | 0.6 | (1/164) | 19.5 | (32/164) | 0 | (0/164) |
| Overall | 4.3 | (382/8,901) | 4.0 | (355/8,903) | 3.4 | (304/8,903) | 0.3 | (26/8,903) | 1.7 | (153/8,902) | 5.3 | (470/8,902) | 0.7 | (63/8,902) |
| Range | 0 to 37.4 | 0 to 17.0 | 0.8 to 14.9 | 0 to 2.7 | 0 to 10.5 | 0 to 19.5 | 0 to 4.0 | |||||||
1DA = displaced abomasum.
2CK = clinical ketosis.
DISCUSSION
In the last three decades, research results addressing SCK and periparturient diseases represented primarily published data from the United States, Canada, and Western Europe (McArt et al., 2012; Suthar et al., 2013; Raboisson et al., 2014). The majority of reports usually based on investigations conducted in regional and small-sized dairy farms of individual countries (Oetzel, 2004; McLaren et al., 2006; Ospina et al., 2010; Garro et al., 2014). Studies involving a greater number of animals and covering wider geographic areas were presented earlier by Chapinal et al. (2011) and Suthar et al. (2013), but still considered only SCK occurrence within Europe and the United States, respectively. Different study designs and scopes (such as pre- or postpartum reclassification of animals, herd- or cow-level investigations, sampling of blood, milk or urine, and defining SCK at various BHBA thresholds) do not always allow a straightforward comparison of literature data across farms, regions, and countries, as recently described in a meta-analysis evaluating 23 SCK publications by Raboisson et al. (2014). Furthermore, data on global SCK prevalence obtained by a standardized protocol for sampling and defining SCK are scarce. Therefore, we have investigated the prevalence of SCK and typical production-related disorders in early lactating dairy cows in various countries across the world other than those in North America and Western Europe. By using the same validated tool for on-site BHBA testing in all cows, diagnosing SCK at the generally accepted blood BHBA cut-off concentration of 1.2 mmol/L, and sampling cows in the very early lactation (days 2 to 21 p.p.) make the results presented here comparable to studies performed in other countries. Many countries worldwide refer to the thresholds for classification of SCK as obtained in North America and Western Europe. Although individual differences in setting thresholds might exist, the prominent threshold of 1.2 mmol/L allows an estimation of consequences for health and animal performance.
The overall SCK prevalence of the present survey was in a comparable range to observations from a European evaluation of Suthar et al. (2013). In addition, the variation in the global SCK rates presented here is considerable (approximately 8% to 40%) and is similar to findings reported for the United States and Europe (McArt et al., 2012; Suthar et al., 2013; Garro et al., 2014). The majority of our investigated cows with SCK were multiparous confirming the assumptions that the risk of developing SCK increases with parity number as milk production rises concomitantly (Duffield et al., 1997). Based on an analysis of a variety of publications, Oetzel (2004) considered a prevalence rate of 15% representative for SCK in dairy herds but emphasized to consider an SCK prevalence of 10% already as an alert level. In general, the SCK incidence in our investigations was similar to the range of findings in Western Europe and North America. In the present study, only 2 out of 12 countries (Australia and Colombia) had an average SCK prevalence of less than 10%, whereas the remaining countries had greater rates. Four countries (China, New Zealand, Ukraine, and Thailand) revealed even SCK rates up to 40%. However, this does not exclude that SCK may also occur at greater rates in the countries with low SCK prevalence in the present study, which might be attributed to the random selection of farms and animals. In general, SCK in early lactating dairy cows is globally present. Further explanations for differences in SCK incidence might be greater milk production in some countries resulting in a more negative energy balance, geographic and regional differences of management, and dairy cow feeding as well as individual cow variation. The greatest average SCK prevalence of all countries was observed in New Zealand. One explanation might be that dairy cows in New Zealand are mostly kept under extensive pasture-based production conditions with low or even zero concentrate supplementation (Compton et al., 2014). Along with the genetic progress in dairy cow breeding, milk production in export-oriented countries such as New Zealand probably increased and aggravated the energy deficiency in early lactating cows (Capper et al., 2009; Bruckmaier and Gross, 2017). In another study, Compton et al. (2014) observed a SCK prevalence of approximately 17% in New Zealand dairy cows, which would be still in the upper range when compared with the present evaluations. The difference just implies the possible variation due to the selection of farms and animals.
Interestingly, the occurrence of postpartum diseases in the present study was much lower compared with literature reports. Our observations on average prevalence rates of retained placenta, mastitis, lameness, and metritis were approximately half of the values reported in a European study by Suthar et al. (2013). At first sight, these results were surprising considering the fact that SCK prevalence rates of this investigation were in agreement with other reports published. The cows diagnosed SCK in the present study did obviously not develop health disorders at this early sampling stage. However, results of the concomitant presence of SCK and further production diseases are inconsistent. Although we observed the greatest SCK prevalence on farms in New Zealand, postpartum diseases occurred only at less than 1% in cows diagnosed SCK. On the other hand, the SCK prevalence data in Russia were only moderately increased (14.1%), but many health disorders were diagnosed at the same time (milk fever 37.4%, retained placenta 17.0%, mastitis 14.9%, lameness 10.5%). In a recent study by Zbinden et al. (2017), high-yielding cows fed only herbage showed blood BHBA concentrations approaching that representative for CK, however, not necessarily followed by the occurrence of diseases. Irrespective of the concomitant presence of production diseases in cows with SCK, elevated BHBA concentrations ≥ 1.2 mmol/L undoubtedly increase the risk of subsequently developing further health disorders (Duffield et al., 2009; Ospina et al., 2010) and simultaneously reduced performance in terms of milk yield and reproduction (Dohoo and Martin, 1984; Walsh et al., 2007; Duffield et al., 2009; McArt et al., 2012). Management and environmental conditions are crucial factors that affect the ability of cows to cope successfully with the imposed metabolic load. These circumstances cannot be effectively recorded in a field study like the present one. Furthermore, one might speculate on regional differences in terms of breed and feeding system affecting the development of health disorders. The occurrence of DA was reported to be relatively rare in dairy cattle in New Zealand and Australia (Jubb et al., 1991; Compton et al., 2014), which is assumed to be associated with the predominantly extensive farming on pasture where important risk factors for DA such as low locomotion activity are not important. However, prevalence of DA concomitant with SCK in the present study was, in particular, low in countries with a more intensive milk production in confined housing systems (e.g., China, Russia, Thailand, and Ukraine). Similar observations were made for mastitis and the occurrence of lameness. Although lameness may also occur in cattle on pasture, it appears to be less frequent and to be usually based on a different etiology (Chesterton et al., 1989; Laven and Holmes, 2008). In contrast, we observed that mastitis prevalence in cows with SCK can be elevated independently if cows were kept on pasture or indoors.
In conclusion, findings of the present study showed that SCK in dairy cows is a global issue and can be observed at various rates in many countries over the world. Possibly due to the diagnosis of SCK and clinical examination at a very early stage of lactation, concomitant postpartum diseases in cows diagnosed for SCK have not yet developed at this stage. An appropriate management is crucial for avoiding a deterioration of animals’ metabolic status with the associated negative impacts on health and performance. The results of the present study support the hypothesis that the association between SCK and postpartum diseases is complex and that based on an occasional single determination of blood BHBA concentrations alone, a reliable and robust prediction of further production diseases is not possible.
Footnotes
The authors are grateful to all involved veterinarians, farmers, and staff on the different farms and countries. Furthermore, we thank Marion Ocak for her contribution to the statistical analysis and Tanja Knoppe for data processing and literature research. This study was supported by Bayer Animal Health GmbH, Leverkusen, Germany.
LITERATURE CITED
- Andersson L. 1988. Subclinical ketosis in dairy cows. Vet. Clin. North Am. Food Anim. Pract. 4:233–251. [DOI] [PubMed] [Google Scholar]
- Bruckmaier R.M., and Gross J.J.. 2017. Lactational challenges in transition dairy cows. Anim. Prod. Sci. 57:1471–1481. doi:10.1071/AN16657 [Google Scholar]
- Capper J.L., Cady R.A., and Bauman D.E.. 2009. The environmental impact of dairy production: 1944 compared with 2007. J. Anim. Sci. 87:2160–2167. doi:10.2527/jas.2009-1781 [DOI] [PubMed] [Google Scholar]
- Chapinal N., Carson M., Duffield T.F., Capel M., Godden S., Overton M., Santos J.E., and LeBlanc S.J.. 2011. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 94:4897–4903. doi:10.3168/jds.2010-4075 [DOI] [PubMed] [Google Scholar]
- Chesterton R.N., Pfeiffer D.U., Morris R.S., and Tanner C.M.. 1989. Environmental and behavioural factors affecting the prevalence of foot lameness in New Zealand dairy herds – a case-control study. N. Z. Vet. J. 37:135–142. doi:10.1080/00480169.1989.35587 [DOI] [PubMed] [Google Scholar]
- Compton C.W., McDougall S., Young L., and Bryan M.A.. 2014. Prevalence of subclinical ketosis in mainly pasture-grazed dairy cows in New Zealand in early lactation. N. Z. Vet. J. 62:30–37. doi:10.1080/00480169.2013.823829 [DOI] [PubMed] [Google Scholar]
- DeGaris P.J., and Lean I.J.. 2008. Milk fever in dairy cows: a review of pathophysiology and control principles. Vet. J. 176:58–69. doi:10.1016/j.tvjl.2007.12.029 [DOI] [PubMed] [Google Scholar]
- Dohoo I.R., and Martin S. W.. 1984. Subclinical ketosis: prevalence and associations with production and disease. Can. J. Comp. Med. 48:1–5. [PMC free article] [PubMed] [Google Scholar]
- Duffield T. 2000. Subclinical ketosis in lactating dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 16:231–253. [DOI] [PubMed] [Google Scholar]
- Duffield T.F., Kelton D.F., Leslie K.E., Lissemore K.D., and Lumsden J.H.. 1997. Use of test day milk fat and milk protein to detect subclinical ketosis in dairy cattle in Ontario. Can. Vet. J. 38:713–718. [PMC free article] [PubMed] [Google Scholar]
- Duffield T.F., Leslie K.E., Sandals D., Lissemore K., McBride B.W., Lumsden J.H., Dick P., and Bagg R.. 1999. Effect of a monensin-controlled release capsule on cow health and reproductive performance. J. Dairy Sci. 82:2377–2384. doi:10.3168/jds.S0022-0302(99)75488-3 [DOI] [PubMed] [Google Scholar]
- Duffield T.F., Lissemore K.D., McBride B.W., and Leslie K.E.. 2009. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 92:571–580. doi:10.3168/jds.2008-1507 [DOI] [PubMed] [Google Scholar]
- Garro C. J., Mian L., and Cobos Roldán M.. 2014. Subclinical ketosis in dairy cows: prevalence and risk factors in grazing production system. J. Anim. Physiol. Anim. Nutr. (Berl). 98:838–844. doi:10.1111/jpn.12141 [DOI] [PubMed] [Google Scholar]
- Gross J., van Dorland H.A., Bruckmaier R.M., and Schwarz F.J.. 2011. Performance and metabolic profile of dairy cows during a lactational and deliberately induced negative energy balance by feed restriction with subsequent realimentation. J. Dairy Sci. 94:1820–1830. doi:10.3168/jds.2010–3707 [DOI] [PubMed] [Google Scholar]
- Iwersen M., Falkenberg U., Voigtsberger R., Forderung D., and Heuwieser W.. 2009. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J. Dairy Sci. 92:2618–2624. doi:10.3168/jds.2008-1795 [DOI] [PubMed] [Google Scholar]
- Jubb T.F., Malmo J., Davis G.M., and Vawser A.S.. 1991. Left-side displacement of the abomasum in dairy cows at pasture. Aust. Vet. J. 68:140–142. [DOI] [PubMed] [Google Scholar]
- Laven R.A., and Holmes C.W.. 2008. A review of the potential impact of increased use of housing on the health and welfare of dairy cattle in New Zealand. N. Z. Vet. J. 56:151–157. doi:10.1080/00480169.2008.36827 [DOI] [PubMed] [Google Scholar]
- LeBlanc S. J. 2010. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Develop. 56(suppl):S29–S35. [DOI] [PubMed] [Google Scholar]
- LeBlanc S.J., Duffield T.F., Leslie K.E., Bateman K.G., TenHag J., Walton J.S., and Johnson W.H.. 2002. The effect of prepartum injection of vitamin E on health in transition dairy cows. J. Dairy Sci. 85:1416–1426. doi:10.3168/jds.S0022-0302(02)74209-4 [DOI] [PubMed] [Google Scholar]
- McArt J.A.A., Nydam D.V., and Oetzel G.R.. 2012. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 95:5056–5066. doi:10.3168/jds.2012-5443 [DOI] [PubMed] [Google Scholar]
- McArt J.A.A., Nydam D.V., and Overton M.W.. 2015. Hyperketonemia in early lactation dairy cattle: a deterministic estimate of component and total cost per case. J. Dairy Sci. 98:2043–2054. doi:10.3168/jds.2014–8740 [DOI] [PubMed] [Google Scholar]
- McLaren C.J., Lissemore K.D., Duffield T.F., Leslie K.E., Kelton D.F., and Grexton B.. 2006. The relationship between herd level disease incidence and a return over feed index in Ontario dairy herds. Can. Vet. J. 47:767–773. [PMC free article] [PubMed] [Google Scholar]
- Oetzel G. R. 2004. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. North Am. Food Anim. Pract. 20:651–674. doi:10.1016/j.cvfa.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Ospina P.A., Nydam D.V., Stokol T., and Overton T.R.. 2010. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: critical thresholds for prediction of clinical diseases. J. Dairy Sci. 93:546–554. doi:10.3168/jds.2009–2277 [DOI] [PubMed] [Google Scholar]
- Pérez-Cabal M.A., de los Campos G., Vazquez A.I., Gianola D., Rosa G.J., Weigel K.A., and Alenda R.. 2009. Genetic evaluation of susceptibility to clinical mastitis in Spanish Holstein cows. J. Dairy Sci. 92:3472–3480. doi:10.3168/jds.2008-1978 [DOI] [PubMed] [Google Scholar]
- Pineda A. and Cardoso F.C.. 2015. Technical note: validation of a handheld meter for measuring β-hydroxybutyrate concentrations in plasma and serum from dairy cows. J. Dairy Sci. 98:8818–8824. doi:10.3168/jds.2015-9667 [DOI] [PubMed] [Google Scholar]
- Raboisson D., Mounié M., and Maigné E.. 2014. Diseases, reproductive performance, and changes in milk production associated with subclinical ketosis in dairy cows: a meta-analysis and review. J. Dairy Sci. 97:7547–7563. doi:10.3168/jds.2014–8237 [DOI] [PubMed] [Google Scholar]
- Rollin E., Berghaus R.D., Rapnicki P., Godden S.M., and Overton M.W.. 2010. The effect of injectable butaphosphan and cyanocobalamin on postpartum serum beta-hydroxybutyrate, calcium, and phosphorus concentrations in dairy cattle. J. Dairy Sci. 93:978–987. doi:10.3168/jds.2009-2508 [DOI] [PubMed] [Google Scholar]
- Rutherford A.J., Oikonomou G., and Smith R.F.. 2016. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. J. Dairy Sci. 99:4808–4815. doi:10.3168/jds.2015-10154 [DOI] [PubMed] [Google Scholar]
- Sheldon I.M., Lewis G.S., LeBlanc S., and Gilbert R.O.. 2006. Defining postpartum uterine disease in cattle. Theriogenology. 65:1516–1530. doi:10.1016/j.theriogenology.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Suthar V. S., Canelas-Raposo J., Deniz A., and Heuwieser W.. 2013. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 96:2925–2938. doi:10.3168/jds.2012-6035 [DOI] [PubMed] [Google Scholar]
- Thomsen P.T., Munksgaard L., and Tøgersen F.A.. 2008. Evaluation of a lameness scoring system for dairy cows. J. Dairy Sci. 91:119–126. doi:10.3168/jds.2007-0496 [DOI] [PubMed] [Google Scholar]
- Walsh R.B., Walton J.S., Kelton D.F., LeBlanc S.J., Leslie K.E., and Duffield T.F.. 2007. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J. Dairy Sci. 90:2788–2796. doi:10.3168/jds.2006-560 [DOI] [PubMed] [Google Scholar]
- Zbinden R.S., Falk M., Münger A., Dohme-Meier F., van Dorland H.A., Bruckmaier R.M., and Gross J.J.. 2017. Metabolic load in dairy cows kept in herbage-based feeding systems and suitability of potential markers for compromised well-being. J. Anim. Physiol. Anim. Nutr. (Berl). 101:767–778. doi:10.1111/jpn.12498 [DOI] [PubMed] [Google Scholar]