Abstract
The aim of this observational study is to identify risk factors associated with body weight (BW) variability in three data sets (DS) in commercial conditions. A total of 1,009 (DS1), 460 (DS2), and 1304 (DS3) male and female crossbreed pigs (Pietrain × [Landrace × Large White]), respectively, were included in each trial. Pigs were periodically weighed until slaughter. Then, variables such as length of gestation, length of lactation, parity, litter size, sex, birth BW, and ADG were considered. Pigs remaining on the farm after two loads to the slaughterhouse were defined as last group of animals sent to slaughterhouse (LGS). Descriptive statistics of variability were calculated, and a risk analysis approach was used to look for the factors related to LGS. A multiple logistic regression was performed to identify all variables that were significant (P < 0.05). The risk ratio (RR), odds ratio (OR), and population attributable risk (PAR) were calculated for all of the significant variables after transforming all of them into binary factors using the 25th percentile as the cut-off point. Results showed that the major part of the variability (as CV) comes from birth (20% to 25%) and increased only a little during lactation and 14-d post weaning. From this point onwards, CV tended to decrease, as pigs got closer to the marketing weight (down 11.5% to 12.7%). Regarding the indicators selected, RR, OR, and PAR presented similar trends in the three DS studied. Therefore, for the variables finally included, these indicators had their minimum values at the start of the cycle and then gradually increased at the end. Those results, based on an epidemiological approach, suggest that the closer to the end of the cycle the greater the probability for a light piglet of being/becoming LGS. It might be explained by the shorter available time to efficiently implement preventive measures aimed to improve the performance of delayed pigs and, thus, reducing variability.Those results, based on an epidemiological approach, make sense as the probability for a light piglet to be a LGS increases the closer to the end of the cycle, due to the short time to implement preventive measures and increase the performance of delayed pigs and reduce variability. The differential PAR associated with both, the nursery and the growing period, was 1.7% and 1.5% for DS1, 5.1% and 3.1% for DS2, and 3.7% and 2.8% for DS3. For the lactation period, the results were 4.3% for DS2 and 4.5% for DS3. Results suggest that the most critical periods, in relation to retardation of growth in swine, are lactation and nursery. Implementing measures that maintain risk factors under or above thresholds, especially in the initial phases of growth, will reduce the percentage of LGS pigs and positively affect the overall homogeneity of the batch.
Keywords: correlation, growth curves, pigs, risk factors, variability, coefficient of variation
INTRODUCTION
Body weight (BW) variability in swine production hinders farm efficiency and occupation time, mainly in regard to the growing–finishing facilities, being a limiting factor for the current all-in-all-out swine production systems (Hardy, 1998; Patience et al., 2004). Thus, pigs with a slow growth rate are expected to reach market BW (MBW) later than their faster counterparts, reducing the pig producer’s income and efficiency (Douglas et al., 2014). Most of the economic consequences of a higher variability among cohorts have to do with the quality classification and quotation of the carcasses, mainly due to the lightest pigs within a same batch, which are severely depreciated (Larriestra et al., 2006; Douglas et al., 2014). Furthermore, although for sanitary reasons it is recommended to maintain contemporary pigs in the same batch (Backstrom and Bremer, 1976; Maes et al., 2004), this could negatively influence BW homogeneity since all pigs will be managed as a group, independently of their BW and age associated with each batch farrowing dispersion. Certainly, there exist many variables that may affect pig performance, and, generally, pigs with a delayed growth are the consequence of several factors, such as environment, nutrition, and genetic potential, among others (Botermans et al., 2000; Georgsson and Svendsen, 2002; Quiniou et al., 2002). The growing–fattening is the most expensive period of the pig’s life, accounting for 65% of the total cost of a pig of 109-kg BW (SIP Consultors, 2016). Then, to identify potential risk factors associated with the occurrence of pigs with a delayed growth could be a good strategy to reduce production costs, especially if most of them can be easily modified in order to improve batch homogeneity. Consequently, reducing BW variability will increase the overall efficiency by reducing occupation time of the nursery and growing–fattening facilities. Hence, the aim of the present observational study is to identify risk factors associated with pig’s growth retardation and their relation with BW variability and to quantify the population attributable risk (PAR) in different phases of production in a sequential approach, in three populations of pigs reared in commercial conditions through a risk analysis approach.
MATERIALS AND METHODS
The present work was conducted under the approval of the Animal Ethics Committee of the Universitat Autònoma de Barcelona and was in compliance with the European Union guidelines for the care and use of research animals (European Parliament, 2010).
Three independent populations of pigs were studied in Catalonia (Spain) in commercial conditions. A summary of each data set (DS) is shown in Table 1.
Table 1.
Summary of the three data sets studied, containing information regarding the number of pigs used, the genetic line of sows and piglets, the average length of lactation, and type of management
| Item | Data set 1 | Data set 2 | Data set 3 |
|---|---|---|---|
| Number of pigs, n | 1009 | 460 | 1304 |
| Data collection | December 2013–June 2014 | January 2014–August 2014 | September 2015–April 2016 |
| Pig crossbred line | (P × [Ld × Lw]) | (P × [Ld × Lw]) | (P × [Ld × Lw]) |
| Sex | Males and females | Males and females | Males and females |
| Sow line | Ld × Lw (nucleus) | Ld × Lw (Hermitage) | Ld × Lw (Hypor) |
| Length of lactation, d | 21.0 ± 1.54 | 24.9 ± 2.10 | 21.4 ± 2.15 |
| Type of management | Weekly | 3 wk | 4 wk |
| BW recording | Weaning to slaughter | Birth to slaughter | Birth to slaughter |
In Data set 1 (DS1), pigs were monitored from weaning to slaughter. From weaning to the end of the nursing period (61 d of age, 5.5 wk), the pigs were allocated in a weanling unit facility, and during the growing–finishing period, all piglets were moved to a growing–finishing facility until slaughter.
In Data sets 2 (DS2) and 3 (DS3), pigs were monitored from birth until slaughter. From birth to the end of the nursery period (63 d of age, 9 wk), all animals were in the same facility farrowing unit, and at weaning, all piglets were moved without transport to the weanling unit located within the same farm for the entire nursery period. From this period onwards, pigs were moved to an external growing–finishing facility where they were reared until slaughter.
Animals, Housing, Management, and Diets
In DS1, 1,009 crossbred piglets (Pietrain × [Landrace × Large White]) from approximately 100 litters were used. Animals came from a commercial farm of 2,500 Landrace × Large White sows (Nucleus S.A.S; France) with a weekly batch management production system.
In DS2, a total of 460 crossbred piglets (Pietrain × [Landrace × Large White]) from nearly 40 litters were used. All animals were obtained from a commercial farm of approximately 350 Landrace × Large White sows (Hermitage, Gepork; Spain) that follows a 3-wk batch management production system.
Lastly, in DS3, a total of 1,304 crossbred piglets (Pietrain × [Landrace × Large White]) from 110 litters were used. All animals were acquired from a commercial farm of 500 Landrace × Large White sows (Hypor, Hendrix-Genetics, Netherlands) that follows a 4-wk batch management production system.
In all DS, piglets were cross-fostered by number in the following 24 to 48 h after farrowing. Next, piglets were processed and individually identified by conventional ear tags.
Regarding weaning, in DS1, it was set at 21.0 ± 1.54 d. Immediately thereafter, all piglets were transferred to a nursery facility and housed in three different rooms of 12 pens with 28 animals per pen. Free access to water and feed was guaranteed with a nipple-drinker and a commercial, metal nonlidded hopper with a capacity to feed six animals at a time, respectively.
In DS2, piglets were weaned at 24.9 ± 2.10 d and were also transferred to a nursery accommodation. At this point, all piglets were housed in two weanling rooms of 20 pens with 11 animals per pen. Each pen was equipped with a nipple water drinker and a commercial, INOX-lidded hopper with a capacity to feed three animals at a time.
For DS3, piglets were weaned at 21.4 ± 2.15 d of age, on average, and moved to a nursery unit and housed in four rooms of 24 pens with 23 to 24 animals per pen. Each pen was equipped with a nipple-drinker and a commercial, plastic nonlidded hopper with a capacity to feed five animals at a time.
All nursery accommodations were equipped with central heating and forced ventilation with a cooling system and completely slatted plastic floors. Thereafter, the animals of the three populations were moved to their corresponding external growing–finishing farm. In that period, the different groups of pigs were not maintained in order to not interfere in the routine of the farms. Instead, pigs were again distributed in their respective farms according to sex and approximate BW (according to the experience of the stock workers). Animal density within pens during the growing–fattening phase was 13 pigs per pen (80 pens), 10 to 11 pigs per pen (40 pens), and 13 pigs per pen (80 pens) for DS1, DS2, and DS3, respectively.
To guarantee free access to feed and water for the animals, all pens were equipped with a nipple-drinker and a commercial hopper-feeder with an additional nipple-drinker inside (DS1 and DS3) or a nipple and a concrete hopper with two feeder spaces (DS2).
Regarding the dimensions of each pen, these were 5, 3.2, and 4.5 m2 for the nursery and 9, 7.5, and 8.5 m2 for the growing–finishing facilities in DS1, DS2, and DS3, respectively; in all cases, it was above the minimum space per piglet per pig set by European legislation based on live weight (Council Directive 2008/120/EC of December 2008). All growing–finishing facilities were equipped with natural ventilation and completely slatted concrete floors. Finally, during the nursery and growing–finishing phases, pigs were handled in an all-in-all-out system.
Finally, all diets (Table 2) were offered ad libitum, in pelleted (DS1 and DS3, except for the creep-feed) and mash (DS2) form and formulated to meet or exceed the FEDNA (2013) nutrient requirements.
Table 2.
Summary of the diets (as dry matter) offered to the animals along the productive cycle for the three data sets.
| Nursery | Growing–Fattening | ||||||
|---|---|---|---|---|---|---|---|
| Data sets 1 and 3 | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 |
| Days | 10 | 10 | 20 | 7 | 7 | 40 | 40 onward |
| NE, MJ/kg | 10.8 | 10.6 | 10.6 | 10.4 | 10.0 | 10.2 | 10.2 |
| CP, % | 22.0 | 19.5 | 18.5 | 17.0 | 16.0 | 15.5 | 14.0 |
| d-Lys, % | 1.39 | 1.27 | 1.15 | 1.08 | 1.05 | 0.95 | 0.89 |
| Nursery | Growing–Fattening | ||||||
| Data set 2 | Diet 1* | Diet 2 | Diet 3 | Diet 4 | — | Diet 5 | — |
| Days | 2–3 | 8 | 27 | 64 | — | 64 onwards | — |
| NE, MJ/kg | 11.0 | 10.7 | 10.4 | 9.9 | — | 10.0 | — |
| CP, % | 20.2 | 19.1 | 18.0 | 16.0 | — | 16.0 | — |
| d-Lys, % | 1.37 | 1.32 | 1.20 | 0.99 | — | 0.95 | — |
These diets only refer to the piglets per pigs.
*In data set 2, creep-feed (Diet 1) was offered simultaneously to Diet 1 and for a few days; on the other hand, in data sets 2 and 3, it was offered as a single diet for 10 d.
BW Recording and Selection for Slaughter
In all DS, pigs were periodically and individually weighed throughout the whole production cycle until the day before each group of animals was sent to slaughter, once they reached their MBW, fixed at 103 kg. For the pigs’ BW recording, all animals were weighed using a model Baxtran BR30 scale until the end of the nursery period; on the other hand, from the growing period until slaughter, a model Veserkal Utilcell SWIFT scale was used.
In all cases, the selection for slaughter was performed by picking up the animals that had reached their slaughter weight (103 kg) the day before slaughtering and fasting them overnight. The same procedure was conducted twice more until the finishing barn was emptied. In all three DS, the time elapsed between the first and last load to slaughter was similar, with 42, 44, and 42 d for DS1, DS2, and DS3, respectively. The time from the first load to slaughter to the second one was 20, 23, and 25 d for DS1, DS2, and DS3, respectively. Finally, the time from the second load to the third one was 22, 21, and 17 d for DS1, DS2, and DS3, respectively. In the last truck, the number of pigs was lower compared with the previous two loads, and some of them also did not reach their commercial MBW.
Calculations and Statistical Analyses
The SAS statistical package (SAS Institute Inc., Cary, NC) was used to analyze the data.
Descriptive statistics were calculated for the three DS using the MEANS procedure. All piglets from the three DS were included in the analysis.
In the present study, pigs remaining in the farm after two loads to the slaughterhouse were defined as delayed pigs or last group sent to slaughterhouse (LGS). Epidemiological studies are commonly used in veterinary sciences to know the risk factors associated with the prevalence of a disease (Ruiz-Fons et al., 2008).
The same approach is applied in the present study in order to identify the risk factors associated with the occurrence of LGS within a population of pigs.
Pigs that did not survive until the finishing period were excluded from the analysis because the interest was to analyze the potential risk factors associated with a slow growth rate throughout the productive cycle.
The percentage of pigs defined as LGS was 10%, 21%, and 20% for DS1, DS2, and DS3, respectively. Variables such as length of gestation, length of lactation, parity, litter size, sex, birth BW (BBW), and ADG (0–7, 7–14, 0–28, 21–42, 28–42, 21–64, and 65–83 d) for the three DS were considered. Afterwards, a multiple logistic regression was performed to identify and rank all variables that were significant (P < 0.05) in the three DS studied by using the LOGISTIC procedure (Table 3). Multicollinearity was checked in all explanatory variables included in the final model for each DS. All variables finally included were transformed into binary factors using the 25th percentile as the cut-off point. In the analysis of the association between a binary factor, F, and a binary response, C, several indicators can be used. Thus, the risk ratio (RR), odds ratio (OR), and PAR were calculated since they are the most common.
Table 3.
Summary of the principal risk indicators (and the corresponding thresholds) calculated for the three data sets studied
| Item | RR | OR | PAR (%) | Global PAR (%) |
|---|---|---|---|---|
| Data set 1 | ||||
| BW21 (<4.9 kg) | 6.65 | 8.72 | 5.70 | 9.22 |
| ADG21–61 d (<280 g/d) | 6.93 | 9.42 | 5.38 | |
| ADG61–81 d (<604 g/d) | 13.77 | 19.74 | 7.36 | |
| Data set 2 | ||||
| Birth BW (<1.3 kg) | 2.18 | 2.95 | 3.35 | 15.55 |
| Lactation length (<23 d) | 1.46 | 1.65 | 1.93 | |
| ADG0–28 d (<190 g/d) | 2.20 | 2.95 | 4.46 | |
| ADG28–63 d (<271 g/d) | 4.60 | 8.73 | 10.32 | |
| ADG63–85 d (<505 g/d) | 4.81 | 9.31 | 10.62 | |
| Data set 3 | ||||
| Birth BW (<1.2 kg) | 2.01 | 2.49 | 3.71 | 14.9 |
| Lactation length (<19 d) | 1.53 | 1.73 | 1.44 | |
| ADG7–14 d (<140 g/d) | 2.74 | 3.79 | 5.76 | |
| ADG21–64 d (<300 g/d) | 4.79 | 8.66 | 9.24 | |
| ADG64–83 d (<550 g/d) | 5.70 | 11.21 | 10.26 | |
Herein are presented the values of the values of risk ratio (RR), odds ratio (OR), and population attibutable risk (PAR). Furthermore, the global PAR is also presented in the last column, in which the reduction in the prevalence of LGS in the absence of all of the risk factors is expressed. BW21 refers to BW at weaning.
The association between a binary risk factor, F, and a binary response, C, can be measured from the counts given in a contingence table:
| LGS (C = 1) | No LGS(C = 0) | ||
|---|---|---|---|
| Exposed (F = 1) | a = n11 | b = n10 | a + b = n1 |
| Unexposed (F = 0) | c = n01 | d = n00 | c + d = n0 |
With these notations, the rates are as follows:
Risk rate:
| (1) |
Values of RR >1 mean that the risk in the exposed group is greater than that in the unexposed group.
Odds ratio:
| (2) |
The odds = P/(1 − P) is the rate between a probability P and its complementary 1 − P, which means the opportunities of the event to be a LGS over the opportunities of its contrary. So, the odds ratio is the quotient of odds in the exposed group, with respect to the odds in the unexposed group. A value of OR >1 means that the odds in the exposed group are higher than the odds in the unexposed group. These terms are closely related to logistic regression, which precisely models the logarithm of OR as a linear function of the factors.
Population attributable risk:
| (3) |
PAR is the reduction in incidence that would be observed if the population was entirely unexposed. The computation of PAR depends on the kind of study. In our cohort, retrospective observational design, incidence and prevalence coincide, and PAR can be estimated as in equation 3, that is, the difference between the prevalence in total population minus the prevalence in the unexposed subjects. Therefore, PAR informs concerning how much the prevalence will decrease (in %) if the population is entirely unexposed to that risk factor. Thus, when considering factors that are sequential in time (F1, F2, F3, …, Fj), the PAR differential can be computed, which is the PAR due to factor Fj in the population unexposed to all of the previous risks. To compute this differential PAR, sequentially, the population exposed to the previous risks is omitted, and then, the new PAR is calculated.
Furthermore, BW data for each pig registered throughout the entire experimental period were adjusted to the following double exponential Gompertz function, described in previous studies, such as in Winsor (1932), Fialho (1999), Schinkel et al. (2003), and Casas et al. (2010), by using the NLIN procedure:
| (4) |
where A, b, and c are the parameters (constants) of the curve, and t is the time (measured in d). Only curves that met their convergence criteria were considered; otherwise, those curves were discarded for the study. The predicted time to reach a MBW of 103 kg (T103) was calculated using the above formula.
Finally, the Spearman’s rank correlation coefficient (rho, ρ) was also used to estimate the correlations between the BW categories of the piglets at different stages of production and the expected T103 using the CORR procedure.
In all statistical analyses, significant differences were declared at P ≤ 0.05, whereas 0.05 < P ≤ 0.10 differences were considered near-significant trends.
RESULTS
In this section, the main results for the three DS are presented, starting with the descriptive statistics of variability across all phases of production (Table 4) and the farrowing performance for DS2 and DS3 (Table 5).
Table 4.
Descriptive statistics for DS1, DS2, and DS3, along the productive cycle
| Item | n | Mean | SD | CV | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Data set 1 | ||||||
| BW, kg | ||||||
| Weaning, 21 d | 1,009 | 5.89 | 1.58 | 26.88 | 1.52 | 11.02 |
| 21 d post weaning, 42 d | 986 | 10.37 | 2.30 | 22.16 | 2.50 | 18.54 |
| End nursery period, 61 d | 957 | 19.36 | 3.63 | 18.73 | 8.68 | 30.62 |
| Growing period, 81 d | 952 | 32.91 | 5.70 | 17.33 | 14.50 | 49.50 |
| Fattening period, 116 d | 949 | 57.66 | 8.63 | 14.97 | 21.60 | 78.80 |
| Finishing, 145 d | 935 | 81.96 | 10.20 | 12.45 | 44.00 | 109.40 |
| Slaughter*, 159 d | 933 | 93.68 | 10.65 | 11.37 | 52.00 | 122.80 |
| ADG, kg/d | ||||||
| Nursery, 21–61 d | 957 | 0.33 | 0.07 | 21.22 | 0.14 | 0.57 |
| Growing, 61–116 d | 945 | 0.70 | 0.11 | 15.13 | 0.12 | 1.00 |
| Finishing, 116–159 d | 932 | 0.84 | 0.11 | 13.67 | 0.26 | 1.20 |
| Total, 21–159 d | 933 | 0.64 | 0.07 | 11.30 | 0.34 | 0.83 |
| Time to 103 kg, d | ||||||
| T103† | 928 | 173.56 | 16.44 | 9.47 | 139.85 | 275.65 |
| Data set 2 | ||||||
| BW, kg | ||||||
| Birth | 460 | 1.60 | 0.33 | 20.68 | 0.60 | 2.60 |
| Weaning, 28 d | 394 | 7.21 | 1.75 | 24.26 | 3.10 | 11.90 |
| 14 d post weaning, 42 d | 381 | 9.37 | 2.29 | 24.43 | 3.40 | 14.70 |
| End nursery period, 63 d | 369 | 18.67 | 4.40 | 23.56 | 6.70 | 27.90 |
| Growing period, 85 d | 367 | 31.50 | 7.11 | 22.55 | 11.00 | 46.00 |
| Growing, 106 d | 372 | 48.04 | 8.90 | 18.53 | 19.50 | 65.10 |
| Fattening, 127 d | 371 | 65.46 | 10.32 | 15.77 | 30.00 | 88.50 |
| Finishing period, 148 d | 369 | 83.91 | 11.52 | 13.73 | 43.50 | 111.50 |
| Slaughter‡, 170 d | 364 | 99.39 | 12.50 | 12.58 | 50.50 | 133.00 |
| ADG, kg/d | ||||||
| Lactation, 0-Weaning | 379 | 0.20 | 0.06 | 28.86 | 0.05 | 0.36 |
| Nursery, 28–63 d | 369 | 0.32 | 0.10 | 30.23 | −0.02 | 0.52 |
| Growing, 63–127 d | 358 | 0.73 | 0.11 | 15.40 | 0.32 | 0.99 |
| Finishing, 127–170 d | 364 | 0.79 | 0.13 | 16.68 | −0.07 | 1.12 |
| Total, 0–170 d | 343 | 0.58 | 0.07 | 12.62 | 0.29 | 0.77 |
| Time to 103 kg, d | ||||||
| T103† | 349 | 175.09 | 17.68 | 10.10 | 141.26 | 272.51 |
| Data set 3 | ||||||
| BW, kg | ||||||
| Birth | 1,304 | 1.41 | 0.36 | 25.92 | 0.44 | 2.62 |
| Weaning, 21 d | 1,102 | 5.52 | 1.67 | 30.30 | 1.48 | 11.56 |
| 21 d post weaning, 42 d | 1,029 | 9.98 | 2.74 | 27.43 | 1.10 | 18.50 |
| End nursery period, 64 d | 1,049 | 20.58 | 4.51 | 21.89 | 4.00 | 34.30 |
| Growing period, 83 d | 1,063 | 32.27 | 6.25 | 19.36 | 13.80 | 52.50 |
| Growing period, 104 d | 1,059 | 47.90 | 8.10 | 16.82 | 23.60 | 71.30 |
| Fattening, 125 d | 1,058 | 66.10 | 9.97 | 15.10 | 20.80 | 96.80 |
| Finishing period, 146 d | 1,056 | 83.10 | 11.07 | 13.33 | 43.40 | 117.0 |
| Slaughter‡, 163 d | 1,053 | 95.14 | 12.10 | 12.70 | 50.60 | 130.90 |
| ADG, kg/d | ||||||
| Lactation, 0-Weaning | 1,099 | 0.19 | 0.07 | 38.43 | 0.01 | 0.45 |
| Nursery, 21–64 d | 1,040 | 0.35 | 0.08 | 24.10 | 0.02 | 0.67 |
| Growing, 64–125 d | 1,042 | 0.74 | 0.11 | 14.88 | 0.13 | 1.11 |
| Finishing, 125–163 d | 1,053 | 0.76 | 0.13 | 17.13 | 0.08 | 1.54 |
| Total, 0–163 d | 1,026 | 0.57 | 0.07 | 12.83 | 0.31 | 0.79 |
| Time to 103 kg, d | ||||||
| T103† | 1,024 | 174.40 | 19.44 | 11.14 | 132.83 | 296.13 |
The variables considered are especially the BW, ADG, and T103 (time to reach MBW). The information includes the mean, SD, CV, and the minimum and maximum values for each variable.
*Corresponds to the last measurements with all pigs present in the finishing barn but 10 more days elapsed/passed, approximately, until pigs were sent to slaughter.
†The time to reach market body weight (MBW) was estimated (not measured) from the Gompertz model.
‡Corresponds to the last measurement just before the first group of animals was sent to slaughter the next day.
Table 5.
Farrowing performance for data sets 2 and 3
| Number of sows | Mean | SD | Min | Max | |
|---|---|---|---|---|---|
| Data set 1* | |||||
| — | — | — | — | — | |
| Data set 2 | |||||
| Parity | 37 | 4.3 | 1.68 | 1.00 | 7.00 |
| TB | 37 | 14.70 | 2.43 | 9.00 | 20.00 |
| LB | 37 | 13.58 | 2.19 | 9.00 | 19.00 |
| SB | 37 | 1.12 | 1.20 | 0.00 | 5.00 |
| MM† | 37 | — | — | — | — |
| Data set 3 | |||||
| Parity | 110 | 3.77 | 2.50 | 1.00 | 10.00 |
| TB | 110 | 14.90 | 3.91 | 6.00 | 24.00 |
| LB | 110 | 12.62 | 3.76 | 2.00 | 21.00 |
| SB | 110 | 2.08 | 2.07 | 0.00 | 9.00 |
| MM | 110 | 0.20 | 0.62 | 0.00 | 4.00 |
The information includes the mean, SD, CV, and the minimum and maximum values for each variable.
LB = live born; MM = mummified; SB = stillborn; TB = total born.
*Not available.
†Not available.
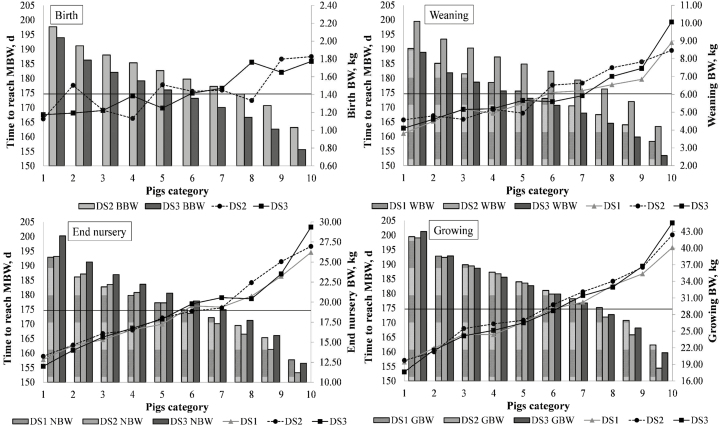
Next, the principal risk factors related to slow growth are presented for all DS (Table 3). Additionally, the results regarding the time to reach MBW for each pig category (based on live weight) are summarized in Figure 1.
Figure 1.
Distribution of each 10% of pigs, sorted from heaviest (1) to lightest (2) in data set (DS)1, DS2, and DS3 at birth, weaning, end of nursery, and growing period. The horizontal line relates to the average number of days to reach MBW (t103, 174.6 d). Birth refers to 0 d of age; weaning refers to 21 d of age (DS1 and DS3) or 28 d of age (DS2); end nursery refers to around 64 d of age; growing refers to around 83 d of age; and finishing refers to 125 d of age (116 d for DS1). DS1, data set 1; DS2, data set 2; DS3, data set 3; BBW, birth BW; WBW, weaning BW; NBW, body weight at the end of nursery period; GBW, growing BW.
Concerning the productive parameters and mortality, the results obtained remained within the normal ranges observed in standard production values in the Spanish (and by extension European) pig industry. Mortality was around 5.55%, 6.33%, and 3.50% for the nursery period and 2.0%, 1.5%, and 1.0% for the growing–finishing phase in DS1, DS2, and DS3, respectively.
Also, in this section, the results are presented for all three DS combined or only focused on one particular DS when necessary.
Descriptive Statistics of Variability and Farrowing Performance
Regarding BW, in all three DS, it is observed that the mean and SD increased with age until pigs were slaughtered (Table 4). However, CV presented the highest value at early stages of production (26.9% at weaning for DS1, 24.5% at 14-d post weaning for DS2, and 30.30% at weaning for DS3) and then gradually decreased to 11.4%, 12.6%, and 12.7% at days 159, 170, and 163 in DS1, DS2, and DS3, respectively (when the first group of animals was sent to slaughter). Despite the fact that in DS2 and DS3, the CV increased to 24.4% at 14-d post weaning and 30.3% at weaning, respectively, the highest portion of variability came directly from birth (as can be observed in DS2 and DS3, with a CV of around 21% and 26%, respectively) and then tends to decrease from weaning or 14-d post weaning until the end of the cycle.
In terms of ADG, the pattern observed is quite similar to what occurred with BW; excluding the whole-period values (from weaning in DS1 or birth until slaughter in DS2 and DS3), the mean and SD increased throughout the cycle. The highest CV was generally observed during the nursery period (21.2%, 30.2%, and 24.1%) and then decreased until the finishing phase in DS1, DS2, and DS3, respectively (but it was also high during lactation, with values ranging from 28.9% to 38.4% in DS2 and DS3). Furthermore, considering the time to reach the MBW (T103), the results were also very similar for the three DS studied.
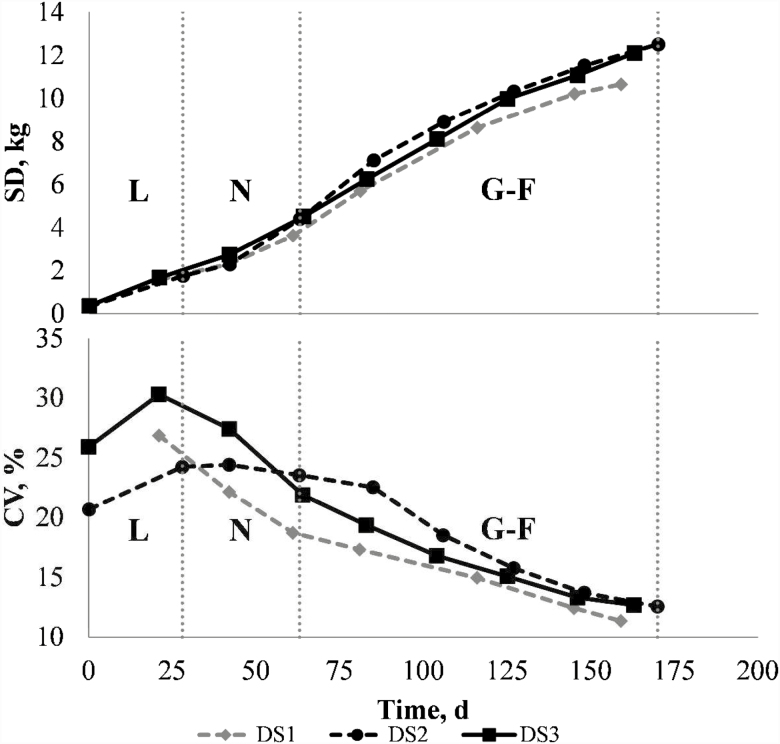
Some of the information in Table 4, regarding the trends for BW in terms of SD and CV over time, is graphically summarized in Figure 2 for all DS studied. It is observed that the trends in the three populations follow a similar pattern, that is, the increase of SD and the decrease of CV according to the age of pigs.
Figure 2.
Evolution of BW along productive cycle but represented in terms of SD (above) or CV (below). L, for Lactation; N, Nursery; G–F, Growing–finishing. DS1, data set 1; DS2, data set 2; DS3, data set 3.
Finally, regarding farrowing performance (Table 5), the results were similar in DS2 and DS3 (no data were available for DS1). However, the number of total born was a little higher in DS3, showing a lower birth weight on average (Table 4). The number of stillborn piglets was also numerically higher in DS3, when compared with DS2, with one sow presenting nine stillborn piglets.
Risk Factors
None of the variables finally included in the logistic models presented collinearity (VIF < 5). Each risk factor has a threshold below which the population of pigs is exposed to that particular risk (see MATERIALS AND METHODS), leading to an increase in the probability of being in the LGS (Table 3). In the case of DS1, the lactation period was not considered since data were not available. Thus, information only refers to weaning onwards; also, sow variables were not considered. Regarding the other two DS (DS2 and DS3), more data were available and risk factors related to the sows and their offspring were studied, comprising information from birth onwards.
First of all, similar trends can be observed in the three populations of pigs. In all cases, RR and OR tended to increase over time by adding factors related to the different phases of production. In the cases of DS2 and DS3, results of RR are more similar (ranging from RR values of 1.46 or/and 1.53 up to 4.81 or/and 5.70, respectively). For DS1, however, although the trend was similar to the other two DS, there was a difference in the magnitude of the values (RR values ranging from 6.65 to 13.77). In the case of OR, the trend was quite similar to that observed in RR. Regarding ADG during lactation, the periods included were a little bit different between DS2 and DS3 (190 g/d for ADG from 0 to 28 d in DS2 or 140 g/d for ADG from 7 to 14 d in DS3). In the case of BBW, the cut-off points were similar for DS2 and DS3 (1.3 and 1.2 kg, respectively). Moreover, the cut-off points for ADG during the nursery period were similar in DS1 and DS2 (280 and 271 g/d) and slightly higher in DS3 (300 g/d). Looking at the ADG for the growing period, the cut-off points were established at 604, 505, and 550 g/d for DS1, DS2, and DS3, respectively. In the case of DS1, the BW at weaning was entered in the analysis, with a cut-off point of 4.9 kg. Finally, for DS2 and DS3, lactation length was also considered, with cut-off points of 23 and 19 d, respectively.
In the three DS studied, the results for PAR also follow the same trend, the risk factors closer to slaughter being the major responsible factors in the reduction of the prevalence to that particular risk if it were not present. Then, PAR ranged from 5.70 to 7.36 in DS1, from 1.93 to 10.62 in DS2, and, finally, from 1.44 to 10.26 in DS3. Regarding the differential PAR, the results are also pretty similar in the three DS studied, and the highest reduction is generally observed during lactation and nursery (Table 6). The growing–fattening periods presented the lowest differential PAR. Finally, regarding the global PAR (that is, the reduction in the prevalence of LGS in the absence of all the risk factors), it was quite similar for DS2 and DS3 (15.6% and 14.9%) and slightly lower in DS1 (9.2%) (Table 3). So, if all risk factors in each DS were eliminated, the percentage of LGS would be 1.0% instead of 10% for DS1, 5.5% instead of 21% for DS2, and, lastly, 5.1% instead of 20% for DS3, respectively.
Table 6.
Results for the differential PAR in the three data sets studied, which is the PAR due to a factor in the population unexposed to all previous risks
| Period | Differential PAR (%) | ||
|---|---|---|---|
| Data set 1 | Data set 2 | Data set 3 | |
| Lactation | — | 4.3 | 4.5 |
| Nursery | 1.7 | 5.1 | 3.7 |
| Growing–fattening | 1.5 | 3.1 | 2.8 |
The lactation period comprises from 0 to 21 d (DS3) or/and 28 d (DS2); nursery comprises from 21/28 to 63 d; and growing period comprises from 64 to 83 d.
Time to Sacrifice Regarding BW Category and Production Phase
The values of T103 were 173.6 ± 16.44 d, 175.1 ± 17.68 d, and 174.4 ± 19.44 d for DS1, DS2, and DS3, respectively (Table 4).
In Figure 1, four series of two-axis graphs are presented in order to illustrate the relationship between the BW category in different stages of production and the time needed to reach MBW for the three DS studied. Regarding the y-axis, the scale on the left-hand side corresponds to the time to reach 103 kg (T103), represented by lines, whereas the scale on the right-hand side corresponds to the 10 categories of BW represented in a bar chart.
When the entire pig population is sorted and categorized into 10 groups of an equal number of animals (based on their BW) from the heaviest (Group 1) to the lightest (Group 10), a clear pattern can generally be observed; in terms of time taken to reach MBW, pigs in the heavier categories were below average (horizontal line); meanwhile, pigs in the lighter categories were above the average and more time was needed to reach the same point. However, this trend was slightly different depending on the production phase (birth, weaning end of nursery, and start of growing period).
Thus, in DS1, DS2, and DS3 (Figure 1), when the effect of sorting the animals at weaning or at the end of the nursery period, regarding the number of days to reach MBW was compared, the results were not exactly the same. Better BW at weaning does not guarantee that pigs will reach MBW earlier than will their littermates. This fact was observed in the three DS, but especially in DS2; in contrast, the closer to the end of the cycle, the classification of pigs was performed (i.e., BW at the end of the nursery period or during the growing period), the more defined was the pattern observed (it being more probable that heavier pigs reach MBW earlier than do lighter pigs).
For BW at birth (BBW), it was clearly observed that pigs in their first stage of life do not necessarily behave as expected, concerning the number of days needed to reach MBW, depending on their BW category (DS2 and DS3). The increase in this relation, then, is stronger from weaning onwards, when the time pigs need to reach MBW is more related to the BW category they belong to. The Spearman correlation coefficients showed the same trend, increasing the correlation between the BW of the animals and the time to reach MBW (T103) with age (Table 7). Consequently, the correlations ranged from around 0.30 to 0.85–0.89 (P < 0.0001) from birth to the finishing period (around day 125) for all DS (no birth data available for DS1).
Table 7.
A summary of Spearman correlations between BW at different periods with the time to reach MBW (T103) for the three data sets studied
| Item | Birth-T103 | Weaning-T103 | Nursery-T103 | Growing-T103 | Finishing-T103 |
|---|---|---|---|---|---|
| Data set 1* | — | ρ = −0.49 | ρ = −0.68 | ρ = −0.74 | ρ = −0.85 |
| — | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | |
| Data set 2 | ρ = −0.36 | ρ = −0.51 | ρ = −0.70 | ρ = −0.78 | ρ = −0.89 |
| (P <.0001) | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | |
| Data set 3 | ρ = −0.31 | ρ = −0.46 | ρ = −0.71 | ρ = −0.77 | ρ = −0.89 |
| (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P < 0.0001) |
Birth refers to 0 d of age; weaning refers to 21 d of age (DS1 and DS3) or 28 d of age (DS2); nursery refers to around 64 d of age; growing refers to around 83 d of age; and finishing refers to 125 d of age (116 d for DS1).
*No data available for birth.
ρ = rho or Spearman correlation coefficient.
DISCUSSION
An unequal growth rate within a group of pigs leads to an increase in BW variability, resulting in a decrease of the global efficiency throughout the productive cycle. The literature reported some factors responsible for this variability, like BBW (Quiniou et al., 2002; Fix et al., 2010), suckling position during lactation, sex, season of birth, weaning BW (Paredes et al. 2012, 2014), environment, nutrition, and genetic potential (Botermans et al., 2000; Georgsson and Svendsen, 2002; Quiniou et al., 2002). Nevertheless, it is very challenging to study and quantify all of the factors involved in BW variability in one trial because of the several sources of variation potentially interacting at the same time. Therefore, an epidemiological approach was conducted to highlight numerous risk factors (BBW, day of lactation, weaning BW, and ADG) related to the occurrence of LGS in commercial conditions.
To observe the evolution of the variability, the CV is the statistic preferred, as it is corrected by the mean, allowing the comparison of pigs’ variability at different moments or phases of production. Thus, the variability of pigs tends to decrease with age (in line with results obtained by Patience et al. (2004) and López-Vergé et al. (2015)); thus, the major portion of variability generally comes from birth, although it still increases a little during lactation or even 14-d post weaning (Table 4). The decrease of variability with age could be a consequence of the different management practices implemented in the farms, like cross-fostering as one of the most obvious (Patience et al., 2004), but also others like segregation by BW or gender. Sows are highly prolific animals, when compared with other farm animals, leading to a wide variation in BBW, and this is more exacerbated with the use of modern hyper-prolific sow breeds (Foxcroft et al., 2007). In recent years, litter size has increased and the piglet’s average BBW has decreased (Quiniou et al., 2002; Devillers et al., 2007; Wolf et al., 2008). Our results are also in line with other recent works (Beaulieu et al., 2010; Baxter et al., 2013; Rutherford et al., 2013).
BBW affects future pig performance and survivability (Neal and Irvin, 1991; Wattanaphansak et al., 2002; Paredes et al., 2012; Panzardi et al., 2013; Douglas et al., 2014). Therefore, when BBW is below a critical threshold, it may negatively affect the subsequent postweaning growth of pigs. To maximize the piglets’ BBW in order to improve their growth rate and diminish the time to marketing, weight is a major issue. Consequently, BBW was included as a risk factor to predict pig growth rate to slaughter in the present study.
Variability in postnatal growth may arise from management deficiencies, but it can also be a result of pigs born small or growing markedly slower (defined as LGS in the present work). To slow the growth rate of heavier pigs to reduce variability is not an option, but raising the growth rate of small pigs, exploiting their ability to compensate (Handel and Stickland, 1988) will be a preferred approach. Only farrowing performance for DS2 and DS3 was available. In the present work, the farrowing performance information for DS2 and DS3 is similar to the average Spanish numbers, with 14.6, 13.5, and 1.16 total born, live-born, and stillborn piglets per sow, respectively (Bdporc, 2016), confirming the representativeness of our data. It is worth mentioning that the BBW only included piglets that survived until slaughter; otherwise, they were discarded for the study (see MATERIALS AND METHODS). The RR results were similar in DS2 and DS3, partly because it shared almost the same information (RR values ranging from 1.46 or 1.53 up to 4.81 or 5.70, respectively). The 25th percentile was defined as the threshold for all risk factors finally included. Consequently, piglets born below that threshold (1.3 or 1.2 kg for DS2 and DS3, respectively) were not only light at weaning, but also at slaughter; thus, those piglets weighed 1.49 and 1.15 kg less at weaning and 8.70 and 8.24 kg less at slaughter, respectively, than did piglets born in the higher percentiles. These findings are in line with the results of Panzardi et al. (2013), when it was observed that piglets born with ≤1.3 kg weighed 1.2 kg less at weaning than did piglets born >1.3 kg. In fact, they concluded that 1.3 kg was the critical value to ensure a good preweaning and postweaning growth (but they did not follow the pigs to slaughter). However, other cut-off points could be explored, like a BBW <2 SD from the average, resulting in a cut-off point below 1 kg, as proposed by Paredes et al. (2012). Regarding BBW, in the current study, piglets born below 1.3 or 1.2 kg in DS2 and DS3, respectively, had 1.46 to 1.53 times more risk to be a LGS. These probabilities should increase as the threshold decreases. Furthermore, in the case of DS1, the trend was similar, but the magnitude of the values differed because information regarding birth and lactation was not available. Concerning OR, the results were equivalent to those observed with RR. In the case of DS3, piglets were weighed up to four times during lactation and only twice for DS2, leading to differences in the threshold of the significant variables finally included. Lactation length also appeared to be a significant risk factor affecting the growth rate of piglets. Therefore, previous results from our group (López-Vergé et al., 2017) showed that by increasing lactation length, the pig’s BW increased linearly at weaning and, more interestingly, also increased previously to slaughter. Thus, weaning BW is useful to predict future performance as well as time to MBW (Mahan and Lepine, 1991; Tokach et al., 1992). In DS1, with no available data for BBW, BW at weaning was observed to be a significant risk factor in DS1 and was finally included in the analysis. Therefore, piglets <4.87 kg had almost seven times more risk to be a LGS than did piglets with a higher BW at weaning, meaning that weaning BW is indeed a good predictor of future performance. Nevertheless, other studies did not find positive effects of increasing the weaning weight in the growth to slaughter (Kim et al., 2001; English and Bilkei, 2004; Morise et al., 2011; Douglas et al., 2014).
The parity of the sows was another factor analyzed (DS2 and DS3), and the results showed a higher probability to produce LGS pigs for gilts than for sows. Dam parity appears to be a good predictor of piglet survival and growth; moreover, the risk of pigs with slower growth in gilt progeny is higher relative to sow progeny (Larriestra et al., 2006). Parity was not considered a risk factor per se (concerning gilts) in our results.
Concerning ADG, it is directly related to BW, partly driving the ADG of the pigs. Therefore, BBW constitutes the first step to ensure a good ADG onwards, although it is not the only one. As a result, other factors may have an influence modulating ADG, depending on the phase of production. During lactation, cross-fostering is a widely used technique to deal with large litter sizes and high BW variability within litters (Baxter et al., 2013) and increase piglet growth rates (Wattanaphansak et al., 2002). Recently, Huting et al. (2017) reported that cross-fostering improves the performance of light piglets when they are fostered uniformly, and this weight advantage was maintained to slaughter. Several practices are known to decrease ADG in this period, such as tooth clipping (Brown et al., 1996) or castration in the first 3 d after birth (Kielly et al., 1999). In the present study, however, none of the piglets were castrated because the MBW (fixed at 103 kg) made it unnecessary. Moreover, creep-feed stimulates intake and postweaning growth in piglets that eat it during lactation, as Bruininx et al. (2002), Carstensen et al. (2005), Sulabo et al. (2010), and Blavi et al. (2015) pointed out. Nevertheless, there are studies that argue that creep-feed has no effect (Morrison et al., 2008). The current weaning management routines are also important; as an example, in DS2 and DS3, pigs were managed in three- and four-batch management systems, leading to a huge variation in piglet age at weaning and, consequently, in BW and their adaptive status of their gut to the diets offered in the nursery. In weekly management systems, the variation in age and BW is lower (Table 4, DS1). After weaning, the first week is crucial in order to achieve an adequate subsequent ADG (Tokach et al., 1992).
Beyond lactation, aspects related to space allowance (Flohr et al., 2016) as well as dominance status (social hierarchy) and access to the feed will be more important factors taking place, affecting the ADG of pigs, among other variables. Again, the BW itself will continue to drive the ADG of most of the pigs so that heavier animals will present a higher ADG. Feed intake was not measured in the current study so that we can only assume a higher intake for heavier animals leading to greater growth (van Milgen, 2008). All pigs exposed to the previous risk factors (Table 3) could be considered a population at risk; PAR informs how much the prevalence will decrease (in %) if the population was entirely unexposed to a particular risk factor. Thus, the higher the risk (expressed as RR or OR), the higher the decrease in the prevalence for that risk. In the end, the differential PAR also followed the same trend for the three DS studied, despite the fact that DS1 had no data available regarding birth. The PAR results pointed out that the most critical periods, in relation to retardation of growth rate in swine, are lactation and nursery. Our results showed that animals with a higher BW invest fewer days to reach MBW, in agreement with previous works (Powell and Aberle, 1980; Quiniou et al., 2002; Le Dividich et al., 2003; Smith et al., 2007; Rehfeldt et al., 2008; Douglas et al., 2014). Nevertheless, this evidence varies, depending on the phase of production. Hence, a trend can be observed in terms of the number of days it takes to reach MBW. When pigs are categorized into groups of 10%, from the heaviest to the lightest, it is observed that pigs with a higher BW generally take fewer days to reach MBW than do pigs with a lower BW (Figure 1). However, considering the different phases in the swine cycle, the closer to the end of the cycle the classification of pigs is made (i.e., BW at the end of the nursery period), the more defined the pattern is, as is shown in Figure 1 and the Spearman correlation coefficients in Table 6. In fact, for the three DS, the two charts at the bottom of Figure 1 have very similar shapes, but it is clearly different from the two upper graphs (weaning and birth). It follows that, for instance, the probability of a piglet to recover or to change BW category is higher at birth than at the end of the nursery period. It is also worth to mention that the three DS reached similar conclusions. The simplest interpretation would be to try to maximize BW throughout the productive cycle, considering that beyond weaning, the movement between BW categories is less common. As the correlation between BW and time to MBW increases along the cycle, early strategies to maximize pigs’ BW in the previous phases of production should be worth improving the BW in subsequent stages and, lastly, decreasing the time to MBW.
In summary, results indicate that the largest portion of BW variability in the swine industry comes directly from birth, and it slightly increases through lactation and the first day post weaning; from this point until slaughter, variability tends to decrease. The risk analysis results suggest that BBW, lactation length, weaning BW, and ADG are important factors to take into account in order to decrease the probability of obtaining LGS pigs. It was also observed that from weaning onwards, the piglets’ category is maintained (due to a stronger correlation between BW at weaning and subsequent phases of production), making it difficult for lighter piglets to catch up with their weightier counterparts. Finally, the present study presents some insights suggesting that the most critical periods in relation to retardation of growth rate are lactation and nursery.
This manuscript has been proofread by Mr Chuck Simmons, a native, English-speaking university professor of English.
LITERATURE CITED
- Backstrom L., and Bremer H.. 1976. Disease recording of pigs at slaughter as a method of preventive medicine in the swine production. Svensk Veterinärtidning Tidningen 28:312–336. [Google Scholar]
- Banco de datos de referencia del porcino español (BdPorc).. 2016. [accessed February, 2018]. http://www.bdporc.irta.es/informes/PartPublica/Datos%20publicos%20Anyo%202016.htm.
- Baxter E. M., Rutherford K. M. D., D’Eath R. B., Arnott G., Turner S. P., Sandøe P., Moustsen V. A., Thorup F., Edwards S. A., and Lawrence A. B.. 2013. The welfare implications of large litter size in the domestic pig II: management factors. Anim. Welf. 22:219–238. doi: 10.7120/09627286.22.2.219 [DOI] [Google Scholar]
- Beaulieu A. D., Aalhus J. L., Williams N. H., and Patience J. F.. 2010. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88:2767–2778. doi: 10.2527/jas.2009-2222. [DOI] [PubMed] [Google Scholar]
- Blavi L., Solà-Oriol D., and Pérez J. F.. 2015. Effect of supplementary feeding strategies during the suckling period to improve weanling performance. In: Proceedings of the 13th Digestive Physiology of the Pig Symposium;Kliczków, Poland p. 49. [Google Scholar]
- Botermans A. M., Georgsson L., Weströn B. R., Olson A., and Svendsen J.. 2000. Effect of feeding environment on performance, injuries, plasma cortisol and behavior in growing-finishing pigs: studies on individual pigs housed in groups. Acta Agric. Scand. Sect. A. 50:250–262. doi: 10.1080/090647000750069449 [DOI] [Google Scholar]
- Brown J. M. E., Edwards S. A., Smith W. J., Thompson E., and Duncan J.. 1996. Welfare and production implication of teeth clipping and iron injection in outdoor systems in Scotland. Prev. Vet. Med. 27:95–105. doi:10.1016/0167-5877(96)01013-6 [Google Scholar]
- Bruininx E. M., Binnendijk G. P., van der Peet-Schwering C. M., Schrama J. W., den Hartog L. A., Everts H., and Beynen A. C.. 2002. Effect of creep feed consumption on individual feed intake characteristics and performance of group-housed weanling pigs. J. Anim. Sci. 80:1413–1418. doi:10.2527/2002.8061413x [DOI] [PubMed] [Google Scholar]
- Carstensen L., Ersbøll A. K., Jensen K. H., and Nielsen J. P.. 2005. Escherichia coli post-weaning diarrhoea occurrence in piglets with monitored exposure to creep feed. Vet. Microbiol. 110:113–123. doi: 10.1016/j.vetmic.2005.07.011 [DOI] [PubMed] [Google Scholar]
- Casas G. A., Rodríguez D., and Afanador G.. 2010. Propiedades matemáticas del modelo de Gompertz y su aplicación al crecimiento de los cerdos [accessed May, 2016]. http://www.rccp.udea.edu.co/index.php/ojs/article/view/455/561.
- Devillers N., Farmer C., Le Dividich J., and Prunier A.. 2007. Variability of colostrum yield and colostrum intake in pigs. Animal 1:1033–1041. doi: 10.1017/S175173110700016X [DOI] [PubMed] [Google Scholar]
- Douglas S. L., Edwards S. A., and Kyriazakis I.. 2014. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 92:2280–2288. doi: 10.2527/jas.2013-7388 [DOI] [PubMed] [Google Scholar]
- English J. G. H., and Bilkei G.. 2004. The effect of litter size and littermate weight on pre-weaning performance of low-birth-weight piglets that have been crossfostered. Anim. Sci. 79:439–443. doi:10.1017/S1357729800090305 [Google Scholar]
- European Parliament. Legislation for the protection of animals used for scientific purposes. 2010. [accessed July 6, 2018]. http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm
- FEDNA.. 2013. Fundación española desarrollo nutrición animal. In: De Blas C., Gasa J., and Mateos G.G., editors. Necesidades nutricionales para ganado porcino. 2nd ed. Madrid, Spain: FEDNA. p. 109. [Google Scholar]
- Fialho F. B. 1999. Interpretação da Curva de Crescimento de Gompertz. Comunicado técnico. Embrapa-CBPSA 237:1–4. [Google Scholar]
- Fix J. S., Cassady J. P., Holl J. W., Herring W. O., Culbertson M. S., and See M. T.. 2010. Effect of piglet birth weight on survival and quality of commercial market swine. Livest. Sci. 132:98–106. doi: 10.1016/j.livsci.2010.05.007 [DOI] [Google Scholar]
- Flohr J. R., Tokach M. D., DeRouchey J. M., Woodworth J. C., Goodband R. D., and Dritz S. S.. 2016. Evaluating the removal of pigs from a group and subsequent floor space allowance on the growth performance of heavy weight finishing pigs. J. Anim. Sci. 94:4388–4400. doi: 10.2527/jas2016-0407 [DOI] [PubMed] [Google Scholar]
- Foxcroft G., Bee G., Dixon W., Hahn M., Harding J., Patterson J., Putman T., Sarmento S., Smit M., Tse W., et al. 2007. Consequences of selection for litter size on piglet development. In: Wiseman J, et al. , editors. Paradigms of pig science. Nottingham (UK): Nottingham University Press; p. 207−229. [Google Scholar]
- Georgsson L., and Svendsen J.. 2002. Degree of competition at feeding differentially affects behavior and performance of group-housed growing-finishing pigs of different relative weights. J. Anim. Sci. 80:376–383. doi:10.2527/2002.802376x [DOI] [PubMed] [Google Scholar]
- Handel S. E., and Stickland N. C.. 1988. Catch-up growth in pigs—a relationship with muscle cellularity. Anim. Prod. 47:291–295. doi:10.1017/S000335610000338X [Google Scholar]
- Hardy B. 1998. Management of large units. In: Wiseman J., Varley M.A., and Chadwich J.P., editors. Progress in pig science. Cambridge (UK): University Press; p. 561−581. [Google Scholar]
- Huting A. M. S., Almond K., Wellock I., and Kyriazakis I.. 2017. What is good for small piglets might not be good for big piglets: the consequences of cross-fostering and creep feed provision on performance to slaughter. J. Anim. Sci. 95:4926–4944. doi: 10.2527/jas2017.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielly J., Dewey C. E., and Cochran M.. 1999. Castration at 3 days of age temporarily slows growth of pigs. Swine Health Prod. 7:151–153. [Google Scholar]
- Kim J. H., Heo K. N., Odle J., Han K., and Harrell R. J.. 2001. Liquid diets accelerate the growth of early-weaned pigs and the effects are maintained to market weight. J. Anim. Sci. 79:427–434. doi:10.2527/2001.792427x [DOI] [PubMed] [Google Scholar]
- Larriestra A. J., Wattanaphansak S., Neumann E. J., Bradford J., Morrison R. B., and Deen J.. 2006. Pig characteristics associated with mortality and light exit weight for the nursery phase. Can. Vet. J. 47:560–566. [PMC free article] [PubMed] [Google Scholar]
- Le Dividich J., Martineau G. P., Madec F., and Orgeur P.. 2003. Saving and rearing underprivileged and supernumerary piglets, and improving their health at weaning. In: Pluske J.R., et al., editors. Weaning the pig, concepts and consequences. Wageningen (Netherlands):Wageningen Pers; p. 361−383. [Google Scholar]
- López-Vergé S., Solà-Oriol D., and Gasa J.. 2015. Is the lactation period the main variable responsible for reducing the efficiency of the swine production?J. Anim. Sci. 93, Suppl. s3/J. Dairy Sci. Vol. 98, Suppl p.184. [Google Scholar]
- López-Vergé S., Solà-Oriol D., Bonet J., Coma J., and Gasa J.. 2017. Effect of the lactation length of piglets on their later performance. J. Anim. Sci. 95(supplement2):31–32. [Google Scholar]
- Maes D. G., Duchateau L., Larriestra A., Deen J., Morrison R. B., and de Kruif A.. 2004. Risk factors for mortality in grow-finishing pigs in Belgium. J. Vet. Med. B. Infect. Dis. Vet. Public Health 51:321–326. doi: 10.1111/j.1439-0450.2004.00780.x [DOI] [PubMed] [Google Scholar]
- Mahan D. D., and Lepine A. J.. 1991. Effect of pig weaning weight and associated nursery feeding programs on subsequent performance to 105 kilograms body weight. J. Anim. Sci. 69:1370. [DOI] [PubMed] [Google Scholar]
- van Milgen J., Valancogne A., Dubois S., Dourmad J.Y., Sève B., and Noblet J.. 2008InraPorc: a model and decision support tool for the nutrition of growing pigs. Anim. Feed Sci. Technol. 143:387–340. doi:10.1016/j.anifeedsci.2007.05.020 [Google Scholar]
- Morise A., Sève B., Macé K., Magliola C., Le Huërou-Luron I., and Louveau I.. 2011. Growth, body composition and hormonal status of growing pigs exhibiting a normal or small weight at birth and exposed to a neonatal diet enriched in proteins. Br. J. Nutr. 105:1471–1479. doi: 10.1017/S0007114510005386 [DOI] [PubMed] [Google Scholar]
- Morrison R., Pluske J., Smits R., Henman D., and Collins C.. Creep feeding, weaning age interactions with creep feeding. In: Feed Intake Innovations. Australia: Cooperative Research Centre; 2008. Section 2B, Report 3-3. [Google Scholar]
- Neal S. M., and Irvin K. M.. 1991. The effects of crossfostering pigs on survival and growth. J. Anim. Sci. 69:41–46. doi:10.2527/1991.69141x [DOI] [PubMed] [Google Scholar]
- Panzardi A., Bernardi M. L., Mellagi A. P., Bierhals T., Bortolozzo F. P., and Wentz I.. 2013. Newborn piglet traits associated with survival and growth performance until weaning. Prev. Vet. Med. 110:206–213. doi: 10.1016/j.prevetmed.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Paredes S. P., Jansman A. J., Verstegen M. W., Awati A., Buist W., den Hartog L. A., Van Hees H. M., Quiniou N., Hendriks W. H., and Gerrits W. J.. 2012. Analysis of factors to predict piglet body weight at the end of the nursery phase. J. Anim. Sci. 90:3243–3251. doi: 10.2527/jas.2011-4574 [DOI] [PubMed] [Google Scholar]
- Paredes S. P., Jansman A. J., Verstegen M. W., den Hartog L. A., van Hees H. M., Bolhuis J. E., van Kempen T. A., and Gerrits W. J.. 2014. Identifying the limitations for growth in low performing piglets from birth until 10 weeks of age. Animal 8:923–930. doi: 10.1017/S175173111400069X [DOI] [PubMed] [Google Scholar]
- Patience J. F., Engele K., Beaulieu A. D., Gonyou H. W., and Zijlstra R. T.. 2004. Variation: costs and consequences. In:Advances in pork production. Proceedings of the Banff Pork Seminar, Advances in Pork Production 15:257–266. [Google Scholar]
- Powell S. E., and Aberle E. D.. 1980. Effects of birth weight on growth and carcass composition of swine. J. Anim. Sci. 52:748−756. doi:10.2527/jas1980.505860x [DOI] [PubMed] [Google Scholar]
- Quiniou N., Dagorn J., and Gaudre D.. 2002. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 78:63–70. doi: 10.1016/S0301-6226(02)00181-1 [DOI] [Google Scholar]
- Rehfeldt C., Tuchscherer A., Hartung M., and Kuhn G.. 2008. A second look at the influence of birth weight on carcass and meat quality in pigs. Meat Sci. 78:170–175. doi: 10.1016/j.meatsci.2007.05.029 [DOI] [PubMed] [Google Scholar]
- Ruiz-Fons F., Vidal D., Vicente J., Acevedo P., Fernández-de-Mera I. G., Montoro V., and Gortázar C.. 2008. Epidemiological risk factors of Aujeszky’s disease in wild boar (Sus scrofa) and domestic pigs in Spain. Eur. J. Wildlife Res. 54:549–555 [Google Scholar]
- Rutherford K. M. D., Baxter E. M., D’Eath R. B., Turner S. P., Arnott G., Roehe R., Ask B., Sandøe P., Moustsen V. A., Thorup F., et al. 2013. The welfare implications of large litter size in the domestic pig I: biological factors. Anim. Welf. 22:199–218. doi: 10.7120/09627286.22.2.199 [DOI] [Google Scholar]
- Schinkel A., Ferrell P. J., Einstein M. E., Pearce S. A., and Boyd R. D.. Analysis of pig growth from birth to sixty days of age. In: Swine Research Report. West Lafayette (IN): Purdue University; 2003. p. 57–67. doi:10.15232/S1080-7446(15)31276-6 [Google Scholar]
- SIP Consultors.. 2016. Informe Consolidado—España 2016 [accessed May 15, 2018]. http://agricultura.gencat.cat/web/.content/de_departament/de02_estadistiques_observatoris/08_observatoris_sectorials/04_observatori_porci/informes_periodics_2017/E2_informe_economic_2017/fitxer_estatic/Informe-Economic2017.pdf.
- Smith A. L., Stalder K. J., Serenius T.V., Baas T. J., and Mabry J.W.. 2007. Effect of birth weight on weights at weaning and 42 days post weaning. J. Swine Health Prod. 15:213–218. [Google Scholar]
- Sulabo R. C., Jacela J. Y., Tokach M. D., Dritz S. S., Goodband R. D., DeRouchey J. M., and Nelssen J. L.. 2010. Effects of lactation feed intake and creep feeding on sow and piglet performance. J. Anim. Sci. 88:3145–3153. doi: 10.2527/jas.2009-2131 [DOI] [PubMed] [Google Scholar]
- Tokach M. D., Pettigrew J. E., Crooker B. A., Dial G. D., and Sower A. F.. 1992. Quantitative influence of lysine and energy intake on yield of milk components in the primiparous sow. J. Anim. Sci. 70:1864–1872. doi:10.2527/1992.7061864x [DOI] [PubMed] [Google Scholar]
- Wattanaphansak S., Luengyosluechakul S., Larriestra A., and Deen J.. 2002. The impact of cross-fostering on swine production. Thai J. Vet. Med. 32:101–106. [Google Scholar]
- Winsor C. P. 1932. The gompertz curve as a growth curve. Proc. Natl. Acad. Sci. USA 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Žáková E., and Groeneveld E.. 2008. Within-litter variation of birth weight in hyperprolific Czech large white sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest. Sci. 115:195–205. doi: 10.1016/j.livsci.2007.07.009 [DOI] [Google Scholar]