Introduction
Kaposi Sarcoma (KS) is an angioproliferative neoplasm driven by human herpes virus 8 (HHV-8), classically described in HIV populations, although also occurring in HIV-negative patients (classic, endemic, and iatrogenic). Treatment for limited cutaneous lesions consists of skin-directed local therapy, such as intralesional chemotherapy, radiation therapy, and topical agents. For HIV-related KS, antiretroviral therapy is critical and sometimes may induce regression on its own. For more bulky disease or visceral involvement, systemic therapies are indicated, with pegylated doxorubicin often used as first-line treatment. Recent reports suggest that checkpoint immunotherapy may be promising in both HIV and non–HIV-associated KS.1,2 In 17 patients with HIV-associated KS, checkpoint inhibitors show antitumor activity.1 An interim analysis of 10 patients in a phase II study of nivolumab and ipilimumab found promising activity in progressive classic KS, with a 50% objective response rate.2 Here we report a case of chemorefractory, metastatic, HIV-negative KS that was treated successfully with ipilimumab and nivolumab. Additionally, the patient had baseline immune-mediated dermatoses, psoriasis, and bullous pemphigoid that were managed during immunotherapy treatment.
Case report
A 78-year-old-man with a history of psoriasis, bullous pemphigoid, and 8 years of HIV-negative KS presented with progression of KS with metastases to the lung and soft tissues, confirmed by biopsy that showed spindle cell proliferation with HHV-8 and CD31 positivity on immunohistochemistry staining. The development of his KS was thought to be secondary to immunosuppression for psoriasis, including methotrexate and a tumor necrosis factor inhibitor, which were discontinued after development of KS. The initial diagnosis was KS of the skin and stomach, and the patient was treated with pegylated doxorubicin, paclitaxel, radiation therapy, sorafenib, and etoposide. He underwent a below-the-knee amputation of his left foot for a refractory, ulcerating KS mass. He experienced periods of disease control, but eventually had new enlarging lung and left leg subcutaneous metastases. He underwent 4 cycles of ipilimumab, 1 mg/kg, and nivolumab, 3 mg/kg, which markedly reduced the tumor burden in the skin and lungs within one month (Fig 1). Ipilimumab inhibits cytotoxic T-lymphocyte–associated protein 4, whereas nivolumab inhibits programmed cell death protein 1. After treatment with ipilimumab and nivolumab for 4 cycles, the patient has remained on monthly nivolumab maintenance, per protocol, with continuing resolution of metastases without any new lesions.
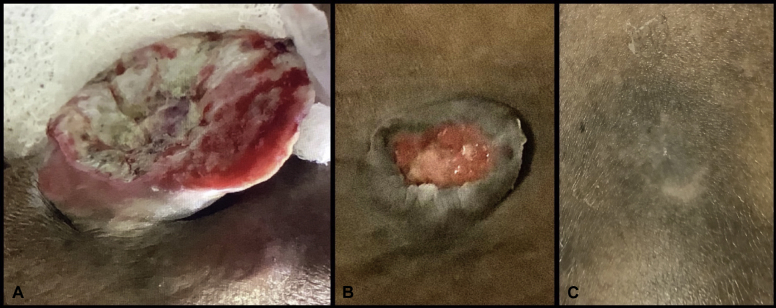
Fig 1.
KS regression. A, New KS mass that developed on the leg after below-the-knee amputation, before initiation of immunotherapy. B, Decrease in size of mass 35 days after initiation of immunotherapy. C, Remaining scar 120 days after complete resolution of KS mass.
His other dermatologic conditions were successfully managed without oral steroids throughout immunotherapy treatment. He experienced a psoriasis flare, successfully managed with sulfasalazine 2000 mg twice a day, acitretin 50 mg/d, and triamcinolone 0.1% ointment twice a day (Fig 2). His bullous pemphigoid has been well controlled with clobetasol 0.05% ointment. Facial seborrheic dermatitis has been well controlled with hydrocortisone 2.5% cream twice a day, and pre-existing facial verruca vulgaris resolved in the setting of immunotherapy without other treatment.
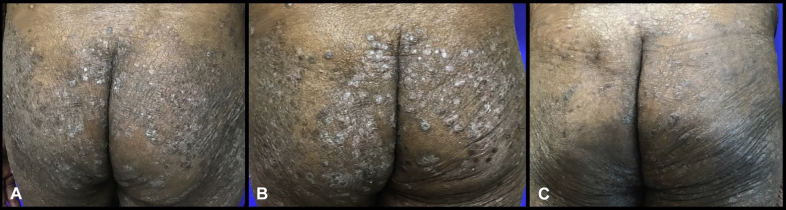
Fig 2.
Psoriasis treatment. A, Psoriasis before initiation of immunotherapy. B, Psoriasis flare 11 days after initiation of immunotherapy. C, Improvement in psoriasis with sulfasalazine, 2000 mg twice a day, acitretin, 50 mg/d, and triamcinolone 0.1% ointment twice a day, while on immunotherapy.
Discussion
Immunotherapy is a promising new treatment option for HIV and non–HIV-associated KS.1,2 Our case shows successful treatment of HIV-negative, metastatic KS with ipilimumab and nivolumab, followed by maintenance nivolumab. Recent advances in immunotherapy have shown marked benefit in number of skin cancers, including melanoma, Merkel cell carcinoma, and mycosis fungoides.3 The pathogenesis of KS is thought to be due to immunosuppression in the setting of HHV-8 infection. KS tumors may fail to activate T cells because of lack of expression of costimulatory molecules CD80 and CD86,4 or because of functional impairment of HHV-8–infected skin infiltrating dendritic cells.5 In the absence of activated T cells, CD8+ T cells do not kill KS-infected cells to combat KS tumor proliferation. Therefore, checkpoint inhibitors may work to combat KS by disinhibiting adaptive immunity and enabling cytotoxic killing of KS-infected cells.
Physicians need to be aware of this newly reported treatment of KS with ipilimumab and nivolumab and how management of other immune-mediated concomitant skin disease, such as psoriasis and bullous pemphigoid, is possible during immunotherapy. This is particularly pertinent given the increased incidence of autoimmune bullous diseases in patients with non–HIV-associated KS.6 As immunotherapies are being increasingly developed, we must find ways to leverage therapeutic benefits in rare diseases such as KS. Further trials are warranted to explore checkpoint inhibition in KS, especially whether cytotoxic T-lymphocyte–associated protein 4 inhibition enhances programmed cell death protein 1 inhibition to lead to further cytotoxic T-cell destruction of KS-infected cells.
Acknowledgments
Drs Bui and Zaba provided patient care, manuscript supervision, and manuscript editing. Tabata provided manuscript writing and manuscript editing. Dr Novoa provided histopathologic interpretation for diagnosis of KS and production of histology image.
Footnotes
Drs Bui and Zaba contributed equally to this report.
Funding sources: None.
Conflicts of interest: Dr Bui is a consultant to NewBay Pharma. The rest of the authors have no other conflicts of interest to disclose.
References
- 1.Galanina N., Goodman A.M., Cohen P.R., Frampton G.M., Kurzrock R. Successful treatment of HIV-associated Kaposi sarcoma with immune checkpoint blockade. Cancer Immunol Res. 2018;6:1129–1135. doi: 10.1158/2326-6066.CIR-18-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zer A., Icht O., Joseph L. A phase II single-arm study of nivolumab and ipilimumab (Nivo/Ipi) in previously treated classic Kaposi sarcoma (CKS) J Clin Oncol. 2019;37:11064. [Google Scholar]
- 3.Khodadoust M.S., Rook A.H., Porcu P. Pembrolizumab in relapsed and refractory mycosis fungoides and Sezary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38:20–28. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foreman K.E., Wrone-Smith T., Krueger A.E., Nickoloff B.J. Expression of costimulatory molecules CD80 and/or CD86 by a Kaposi's sarcoma tumor cell line induces differential T-cell activation and proliferation. Clin Immunol. 1999;91:345–353. doi: 10.1006/clim.1999.4712. [DOI] [PubMed] [Google Scholar]
- 5.Rappocciolo G., Jais M., Piazza P.A., DeLucia D.C., Jenkins F.J., Rinaldo C.R. Human herpesvirus 8 infects and replicates in Langerhans cells and interstitial dermal dendritic cells and impairs their function. J Virol. 2017;91 doi: 10.1128/JVI.00909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourlaki A., Genovese G., Guanziroli E., Scoppio B.M., Berti E., Brambilla L. Autoimmune bullous diseases in non-HIV Kaposi's sarcoma: a retrospective study in a large cohort of patients. J Eur Acad Dermatol Venereol. 2018;32:1777–1783. doi: 10.1111/jdv.15051. [DOI] [PubMed] [Google Scholar]