Abstract
Shrub encroachment on grasslands is a worldwide issue and sheep are a potential tool for mitigating shrub encroachment. Many shrubs, however, contain bitter-tasting compounds that may deter grazers. Cattle and sheep commonly graze rangelands, but of the two, sheep have a greater tolerance for bitter compounds and would be expected to consume more bitter-tasting vegetation. We hypothesized that sheep could detect (i.e., taste) bitter-tasting compounds and the sensitivity to these compounds would vary from animal to animal. The objective of this study was to determine whether sheep could detect the bitter-tasting compound phenylthiocarbamide (PTC), and if so, what PTC concentration would elicit an avoidance response. Using a crossover study design, mature Rambouillet and Targhee rams (n = 30) were subjected in randomized order to various PTC concentrations mixed in the drinking water (PTC solution). In trials 1 and 2 (n = 15/trial), 0.20, 0.56, 1.57, 4.39, and 12.29 mM and 0.20, 0.43, 0.94, 2.03, and 4.39 mM of PTC were tested, respectively. On test days, PTC solution (trial 1: 1.5 kg; trial 2: 3.0 kg) and water (same amounts) were offered for ad libitum intake in a side-by-side presentation for 1 h in trial 1 and 2 h in trial 2. Each test day was followed by a rest day where PTC solution was replaced with water to limit potential carry over effects into the next test day. Consumption of PTC solution for each PTC concentration was expressed as the percentage of PTC solution intake of total morning fluid intake. There was no effect (P > 0.74) of sequence that rams received PTC solutions on PTC consumption during either trial. As PTC concentration increased, percentage of PTC solution intake decreased (P ≤ 0.01) for both trials. The greatest decrease in percentage of PTC solution intake occurred between 1.57 and 4.39 mM (58%) for trial 1 and 2.03 and 4.39 mM (72%) for trial 2. In trial 2, the least percentage of PTC solution intake was the 4.39 mM PTC concentration, which was different (P ≤ 0.05) from lesser PTC concentrations. All other PTC concentrations did not differ (P > 0.05) from each other in percentage intake. This research suggests rams could taste the PTC, and the concentration at which PTC solution was avoided varied across rams. It may be possible to select sheep, based on demonstrated avoidance of PTC, for targeted grazing applications to manipulate vegetation toward range management goals.
Keywords: bitterness avoidance, phenylthiocarbamide, sagebrush, sheep
INTRODUCTION
Overgrowth of mountain big sagebrush (Artemisia tridentate Nutt. ssp. vaseyana) can lead to a reduction in rangeland plant diversity, carrying capacity, and wildlife abundance (Johnson et al., 1996; Launchbaugh, 2003). Sagebrush can be controlled or eliminated by plowing, burning, and spraying (Wambolt and Payne, 1986), but these methods can be expensive and have potential undesirable effects on rangelands. One method of control that has received minimal attention, and may be more sustainable, is reduction of sagebrush with grazing sheep. Using fecal analysis over several experiments, Snowder et al. (2001) indicated that the dietary preference for sagebrush in sheep has a heritability of 0.28, suggesting that selection against bitterness avoidance in sheep breeding programs may be feasible. Furthermore, Ferreira et al. (2013) identified a set of novel genes for bitter taste receptors in sheep, suggesting that sheep may be genetically predisposed to select or avoid plants with bitter or noxious tastes.
Sheep are adaptive selective grazers (Launchbaugh et al., 2001) with varying dietary preference for consuming sagebrush (Bork et al., 1998; Snowder et al., 2001; Seefeldt, 2005). Several factors can be attributed to an individual’s diet preference/selection including learned behaviors, taste preference, postdigestive feedback, and their ability to detoxify secondary metabolites. Many toxic forages have a bitter taste, but the toxicity and the correlation of bitter taste to toxicity varies (Cedarleaf et al., 1983; Johnson et al., 1985). Avoidance to bitter tasting plants is a mechanism sheep utilize to limit toxin ingestion (Launchbaugh et al., 2001). Early research on the primary taste groups of sweet, sour, salty, and bitter in sheep suggested that bitterness may be the most sensitive (Goatcher and Church, 1970a). Additional studies indicated that sheep can taste and(or) sense bitterness when mimicked by addition of compounds, like quinine, when added to drinking water (Goatcher and Church, 1970a; Favreau et al., 2010), and lithium chloride, when added to forages (Launchbaugh and Provenza, 1994).
Phenylthiocarbamide (PTC) is a compound, not found in nature, that mimics bitter tastes found in food (Blakeslee and Salmon, 1935; Barnicot et al., 1951; Lee and O’Mahony, 1998), and has been used in bitter taste research in humans (Blakeslee, 1932; Fox, 1932; Harris and Kalmus, 1949) and mice (Lush, 1986; Nelson et al., 2003). In humans, PTC thresholds have been suggested to be heritable (h2 = 0.55) (Morton et al., 1981). It has also been suggested that PTC avoidance is influenced by postdigestive factors (Nelson et al., 2003), similar to the preferences of sheep grazing bitter/toxic forages (Launchbaugh et al., 2001).
This study focused on bitter taste avoidance (Parker, 2003) by the addition of PTC in water. We hypothesized that sheep could detect bitter-tasting compounds and the sensitivity would vary from animal to animal. The objective of this study was to determine whether sheep could detect the bitter tasting compound, phenylthiocarbamide, and if so, what PTC concentration would elicit an avoidance response.
MATERIALS AND METHODS
Animals
All animal procedures were approved by an Institutional Animal Care and Use Committee (USDA, ARS, Dubois, ID) in accordance with the USDA, APHIS Animal Welfare Regulations (2013; 9 C.F.R. § 2.30-2.38 2013) and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010). Two trials were conducted at the USDA, ARS U.S. Sheep Experiment Station (USSES) located near Dubois, Idaho in the spring of 2018. In trial 1, yearling Rambouillet (n = 7) and Targhee (n = 8) rams (initial BW = 76.6 ± 5.7 and 83.7 ± 9.1 kg, respectively) were used; while in trial 2, yearling Rambouillet (n = 6) and Targhee (n = 9) rams (initial BW = 83.0 ± 9.7 and 93.5 ± 9.14 kg, respectively) were used. For the duration of both trials, rams were housed indoors in individual pens, so feed and water intake could be monitored under controlled conditions of 10 °C with a 12 h light:dark cycle. Additionally, feed and water were withheld from rams from 1700 to 0700 h each day during the trials. For each trial, rams were randomly allotted within breed to alternate pens throughout the barn. Both trials were divided into two phases; an acclimation phase, where rams were adjusted to the pens and daily feed and fluid delivery routines, and a testing period, where the phenylthiocarbamide (PTC) treatments were delivered.
Experimental Design
Both trials were conducted as a cross-over design consisting of five PTC treatments with individual rams receiving a different PTC concentration each test day. In order for all rams to be tested in a day, rams were randomized to five testing blocks consisting of three rams each. Each block was randomly assigned a PTC testing sequence, which consisted of the order in which rams received their PTC treatments over the five test days (Table 1).
Table 1.
Sequence in which blocks of rams received each PTC solution concentration for both trials
| Test day | Trial 1—PTC concentrations (mM) | ||||
|---|---|---|---|---|---|
| 0.20 | 0.56 | 1.57 | 4.39 | 12.29 | |
| 1 | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| 2 | Group 2 | Group 5 | Group 4 | Group 1 | Group 3 |
| 3 | Group 5 | Group 3 | Group 1 | Group 2 | Group 4 |
| 4 | Group 3 | Group 4 | Group 2 | Group 5 | Group 1 |
| 5 | Group 4 | Group 1 | Group 5 | Group 3 | Group 2 |
| Trial 2—PTC concentrations (mM) | |||||
| 0.20 | 0.43 | 0.94 | 2.03 | 4.39 | |
| 1 | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| 2 | Group 2 | Group 5 | Group 4 | Group 1 | Group 3 |
| 3 | Group 5 | Group 3 | Group 1 | Group 2 | Group 4 |
| 4 | Group 3 | Group 4 | Group 2 | Group 5 | Group 1 |
| 5 | Group 4 | Group 1 | Group 5 | Group 3 | Group 2 |
Each group consisted of 3 rams with a total of 15 rams tested on each test day per trial.
Each trial consisted of a 5-d acclimation phase followed by the PTC testing. During the acclimation phase of both trials, rams received alfalfa pellets (Table 2) at a rate of 1.9% of BW (as fed basis) at 0700 h. Thirty minutes after feeding, feed was removed, and refusals weighed. Immediately following feed removal, two buckets filled with water (1.5 kg, trial 1; 3.0 kg, trial 2) were placed in each pen and rams were allowed access to the water for 1 h in trial 1 and 2 h in trial 2. Buckets were placed side-by-side in a holding rack and given a designation of left and right side. At the end of the first water consumption period, buckets were removed from each pen and water refusals weighed for each bucket and discarded. After removal of buckets, pens were cleaned, and rams were given their daily feed and water at approximately 0900 h for trial 1 and 1000 h for trial 2. In trial 1, rams received alfalfa pellets (Table 2) fed at a rate of 2.8% of BW (as fed basis), 45 g of a mineral mix (Table 2), and 5 kg of water in a single bucket. At approximately 1230 h, the water bucket was removed, refusals weighed, and discarded. An additional 4 kg of water was offered, and at 1700 h, all feed and water were removed, refusals weighed, and discarded. Whereas in trial 2, rams received alfalfa pellets fed at a rate of 2.8% of BW (as fed basis), 45 g of a mineral mix, and two buckets with each containing 4 kg of water were offered, and at 1700 h, all feed and water were removed, refusals weighed, and discarded.
Table 2.
Alfalfa pellets and mineral supplement component analysis (DM basis)
| Item | Alfalfa pelletsa | Mineral supplementb |
|---|---|---|
| Dry matter, % | 100 | 100 |
| Crude protein, % | 17.4 | — |
| Acid detergent fiber, % | 36.8 | — |
| Total digestible nutrients | 54.8 | — |
| Ca, % | 1.79 | 0.85 |
| P, % | 0.22 | 0.002 |
| K, % | 2.09 | 0.03 |
| Mg, % | 0.29 | 0.06 |
| S, % | 0.28 | 0.07 |
| Na, % | 0.16 | 95.0 |
| Zn, mg/kg | 22.6 | 1 |
| Fe, mg/kg | 717 | 300 |
| Mn, mg/kg | 50 | 5 |
| Cu, mg/kg | 7.8 | 3 |
| Mo, mg/kg | 2.17 | — |
aComponent analysis of alfalfa pellets conducted by Ward Laboratories (Kearney, NE).
bMineral supplement formulated by Redmond Agriculture (Redmond, UT). Product name “10 Fine Premium Mineral Salt.”
The PTC was chosen as a bitter tasting agent to mimic the attributes of monoterpenoids, which are often found in toxic shrubs. It is unknown what PTC concentration mimics the degree of bitterness in plants; therefore, PTC concentrations for trial 1 were chosen over a large range, then adjusted for trial 2 to better meet the objectives of the study. In trial 1, some individuals consumed all fluid in either bucket offered during the testing times. Therefore, in order to limit thirst as a potential factor in consumption, the volume of water and PTC solution offered were increased while the time allotted for consumption was also increased for trial 2.
The test phase for both trials consisted of test days where PTC solutions and water (in separate buckets) were delivered after the morning feeding, and each test day was followed by a rest day where only water was delivered to minimize potential carry-over effects of PTC from the previous test day. Tap water (water) from the USSES well was used for this study. For test and rest days, the same procedures relative to timing of feed delivery, number of buckets, and total amounts of fluid delivered were followed as per the acclimation phase. On a test day, each ram block received one of the five concentrations of PTC solutions (trial 1: 0.20, 0.56, 1.57, 4.39, or 12.29 mM delivered in a total volume of 1.5 kg; trial 2: 0.20, 0.43, 0.94, 2.03, or 4.39 mM delivered in a total volume of 3.0 kg) in one bucket, and water only (trial 1: 1.5 kg; trial 2: 3.0 kg) in the other bucket. The location (left or right) of the PTC solution bucket was alternated between test days. On the subsequent rest day, no PTC solution was administered and was replaced with water. For both trials, PTC (Sigma P7629, Sigma–Aldrich, Saint Louis, MO) was dissolved in absolute ethanol then diluted with water to the desired concentrations for delivery.
Statistical Analysis
For all fluid intake variables analyzed within a trial, data were analyzed using PROC MIXED procedures of SAS (Statistical Analysis System, SAS Institute, Inc., Version 9.4, Cary, NC). The model included treatment (PTC concentration), sequence (order PTC concentrations were administered to rams), and period (day that PTC was administered within the sequence) with a random statement that included ram within sequence. Means are reported as least squares means, and mean comparisons were made using pair-wise contrasts (PDIFF). Significance was set at P ≤ 0.05.
Due to the variation in avoidance to PTC observed within and across PTC concentrations for each trial (Table 3), individual rams were further classified into consumer groups based upon total PTC intake (g) over the five test days. Consumer group differentiation was determined by 0.5 standard deviation of the population mean to divide rams into high (≥0.5 SD), medium (<0.5 to >−0.5 SD), or low (≤−0.5 SD) PTC consumers (Table 4). One objective of this study was to evaluate variation among individual rams. To test the variation observed, linear regression using PROC GLM for analysis by consumer group with the independent variable being PTC concentration and dependent variable included percent of PTC solution intake of test fluid intake. Orthogonal and paired contrasts were used to test coincidence of regression lines (slope and intercept analyzed together), as well as slopes, and intercepts individually between PTC consumption groups.
Table 3.
Descriptive statistics of variation observed across phenylthiocarbamide (PTC) concentration categories within each trial where values are represented as percentage of PTC solution intake of total fluid offered and percentage of water intake of total fluid offered
| Trial 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| PTC concentration, mM | Mean ± SD | Minimum | Maximum | Coefficient of variation | ||||
| PTC | Water | PTC | Water | PTC | Water | PTC | Water | |
| 0.20 | 54.5 ± 36.0 | 67.3 ± 35.9 | 0.5 | 1.0 | 95.7 | 100 | 66.1 | 53.3 |
| 0.56 | 39.0 ± 33.5 | 65.0 ± 35.8 | 0.4 | 1.0 | 93.1 | 100 | 86.0 | 55.0 |
| 1.57 | 30.9 ± 32.4 | 71.5 ± 32.5 | 0.5 | 8.2 | 94.7 | 100 | 105.0 | 45.4 |
| 4.39 | 7.2 ± 9.1 | 81.2 ± 26.9 | 0.3 | 0.6 | 32.2 | 100 | 126.7 | 33.1 |
| 12.29 | 3.6 ± 4.0 | 77.7 ± 26.0 | 0.7 | 25.9 | 14.3 | 100 | 112.1 | 33.5 |
| Trial 2 | ||||||||
| 0.20 | 41.9 ± 33.8 | 92.7 ± 10.1 | 0.3 | 69.1 | 97.6 | 100 | 80.8 | 10.9 |
| 0.43 | 29.9 ± 23.9 | 88.0 ± 22.0 | 0.3 | 28.6 | 90.9 | 100 | 79.7 | 25.0 |
| 0.94 | 40.5 ± 34.5 | 88.0 ± 18.3 | 0.5 | 39.6 | 95.8 | 100 | 85.1 | 20.8 |
| 2.03 | 33.1 ± 27.9 | 89.5 ± 17.3 | 0.3 | 53.0 | 89.8 | 100 | 84.3 | 19.3 |
| 4.39 | 11.5 ± 15.4 | 90.2 ± 16.7 | 0.5 | 49.2 | 57.1 | 100 | 133.3 | 18.5 |
All units are expressed as percentages.
Table 4.
Descriptive statistics of phenylthiocarbamide (PTC) consumption categories based on rankings of total PTC consumption by individual rams across the five test days within a trial
| Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|
| Ram ID | Consumption group | Total PTC intake (g) | Ram ID | Consumption group | Total PTC intake (g) |
| T6581 | High | 1.056 | T6886 | High | 2.103 |
| S0791 | High | 0.876 | T6342 | High | 1.389 |
| S1497 | High | 0.769 | S0801 | High | 1.318 |
| S0583 | Medium | 0.408 | T6884 | High | 1.236 |
| T6406 | Medium | 0.384 | T6093 | High | 1.163 |
| S1500 | Medium | 0.305 | T6516 | Medium | 0.932 |
| T6885 | Medium | 0.294 | T6313 | Medium | 0.781 |
| T6578 | Medium | 0.281 | S1499 | Medium | 0.737 |
| T6502 | Low | 0.207 | T6582 | Medium | 0.711 |
| T6883 | Low | 0.205 | T6299 | Medium | 0.697 |
| T6297 | Low | 0.204 | S1069 | Low | 0.452 |
| S1501 | Low | 0.166 | S0912 | Low | 0.327 |
| S1125 | Low | 0.106 | S1498 | Low | 0.177 |
| S1124 | Low | 0.104 | T6580 | Low | 0.091 |
| T6401 | Low | 0.082 | S1126 | Low | 0.035 |
| Mean ± SD for all rams | 0.363 ± 0.299 | 0.810 ± 0.567 | |||
Thresholds determined by mean ± (0.5 × SD).
RESULTS AND DISCUSSION
We hypothesized sheep could detect PTC when mixed in water and that sensitivity would be different among individuals. Unlike in human studies (Fox, 1932; Blakeslee and Salmon, 1935), rams are unable to verbally express if they can detect PTC. Although behavioral data were not quantified in this experiment, PTC concentrations where the PTC solution was consumed less than water negative behavioral reactions were observed during the study (e.g., smacking lips and shaking their head after tasting the PTC), particularly with the highest PTC concentrations (data not shown). Furthermore, as PTC concentration increased, mean intake of the PTC solutions decreased (Tables 5 and 6). Individual reactions and intake of PTC solution, taken altogether, suggest that rams could detect PTC, and that animals varied in sensitivity to detection of PTC.
Table 5.
Mean fluid intakes of rams receiving either water or phenylthiocarbamide (PTC) during a test period for trial 1
| PTC concentration, mM | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0.20 | 0.56 | 1.57 | 4.39 | 12.29 | Pooled SE | Treatment | Sequence | Period |
| Total test fluid intake as a percentage of total offered (1.5 kg water and 1.5 kg PTC solution) | 60.9a | 52.0a,b | 51.2a,b | 44.2b | 40.7b | 6.3 | 0.02 | 0.74 | 0.33 |
| Water intake as a percentage of total test fluid intake | 57.6a | 64.5a,b | 72.1b | 88.4c | 95.9c | 5.0 | 0.0001 | 0.39 | 0.11 |
| PTC solution intake as a percentage of total test fluid intake | 42.4a | 35.5a,b | 27.9b | 11.6c | 4.1c | 5.0 | 0.0001 | 0.39 | 0.11 |
| Afternoon water intake as a percentage of total offered (9 kg) | 92.5 | 91.4 | 91.6 | 89.5 | 92.9 | 3.2 | 0.61 | 0.68 | 0.03 |
| Total fluid intake as a percentage of total fluid offered (12 kg) | 84.6a | 81.5b | 81.5b | 78.2c | 79.9b,c | 2.6 | 0.003 | 0.54 | 0.002 |
Treatment refers to PTC concentrations, sequence is the order PTC concentrations that were administered to rams, and period is the day PTC was administered within the sequence.
a,b,cMeans with different superscripts within a response and across PTC concentrations are different (P ≤ 0.05).
Table 6.
Mean fluid intakes of rams receiving either water or phenylthiocarbamide (PTC) during a test period for trial 2
| PTC concentration, mM | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0.20 | 0.43 | 0.94 | 2.03 | 4.39 | Pooled SE | Treatment | Sequence | Period |
| Total test fluid intake as a percentage of total offered (1.5 kg water and 1.5 kg PTC solution) | 67.3a | 58.9b | 64.3a,b | 61.3a,b | 50.8c | 4.5 | 0.0002 | 0.86 | 0.02 |
| Water intake as a percentage of total test fluid intake | 73.6a | 75.9a | 72.9a | 76.3a | 90.3b | 4.8 | 0.01 | 0.98 | 0.87 |
| PTC solution intake as a percentage of total test fluid intake | 26.4a | 24.1a | 27.1a | 23.7a | 9.7b | 4.8 | 0.01 | 0.98 | 0.87 |
| Afternoon water intake as a percentage of total offered (9 kg) | 86.2 | 91.7 | 94.1 | 95.6 | 95.9 | 2.7 | 0.06 | 0.07 | 0.01 |
| Total fluid intake as a percentage of total fluid offered (12 kg) | 78.1 | 77.7 | 81.3 | 80.9 | 76.6 | 2.5 | 0.25 | 0.46 | 0.005 |
Treatment refers to PTC concentrations, sequence is the order PTC concentrations were administered to rams, and period is the day PTC was administered within the sequence.
a,bMeans with different superscripts within a variable and across PTC concentrations are different (P ≤ 0.05).
Consumption of PTC solution expressed as a percentage of total morning fluid intake is depicted in Tables 5 and 6 (trials 1 and 2, respectively). In trial 1, as each solution increased in PTC concentration the intake of PTC solution decreased (P < 0.001). The greatest decrease in percentage of PTC solution intake was observed between 1.57 and 4.39 mM (58%) in trial 1. As PTC concentration increased, PTC solution intake decreased, and water intake increased (P < 0.001).
In trial 2, the greatest decrease in percentage of PTC solution intake was observed between 2.03 and 4.39 mM (72%). There was also a treatment effect (P < 0.01) on PTC solution intake but a slightly different trend was observed than in trial 1. The intake of the 0.20, 0.43, 0.94, and 2.03 concentrations were all similar (P > 0.05), but the intake of the 4.39 mM concentration was different (P ≤ 0.05) than the rest. Similar to observations from trial 1, intake of the water increased as PTC increased in trial 2. The limited dose response in trial 2 may be due to the smaller differences between PTC concentration levels for that trial and/or the increase in total morning fluid offered. Trial 1 PTC concentrations were chosen at approximately multiples of three; whereas in trial 2 PTC concentration were chosen at approximately multiples of two. Trial 1 and trial 2 results indicated that the greatest decrease in PTC solution consumption occurred when PTC concentrations increased from 1.57 to 4.39 mM, which suggests a threshold within this range may have possible implications in determining bitter taste avoidance in sheep.
The lowest PTC concentration (0.20 mM) for both trials had lower intake than the water. This observation suggests PTC is detectable and avoidance begins for some rams below 0.20 mM. This is supported by the observation that minimum consumption of the 0.20 mM PTC solution were 0.5% and 0.3% of total fluid intake for trials 1 and 2, respectively (Table 3). It should also be noted, however, that within the 0.20 mM PTC concentration that maximum PTC solution consumption was >95% for trials 1 and 2 (Table 3). In trial 2, the mean consumption of the 0.20 mM PTC concentration was approximately half of that observed in trial 1. This difference could be attributed to the amount of time allotted to consume test fluid. If a ram chose to avoid a particular PTC concentration, there would be more time in trial 2 to consume water postavoidance.
Nelson et al. (2003) observed a similar inverse relationship between PTC concentration and average intake when PTC solutions were administered to mice. Similarly, Goatcher and Church (1970a) administered increasing concentrations of quinine (a bitter-mimicking agent) in drinking water to rams alongside a control of water and observed an inverse relationship between concentration of quinine and percent of quinine solution of fluid intake. Goatcher and Church (1970b) further studied the sensitivity to quinine in a subsequent study, and when analyzed on an individual basis. Similar to this study, considerable variation from the mean was observed in percentage of solution intake of test fluid intake for each concentration. This large degree of difference in sensitivity among individuals has also been observed in human research and has led to the categorization of individuals into tasters, and nontasters (Fox, 1932; Blakeslee and Salmon, 1935). Research in humans has typically placed participants into upper or lower thresholds to categorize tasters, nontasters, and super tasters, which was originally suggested by Bartoshuk et al. (1994). Tasters are categorized as “tasters” if they can detect PTC at a low concentration and as “nontasters” when detection is not until they consume a high concentration (Blakeslee and Salmon, 1935; Harris and Kalmus, 1949). The lack of standardization of testing sensitivity to PTC has produced inconsistent conclusions (Tepper, 2008). In this study, some individuals (tasters) consumed less than 1% of the 0.20 mM PTC concentration, and other individuals (nontasters) consumed >95% in both trials. Including the observations made by Goatcher and Church (1970a, 1970b) and those from this study, sheep might fall into similar categories as humans.
While it is known that sheep will tolerate bitter-tasting compounds (Provenza et al., 1992; Launchbaugh et al., 2001), there is no previous literature indicating PTC tolerance thresholds in sheep. In human research, PTC categories have been suggested to be associated with bitterness intensity perception (Blakeslee and Salmon, 1935; Bartoshuk et al., 1994; Drewnowski and Rock, 1995). However, quinine sensitivity and PTC sensitivity in humans are variable where some individuals perceive quinine as being more bitter than PTC and some individuals perceive PTC as being more bitter than quinine (Blakeslee and Salmon, 1935; Frank and Korchmar, 1985). The bitter tasting compound PTC contains a thiocyanate moiety (Bartoshuk et al., 1994), which is similar to isothiocyanates. Isothiocyanates are produced during the breakdown of glucosinolates, commonly found in bitter tasting vegetables (Ettlinger and Lundeen, 1957). Quinine and PTC both elicit bitter tastes, but likely due to its’ chemical makeup, PTC sensitivity has been linked to glucosinolates preference (Duffy and Bartoshuk, 2000). Goatcher and Church (1970a) observed a similar inverse relationship between increasing quinine solution concentrations and decrease in consumption as described in this study, but sensitivity to quinine or PTC may translate differently to foraging preferences in sheep.
For both trials, there were no sequence effects (P > 0.05) observed for percentage of PTC solution consumed, indicating that the sequence in which rams received the PTC solutions did not affect fluid intakes on subsequent test days (Tables 4 and 5). There was also no sequence effect (P > 0.05) on total fluid intake on rest days, which suggested the effects of PTC dissipate rapidly (data not shown). Relative to both PTC treatment and sequence that rams received it on a test day, there were no treatment or sequence effects (P > 0.05) on the amount of water intake during the rest days for the morning, afternoon, and total fluid intake. The average percentage of water intake on the rest days during the morning, afternoon, and total for the day were 56.8% ± 7.7%, 56.8% ± 3.5%, and 82.6% ± 3.0%, respectively for trial 1 and 76.6% ± 4.7%, 90.1% ± 3.0%, and 84.3% ± 2.3 %, respectively for trial 2.
A great deal of variation in PTC-solution intake was observed between rams (Table 4). In trial 1, the ram with the greatest intake of PTC consumed 9.7-fold more PTC than the ram with the lowest intake (1.06 vs. 0.109 g, respectively). For trial 2, the magnitude of difference was much greater at 60-fold (2.10 vs. 0.0348 g PTC, respectively). Based on the variation between rams within each trial, rams were grouped according to total (g) PTC intake (Table 4). In trial 1, the high intake group consisted of three individuals, medium consisted of five, and low consisted of seven, where in trial 2, all groups consisted of five individuals.
Similar to the sensitivity observed in this study, sensitivity to consuming bitter shrubs has also been observed in grazing sheep. Snowder et al. (2001) determined percentage of sagebrush consumed in the diet of 549 ewes was 10.3–31.9% for September and 23.7–42.3% for October. The September and October measurements were highly correlated (r2 = 0.91), where the highest consumers in September were also the highest consumers in October, similar to this study, where the individuals in the high consumer group consistently consumed the most PTC solution.
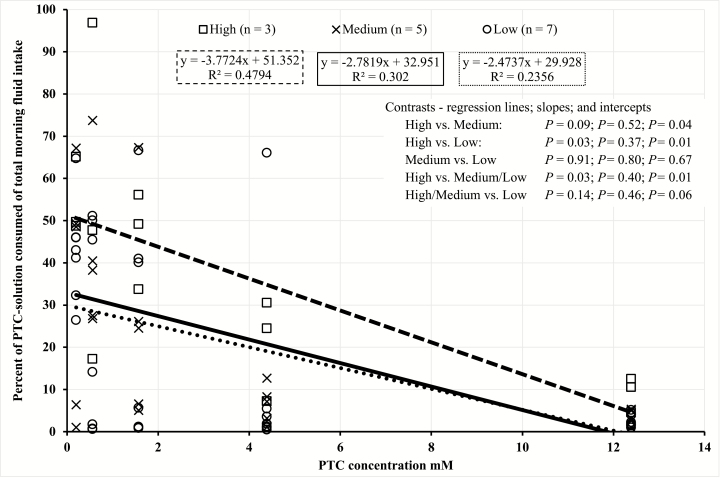
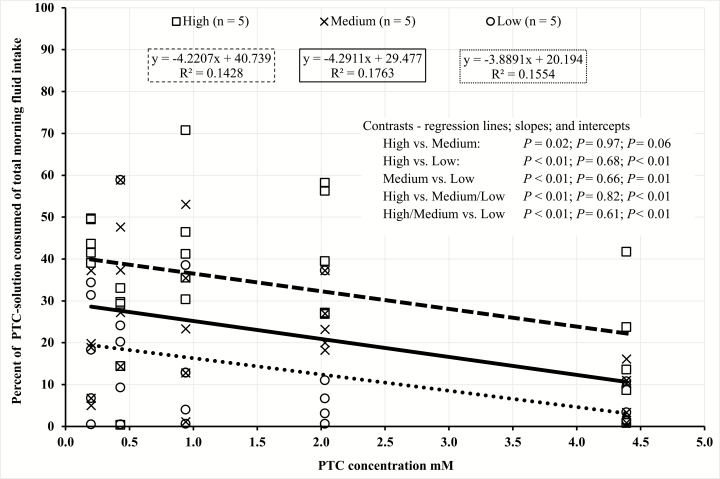
Variation was also observed in total daily fluid intake among the rams in this study. Based on this observation, to account for individual total fluid intake variation, we used the percent of PTC solution intake of total morning fluid intake (Figures 1 and 2, and Tables 5 and 6). Regression analyses were performed on each consumer group within each trial based on percentage of PTC solution intake of total morning fluid intake (Figures 1 and 2). The slopes of each consumer group within each trial did not differ (P > 0.05), suggesting that the rate of avoidance between consumer groups was not different. However, most of the intercepts differed across consumer groups (Figures 1 and 2), which suggests that the point of avoidance as PTC concentration increases is different between groups.
Figure 1.
Trial 1 linear regressions of phenylthiocarbamide (PTC) consumption by total PTC intake categories (high: □ − − −; medium: × ——; low: ○ ∙ ∙ ∙ ∙) for Rambouillet and Targhee rams administered five PTC concentrations (0.20, 0.56, 1.57, 4.39, and 12.29 mM) suspended in 1.5 kg of water. Paired contrast made between high vs. low, medium vs. low, and low vs. high. Orthogonal contrast made between high/medium vs. low and high vs. medium/low.
Figure 2.
Trial 2 linear regressions of phenylthiocarbamide (PTC) consumption by total PTC intake categories (high: □ − − −; medium: × ——; low: ○ ∙ ∙ ∙ ∙) for Rambouillet and Targhee rams administered five PTC concentrations (0.20, 0.43, 0.94, 2.03, and 4.39 mM) suspended in 3.0 kg of water. Paired contrast made between high vs. low, medium vs. low, and low vs. high. Orthogonal contrast made between high/medium vs. low and high vs. medium/low.
In trial 1, within the medium and low groups, no individual consumed more than 5% of the highest PTC concentration (12.29 mM) offered. Because the point at which the greatest avoidance within the population was observed between the 1.57 and 4.39 mM PTC concentrations in trial 1, the 12.29 mM concentration was eliminated for trial 2. Furthermore, because the range of PTC concentrations for trial 2 was smaller than that of trial 1, the amount of PTC-solution and water offered in the morning and the time allotted for intake were increased. These changes made from trial 1 to trial 2 were to encourage those individuals that were willing to consume greater concentrations of PTC to differentiate themselves from the population. Although PTC concentrations, total fluid offered, and duration that the PTC solution was available to the rams varied between trials, a similar individual variation in PTC solution intake was still observed in trial 2 compared with trial 1 (Figures 1 and 2). These results suggest that PTC intake is related to the individual ram’s preference for, or avoidance to, bitter taste and that PTC can be used as a bitter-mimicking agent to determine sensitivity to bitterness among individuals.
To date, this is the first study to use PTC to test for sensitivity of bitterness among sheep. Sheep have displayed the ability to identify the presence of terpenes when fed in a mixed ration (Villalba et al., 2006; Mote et al., 2007), which suggests that part of diet selection is sensory and is related to taste and(or) aroma. Dziba and Provenza (2008) reported that in lambs offered varying concentrations of monoterpenoids (camphor, p-cymeme,1–8 cineole, methacrolein; commonly found in Big Mountain sagebrush) mixed into their diets, intake rates of the mixed diet in relation to monoterpene concentration varied. There was no difference in the percentage of time spent eating among the groups (high concentration = 4.65% terpene, medium concentration = 3.10%, and low concentration = 1.55%), but the medium group consumed less than the low group and the high group consumed less than the medium group. Furthermore, Dziba and Provenza (2008) suggested that lambs regulate feed intake of bitter vegetation to prevent consuming a toxic dose of terpenes. Although total amount of forages consumed differed between the medium and high group, both groups stopped consuming feed when they reached approximately 28 g of monoterpenoids per day (Dziba and Provenza, 2008). Launchbaugh et al. (1993) observed similar behavior when lithium chloride was mixed in diets fed to lambs. Despite the concentration at which lithium chloride was fed, lambs regulated their total intake to not exceed concentrations of 62.7 ± 4.5 mg/kg lithium chloride per day. Regulating intake of bitter-tasting compounds is likely a developed mechanism that sheep use to avoid forages that have negative postingestion qualities (Provenza and Balph, 1987). While postingestion feedback is one mechanism used by ruminants in forage selection, it is likely not the only deciding factor.
Launchbaugh (2001) suggested that foraging behaviors can be learned from mimicking maternal and herd behavior, and taste memory from suckling. Nolte and Provenza (1992) observed that feeding onion-flavored milk to orphan lambs resulted in a preference for onion-flavored feeds later in life. Some literature suggests that bitterness is likely not the apparent causative factor when consuming toxic forages, but rather postingestive feedback mechanisms (Provenza et al., 1992; Launchbaugh et al., 2001). Future selection or determent of a forage is associated with the memory of that taste and the digestive feedback; however, memory and tolerance vary between individuals and each individual perceives cost/benefit from a forage differently (Sclafani, 1991; Provenza et al., 1992; Launchbaugh et al., 2001). Differences in memory of a forage is likely linked to the individual’s physiologic ability to suppress the toxic effects (Provenza et al., 1992). Because terpenoids contain bitter-tasting compounds, variation in bitter preference between individuals may not only translate to forage selection but may also be correlated with the individual’s ability to suppress toxins. Toxic shrub intake is likely driven by several phenotypic (Mennella et al., 2005; Dziba et al., 2007; Ginane et al., 2011) and genotypic factors (Chandrashekar et al., 2000; Bufe et al., 2005).
Results from this study indicate that there are sensitivity differences between individual’s preference for consuming bitter tasting compounds in sheep. The variation in bitterness intake may translate to foraging preferences while grazing, where rams with greater tolerance for bitter taste may consume plants with higher concentrations of bitter tasting compounds, such as monoterpenoids. Similarly, humans that are categorized as nontasters consume more anti-oxidant rich vegetables with bitter attributes than tasters (Garcia-Bailo et al., 2009).
Utilizing sheep as a grazing tool to reduce sagebrush canopy has been suggested to entail long-term and high-intensity grazing applications (Seefeldt, 2005); however, sheep grazing may be a good tool for suppressing sagebrush canopy growth and decrease shrub encroachment on grasslands. Moffet et al. (2015) suggested that during a rangeland life cycle in a mountain big sagebrush ecosystem, the greatest forage productivity and optimal wildlife habitat conditions occur 5–15 years postfire. Furthermore, productivity of rangeland decreases as sagebrush canopies become overgrown. Johnson et al. (1996) suggested that the greatest ecological diversity in mountain big sagebrush ecosystems occurs when the sagebrush canopy makes up approximately 15% of total plant composition, and the greatest herbaceous production occurs when the sagebrush canopy makes up 11–17% of total plant composition. Because diet selection is moderately heritable in sheep (h2 = 0.28) (Snowder et al., 2001), selection for sheep that have a higher tolerance for bitter tasting compounds may translate to sagebrush canopy growth suppression on rangeland, and therefore, extend the optimal ecological productivity-time period beyond 5–15 years postfire (Moffet et al., 2015).
ACKNOWLEDGMENTS
The authors would like to acknowledge the support for this research provided by the USDA-ARS-Sheep Experiment Station employees, Mark Williams, Taylor Hudson, and Ashleigh Redman. The authors would also like to thank Dr William Price, University of Idaho, College of Agriculture and Life Sciences for his assistance with the statistical analysis.
LITERATURE CITED
- Barnicot N. A., Harris H., and Kalmus H.. . 1951. Taste thresholds of further eighteen compounds and their correlation with P.T.C thresholds. Ann. Eugen. 16:119–128. doi: 10.1111/j.1469-1809.1951.tb02464.x [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. M., Duffy V. B., and Miller I. J.. . 1994. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol. Behav. 56:1165–1171. doi: 10.1016/0031-9384(94)90361-1 [DOI] [PubMed] [Google Scholar]
- Blakeslee A. F. 1932. Genetics of sensory thresholds: taste for phenyl thio carbamide. Proc. Natl. Acad. Sci. USA. 18:120–130. doi: 10.1073/pnas.18.1.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee A. F., and Salmon T. N.. . 1935. Genetics of sensory thresholds: individual taste reactions for different substances. Proc. Natl. Acad. Sci. USA. 21:84–90. doi: 10.1073/pnas.21.2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork E. W., West N. E., and Walker J. W.. . 1998. Cover components on long-term seasonal sheep grazing treatments in three-tip sagebrush steppe. J. Range Management. 51(3):293–300. doi: 10.2307/4003414 [DOI] [Google Scholar]
- Bufe B., Breslin P. A., Kuhn C., Reed D. R., Tharp C. D., Slack J. P., Kim U. K., Drayna D., and Meyerhof W.. . 2005. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 15:322–327. doi: 10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarleaf J. D., Welch B. L., and Brotherson J. D.. . 1983. Seasonal-variation of monoterpenoids in big sagebrush [Artemisia-Tridentata]. J. Range Manag. 36:492–494. doi: 10.2307/3897950 [DOI] [Google Scholar]
- Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., and Ryba N. J.. . 2000. T2Rs function as bitter taste receptors. Cell 100:703–711. doi: 10.1016/s0092-8674(00)80706-0 [DOI] [PubMed] [Google Scholar]
- Drewnowski A., and Rock C. L.. . 1995. The influence of genetic taste markers on food acceptance. Am. J. Clin. Nutr. 62:506–511. doi: 10.1093/ajcn/62.3.506 [DOI] [PubMed] [Google Scholar]
- Duffy V. B., and Bartoshuk L. M.. . 2000. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 100:647–655. doi: 10.1016/s0002-8223(00)00191-7 [DOI] [PubMed] [Google Scholar]
- Dziba L. E., and Provenza F. D.. . 2008. Dietary monoterpene concentrations influence feeding patterns of lambs. App. Anim. Behav. Sci. 109(1):49–57. doi: 10.1016/j.applanim.2007.02.003 [DOI] [Google Scholar]
- Dziba L. E., Provenza F. D., Villalba J. J., and Atwood S. B.. . 2007. Supplemental energy and protein increase use of sagebrush by sheep. Small Rumin. Res. 69(1–3):203–207. doi: 10.1016/j.smallrumres.2005.12.013 [DOI] [Google Scholar]
- Ettlinger M. G., and Lundeen A. J.. . 1957. First synthesis of a mustard oil glucoside; the enzymatic Lossen rearrangement. J. Am. Chem. Soc. 79:1764–1765. doi: 10.1021/ja01564a066 [DOI] [Google Scholar]
- FASS 2010. Guide for the care and use of agricultural animals in agricultural research and teaching, 3rd edn Savoy (IL): Federation of Animal Science Societies; Available from https://www.aaalac.org/about/ag_guide_3rd_ed.pdf [Google Scholar]
- Favreau A., Baumont R., Duncan A. J., and Ginane C.. . 2010. Do sheep use umami and bitter tastes as cues of post-ingestive consequences when selecting their diet? App. Anim. Behav. Sci. 125(3–4):115–123. doi: 10.1016/j.applanim.2010.04.007 [DOI] [Google Scholar]
- Ferreira A. M., Araújo S. S., Sales-Baptista E., and Almeida A. M.. . 2013. Identification of novel genes for bitter taste receptors in sheep (Ovis aries). Animal. 7:547–554. doi: 10.1017/S1751731112002030 [DOI] [PubMed] [Google Scholar]
- Fox A. L. 1932. The relationship between chemical constitution and taste. Proc. Natl. Acad. Sci. USA. 18:115–120. doi: 10.1073/pnas.18.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. A., and Korchmar D. L.. . 1985. Gustatory processing differences in PTC tasters and non-tasters: a reaction time analysis. Physiol. Behav. 35:239–242. doi: 10.1016/0031-9384(85)90343-9 [DOI] [PubMed] [Google Scholar]
- Garcia-Bailo B., Toguri C., Eny K. M., and El-Sohemy A.. . 2009. Genetic variation in taste and its influence on food selection. Omi. A J. Integr. Biol. 13:69–80. doi: 10.1201/9780203023433.ch9 [DOI] [PubMed] [Google Scholar]
- Ginane C., Baumont R., and Favreau-Peigné A.. . 2011. Perception and hedonic value of basic tastes in domestic ruminants. Physiol. Behav. 104:666–674. doi: 10.1016/j.physbeh.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Goatcher W., and Church D.. . 1970a. Review of Some Nutritional Aspects of the Sense of Taste. J. Anim. Sci. 31:973–981. doi: 10.2527/jas1970.315973x [DOI] [PubMed] [Google Scholar]
- Goatcher W., and Church D.. . 1970b. Taste responses in ruminants. II. Reactions of sheep to acids, quinine, urea and sodium hydroxide. J. Anim. Sci. 30:784–790. doi: 10.2527/jas1970.305784x [DOI] [PubMed] [Google Scholar]
- Harris H., and Kalmus H.. . 1949. The measurement of taste sensitivity to phenylthiourea (PTC). Ann. Eugen. 15:24–31. doi: 10.1111/j.1469-1809.1949.tb02419.x [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Molyneux R. J., and Merrill G. B.. . 1985. Chemistry of toxic range plants—variation in pyrrolizidine alkaloid content of Senecio, Amsinckia, and Crotalaria species. J. Agric. Food Chem. 33(1):50–55. doi: 10.1021/jf00061a015 [DOI] [PubMed] [Google Scholar]
- Johnson K. H., Olson R. A., and Whitson T. D.. . 1996. Composition and diversity of plant and small mammal communities in tebuthiuron-treated big sagebrush (Artemisia tridentata). Weed Technol. 10(2):404–416. doi: 10.1017/s0890037x0004015x [DOI] [Google Scholar]
- Launchbaugh K. L. 2003. Prescription grazing for rangeland weed management: a new look at an old tool. Rangelands. 25(6):43–47. doi: 10.2458/azu_rangelands_v25i6_frost [DOI] [Google Scholar]
- Launchbaugh K. L., and Provenza F. D.. . 1994. The effect of flavor concentration and toxin dose on the formation and generalization of flavor aversions in lambs. J. Anim. Sci. 72:10–13. doi: 10.2527/1994.72110x [DOI] [PubMed] [Google Scholar]
- Launchbaugh K. L., Provenza F. D., and Burritt E. A.. . 1993. How herbivores track variable environments: response to variability of phytotoxins. J. Chem. Ecol. 19:1047–1056. doi: 10.1007/BF00987367 [DOI] [PubMed] [Google Scholar]
- Launchbaugh K. L., Provenza F. D., and Pfister J. A.. . 2001. Herbivore response to anti-quality factors in forages. J. Range Management 54(4):431–440. doi: 10.2307/4003114 [DOI] [Google Scholar]
- Lee H. D., and O’Mahony M.. . 1998. PTC and PROP behave differently in tests of discrimination from their solvents. Chem. Senses 23:403–410. doi: 10.1093/chemse/23.4.403 [DOI] [PubMed] [Google Scholar]
- Lush I. E. 1986. Differences between mouse strains in their consumption of phenylthiourea (PTC). Heredity. 57(3):323–323. doi: 10.1038/hdy.1986.129 [DOI] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., and Reed D. R.. . 2005. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115:e216–e222. doi: 10.1542/peds.2004-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffet C. A., Taylor J. B., and Booth D. T.. . 2015. Postfire shrub cover dynamics: a 70-year fire chronosequence in mountain big sagebrush communities. J. Arid Environ. 114:116–123. doi: 10.1016/j.jaridenv.2014.12.005 [DOI] [Google Scholar]
- Morton C. C., Cantor R. M., Corey L. A., & Nance W. E.. . 1981. A genetic analysis of taste threshold for phenylthiocarbamide. Acta. Genet. Med. Gemellol. (Roma). 30(1):51–57. doi: 10.1017/s0001566000006619 [DOI] [PubMed] [Google Scholar]
- Mote T. E., Villalba J. J., and Provenza F. D.. . 2007. Relative availability of tannin- and terpene-containing foods affects food intake and preference by lambs. J. Chem. Ecol. 33:1197–1206. doi: 10.1007/s10886-007-9305-2 [DOI] [PubMed] [Google Scholar]
- Nelson T. M., Munger S. D., and Boughter J. D. Jr. 2003. Taste sensitivities to PROP and PTC vary independently in mice. Chem. Senses 28:695–704. doi: 10.1093/chemse/bjg062 [DOI] [PubMed] [Google Scholar]
- Nolte D. L., and Provenza F. D.. . 1992. Food preferences in lambs after exposure to flavors in milk. App. Anim. Behav. Sci. 32(4):381–389. doi: 10.1016/S0168-1591(05)80030-9 [DOI] [Google Scholar]
- Parker L. A. 2003. Taste avoidance and taste aversion: evidence for two different processes. Learn. Behav. 31:165–172. doi: 10.3758/bf03195979 [DOI] [PubMed] [Google Scholar]
- Provenza F. D., and Balph D. F.. . 1987. Diet learning by domestic ruminants—theory, evidence and practical implications. App. Anim. Behav. Sci. 18(3–4):211–232. doi: 10.1016/0168-1591(87)90218-8 [DOI] [Google Scholar]
- Provenza F. D., Pfister J. A., and Cheney C. D.. . 1992. Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Management 45(1):36–45. doi: 10.2307/4002523 [DOI] [Google Scholar]
- Sclafani A. 1991. The hedonics of sugar and starch. In: R. C. Bolles, editor, The hedonics of taste. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc. p. 59–87. [Google Scholar]
- Seefeldt S. S. 2005. Consequences of selecting Rambouillet ewes for mountain big sagebrush (Artemisia tridentata ssp vaseyana) dietary preference. Rangeland Ecol. Manage. 58(4):380–384. doi: 10.2458/azu_rangelands_v58i4_seefeldt [DOI] [Google Scholar]
- Snowder G. D., Walker J. W., Launchbaugh K. L., and Van Vleck L. D.. . 2001. Genetic and phenotypic parameters for dietary selection of mountain big sagebrush (Artemisia tridentata Nutt. ssp. vaseyana [Rydb] Beetle) in Rambouillet sheep1. J. Anim. Sci. 79:486–492.doi: 10.2527/2001.792486x [DOI] [PubMed] [Google Scholar]
- Tepper B. J. 2008. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 28:367–388. doi: 10.1146/annurev.nutr.28.061807.155458 [DOI] [PubMed] [Google Scholar]
- Villalba J. J., Provenza F. D., and Olson K. C.. . 2006. Terpenes and carbohydrate source influence rumen fermentation, digestibility, intake, and preference in sheep. J. Anim. Sci. 84:2463–2473. doi: 10.2527/jas.2005-716 [DOI] [PubMed] [Google Scholar]
- Wambolt C. L., and Payne G. F.. . 1986. An 18-year comparison of control methods for Wyoming big sagebrush in Southwestern Montana. J. Range Management 39(4):314–319. doi: 10.2307/3899770 [DOI] [Google Scholar]