Abstract
The prenatal environment has been shown to have significant effects on the lifelong health of offspring in humans and other species. Such effects have not been studied extensively in avian species but could prove important, especially in the case of severe feed restriction imposed on broiler breeder hens to prevent obesity and reduce rate of lay. Feed restriction can potentially affect not only nutrient supply to the embryo but stress hormone levels within the hen. This study investigated the impact of nutrient restriction of the breeder hen on growth rate and immune responses in the progeny with the objective to measure the impact of feed restriction of broiler breeder hens on growth and immune response of the progeny. Broiler breeder hens were feed restricted from 24 wk of age and maintained at three bodyweights; 3.4, 3.6, and 4.0 kg until 43 wk of age and behavioral and physiological measures of stress recorded. Chicks were hatched from each hen treatment and at day 7 vaccinated for infectious bronchitis virus (IBV) and at 16, 18, and 20 d old given an immune challenge of lipopolysaccharide. Growth and immune responses of these birds were then recorded. Sex ratio was affected by hen bodyweight, with a significantly increased proportion of males hatched from heavy hens. Growth rate from 35 to 42 d of age was reduced in male progeny from low bodyweight hens. Female progeny from heavy hens responded to an immune challenge by reduced live weight and increased heterophil: lymphocyte ratio, suggesting a more robust immune response in these birds than in the progeny from lower bodyweight hens. Overall, progeny from heavy hens had increased antibodies at day 35 to the vaccination of IBV compared with progeny of low bodyweight hens, also suggesting an improved immune response in these birds. Breeder hens restricted to the lowest feed level showed behaviors indicative of increased stress (object pecking) and an increased heterophil: lymphocyte ratio. Feed restriction of broiler breeder hens increased indices of stress in hens and resulted in offspring that have reduced growth rate and immune response in a sex-dependent way.
Keywords: broiler breeder, feed restriction developmental programming, immune response, meat chicken, stress
INTRODUCTION
The concept that early life events affect health was first demonstrated during the Dutch famine (Barker, 2004). Early “events” occur during embryonic/fetal development, or early postnatal life and “impacts” can be metabolic, physiological, behavioral, and immunological (Chmurzynska, 2010). The major environmental factor studied to date has been maternal nutrition using intrauterine growth-restriction (IGUR) (Wu et al., 2004) and dietary composition and level (Armitage et al., 2005; Langley-Evans, 2006), with studies confirming the reprogramming effects of maternal diet on progeny growth and health. Animals reprogrammed by early nutrition display “metabolic syndrome,” which can include diabetes and hypertension (Langley-Evans, 2006; Elahi et al., 2009). Recently attention has been directed to the effects of maternal stress reprogramming on immunological functioning, with evidence of alterations in immunity in offspring of stressed mothers in multiple species, from rodents (Kay, 1998) to livestock (Couret et al., 2009; He et al., 2014)
Although developmental programming stress studies have been carried out in several species, there is limited research in meat chickens. Mothers of meat chickens, breeder hens, are selected for rapid, efficient growth of their progeny, leading to detrimental effects on their production of fertile eggs. The subsequent management practice of feed restriction of hens leads to chronic hunger and stress (de Jong et al., 2002; Mench, 2002; de Jong et al., 2003). Breeder hens are exposed to both under-nutrition and stress, likely affecting growth and immunity of their offspring, through developmental programming. The aim of this study was to measure the impact on the growth and immunity of progeny from breeder hens feed restricted to differing bodyweights. We hypothesized that hens kept at the highest level of feed restriction and lowest bodyweight would show increased stress and have offspring with reduced growth and immunity.
MATERIALS AND METHODS
Animal Husbandry
All animal use was approved by the animal ethics committees of The University of Adelaide (S-2014-121) and The Department of Primary Industries and Regions, South Australia (PIRSA) (#14/14). A total of 36 Cobb 500 broiler breeder hens were maintained in groups of 12 at a commercial breeder facility in group pens. Hens were maintained at three different bodyweights, low, medium, and high, through feed restriction on a commercial breeder hen diet. Hens were separated into treatment groups at 24 wk of age and remained in these groups for the duration of the experiment until 43 wk of age. Birds were fed once a day at a level that would allow for a difference in bodyweight between groups to be maintained. Feed intake for hens was increased from 21 wk of age from an average of 112 g/bird/d to 31 wk of age where birds were maintained at the maintenance needed to maintain the desired bodyweights from each treatment. Low bodyweight’ hens were maintained on an average at 3.4 ± 0.1 kg and fed 140 g of feed/hen/d, “medium-bodyweight” hens were maintained on an average at 3.5 ± 0.1 kg and fed 145 g of feed/hen/d and “high-bodyweight” hens were maintained on an average at 3.9 ± 0.1 kg and fed 160 g of feed/hen/d. Hens were weighed weekly to ensure that correct bodyweights were maintained and feed intake of each group was adjusted to maintain bodyweight differences between groups.
Eggs were collected over a 2-wk period from each group of breeder hens. Eggs from each hen treatment collected over the 2-wk collection period were randomized and incubated at 38° and 55% humidity from days 0 to 15, and then 36.7°C and 60% humidity until hatch at day 21 at Roseworthy Campus, The University of Adelaide. After hatch viable chicks were weighed, ID tagged and placed into group-rearing pens. Birds from each hen treatment group were placed in pens together with chicks from the same hen group over three replicates.
Standard husbandry procedures were followed with chicks placed in a shed at 25°C with heat lamps in each pen and a light cycle of 23 h light for the first 24 h. From the second day, the light cycle was changed to 16 h light and the shed temperature adjusted accordingly. Chicks were fed ad libitum a standard commercial meat bird starter diet (Ridley Turkey and Meat Chicken Grower) until 3 wk of age and a commercial meat bird finisher diet (Ridley Turkey and Meat Chicken Finisher) until 6 wk old. All birds were given an ocular vaccination for infectious bronchitis virus (IBV) at day 7, weighed weekly, and feed intake recorded.
Hen Behavior Analysis
Breeder hens were observed daily over 1-h periods in the morning during the 2-wk egg collection. Behaviors were recorded using an ethogram at 30-s intervals and the number of birds displaying each behavior at each time point was recorded. The total numbers of observations for each behavior and hen group were then added to give an overall total. The behaviors used (Table 1) were adapted from a previous study on breeder hen behavior (de Jong et al., 2003).
Table 1.
Recorded broiler breeder hen behaviors using an ethogram, at 30-s intervals, for 1 h, daily over 2 wk of lay
| Behavior category | Observed behaviors |
|---|---|
| Walking | walking/running |
| Sitting | sitting still |
| Standing | standing still |
| Peck drinker | peck drinker (not drinking) |
| Foraging | pecking/scratching litter |
| Comfort | preening, nibbling, stretching, and wing flapping |
| Peck object | peck object, peck cage (not including drinker) |
| Aggression | peck another bird/fighting |
| Other | any other observed behavior |
Heterophil:Lymphocyte Counts
After blood collection, a drop of blood was placed onto a glass slide and smeared across the slide to create a monolayer of cells. The slide was then fixed in 70% methanol for 60 s and allowed to dry and later stained with Wright-Giemsa Stain using a Hematek Stain Pak (Bayer) Automatic Stainer. All slides were counted at 40× magnification three times by the same counter, under a blinded analysis. Cells counted were lymphocytes, heterophils, monocytes, basophils, and eosinophils until a total of 100 cells were counted. A ratio of heterophils to lymphocyte cells was then calculated for each slide.
Sampling
A blood sample was collected from 18 broiler progeny of each hen treatment group (n = 54). Samples were collected when birds were 21, 35, and 42 d old. Birds were sampled via the brachial vein using a 23-gauge needle and 2-mL syringe into 4-mL lithium heparin tubes and stored on ice. Samples were centrifuged at 2000 g for 5 min and plasma collected and stored at −20°C.
Meat birds were grown until 42 d old and at day 42, 69 birds (23 per hen group) were euthanized. Birds were dissected and gross organ weight recorded for duodenum, jejunum, ileum, liver, heart, proventriculus, gizzard, spleen, and bursa. Duodenum, jejunum, ileum, gizzard, and proventriculus samples were opened and flushed of digesta prior to weighing and sampling. Tissue samples of the duodenum, jejunum, ileum, liver, spleen, and bursa were then collected, snap frozen in liquid nitrogen, and stored at −80°C.
Lipopolysaccharide Injections
Half of the remaining chicks (n = 72) were given three injections of a lipopolysaccharide (LPS) injection on alternate days, at 16, 18, and 20 d of age. Birds in each treatment group received an immune challenge injection of LPS Escherichia coli O55:B5 (Sigma-Aldrich) at a dose rate of 0.5 mg/kg bodyweight. Dose was determined after a review of the literature and the protocol was taken from Tan et al. (2014). The dose, however, was reduced to 0.5 from 1.0 mg/kg to reduce the risk of making the birds ill. Prior to injection, birds were weighed to determine dose rate and the injection site was disinfected using 70% ethanol. The birds were given an intra-peritoneal injection beneath the keel bone, 1 to 2 mm under the skin using a 23-gauge needle and 1-mL syringe. The remaining chicks (n = 74) in each hen treatment group did not receive the injection of LPS.
Yolk Immunoglobulin Y
Both egg yolk and serum samples were tested for Immunoglobulin Y (IgY) concentration. Egg yolk samples were collected from 20 eggs from low medium and heavy bodyweight hens (n = 60) over 1 wk. Medium hens were excluded from analysis and only low and heavy hen yolks were tested, due to the larger difference in the hen feed intake between these two groups. The eggs were weighed and the yolk collected and stored at −20°C. To test IgY concentrations in the yolk and serum samples, a 96 well IgG (Chicken) ELISA kit was used (Abnova, Sapphire Bioscience) and standard kit procedure was followed.
For the egg yolk ELISA, IgY was first extracted using an adapted method (Hamel et al., 2006). Briefly, the yolk was defrosted and twice the amount of Dulbecco’s PBS (Sigma-Aldrich) was added to the yolk and shaken vigorously. An equal amount of chloroform (Sigma-Aldrich) was then added and mixed thoroughly to produce a thick emulsion which was centrifuged at 1,000 g for 30 min at room temperature. After centrifuging, three distinct layers were visible with IgY present in the watery top layer, which was then removed and aliquoted into 1-mL tubes and stored at −20°C until analysis.
Yolk and Plasma Corticosterone
Corticosterone was extracted from yolks (n = 40) using previously described methods (Cook et al., 2009). Briefly, the egg yolk (~0.1 g) was taken and 0.5 mL of distilled water was added and vortexed until mixed. The mixture was extracted with 3-mL hexane:diether (30:70 ratio) and vortexed and left to settle before snap freezing in an ethanol/dry ice bath. The supernatant was collected, dried, and 1 mL of ethanol was added to the samples which were frozen at −80°C overnight. The samples were centrifuged the next day and the supernatant taken and dried once more. The samples were then thawed, resuspended in 500 µL of phosphate buffer saline, and analyzed.
Extracted yolk and plasma samples from progeny birds at 23 (n = 53) and 42 d old (n = 54) were analyzed for corticosterone concentrations. Samples were analyzed at the University of Western Australia, Animal Biology Department using a validated Radio Immuno Assay Corticosterone 125I RIA KIT (MP Biomedical, Orangeburg, NY).
IBV Antibody Analysis
Plasma samples collected at 35 d of age from offspring of heavy (n=15) and low (n =17) bodyweight hens were analyzed for IBV antibodies, after vaccination at day 7. Samples were analyzed by ACE Laboratory Services (Bendigo, Victoria) using an IBV (Ab) ELISA (BioCheck).
Statistical Analysis
Statistical analysis was performed using the IBM SPSS statistical program version 21. Hen behavior, yolk IgY concentration, plasma IBV titres, yolk and plasma corticosterone, and H:L cell counts were analyzed using a general linear model (GLM) with fixed effects of hen bodyweight, gender, and LPS treatment and interactions fitted into the model. Bodyweight was analyzed for each bird using repeated measures with the same parameters fitted into the model. Organ weights were analyzed using a GLM, with day 42 bodyweight fitted into the model as a co-variate. A Fisher’s exact test was used to determine the sex ratio of the progeny from each hen bodyweight group. A P value of <0.05 was deemed significant, with a P value of >0.05 and <0.10 considered a trend. All data presented are the mean ± SEM.
RESULTS
Hen Behavior
Forage and pecking behaviors differed between hen bodyweight groups (P < 0.05) (Table 2). Hens kept at the lowest bodyweight were observed foraging the least number of times (4.8 ± 2.7) compared with medium (23.6 ± 2.8) and high bodyweight hens (13.4 ± 3.1). Low bodyweight hens also pecked objects in the cage more (184 ± 6.6) than medium (137.7 ± 6.8) and high bodyweight hens (132. 8 ± 7.5) (P < 0.05).
Table 2.
Total number of times hens maintained at a low, medium, and heavy bodyweight were observed displaying foraging and pecking behaviors, using an ethogram with observations taken every 30 s, over 1 h, daily for 2 wk
| Hen weight | Forage | SEM | P-value | Peck | SEM | P-value |
|---|---|---|---|---|---|---|
| Low | 4.8a | 2.7 | 0.011 | 184.0a | 6.6 | <0.001*** |
| Medium | 23.6b | 2.8 | 0.007 | 137.7b | 6.8 | 0.156 |
| High | 13.4c | 3.1 | 0.011 | 132.8b | 7.5 | <0.001*** |
Values are means ± SEM, n = 36. Means in a column with superscripts differ, P < 0.05
Hen Heterophil:Lymphocyte Counts
The H:L counts of white blood cells at 31 wk of age approached statistical significance (P = 0.06). Low bodyweight hens had a greater H:L ratio (1.03 ± 0.1) compared with hens maintained at medium (0.67 ± 0.1) and high bodyweights (0.59 ± 0.1).
Yolk and Progeny IgY
No statistical significance (P > 0.05) was found in egg yolk IgY concentration (ng/mL) in eggs taken from heavy (64.6 ± 6.6) and low bodyweight hens (66.5 ± 6.7). There was also no statistical difference (P > 0.05) in blood serum IgY (ng/mL) taken on day 23 from progeny of low (12.6 ± 3.0) and high bodyweight hens (13.9 ± 3.0).
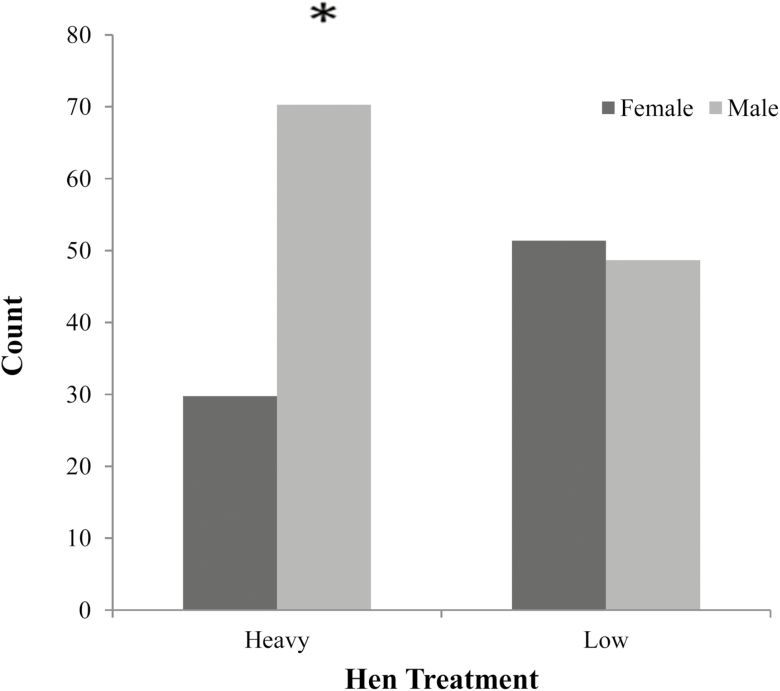
Progeny Sex Ratio
The sex ratio of progeny from low and heavy bodyweight hens was significantly different (P < 0.048). A greater proportion of males were hatched from heavy bodyweight hens (n = 26), compared with lower bodyweight hen offspring (n = 18). Female hatch rates were opposite and were greater in lower bodyweight hens (n = 19) and reduced in heavy bodyweight hen progeny (n =11) (Figure 1).
Figure 1.
Sex ratio of broiler progeny at hatch from low and heavy bodyweight breeder hens. Significance was evaluated using a chi-square, Fischer’s exact test with significance at P < 0.05.
Yolk and Plasma Corticosterone
A trend towards significance (P = 0.086) was found in yolk corticosterone (ng/g) between offspring of low (90.8 ± 17.7), medium (74.8 ± 21.3), and high (87.3 ± 18.1) hens. The yolk corticosterone concentrations (ng/g) between low and medium bodyweight hens was significantly different (P = 0.045).
At 23 d of age, there was no significant difference (P > 0.05) in plasma corticosterone (ng/mL) in offspring from low (89.4 ± 10.3), medium (95.8 ± 11.2), and high (120.4 ± 17) bodyweight hens. There was a trend towards a significant difference in plasma corticosterone (ng/mL) (P = 0.065) in the interaction between males and females from high and low bodyweight hens. Females from high bodyweight hens had increased corticosterone (139.8 ± 31) compared with females from low bodyweight hens (71.4 ± 13.9). In males, the opposite was seen with decreased corticosterone in males from high bodyweight hens (101 ± 9.8) compared with males from low bodyweight hens (107.5 ± 13.9).
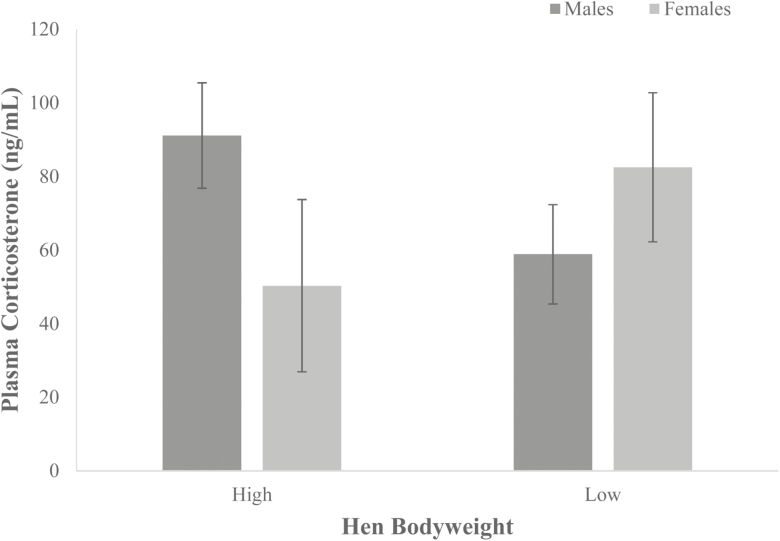
At 42 d of age, there was also no significant difference (P > 0.05) in plasma corticosterone levels (ng/mL) between offspring of low (70.7 ± 7.1), medium (57.3 ± 9.3), and high (55.8 ± 8.3) bodyweight hens. There was a trend towards significance (P = 0.094) in the interaction of hen bodyweight*sex, in plasma corticosterone (ng/mL) in the offspring at 42 d old between males and females from high and low bodyweight hens. The opposite was found to day 23, with corticosterone levels were elevated in females from low bodyweight hens (82.5 ± 11.8) compared with females from high bodyweight hens (50.3 ± 13.6). In males, plasma corticosterone levels were reduced in those from low bodyweight hens (58.8 ± 7.8) compared with male from high (61.3 ± 9.6) bodyweight hens (Figure 2).
Figure 2.
Plasma corticosterone (ng/mL) at 42 d of age in males and females from low (n = 13) and high (n = 11) bodyweight hens. Values are mean ± SEM. Significance was evaluated using a two-way ANOVA.
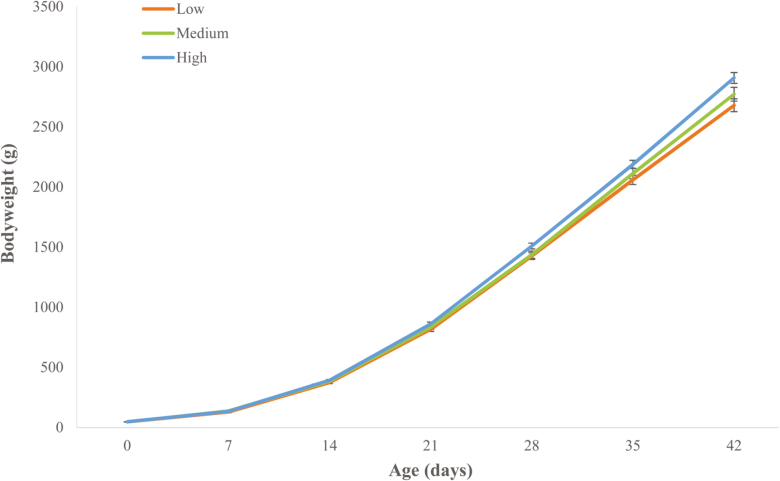
Progeny Growth
A significant three-way interaction (P < 0.001) was found in bodyweight of the progeny, of hen bodyweight, sex, and age. This interaction showed an effect of decreased bodyweight (g) in male progeny from low bodyweight hens (2678.9 ± 52.9) compared with males from heavy bodyweight hens (2906.3 ± 46.1) at 42 d of age (Figure 3).
Figure 3.
Bodyweight (g) of male progeny from low, medium, and heavy hens from hatch (day 0) until 42 d of age. Weight is mean ± SEM. Significance was evaluated using a repeated measures model with significance at P < 0.05.
Immunity Challenge and Response to LPS
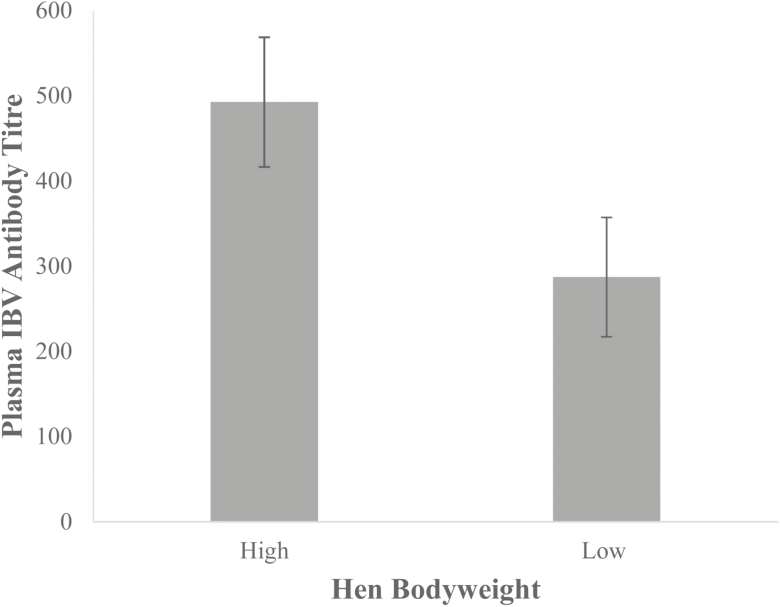
A significant difference (P = 0.05) was found at day 35 in plasma antibodies to IBV between progeny from heavy (n = 15) and low bodyweight hens (n = 17). Antibody titers to the vaccination at day 7 were elevated in progeny from heavy hens (492.7 ± 76.1) compared with progeny from low bodyweight hens (287.3 ± 70.1), as seen in Figure 4.
Figure 4.
Plasma IBV antibody titers at 35 d old in progeny of low (n = 17) and heavy hens (n = 15). Values are means ± SEM. Significance was evaluated using a one-way ANOVA with significance at P < 0.05.
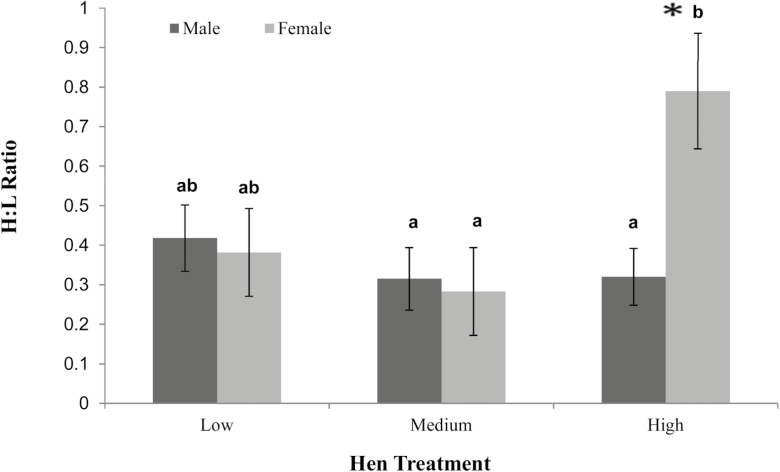
Heterophil:lymphocyte (H: L) ratios at 23 d of age showed significant differences between males and females from heavy hens (P < 0.05). Females from heavy hens had a significantly greater H: L ratio (0.79 ± 0.15) than males from heavy hens (0.32 ± 0.07) as well as females from both low (0.38 ± 0.11) and medium bodyweight hens (0.28 ± 0.11). There was no difference seen between males from heavy (0.32 ± 0.07), medium (0.32 ± 0.08), and low bodyweight hens (0.42 ± 0.08) (Figure 5).
Figure 5.
Heterophil:lymphocyte ratio of males and females from low, medium, and heavy bodyweight hens. Values are means ± SEM (n = 36). Significance was evaluated using a one-way ANOVA with significance at P < 0.05. Labeled means without a common letter differ, P < 0.05.
There was a significant difference (P < 0.05) in bodyweight (g) on day 21 between all progeny injected with LPS (798 ± 8.7) and controls (835.7 ± 8.7). After injection with LPS, a significant difference in bodyweight was observed between males and females on day 21 (P < 0.05). Female bodyweights (g) were lower after LPS injection (786.45 ± 11.7) compared with males that received LPS (842.8 ± 9.2).
There was also a significant interaction of sex*hen bodyweight on day 21 bodyweight (P < 0.05). Females hatched from heavy hens were affected by the LPS challenge, with those given the LPS injection weighing less (755.3 ± 27.2) than control females (860 ± 27.2). Males from heavy hens were not affected in the same way, with males injected with LPS weighing the same (848.5 ± 18.5) as male controls (873.3 ± 19.3).
At dissection at 42 d old, there was a significant difference in spleen weight (P < 0.05) with bodyweight at day 42 fitted as a co-variate. Progeny from heavy bodyweight hens had significantly heavier gross spleen weight at day 42 (3.0 ± 0.2) compared with progeny of medium (2.4 ± 0.2) and low bodyweight hens (2.3 ± 0.2).
DISCUSSION
Hen Feed Restriction and Stress
Hens maintained at a low bodyweight from 24 wk old displayed decreased foraging behavior and increased object pecking. Increased pecking and reductions in comfort behaviors (such as foraging) can indicate chronic hunger in breeder hens, with correlations between these behaviors and other measurements of hunger such as feed intake motivation tests and glucose/NEFA ratio (de Jong et al., 2003). These behaviors, indicative of chronic hunger, were accompanied by changes in the ratio of heterophil to lymphocytes in the blood, an accepted indicator of stress in poultry (Müller et al., 2011). We cannot directly attribute stress in the hens to low feed intake per se as there may be some other component of low bodyweight that is the trigger to stress, but whatever it is, it must ultimately relate to low intake.
Yolk corticosterone levels can reflect plasma corticosterone levels in hens (Henriksen et al., 2011). Surprisingly, hens maintained at a medium level of feed restriction had the lowest yolk corticosterone concentration. These same hens also showed increased foraging behavior, and this together with the yolk corticosterone suggests that medium bodyweight hens were the least stressed, followed by heavy hens. This is likely due to an increased level of feed given to these birds, compared with the lowest bodyweight hens.
IgY Maternal Transfer
No difference was found in yolk IGY antibodies between hen treatments. There was also no significant difference between progeny of hens in plasma IgY, and therefore, it can be assumed that differences in progeny were not due to the transfer of IgY antibodies from the hen to the chick.
Progeny Sex Ratio
Sex ratio of chicks hatched was affected by hen bodyweight, with an increase in males hatched from heavy bodyweight hens. One possible reason behind this is the ability of avian species to modify hatchling sex ratio via changes in production of gonadal steroids, resulting in changes in egg hormone concentrations (Henriksen et al., 2011). Testosterone is thought to be one such hormone behind this ability to manipulate sex ratios, with increases in yolk testosterone linked to increases in male born offspring (Veiga et al., 2004; Rubolini et al., 2005b). As testosterone and corticosterone levels can be influenced by one another; corticosterone levels can be opposite to testosterone in chicken egg yolk (Henriksen et al., 2011). The decreased corticosterone within the yolks from heavy hens may therefore suggest that testosterone levels were elevated within these eggs, resulting in increased male hatchlings; although testosterone was not measured in this study, it should be considered in future work.
Progeny Growth
Hen bodyweight did significantly affect the growth of their offspring, but only in males during the final week of growth, with males from low bodyweight hens significantly lighter than males from heavy mothers. Yolk corticosterone was increased in low bodyweight hens, and elevated corticosterone in hens has been previously linked to decreased growth in their offspring (Janczak et al., 2006; Shini et al., 2009; Ahmed et al., 2014), and could explain the reduced growth in males from low bodyweight hens. Previous avian research has also found reduced growth only in males after corticosterone exposure (Hayward et al., 2006) as well as behavior changes in males (Goerlich et al., 2012). It is possible that males may be more sensitive to changes in corticosterone levels during development and other maternal changes (Rubolini et al., 2005a), and perhaps, this is why only male growth was affected.
Progeny Immunity and Response to LPS Challenge
Hen bodyweight had a significant effect on the antibody titer at 35 d of age of their progeny to the IBV vaccine at day 7. Antibody levels were found to be elevated in progeny from high bodyweight hens, compared with those from low bodyweight hens. This is a significant result as it demonstrates the impact of the hen bodyweight on the ability of the progeny to mount an immune response to a vaccination. The increased antibody titers in progeny of high bodyweight hens suggest an enhanced immune response of these birds in response to the vaccination, compared with progeny of low bodyweight hens.
Spleen weight relative to bodyweight was also increased in progeny from heavy hens and although spleen weight as a measure of immunity is debatable may have affected the antibody titers to IBV. A reduced spleen weight can be an effect of increased stress and corticosterone (Post, 2003) and although not significant, corticosterone within the yolk was slightly elevated in the low bodyweight hens. There is therefore the potential that there was an elevated corticosterone or other glucocorticoid elevated exposure within the low bodyweight eggs. These hens did also have elevated H:L counts and behaviors indicative of stress, which could have affected the immunity of their offspring, including spleen size and weight. This reduced spleen weight may then have gone on to reduce their immune ability as the spleen in avians is a storage organ of lymphocytes (Smith and Hunt, 2004) and their ability to produce the same amount of antibodies as birds from high bodyweight hens.
The impact of maternal stress and elevated glucocorticoids has been demonstrated in multiple species. In rodents, stressed mothers have given birth to offspring with decreased leukocyte counts, reduced B cell proliferation (Kay, 1998; Llorente et al., 2002), and reduced antibodies (Gorczynski, 1992; Sobrian et al., 1997), as seen in this study. Similar findings have also been shown in primates (Coe et al., 1999), with decreased T cell response to antigens in offspring of stressed mothers and decreased white blood cells in piglets from stressed sows (Couret et al., 2009). Therefore, the impact of maternal stress on the immunity of the offspring has been demonstrated across other species and is likely to occur in meat birds, as shown in this study and although the link between larger spleens and increased immunity has not been definitively shown in avians (Smith and Hunt, 2004). Spleen size could have been affected by maternal stress and possibly affected the antibody production in response to the vaccine. Overall, progeny from less feed-restricted heavy bodyweight hens was exhibiting an expected response and may be more sensitive to an immune threat than birds from lower bodyweight hens, under increased feed restriction.
Overall LPS-injected birds had a reduction in bodyweight at day 21, which might be expected as the birds partitioned nutrients to mount an immune response (Klasing, 2007). Voluntary feed intake did not appear to alter between treatments; however, the challenge was at a low dose and showed no effect on bird behavior or feeding. If the challenge dose was elevated, it is likely that feed intake would decrease. Differences in response between birds from low and heavy bodyweight hens were also found, with increased plasma corticosterone in LPS-challenged birds from heavy hens. An increased plasma corticosterone concentration might also be expected as pro-inflammatory cytokines are elevated, along with corticosterone (Lu et al., 2008).
Differences in immune response were also found within gender between progeny from different bodyweight hens. Females from heavy hens challenged with LPS were significantly different from other LPS females, with reduced growth at day 21 after the challenge, increased H:L ratio, and increased plasma corticosterone. Males from heavy hens given LPS showed a similar increase in corticosterone later at day 42, suggesting they may be slower to respond than the females.
Reduced bodyweight and increased corticosterone are part of the immune response to the LPS. The elevation in H:L ratio also suggests the mounting of an immune response, as heterophils are part of the avian immune response (Shini et al., 2010) and are also elevated with increased corticosterone (Shini et al., 2008). These changes were not seen in females from low and medium bodyweight hens, which indicates that these birds were not mounting the same immune response. Therefore, the heavy hen females seem more able to respond to immune problems and although the LPS was low and did not cause any visible illness, these birds may be more likely to be able to defend against an immune challenge.
Reduced bodyweight through feed restriction in broiler breeder hens appears to induce a stress response with changes in behavior and H:L counts, affecting the progeny, possibly related to elevated corticosteroid levels in the yolk of the developing embryo. Progeny from these hens was affected in several ways in a sex-specific way. Males from less feed-restricted hens grew at a greater rate later in life and females from these hens mounted a stronger immune response to an LPS challenge compared with progeny from hens with heavier feed restriction. The results of this study have serious implications for the chicken meat industry both in terms of the welfare of feed-restricted hens but also for the health and productivity of the progeny meat bird. Maintaining breeder hens at a heavier bodyweight may therefore mean that hens are less stressed and could result in improved growth in male broilers and immunity in females as well as overall immune response to vaccination.
Footnotes
The authors would like to acknowledge the Hi-Chick Breeder Company, Bethyl, SA, The Animal Biology Department, The University of Western Australia, ACE Laboratory Services, Bendigo, Victoria, and Associate Professor Kapil Chousalkar.
LITERATURE CITED
- Ahmed A. A., W. Ma Y. Ni S. Wang, and Zhao R.. 2014. Corticosterone in ovo modifies aggressive behaviors and reproductive performances through alterations of the hypothalamic-pituitary-gonadal axis in the chicken. Anim. Reprod. Sci. 146:193–201. doi: 10.1016/j.anireprosci.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Armitage J. A., P. D. Taylor, and Poston L.. 2005. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J. Physiol. 565(Pt 1):3–8. doi: 10.1113/jphysiol.2004.079756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. 2004. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359:1359–1366. doi: 10.1098/rstb.2004.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmurzynska A. 2010. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr. Rev. 68:87–98. doi: 10.1111/j.1753-4887.2009.00265.x [DOI] [PubMed] [Google Scholar]
- Coe C. L., G. R. Lubach, and Karaszewski J. W.. 1999. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol. Neonate 76:301–310. doi: 10.1159/000014172 [DOI] [PubMed] [Google Scholar]
- Cook N. J., R. Renema C. Wilkinson, and Schaefer A. L.. 2009. Comparisons among serum, egg albumin and yolk concentrations of corticosterone as biomarkers of basal and stimulated adrenocortical activity of laying hens. Br. Poult. Sci. 50:620–633. doi: 10.1080/00071660903147424 [DOI] [PubMed] [Google Scholar]
- Couret D., A. Jamin G. Kuntz-Simon A. Prunier, and Merlot E.. 2009. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Vet. Immunol. Immunopathol. 131:17–24. doi: 10.1016/j.vetimm.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Elahi M. M., F. R. Cagampang D. Mukhtar F. W. Anthony S. K. Ohri, and Hanson M. A.. 2009. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 102:514–519. doi: 10.1017/S000711450820749X [DOI] [PubMed] [Google Scholar]
- Goerlich V. C., D. Nätt M. Elfwing B. Macdonald, and Jensen P.. 2012. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm. Behav. 61:711–718. doi: 10.1016/j.yhbeh.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. 1992. Conditioned stress responses by pregnant and/or lactating mice reduce immune responses of their offspring after weaning. Brain. Behav. Immun. 6:87–95. doi: 10.1016/0889-1591(92)90062-S [DOI] [PubMed] [Google Scholar]
- Hamel K. R., Burgess S. C., Pevzner I. Y., and Erf G. F.. 2006. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat line chickens. Poult. Sci. 85(8):1364–1372. doi: 10.1093/ps/85.8.1364 [DOI] [PubMed] [Google Scholar]
- Hayward L. S., J. B. Richardson M. N. Grogan, and Wingfield J. C.. 2006. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. Gen. Comp. Endocrinol. 146:144–148. doi: 10.1016/j.ygcen.2005.10.016 [DOI] [PubMed] [Google Scholar]
- He Z. X., Z. H. Sun W. Z. Yang K. A. Beauchemin S. X. Tang C. S. Zhou X. F. Han M. Wang J. H. Kang, and Tan Z. L.. 2014. Effects of maternal protein or energy restriction during late gestation on immune status and responses to lipopolysaccharide challenge in postnatal young goats. J. Anim. Sci. 92:4856–4864. doi: 10.2527/jas.2014-7904 [DOI] [PubMed] [Google Scholar]
- Henriksen R., T. G. Groothuis, and Rettenbacher S.. 2011. Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS One 6:e23824. doi: 10.1371/journal.pone.0023824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczak A. M., Braastad B. O., and Bakken M.. 2006. Behavioural effects of embryonic exposure to corticosterone in chickens. Appl. Anim. Behav. Sci. 96: 69–82. doi: 10.1016/j.applanim.2005.04.020 [DOI] [Google Scholar]
- de Jong I. C., A. S. van Voorst, and Blokhuis H. J.. 2003. Parameters for quantification of hunger in broiler breeders. Physiol. Behav. 78:773–783. doi: 10.1016/S0031-9384(03)00058-1 [DOI] [PubMed] [Google Scholar]
- de Jong I. C., S. van Voorst D. A. Ehlhardt, and Blokhuis H. J.. 2002. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. Br. Poult. Sci. 43:157–168. doi: 10.1080/00071660120121355 [DOI] [PubMed] [Google Scholar]
- Kay G., N. Tarcic T. Poltyrev, and Weinstock M.. 1998. Prenatal stress depresses immune function in rats. Physiol. Behav. 63:397–402. doi: 10.1016/S0031-9384(97)00456-3 [DOI] [PubMed] [Google Scholar]
- Klasing K. C. 2007. Nutrition and the immune system. Br. Poult. Sci. 48:525–537. doi: 10.1080/00071660701671336 [DOI] [PubMed] [Google Scholar]
- Langley-Evans S. C. 2006. Developmental programming of health and disease. Proc. Nutr. Soc. 65: 97–105. doi: 10.1079/PNS2005478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente E., M. L. Brito P. Machado, and González M. C.. 2002. Effect of prenatal stress on the hormonal response to acute and chronic stress and on immune parameters in the offspring. J. Physiol. Biochem. 58:143–149. doi: 10.1007/BF03179851 [DOI] [PubMed] [Google Scholar]
- Lu Y. C., W. C. Yeh, and Ohashi P. S.. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. doi: 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Mench J. A. 2002. Broiler breeders: feed restriction and welfare. World’s Poultry Science Journal 58: 23–29. doi: 10.1079/WPS20020004 [DOI] [Google Scholar]
- Müller C., Jenni-Eiermann S., and Jenni L.. 2011. Heterophils/lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Functional Ecology 25: 566–576. doi: 10.1111/j.1365-2435.2010.01816.x [DOI] [Google Scholar]
- Post J., J. M. Rebel, and ter Huurne A. A.. 2003. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 82:1313–1318. doi: 10.1093/ps/82.8.1313 [DOI] [PubMed] [Google Scholar]
- Rubolini D., M. Romano G. Boncoraglio R. P. Ferrari R. Martinelli P. Galeotti M. Fasola, and Saino N.. 2005a. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (larus michahellis) chicks. Horm. Behav. 47:592–605. doi: 10.1016/j.yhbeh.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Rubolini D., Romano M., Martinelli R., and Saino N.. 2005b. Effects of elevated yolk testosterone levels on survival, growth and immunity of male and female yellow-legged gull chicks. Behav. Ecol. Sociobiol. 59: 344–352. doi: 10.1007/s00265-005-0057-0 [DOI] [Google Scholar]
- Shini S., P. Kaiser A. Shini, and Bryden W. L.. 2008. Biological response of chickens (gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 149:324–333. doi: 10.1016/j.cbpb.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Shini S., A. Shini, and Huff G. R.. 2009. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol. Behav. 98:73–77. doi: 10.1016/j.physbeh.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Shini S., A. Shini, and Kaiser P.. 2010. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress 13:185–194. doi: 10.3109/10253890903144639 [DOI] [PubMed] [Google Scholar]
- Smith K. G. and Hunt J. L.. 2004. On the use of spleen mass as a measure of avian immune system strength. Oecologia 138:28–31. doi: 10.1007/s00442-003-1409-y [DOI] [PubMed] [Google Scholar]
- Sobrian S. K., V. T. Vaughn W. K. Ashe B. Markovic V. Djuric, and Jankovic B. D.. 1997. Gestational exposure to loud noise alters the development and postnatal responsiveness of humoral and cellular components of the immune system in offspring. Environ. Res. 73:227–241. doi: 10.1006/enrs.1997.3734 [DOI] [PubMed] [Google Scholar]
- Tan J., S. Liu Y. Guo T. J. Applegate, and Eicher S. D.. 2014. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 111:1394–1404. doi: 10.1017/S0007114513003863 [DOI] [PubMed] [Google Scholar]
- Veiga J. P., J. Viñuela P. J. Cordero J. M. Aparicio, and Polo V.. 2004. Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm. Behav. 46:47–53. doi: 10.1016/j.yhbeh.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Wu G., F. W. Bazer T. A. Cudd C. J. Meininger, and Spencer T. E.. 2004. Maternal nutrition and fetal development. J. Nutr. 134:2169–2172. doi: 10.1093/jn/134.9.2169 [DOI] [PubMed] [Google Scholar]