INTRODUCTION
Managing nutrient supply to match demand is critical for sustainable and efficient livestock production. The California Net Energy System (CNES) was developed during the late 1950s and 1960s, as documented in the classic publication of Lofgreen and Garrett (1968). This system was a significant step forward and has served the beef cattle industry well during the past 50 yr. Energy requirements for maintenance (NEm) and gain (NEg), described by Lofgreen and Garrett (1968) as modified by expanding databases (Garrett 1980) and for specific situations [National Research Council (NRC), 1984, 1996, 2000; The National Academies of Sciences, Engineering, and Medicine (NASEM), 2016], are used worldwide as a basis for feeding beef cattle and often other ruminant species as well.
Concepts of developmental programming, or the idea that stressors during critical windows of development can have both short- and long-term consequences in offspring, began to emerge about three decades ago based on human epidemiological studies (Barker, 1992, 2004). Research with animal models, including livestock, has since demonstrated that developmental programming is probably universal and that consequences on offspring growth, development, and health are likely much larger than previously thought (Wu et al., 2006; Caton and Hess, 2010; Funston et al., 2012; Reynolds and Caton, 2012; Greenwood et al., 2017; Hoffman et al., 2017; Reynolds et al., 2017). Maternal stress, particularly nutritional stress, is one of the major drivers of negative consequences of developmental programming in offspring. Livestock can often experience a poor or compromised nutritional environment during gestation. For example, extensive livestock production systems such as those experienced by grazing animals in the Intermountain Region of the Western United States and similar environments throughout the world often result in compromised nutrient supply during gestation. In the United States, livestock can experience a poor nutritional environment during pregnancy as a result of 1) breeding of young dams, which increases competition for nutrients among the maternal and fetal systems; 2) presence of multiple fetuses; 3) selection for increased milk production, which results in competition for nutrients between the mammary gland and gravid uterus; and 4) naturally occurring environmental temperature stress (which complicates nutrient supply and fetal growth) or conditions that compromise feed quality or quantity that coincide with breeding and gestation of livestock (Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010; Funston et al., 2012; Reynolds and Caton, 2012; Reynolds et al., 2017).
Compromised offspring may have altered metabolic and body composition outcomes (Du et al., 2017; Greenwood et al., 2017; Reed and Govoni, 2017; Reynolds and Vonnahme, 2017) at various points during their postnatal growth curves. Metabolic and body composition changes could influence NEm or NEg requirements. The goal of this review was to examine the potential impacts of maternal nutrition and developmental programming on offspring energy requirements.
MATERNAL NUTRITION AND DEVELOPMENTAL PROGRAMMING
Developmental Programming
Growth-restricted or developmentally impaired newborns have an increased risk of health complications throughout life, including metabolic, growth-related, and reproductive complications. Originally referred to as “the Barker hypothesis,” or “fetal programming,” the concept is that poor maternal nutrition (or others types of stress such as young or old maternal age, environmental heat stress, etc.) during critical windows of in utero or early postnatal development can have long-term effects on offspring health and well-being (Barker, 1992; Paneth and Susser, 1995; Armitage et al., 2004; Barker et al., 2004; Wu et al., 2006; Caton and Hess, 2010; Reynolds et al., 2010, 2017; Reynolds and Caton, 2012; Meyer and Caton, 2016). The concept of developmental programming was originally based on epidemiological studies in humans, but evidence of developmental programming of growth and well-being in livestock is found in older published literature and is often referred to as the maternal effect. For example, the crossbreeding experiments of Walton and Hammond with large (Shire) and small (Shetland) horses demonstrated that uterine environment impacts both birth weight and adult size (Hammond, 1927; Walton and Hammond, 1938).
In many species, including livestock, compromised fetal and/or neonatal growth can result in 1) increased neonatal morbidity and mortality; 2) altered postnatal growth; 3) changed body composition (e.g., increased fat, reduced muscle growth); 4) metabolic disorders (e.g., poor glucose tolerance and insulin resistance); 5) cardiovascular disease; and 6) dysfunction of organs and/or organ systems, (including adipose, brain, cardiovascular, endocrine, gastrointestinal, immune, kidney, liver, mammary gland, muscle, pancreas, placenta, and reproductive; Rhind et al., 2001; Sheldon and West, 2004; Wu et al., 2006; Anway et al., 2008; Caton and Hess, 2010; Du et al., 2010; Reynolds et al., 2010; Long et al., 2011, 2012; Shankar et al., 2011; Bartol and Bagnell, 2012; Connor et al., 2012; Meyer et al., 2012a; Reynolds and Caton, 2012; Spencer et al., 2012; Symonds et al., 2012; Jackson et al., 2013; Xiong and Zhang, 2013; Cardoso et al., 2014; Kilcoyne et al., 2014; Schmidt et al., 2014; Zambrano et al., 2014; Meyer and Caton, 2016; Reynolds and Vonnahme, 2016). Clearly, developmental programming can affect multiple organs and/organ systems when assessed in various animal models, including livestock (Caton and Hess, 2010; Reynolds et al., 2010, 2017; Reynolds and Caton, 2012).
Impacts of Maternal Nutrition
Maternal nutrient supply is a major driver of developmental programming events and consequently, offspring outcomes (Wallace, 1948; Wallace et al., 1999; Wu et al., 2006; Caton et al., 2007; Caton and Hess, 2010; Funston et al., 2012; Reynolds and Caton, 2012; Robinson et al., 2013; Vonnahme et al., 2015, Meyer and Caton, 2016; Reynolds et al., 2017; Reynolds and Vonnahme, 2017). Fetal growth trajectory is affected by maternal nutrient intake even from very early stages of embryonic development, when nutrient requirements for conceptus growth are negligible in proportion to maternal needs (NRC, 1996, 2007; Robinson et al., 1999; NASEM, 2016).
Maternal nutrient restriction includes any event that decreases fetal nutrient supply during critical developmental windows (Caton and Hess, 2010; Reynolds and Caton, 2012). Restriction of fetal nutrient supply can result from many things, including compromised maternal nutrient supply, placental dysfunction, deranged maternal metabolism, physiological or environmental extremes, or combinations of the aforementioned or other scenarios. Effects of fetal nutrient restriction during gestation depend on timing, level, and/or duration of compromised nutrition (Reynolds and Caton, 2012; Reynolds et al., 2013; Vonnahme et al., 2015; Zhang et al., 2015). A majority of the data (Reed et al., 2007; Swanson et al., 2008; Vonnahme et al., 2015) demonstrate that maternal nutrient restriction during the last two-thirds of gestation can decrease fetal growth and offspring birth weights in sheep.
Nutrient restriction in beef cattle can decrease birth weights and result in slower postnatal growth (Robinson et al., 2013). Likewise, relative to controls fed at requirements, feeding of low or high levels of metabolizable protein to mature beef cows resulted in decreased birth weights (Sletmoen-Olsen et al., 2000). Others (Martin et al., 2007; Larson et al., 2009) reported that protein supplementation of cows during the last third of pregnancy had little influence on calf birth weights. Conversely, Spitzer et al. (1995) and Stalker et al. (2007) reported that greater body condition during late gestation (a proxy for greater nutrient intake) can increase calf birth weights. Available data are taken to imply that birth weights in sheep, when compared with beef cattle, are more susceptible to maternal nutrient restriction, which could reflect differing placental growth patterns between sheep and cattle (Reynolds et al., 2005; Vonnahme and Lemley, 2012).
Neonates that are growth restricted in utero are at risk of postnatal complications, which may result in poor growth and development and concomitant negative consequences that include poor productivity and reduced longevity later in life (Wu et al., 2006; Caton and Hess, 2010; Funston et al., 2012; Reynolds and Caton, 2012; Reynolds and Vonnahme, 2017). Maternal undernutrition and restricted fetal growth are associated with decreased growth efficiency and altered body composition (Greenwood et al., 1998, 2000; Wu et al., 2006; Caton et al., 2007; Larson et al., 2009; Robinson et al., 2013). Birth weights in cattle are related to postnatal growth performance (Robinson et al., 2013; NASEM, 2016); however, nutrient restriction of dams can alter composition of offspring growth in the absence of birth weight differences (Reynolds and Caton, 2012). Altered postnatal metabolism or growth resulting from perturbed maternal nutrition can result in management challenges for livestock producers because nutritional management decisions are often based on the averages of groups of animals. Therefore, management approaches that mitigate negative effects of developmental programming have the potential to improve efficiency of ruminant livestock production, which will help address the grand challenge of doubling livestock production to feed the projected global population of 9.6 billion by the year 2050 (Reynolds et al., 2015; United Nations News Centre, 2015).
Much of the aforementioned discussion focused on altered total nutritional supply, which is most often achieved via differential intakes; however, a substantial body of literature focuses on supply of specific nutrients and developmental programming outcomes. Across species, the major classes of nutrients (carbohydrates, protein, lipids, vitamins, and minerals) have been investigated in models of developmental programming, and in each case, examples exist where maternal supply of a major nutrient class affected offspring outcomes. In other cases, realimentation or biological plasticity allows for compensation or protection from adverse outcomes.
Research with beef cattle in Nebraska (Funston et al., 2012) demonstrated that protein supplementation during gestation can have long-term positive effects on the offspring, including changes in weaning weight, carcass characteristics, and reproductive traits, when compared with non-supplemented controls. Funston et al. (2012) also showed that such long-term effects are not necessarily foreshadowed by differences in birth weight. When investigating prepartum maternal dietary energy source in beef cows, Radunz et al. (2012) reported that differing maternal dietary energy sources altered offspring adipose tissue development, glucose metabolism, insulin sensitivity, and long-term intramuscular fat deposition. Lan et al. (2013) reported that maternal dietary starch levels in pregnant sheep affected fetal DNA methylation and gene expression. Wang et al. (2015) demonstrated that imprinted gene expression and DNA methyltransferase in calves are influenced by maternal dietary starch. The work of Wang et al. (2015) indicated that epigenetic mechanisms can play a major role in the regulation of offspring responses to altered maternal nutrition, which is supported by recent reviews by Meyer et al. (2012a) and Reynolds et al. (2017). In a review of developmental programming in cattle, Robinson et al. (2013) concluded that fetal programming was pronounced and might explain considerable variation in growth and production traits including body weight, intake, carcass and muscle weights, and lean, fat, and bone weights.
Although global nutrient restriction and excess (driven by intake changes) and altered supply of major nutrient classes can clearly have effects on offspring outcomes, supply of specific nutrients in the maternal diet can also result in changes in offspring outcomes and have long-term consequences. Research from our laboratories investigating supranutritional selenium supply in maternal diets (Caton et al., 2007; Ward et al., 2008; Vonnahme et al, 2010; Camacho et al., 2012; Meyer et al., 2013, Yunusova et al., 2013; Caton et al., 2014a, 2014b) has demonstrated changes in lamb birth weight, growth, nutrient digestion, glucose metabolism and insulin sensitivity, visceral fat content, intestinal vascularity, and endocrine profiles in some but not all studies.
Supplementing ruminally protected arginine to pregnant ewes fed either adequate or nutrient-restricted diets increased the growth trajectory of lambs (Peine et al., 2013, 2018). Lassala et al. (2010, 2011) reported that intravenous administration of l-arginine in nutrient-restricted ewes from day 60 until parturition, or in triplet- and quadruplet-bearing ewes from days 100 to 121 of gestation increased birth weights of lambs. McCoard et al. (2013) demonstrated that intravenous l-arginine in twin-bearing ewes from day 100 of pregnancy until term increased birth weight of females but not males and increased brown adipose stores in both males and females. Supplemental methionine in Holstein cows (Penagaricano et al., 2013) resulted in changes in the transcriptome of flushed embryos, including genes involved in embryonic development and immune responses.
The aforementioned discussion clearly demonstrates that altered maternal nutrition, from global nutrient supply (changing intake) to supply of specific nutrients, can have both short- and long-term consequences on offspring development. Some of these consequences, such as growth and reproductive rates, contribute to variation observed in livestock herds and likely present both recognized and unrecognized management challenges. Matching energy supply to demand in livestock is key to efficient production systems. This is particularly the case in beef cattle where maintenance energy expenditure for the cow herd is one of the large costs experienced by beef cattle producers. The question remains, however, whether maternal nutrition influences or “programs” offspring energy demands in cattle.
ENERGY DEMANDS DURING PREGNANCY
A primary driver of whole-herd beef cattle production efficiencies is reproduction (Dickerson, 1970; Dziuk and Bellows, 1983; Koch and Algeo, 1983). The NASEM (2016) stated:
Undernutrition is a major factor affecting reproductive performance, resulting in delayed attainment of puberty, extended periods of postpartum anestrus, as well as compromised conception rates, embryonic survival, sexual behavior, and, as emerging documentation suggests, compromised developmental programming of offspring.
Beef cow nutrient requirements vary across the normal annual production cycle, with energy requirements being least immediately after weaning a suckling calf, increasing during pregnancy, and being greatest at peak lactation. Lactational demands peak early in beef cows and usually coincide with the annual breeding season, which further compounds nutrient competition between successful breeding and lactation. According to Short et al. (1990), cows partition nutrients to meet priority demands, which he articulated as 1) basal metabolism; 2) food-gathering activities; 3) growth; 4) basic energy reserves; 5) maintenance of pregnancy; 6) lactation; 7) additional energy reserves; 8) estrous cycles and pregnancy establishment; and 9) accumulation of excess energy reserves. Competition for nutrients, particularly in first- and second-calf growing heifers, is a serious management concern in terms of conception and maintenance of pregnancy, cow longevity in the herd, and overall beef system production efficiencies.
Approximately 75% of fetal growth occurs during the last 2 to 3 mo of pregnancy in ruminants (Robinson et al., 1977; NASEM, 2016; Reynolds et al., 2018). The CNES partitions energy demand into NEm and NEg, with energy being used more efficiently for maintenance than for gain. Because efficiency of energy use for maintenance and pregnancy in beef cattle varies similarly with metabolizable energy (ME) concentration in the diet, and for convenience, NASEM (2016) expressed the net energy requirements for pregnancy in terms of NEm. Gravid uterine demand for energy is greater during the last third of pregnancy because energy retention is greater during this time as gravid uterine mass is increasing rapidly. Most energy systems report energy demands of pregnancy to be minimal during the first half of pregnancy because energy retention in the gravid uterus is minimal. Energy requirements associated with advancing pregnancy as reported by the NASEM (2016), NRC (1984, 1996, 2000), and Commonwealth Scientific and Industrial Research Organisation (CSIRO; 1990) are shown in Table 1.
Table 1.
Estimates of NEm (Mcal/d) required for pregnancy in beef cattle†
| Day of gestation | NRC (1996, 2000) | NRC (1984) | CSIRO (1990) |
|---|---|---|---|
| 130 | 0.327 | 0.199 | 0.280 |
| 160 | 0.634 | 0.505 | 0.509 |
| 190 | 1.166 | 1.083 | 0.923 |
| 220 | 2.027 | 1.952 | 1.673 |
| 250 | 3.333 | 2.916 | 3.029 |
| 280 | 5.174 | 3.518 | 5.478 |
†Estimates are based on a calf birth weight of 38.5 kg. Adapted from NASEM (2016).
The data in Table 1 demonstrate that energy requirements increase with advancing stage of pregnancy and are clearly increased during the last half of pregnancy. Under the current paradigm of expressing energy requirements, energy demand is low and practically insignificant during the first half of pregnancy; however, energy demand during the earliest stages of pregnancy must be important to embryonic survival and growth. In fact, a recent review (Bridges et al., 2013) indicated that pregnancy is not limited by fertilization rates but is greatly affected by early embryonic loss, which is largely, but not exclusively, affected by nutritional, environmental, and disease-related stress. Stress can drive nutrient partitioning toward immunological responses that likely create transitory, immunologically driven nutrient supply restriction for growth-related functions, which affect embryonic growth and survival.
Pregnancy is energetically costly (Brody, 1945; Kleiber, 1961; Ferrell et al., 1976; Ferrell and Reynolds, 1987; NASEM, 2016), with NEm increasing 30% to 50% by the end of gestation, about half the ME being attributed to gravid uterine tissues, a quarter to the fetus, and a quarter to the associated increased maternal metabolic activities accompanying pregnancy. Although pregnancy is energetically costly, pregnancy loss and the resulting open female represent even greater energetic (and financial) costs to beef production systems. Likewise compromised offspring at birth exhibits greater short-term morbidity and mortality rates with the potential for other metabolic and reproductive impairments into adult life. A better understanding of the energy requirement during pregnancy, especially transitory energy (and other nutrient) demands during early pregnancy in association with acute stress or challenge events, should provide insight into nutritional and whole-herd management strategies for improving beef cattle production efficiencies.
THE IMPORTANCE OF EARLY PREGNANCY
As stated previously, total energy requirements for pregnancy increase as gestation advances; however, nutrient supply to the developing conceptus is critical for survival and growth starting very early in pregnancy. Caton and Hess (2010) and Meyer and Caton (2016), building on earlier work by Fowden et al. (2006), discussed critical developmental windows and potential effects of maternal nutrient restriction on fetal and postnatal developmental outcomes. Most large-animal models of developmental programming focus on perturbations to the maternal system during mid-to-late gestation and resulting effects on the offspring. During early gestation (days 0 to 50), the conceptus grows from one cell to a fully formed embryo with fully recognizable organ systems and a functional placenta. Tissue-doubling time during this stage of gestation is exceptionally high. Consequently, nutrient supply needs to support rapid growth and development to ensure survival of the embryo and establishment of a viable pregnancy. During the early phase of fetal development, differentiation and vascularization of uteroplacental tissues as well as fetal organogenesis occur, all of which are critical events for normal fetal development (Funston et al., 2010). In addition, dams that undergo stress (nutritional, environmental, etc.) during early, but not late gestation, are likely to produce a normal birth weight offspring that still suffers from poor growth and metabolic problems because of the stress early in pregnancy (Ford et al., 2007; Vonnahme et al., 2007; Reynolds and Caton, 2012).
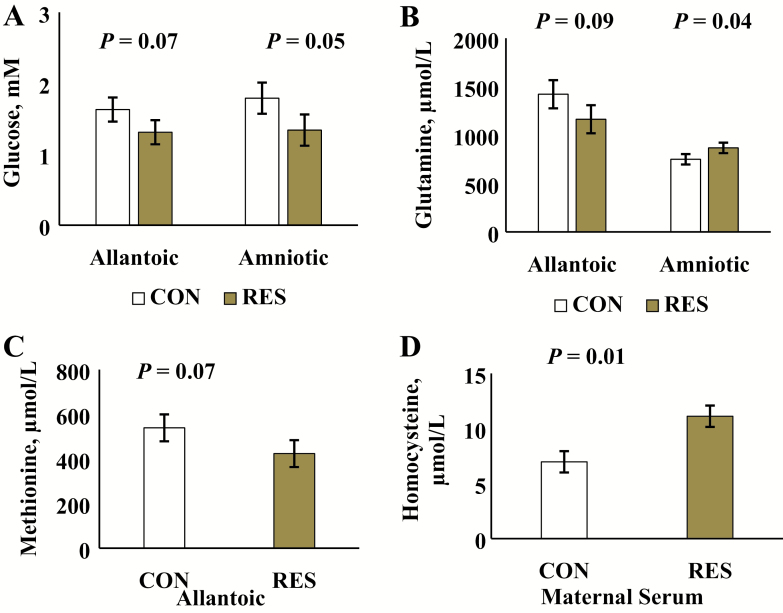
Recently, our laboratory developed an ovariohysterectomy technique (McLean et al., 2016) designed to investigate developmental programming responses to moderate nutrient restriction during the first 50 d of pregnancy. In these studies, postpubertal heifers were fed to gain 0.5 kg/d (control) or −0.08 kg/d (moderate restriction) for the first 50-d postbreeding. At various times during early pregnancy, ovariohysterectomies were conducted and tissues harvested. Results from these studies demonstrated nutrient and metabolite changes in fetal fluids (Figure 1). For example, at day 50 of gestation, glucose, methionine, and glutamine were decreased in allantoic fluids in moderately restricted heifers. Amniotic glucose was also decreased, whereas amniotic glutamine was increased in moderately restricted heifers. Maternal serum homocysteine also was increased in the moderately restricted heifers, suggesting compromised one-carbon metabolism.
Figure 1.
Comparison of (A) glucose concentrations in allantoic and amniotic fluid, (B) glutamine concentrations in allantoic and amniotic fluid, (C) methionine in allantoic fluid, and (D) homocysteine in maternal serum of heifers receiving control (CON) or restricted (RES) dietary treatment from the day of mating (day 0) until day 50 of gestation. Treatments provided for 0.5 kg vs. −0.08 kg of gain/heifer daily between days 0 and 50 of gestation for CON vs. RES heifers, respectively.
Data generated from these studies on fetal muscle from the hind limb and fetal liver at day 50 of gestation revealed that in fetal liver and muscle, a total of 548 and 317 genes, respectively, were differentially expressed as a result of moderate nutrient restriction, of which 201 and 144 genes, respectively, were false-discovery-rate protected. Pathway analysis was performed on the differentially expressed genes to determine the functional categories of pathways or ontologies associated with factors known to affect production efficiencies. In fetal liver, five functional categories of interest were affected by moderate nutrient restriction during the first 50 d of gestation (Crouse et al., 2017b): metabolic pathways (n = 43 genes), protein kinases (n = 47 genes), nucleosome core proteins (n = 22 genes), mRNA splicing (n = 7 genes), and complement/coagulation cascades (n = 6 genes). In fetal muscle, three functional categories of interest were affected by moderate nutrient restriction: skeletal muscle (n = 74 genes), embryogenesis (n = 14 genes), and signaling cascades (n = 18 genes).
Further analyses of our data demonstrated that in fetal liver, nine histone genes were upregulated in moderately nutrient restricted (RES) compared with control (CON) heifers including members of the histone H1, H2A, H2B, and H4 families (Crouse et al., 2017a). The 13 differentially expressed histone-modifying transcripts included genes associated with acetylation and deacetylation, methylation, phosphorylation, and ubiquitination. Of particular note, HDAC10 was 2.67-fold greater (q < 0.05) in liver of RES fetuses. In addition, the histone deacetylase complex gene, CIR1, was 2.22-fold greater (q < 0.05) in liver of RES fetuses. Only one gene associated with histone modifications, SET, was lower (1.77-fold, P = 0.006; q = 0.16) in liver of RES compared with CON fetuses. The SET gene is involved in preventing H4 lysine acetylation. These data imply that maternal nutrient restriction very early in pregnancy initiates developmental programming through epigenetic remodeling of the fetal genome in beef cattle (Crouse et al., 2017a).
In fetal muscle from the hind limb (data from our laboratory), differentially expressed genes include the myogenic genes MYOG and MYOD1 (1.49- and 1.39-fold greater in RES than CON fetuses, respectively), both of which play important roles in skeletal muscle cell differentiation and fiber development. Four members of the Wnt signaling pathway, namely WNT5A, FZD1, APC2, and FZD10, were upregulated in RES fetuses (1.32- to 2.11-fold greater than CON). The Wnt pathway is critical in promoting the differentiation of myocytes from progenitor stem cells. Additional genes upregulated in fetal hind limb muscle of RES compared with CON fetuses included members of the troponin (TNNC1, TNNC2, TNNI1, TNNI2, TNNT1, TNNT2, TPM2), myosin (MYL1, MLY2, MLY4, MLY7, MYL6B, MLY9, MYH8, MYLPF), and actin (ACTA1, ACTA2, ACTG2) families. Therefore, we conclude that early gestation is an important period of myogenic developmental programming, and is sensitive to maternal nutrition in cattle (Ward et al., 2017).
In conclusion, although most investigations of developmental programming events in cattle focus on mid-to-late gestation, recent data from our laboratory indicate that moderate changes in maternal nutrition during the first 50 d of pregnancy can alter nutrient and metabolite concentrations in fetal fluids and gene expression in fetal liver and muscle. Whether these changes alter short- or long-term NEm or NEg requirements remains to be determined.
OFFSPRING MAINTENANCE REQUIREMENTS
Maintenance Requirements
The CNES, as established by Lofgreen and Garrett (1968), used a comparative slaughter technique to measure retained energy (RE) and regressed daily ME intake on daily heat production (HE) in Mcal/d, which was calculated by difference (ME − RE). Estimates of HE were expressed per unit of body weight (BW) raised to the ¾ power as a standard metabolic scaling approach (i.e., metabolic body size). Because of the techniques used in their studies, W more closely represented shrunk body weight (SBW) than live body weight. Lofgreen and Garrett (1968) then regressed calculated daily HE on ME intake using a semi-log regression. When extrapolated back to ME intake = 0, their data estimated fasting FHP as 0.077W0.75.
In the current use of this system (NASEM, 2016), the NEm is computed based on the basal metabolism coefficient of 0.077, which is then adjusted for previous temperature, breed, lactation, gender, and previous plane of nutrition (COMP) as follows:
where
a1 is the basal metabolism coefficient, Mcal/kg0.75/d;
a2 is the acclimatization factor, Mcal/kg0.75/d;
BCS is the body condition score (1 to 9 scale; a proxy for previous plane of nutrition);
BE is the breed factor;
COMP is the NEm adjustment for previous nutrition;
L is the lactation factor;
NEm is the net energy requirement for maintenance, Mcal/d;
SBW is the shrunk body weight, kg (typically 96% of full body weight);
SEX is the gender effect factor (1.15 for bulls or 1 otherwise); and
Tp is previous temperature, °C.
More details regarding adjustment factors and maintenance requirements are provided in the NASEM (2016). As shown earlier, many factors can alter NEm values for cattle, and the NASEM (2016) adjustments reflect the current data at the time of publication.
Developmental Programming of Offspring Maintenance Requirements
Classically designed, definitive studies assessing the effects of maternal plane of nutrition on offspring NEm requirements are not available in the literature. Nonetheless, some of the early investigations into animal energetics and livestock performance provide evidence that compromised offspring at birth underperforms when compared with more normal offspring. For example, Armsby and Fries (1911) reported that “scrub” steers used energy less efficiently than “good” steers. More recently Greenwood et al. (1998) indicated that low-birth-weight lambs had slower growth rates, differing body compositions at a given empty body weight (EBW), and lower RE. In addition, Greenwood et al. (1998) indicated the differences in observed fat and energy content of lambs at 17.5 kg of EBW were attributed to an approximately 30% decrease in maintenance energy requirements for low- compared with high-birth-weight lambs. Robinson et al. (2013) indicated that maintenance energy requirements are less in growth-retarded calves, particularly during the early postnatal phase, and that at any given age, growth-compromised offspring could have different nutrient requirements than their normally growing counterparts.
Whole-animal or specific tissue oxygen consumption reflects energy use. Tissue oxygen consumption reflects energy use and mitochondrial function. Hepatic and small intestinal tissues are major consumers of whole-body energy supply (Koong et al., 1985; Reynolds et al., 1991: Caton et al., 2000). Prezotto et al. (2014) investigated fetal hepatic and small intestinal oxygen consumption at 130 d of gestation from control- and restricted-fed ewes. First-parity ewes were fed a complete pelleted diet at either control (requirements for 140 g of daily growth) or restricted (60% of controls) levels of dietary intake from days 50 to 130 of gestation. At day 130, tissues were harvested and fetal hepatic and small intestinal oxygen consumption measurements were obtained. Data shown in Table 2 (adapted from Prezotto et al., 2014) indicate that both hepatic and small intestinal oxygen consumption in vitro were decreased in fetuses from restricted compared with control fed ewes at 130 d of gestation. Decreases in hepatic oxygen consumption in this study likely resulted from changes in liver mass and not because of changes in oxygen use per unit of tissue; however, small intestinal in vitro oxygen consumption was increased per unit of tissue, suggesting altered tissue energy use. In a follow-up study, with mature ewes fed control-intake, restricted-intake (60% of controls), or restricted-intake plus rumen-protected arginine, in vitro oxygen consumption of hepatic and small intestinal tissues were investigated in 54-d-old offspring (Prezotto et al., 2018). Data shown in Table 3 (adapted from Prezotto et al., 2018) showed that hepatic but not jejunal oxygen consumption was decreased in lambs from nutrient-restricted dams. In fact, hepatic oxygen consumption when expressed per gram of tissue, per whole tissue, or per unit of body weight was decreased in lambs from nutrient-restricted ewes. These data indicate that liver energy use was less in offspring from nutrient-restricted dams. Data from Prezotto et al. (2014, 2018) described earlier are supported by in vitro oxygen consumption data in fetal calves from cows fed control or restricted and then realimented diets (Prezotto et al., 2016). These authors suggested that both cows and fetal calves during gestation can modulate maintenance energy requirements in response to nutrient restriction and realimentation. Their conclusions seem reasonable given known modulations in maintenance energy requirements in nutrient-restricted and then realimented growing cattle, which results in compensatory growth (NASEM, 2016). Existing data indirectly suggest that maintenance requirements of offspring might indeed be programmed by maternal nutritional inadequacies, with a greater likelihood of programming occurring at nutritional extremes and early in the postnatal period. Additional research directed toward assessing the effects of maternal nutrition and developmental programming on offspring maintenance energy requirements will help to delineate these responses and their effects on offspring production outcomes.
Table 2.
Hepatic and small intestinal oxygen consumption in fetal lambs at 130 d of gestation from ewes feed control (CON) or restricted (RES) diets (adapted from Prezotto et al., 2014)
| Treatments† | ||||
|---|---|---|---|---|
| Item | CON | RES | SEM | P-value |
| Liver oxygen consumption | ||||
| µmol/min per mg fresh tissue | 60.3 | 60.5 | 2.24 | 0.76 |
| mol/min per liver | 5.79 | 4.67 | 0.38 | 0.01 |
| Small intestine oxygen consumption | ||||
| µmol/min per mg fresh tissue | 67.1 | 53.3 | 4.2 | 0.009 |
| µmol/min per mg protein | 586 | 780 | 57 | 0.004 |
†Treatments were control (CON; fed at requirements for minimal gain and fetal growth) and restricted (RES) diets fed at 60% of CON.
Table 3.
Hepatic and small intestinal oxygen consumption in 54-d-old lambs from mature ewes fed a control diet (CON) or a restricted (RES) diet with or without supplemental rumen-protected arginine (adapted from Prezotto et al., 2018)
| Treatments† | |||||
|---|---|---|---|---|---|
| Item | CON | RES | RES + ARG | SEM | P-value‡ |
| Liver oxygen consumption | |||||
| mol/min per g fresh tissue | 0.39 | 0.35 | 0.34 | 0.02 | 0.09 |
| mol/min per liver | 181 | 146 | 155 | 10 | 0.04 |
| mol/min per kg BW | 8.0 | 6.7 | 6.8 | 0.4 | 0.02 |
| Small intestine oxygen consumption | |||||
| mol/min per g fresh tissue | 0.35 | 0.38 | 0.35 | 0.02 | 0.60 |
| mol/min per jejunum | 58 | 71 | 60 | 12 | 0.62 |
| mol/min per kg BW | 2.4 | 3.2 | 2.5 | 0.5 | 0.50 |
†Treatments were control (CON; fed at requirements for fetal growth), restricted (RES) diets fed at 60% of CON, and restricted plus supplemental rumen protected arginine (RES + ARG).
‡ P-value is for the contrast of CON vs. RES plus RES + ARG.
OFFSPRING REQUIREMENTS FOR GAIN
Requirements for Gain
Energy requirements for gain are driven by the composition of gain and the resulting RE. The current use of the CNES by nutrient requirement systems for beef cattle (NASEM, 2016) assumes that cattle have a similar body composition at the same degree of maturity. The NRC (1984) medium-framed steer equations are used as the standard reference base from which to compute energy content of gain at various stages of growth and rates of gain for all cattle types. This is accomplished by adjusting the BW of cattle of various body sizes and sexes to a weight at which they are equivalent in body composition to the steers in the Garrett (1980) database. The use of the NRC (1984) medium-framed steer as a standard reference basis to predict NEg values for a wide range of cattle across various breeds, body sizes, implant strategies, and nutritional management systems was assessed in the NASEM (2016) publication. The committee’s conclusion was that this recommended approach worked exceptionally well when plotting predicted RE vs. observed RE, in Mcal/d. When adjusting cattle to fit the Garrett (1980) database, the weight equivalent of the NRC (1984) medium-framed steer (EQSBW) is calculated as:
where
EQSBW is the BW equivalent to the NRC (1984) medium-frame steer;
SBW is the shrunk BW being evaluated;
SRW is the standard reference weight for the expected final body fat; and
FSBW is the final shrunk BW at the expected final body fat.
Within the NASEM system, there are various adjustments made to FSBW, which include decreasing FSBW by 25 to 45 kg for nonuse of an estrogenic implant; increasing FSBW by 25 to 45 kg for use of an implant containing trenbolone acetate plus estrogen; increasing FSBW by 6 to 36 kg for use of a β-adrenergic agonist; increasing FSBW by 25 to 45 kg for extended periods at slow rates of gain; and decreasing FSBW by 25 to 45 kg for continuous use of a high-energy diet from weaning. When problems arise in predicting NEg, they could be related to choosing the wrong FSBW, transitory effects of previous plane of nutrition, gut fill, anabolic implants, variations in NEm requirement, or the ME value assigned to the feed and the dietary NEm and NEg derived from the ME (NASEM, 2016).
Unfortunately, definitive studies that assess NEg requirements of cattle from dams with compromised maternal nutrition are not available in the literature. Considerable data exist, however, regarding the effects of maternal nutrition and developmental programming of muscle and fat accretion. Because NEg is estimated from RE, which depends on the composition of gain, it would seem reasonable that altered body composition at a given age could alter NEg requirements; however, this remains to be determined.
Maternal Undernutrition and Muscle Development
Decreased growth rate and feed efficiency pose a significant economic impact to the beef industry. Clearly, maternal nutritional status is one of the factors programming nutrient partitioning and ultimately growth and development of fetal skeletal muscle (Wallace, 1948; Wallace et al., 1999; Godfrey and Barker, 2000; Rehfeldt et al., 2004; Stickland et al., 2004). Growth-restricted neonates are not only at risk of immediate postnatal complications, but also might be “programmed” to exhibit poor growth and productivity, as well as diseases, later in life (Barker et al., 1993; Godfrey and Barker, 2000). This growth restriction seems to be especially important when fetal muscle development (myogenesis) is adversely affected (Handel and Stickland, 1987a, 1987b; Dwyer et al., 1993). Fetal skeletal muscle has a lower priority for nutrient partitioning compared with the brain and heart in response to challenges during fetal development, rendering fetal muscle particularly vulnerable to nutrient deficiency (Bauman et al., 1982; Close and Pettigrew, 1990). The fetal period is crucial for lifetime skeletal muscle development because no net increase in the number of muscle fibers occurs after birth (Glore and Layman, 1983; Greenwood et al., 2000; Nissen et al., 2003).
Several studies in a range of mammalian species have shown that maternal undernutrition during gestation can significantly decrease the number of muscle fibers and myocyte nuclei in the offspring (Bedi et al., 1982; Wilson et al., 1988; Ward and Stickland, 1991). For example, a lower ratio of secondary to primary myofibers and decreased sizes of the muscle fasciculi were observed in muscle of fetuses gestated in nutrient-restricted ewes (Zhu et al., 2004). Therefore, muscle fiber type development can be influenced by maternal nutritional status depending on the energy needs of the muscle and species observed.
Nutrient restriction of heifers during the first two-thirds of gestation decreased fetal growth and calf birth weight (Micke et al., 2010). Nutrient restriction to 85% of ME compared with 140% in multiparous Angus-Simmental cows resulted in increased expression of myogenic genes MYOG and MYOD1 in offspring of restricted vs. control cows at day 247 of pregnancy (Paradis et al., 2017). Early prenatal nutritional restriction of ewes resulted in a decrease in the number of myofibers but an increased diameter of muscle fibers in offspring at 8 mo of age (Zhu et al., 2006). The finding of enlarged muscle fibers has been confirmed in other muscles in both bovine and ovine fetuses and at 8 mo of age in lambs when nutrient intake of dams was restricted during early gestation (Du et al., 2005, 2010). In addition, lambs born from ewes that were fed restricted diets during early- and mid-gestation had increased subcutaneous fat depots, reduced muscle size, and dysregulated glucose uptake compared with lambs from control ewes (Ford et al., 2007). Ewes that were nutrient-restricted to day 31 of gestation had lambs with decreased muscle fiber density in the triceps brachii compared with lambs of control-fed dams (McCoard et al., 1997).
In the bovine, primary muscle fibers develop during the first 2 mo after conception (Russell and Oteruelo, 1981). Secondary muscle fibers, which make up the majority of muscle fibers, form between 2 and 7 to 8 mo of gestation (Russell and Oteruelo, 1981). The formation of secondary myofibers partially overlaps with the formation of intramuscular adipocytes and fibroblasts (Du et al., 2010). The three cell types, myocytes, adipocytes, and fibroblasts, produce the basic structure of skeletal muscle and form at different time points in gestation. These data clearly show that maternal nutrient restriction during pregnancy can affect muscle development in offspring and that timing of restriction can have differential effects on muscle fiber development and growth as seen in postnatal phenotypic responses of offspring. Moreover, these data are consistent with our recent studies, described in The Importance of Early Pregnancy section, showing altered gene expression in hind limb muscle of fetuses from heifers that were nutrient-restricted during the first 50 d of gestation.
Composition of Gain
Robinson et al. (2013) concluded in their review that:
Fetal programming and related maternal effects are most pronounced and explain substantial amounts of variation for growth-related production characteristics such as BW, feed intake, carcass weight, muscle weights, meat yield, and fat and bone weights at any given age but are less evident when assessed at the same BW and carcass weight.
Body compositional changes resulting from compromised maternal nutrition seem to be more pronounced early in the postnatal and growing phases and less pronounced as offspring approach finished market weight, which is likely a result the high degree of plasticity of cattle body tissues and their ability to recover from early nutritional insults. Nonetheless, examples of differences in carcass composition are prevalent in the literature and are most often reflected by increased body fatness. Growth-restricted offspring may take more time to reach market weight (Funston et al., 2012), which could directly impact total energy required to finish cattle. Unfortunately, many published reports do not contain estimates of days on feed, particularly in response to birth weight or previous plane of maternal nutrition. While investigating effects of maternal nutrition on steer offspring, Underwood et al. (2010) reported maternal nutritional management could alter average daily gain, hot carcass weight, and 12th rib fat thickness at slaughter. Likewise, Radunz et al. (2012) reported that prepartum energy source of cows altered marbling score and intramuscular fat content in offspring at finish. Data from Nebraska (Stalker et al., 2006, 2007; Larson et al., 2009) indicate that steers born to non-protein-supplemented dams had lower dry matter intake and hot carcass weight, and decreased marbling score in some but not all studies.
Clearly, maternal nutritional plane can alter muscle development, even at the very early stages of growth. Tissue plasticity seems to compensate for some of these effects during steer growth and finishing; however, differences often persist until slaughter. Reduction of follicle numbers in the ovaries of offspring from restricted dams observed during gestation in beef heifers was still present at 86 wk of age (Mossa et al., 2013), indicating potential negative effects on fertility. Furthermore, heifers born to non-protein-supplemented dams had lower adjusted 205-d weaning weights, a lower percentage pregnancy after breeding (Martin et al., 2007), and decreased age at puberty (Funston et al., 2010). The degree to which NEg requirements of growing cattle are altered by maternal nutrient supply is not directly assessed in the literature. Additional research in this direction is needed to determine whether NEg requirements are changed in offspring from dams in nutritionally compromised environments.
CONCLUSIONS
Developmental programming is convincingly documented in the literature, can be driven by suboptimal maternal nutrition, and occurs in major livestock species, including beef cattle. Definitive studies that assess the effects of maternal nutrition and the resulting developmental programming events on NEm and NEg requirements in beef cattle have not been conducted. Indirect evidence included within this review suggests it is likely that energy requirements of offspring are affected by maternal nutrition in beef cattle, and that these events are at least partially controlled by epigenetic events during development that persist postnatally. Timing of the maternal nutritional insult(s) is important, and emerging data suggest that early pregnancy is likely much more important than previously thought. Additional research in the area of maternal nutrition and offspring energetics will provide insight that could lead to altered management practices and increased efficiencies of beef cattle production.
Conflict of interest statement. None declared.
Footnotes
Presented as an invited paper at the California Net Energy System: 50th Anniversary Symposium: September 12 to 14, 2018, University of California, Davis.
LITERATURE CITED
- Anway M D, Rekow S.S., and Skinner M.K.. 2008. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics 91:30–40. doi: 10.1016/j.ygeno.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J A, Khan I.Y., Taylor P.D., Nathanielsz P.W., and Poston L.. 2004. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J. Physiol. 561(Pt 2):355–377. doi: 10.1113/jphysiol.2004.072009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armsby H P, and Fries J.A.. 1911. The influence of type and of age upon the utilization of feed by cattle. Bulletin No. 128. Washington (DC): U.S. Department of Agriculture, Bureau of Animal Industry. [Google Scholar]
- Barker D J P. Editor. 1992. Fetal and infant origins of adult disease. London: BMJ Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D J P. 2004. Developmental origins of well being. Philos. Trans. Royal Soc. London. 359:1359–1366. doi: 10.1098/rstb.2004.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D J, Gluckman P.D., Godfrey K.M., Harding J.E., Owens J.A., and Robinson J.S.. 1993. Fetal nutrition and cardiovascular disease in adult life. Lancet 341:938–941. doi: 10.1016/0140-6736(93)91224-A [DOI] [PubMed] [Google Scholar]
- Bartol F F, and Bagnell C.A.. 2012. Lactocrine programming of female reproductive tract development: environmental connections to the reproductive continuum. Mol. Cell. Endocrinol. 354:16–21. doi: 10.1016/j.mce.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Bauman D E, Eisemann J.H., and Currie W.B.. 1982. Hormonal effects on partitioning of nutrients for tissue growth: role of growth hormone and prolactin. Fed. Proc. 41:2538–2544. [PubMed] [Google Scholar]
- Bedi K S, Birzgalis A.R., Mahon M., Smart J.L., and Wareham A.C.. 1982. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br. J. Nutr. 47:417–431. doi: 10.1079/bjn19820053 [DOI] [PubMed] [Google Scholar]
- Bridges G A, Day M.L., Geary T.W., and Cruppe L.H.. 2013. Triennial reproduction symposium: deficiencies in the uterine environment and failure to support embryonic development. J. Anim. Sci. 91:3002–3013. doi: 10.2527/jas.2013-5882 [DOI] [PubMed] [Google Scholar]
- Brody S. 1945. Bioenergetics and growth. New York (NY): Hafner Publishing Co. [Google Scholar]
- Camacho L E, Meyer A.M., Neville T.L., Hammer C.J., Redmer D.A., Reynolds L.P., Caton J.S., and Vonnahme K.A.. 2012. Neonatal hormone changes and growth in lambs born to dams receiving differing nutritional intakes and selenium supplementation during gestation. Reproduction 144:23–35. doi: 10.1530/REP-11-0302 [DOI] [PubMed] [Google Scholar]
- Cardoso R C, Alves B.R., Prezotto L.D., Thorson J.F., Tedeschi L.O., Keisler D.H., Park C.S., Amstalden M., and Williams G.L.. 2014. Use of a stair-step compensatory gain nutritional regimen to program the onset of puberty in beef heifers. J. Anim. Sci. 92:2942–2949. doi: 10.2527/jas.2014-7713 [DOI] [PubMed] [Google Scholar]
- Caton J S, Bauer M. L., and Hidari H.. 2000. Metabolic components of energy expenditure in growing beef cattle. Asian-Australas. J. Anim. Sci. 13:701–710. doi:10.5713/ajas.2000.702. [Google Scholar]
- Caton J S, and Hess B.W.. 2010. Maternal plane of nutrition: impacts on fetal outcomes and postnatal offspring responses. In: Hess B W, Delcurto T., Bowman J.G.P., and Waterman R C, editors. Proceedings of the 4th Grazing Livestock Nutrition Conference 9–10 July, 2010; Estes Park, CO Champaign (IL): Western Section: American Society of Animal Science. p. 104–122. [Google Scholar]
- Caton J S, Meyer A.M., Yunusova R.D., Borowicz P.P., Reynolds L.P., Redmer D.A., Hammer C.J., and Vonnahme K.A.. 2014a. Effects of maternal selenium supply and nutritional plane on offspring intestinal biology. Trace Elements in Man and Animals (TEMA-15) Meeting Abstr. 22–26 June 2014; Orlando, FL. [Google Scholar]
- Caton J S, Neville T.L., Reynolds L.P., Hammer C.J., Vonnahme K.A., Meyer A.M., and Taylor J.B.. 2014b. Biofortification of maternal diets with selenium: postnatal growth outcomes. In Banuelos G, Lin Z., and Yin X, editors. Selenium in the environment and human health. London: CRC Press, Taylor & Francis Group; p. 159–161. [Google Scholar]
- Caton J, Vonnahme K., Reed J., Neville T., Effertz C., Hammer C., Luther J., Redmer D., and Reynolds L.. 2007. Effects of maternal nutrition on birth weight and postnatal nutrient metabolism. Proceedings of International Symposium on Energy and Protein Metabolism 9–13 September 2007 Vichy (France): EAAP Publication No. 124; p. 101–102. doi: 10.3920/978-90-8686-613-7 [DOI] [Google Scholar]
- Close W H, and Pettigrew J.E.. 1990. Mathematical models of sow reproduction. J. Reprod. Fertil. Suppl. 40:83–88. [PubMed] [Google Scholar]
- Connor K L, Vickers M.H., Beltrand J., Meaney M.J., and Sloboda D.M.. 2012. Nature, nurture, or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J. Physiol. 590(9):2167–2180. doi: 10.1113/jphysiol.2011.223305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse M S, Caton J.S., Cushman R.A., McLean K.J., Dahlen C.R., Borowicz P.P., Reynolds L.P., and Ward A.K.. 2017a. Maternal nutrition during the first 50 days of gestation alters expression of histone and histone modifying genes in bovine fetal liver. 36th International Society of Animal Genetics Conference; July 16 to 21, 2017; Dublin, Ireland p 32. [Google Scholar]
- Crouse M S, Caton J.S., Cushman R.A., McLean K.J., Dahlen C.R., Borowicz P.P., Reynolds L.P., and Ward A.K.. 2017b. Moderate nutrient restriction influences transcript abundance of genes impacting production efficiencies of beef cattle in fetal liver, muscle, and cerebrum by d 50 of gestation. Proc. West. Sec. Amer. Soc. Anim. Sci. 68:42–47. doi:10.2527/asasws.2017.0014 [Google Scholar]
- Commonwealth Scientific and Industrial Research Organisation (CSIRO) 1990. Feeding standards for Australian Livestock: ruminants. Melbourne (Australia): CSIRO Publishing. [Google Scholar]
- Dickerson G. 1970. Efficiency of animal production molding the biological components. J. Anim. Sci. 30:849–859. doi: 10.2527/jas1970.306849x [DOI] [Google Scholar]
- Du M, Ford S.P., and Zhu M.J.. 2017. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Anim. Front. 7:5–11. doi: 10.2527/af.2017-0122 [DOI] [Google Scholar]
- Du M, Tong J., Zhao J., Underwood K.R., Zhu M., Ford S.P., and Nathanielsz P.W.. 2010. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 88 (13 Suppl):E51–E60. doi: 10.2527/jas.2009-2311 [DOI] [PubMed] [Google Scholar]
- Du M, Zhu M.J., Olson G.A., Hess B.W., Means W.J., and Ford S.P.. 2005. Nutritional restriction in cows alters the number and volume of fetal myofibers. J. Anim. Sci. 83 (Suppl. 1):388. (Abstr.) [Google Scholar]
- Dwyer C M, Fletcher J.M., and Stickland N.C.. 1993. Muscle cellularity and postnatal growth in the pig. J. Anim. Sci. 71:3339–3343. doi: 10.2527/1993.71123339x [DOI] [PubMed] [Google Scholar]
- Dziuk P J, and Bellows R.A.. 1983. Management of reproduction in beef cattle, sheep and pigs. J. Anim. Sci. 57 (Suppl. 2):355–379. doi: 10.2527/animalsci1983.57Supplement_2355x [DOI] [Google Scholar]
- Ferrell C L, Garrett W.N., Hinman N., and Grichting G.. 1976. Energy utilization by pregnant and non-pregnant heifers. J. Anim. Sci. 42:937–950. doi: 10.2527/jas1976.424937x [DOI] [PubMed] [Google Scholar]
- Ferrell C L, and Reynolds L.P.. 1987. Oxidative metabolism of gravid uterine tissues of the cow. In: Airlie V A, Moe P.W., Tyrrell H.F., and Reynolds P J, editors. Energy Metabolism of Farm Animals: Proceedings of the 10th Symposium; September 1985; EAAP Publication No. 32. New York (NY): Rowman & Littlefield; p. 298–301. [Google Scholar]
- Ford S P, Hess B.W., Schwope M.M., Nijland M.J., Gilbert J.S., Vonnahme K.A., Means W.J., Han H., and Nathanielsz P.W.. 2007. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 85:1285–1294. doi: 10.2527/jas.2005-624 [DOI] [PubMed] [Google Scholar]
- Fowden A L, Giussani D.A., and Forhead A.J.. 2006. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda). 21:29–37. doi: 10.1152/physiol.00050.2005 [DOI] [PubMed] [Google Scholar]
- Funston R N, Martin J L., Adams D.C., and Larson D.M.. 2010. Winter grazing system and supplementation of beef cows during late gestation influence heifer progeny. J. Anim. Sci. 88:4094–4101. doi: 10.2527/jas.2010-3039 [DOI] [PubMed] [Google Scholar]
- Funston R N, Summers A.F., and Roberts A.J.. 2012. Alpharma Beef Cattle Nutrition Symposium: implications of nutritional management for beef cow-calf systems. J. Anim. Sci. 90:2301–2307. doi: 10.2527/jas2011-4568 [DOI] [PubMed] [Google Scholar]
- Garrett W N. 1980. Energy utilization by growing cattle as determined in 72 comparative slaughter experiments. In: Mount L E, editor, Energy Metabolism: Proceedings, 8th Symposium on Energy Metabolism; September 1979; Cambridge, England EAAP Publication No. 26. London (England): Butterworths; p. 3–8. [Google Scholar]
- Glore S R, and Layman D.K.. 1983. Cellular growth of skeletal muscle in weanling rats during dietary restrictions. Growth 47:403–410. [PubMed] [Google Scholar]
- Godfrey K M, and Barker D.J.. 2000. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71 (5 Suppl):1344S–1352S. doi: 10.1093/ajcn/71.5.1344s [DOI] [PubMed] [Google Scholar]
- Greenwood P, Clayton E., and Bell A.. 2017. Developmental programming and beef production. Anim. Front. 7:38–47. doi: 10.2527/af.2017-0127 [DOI] [Google Scholar]
- Greenwood P L, Hunt A.S., Hermanson J.W., and Bell A.W.. 1998. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J. Anim. Sci. 76:2354–2367. doi: 10.2527/1998.7692354x [DOI] [PubMed] [Google Scholar]
- Greenwood P L, Hunt A.S., Hermanson J.W., and Bell A.W.. 2000. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J. Anim. Sci. 78:50–61. doi: 10.2527/2000.78150x [DOI] [PubMed] [Google Scholar]
- Hammond J. 1927. The physiology of reproduction in the cow. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Handel S E, and Stickland N.C.. 1987a. Muscle cellularity and birth weight. Anim. Sci. 44:311–317. doi: 10.1017/S0003356100018687 [DOI] [Google Scholar]
- Handel S E, and Stickland N.C.. 1987b. The growth and differentiation of porcine skeletal muscle fibre types and the influence of birthweight. J. Anat. 152:107–119. [PMC free article] [PubMed] [Google Scholar]
- Hoffman M L, Reed S.A., Pillai S.M., Jones A.K., McFadden K.K., Zinn S.A., and Govoni K.E.. 2017. Physiology and endocrinology symposium: the effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J. Anim. Sci. 95:2222–2232. doi: 10.2527/jas.2016.1229 [DOI] [PubMed] [Google Scholar]
- Jackson L M, Mytinger A., Roberts E.K., Lee T.M., Foster D.L., Padmanabhan V., and Jansen H.T.. 2013. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology 154:1612–1623. doi: 10.1210/en.2012-1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcoyne K R, Smith L.B., Atanassova N., Macpherson S., McKinnell C., van den Driesche S., Jobling M.S., Chambers T.J., De Gendt K., Verhoeven G., . et al. 2014. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc. Natl. Acad. Sci. USA. 111:E1924–E1932. doi: 10.1073/pnas.1320735111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M. 1961. The fire of life: an introduction to animal energetics. New York (NY): Wiley & Sons. [Google Scholar]
- Koch R M, and Algeo J.W.. 1983. The beef cattle industry: changes and challenges. J. Anim. Sci. 57 (Suppl. 2):28–43. doi: 10.2527/animalsci1983.57Supplement_228x [DOI] [Google Scholar]
- Koong L J, Ferrell C.L., and Nienaber J. A.. 1985. Assessment of interrelationships among levels of intake and production, organ size and fasting heat production in growing animals. J. Nutr. 115:1383–1390. doi: 10.1093/jn/115.10.1383 [DOI] [PubMed] [Google Scholar]
- Lan X, Cretney E.C., Kropp J., Khateeb K., Berg M.A., Peñagaricano F., Magness R., Radunz A.E., and Khatib H.. 2013. Maternal diet during pregnancy induces gene expression and DNA methylation changes in fetal tissues in sheep. Front. Genet. 4:49. doi: 10.3389/fgene.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D M, Martin J.L., Adams D.C., and Funston R.N.. 2009. Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny. J. Anim. Sci. 87:1147–1155. doi: 10.2527/jas.2008-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassala A, Bazer F.W., Cudd T.A., Datta S., Keisler D.H., Satterfield M.C., Spencer T.E., and Wu G.. 2010. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J. Nutr. 140:1242–1248. doi: 10.3945/jn.110.125658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassala A, Bazer F.W., Cudd T.A., Datta S., Keisler D.H., Satterfield M.C., Spencer T.E., and Wu G.. 2011. Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J. Nutr. 141:849–855. doi: 10.3945/jn.111.138172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgreen G P, and Garrett W.N.. 1968. A system for expressing net energy requirements and feed values for growing and finishing cattle. J. Anim. Sci. 27:793–806. doi: 10.2527/jas1968.273793x [DOI] [Google Scholar]
- Long N M, Prado-Cooper M.J., Krehbiel C.R., DeSilva U., and Wettemann R.P.. 2011. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle. J. Anim. Sci. 88:3251–3261. doi: 10.2527/jas.2009-2512 [DOI] [PubMed] [Google Scholar]
- Long N M, Tousley C.B., Underwood K.R., Paisley S.I., Means W.J., Hess B.W., Du M., and Ford S.P.. 2012. Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle. J. Anim. Sci. 90:197–206. doi: 10.2527/jas.2011-4237 [DOI] [PubMed] [Google Scholar]
- Martin J L, Vonnahme K.A., Adams D.C., Lardy G.P., and Funston R.N.. 2007. Effects of dam nutrition on growth and reproductive performance of heifers calves. J. Anim. Sci. 85:841–847. doi: 10.2527/jas.2006-337 [DOI] [PubMed] [Google Scholar]
- McCoard S A, Peterson S.W., McNabb W.C., Harris P.M., and McCutcheon S.N.. 1997. Maternal constraint influences muscle fibre development in fetal lambs. Reprod. Fertil. Dev. 9:675–681. doi: 10.1071/R97061 [DOI] [PubMed] [Google Scholar]
- McCoard S, Sales F., Wards N., Sciascia Q., Oliver M., Koolaard J., and van der Linden D.. 2013. Parenteral administration of twin-bearing ewes with L-arginine enhances the birth weight and brown fat stores in sheep. Springerplus 2:684. doi: 10.1186/2193-1801-2-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K J, Dahlen C.R., Borowicz P.P., Reynolds L.P., Crosswhite M.R., Neville B.W., Walden S.D., and Caton J.S.. 2016. Technical note: a new surgical technique for ovariohysterectomy during early pregnancy in beef heifers. J. Anim. Sci. 94:5089–5096. doi: 10.2527/jas.2016-0761 [DOI] [PubMed] [Google Scholar]
- Meyer A M, and Caton J.S.. 2016. Role of the small intestine in developmental programming: impact of maternal nutrition on the dam and offspring. Adv. Nutr. 7:169–178. doi: 10.3945/an.115.010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A M, Caton J.S., Hess B.W., Ford S.P., and Reynolds L.P.. 2012a. Epigenetics and effects on the neonate that may impact feed efficiency. In: Hill R, editor, Feed efficiency in the beef industry. Hoboken (NJ): Wiley-Blackwell; p. 195–224. [Google Scholar]
- Meyer A M, Neville T.L., Reed J.J., Taylor J.B., Reynolds L.P., Redmer D.A., Hammer C.J., Vonnahme K.A., and Caton J.S.. 2013. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J. Anim. Sci. 91:2628–2639. doi: 10.2527/jas.2012-5953 [DOI] [PubMed] [Google Scholar]
- Meyer A M, Reed J.J., Neville T.L., Taylor J.B., Reynolds L.P., Redmer D.A., Vonnahme K.A., and Caton J.S.. 2012b. Effects of nutritional plane and selenium supply during gestation on visceral organ mass and indices of intestinal growth and vascularity in primiparous ewes at parturition and during early lactation. J. Anim. Sci. 90:2733–2749. doi: 10.2527/jas.2011-4524 [DOI] [PubMed] [Google Scholar]
- Micke G C, Sullivan T.M., Soares Magalhaes R.J., Rolls P.J., Norman S.T., and Perry V.E.. 2010. Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim. Reprod. Sci. 117:1–10. doi: 10.1016/j.anireprosci.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Mossa F, Carter F., Walsh S.W., Kenny D.A., Smith G.W., Ireland J.L., Hildebrandt T.B., Lonergan P., Ireland J.J., and Evans A.C.. 2013. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88:92. doi: 10.1095/biolreprod.112.107235 [DOI] [PubMed] [Google Scholar]
- NASEM 2016. Nutrient requirements of beef cattle, 8th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- Nissen P M, Danielsen V.O., Jorgensen P.F., and Oksbjerg N.. 2003. Increased maternal nutrition of sows has no beneficial effects on muscle fiber number or postnatal growth and has no impact on the meat quality of the offspring. J. Anim. Sci. 81:3018–3027. doi: 10.2527/2003.81123018x [DOI] [PubMed] [Google Scholar]
- NRC 1984. Nutrient requirements of beef cattle, 6th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- NRC 1996. Nutrient requirements of beef cattle, 7th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle: update 2000, 7th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- NRC 2007. Nutrients requirements of small ruminants, sheep, goats, cervids and new world camelids. Washington (DC): National Academic Press. [Google Scholar]
- Paneth N, and Susser M.. 1995. Early origin of coronary heart disease (the “barker hypothesis”). BMJ 310:411–412. doi: 10.1136/bmj.310.6977.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis F, Wood K.M., Swanson K.C., Miller S.P., McBride B.W., and Fitzsimmons C.. 2017. Maternal nutrient restriction in mid-to-late gestation influences fetal mRNA expression in muscle tissues in beef cattle. BMC Genomics 18:632. doi: 10.1186/s12864-017-4051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peine J L, Jia G.Q., Van Emon M.L., Neville T.L., Kirsch J.D., Hammer C.J., O’Rourke S.T., Reynolds L.P., and Caton J.S.. 2013. Effects of maternal nutrition and rumen-protected arginine supplementation on ewe and postnatal lamb performance. Proc. West. Sec. Amer. Soc. Anim. Sci. 64:80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peine J L, Jia G., Van Emon M.L., Neville T.L., Kirsch J.D., Hammer C.J., O’Rourke S.T., Reynolds L.P., and Caton J.S.. 2018. Effects of maternal nutrition and rumen-protected arginine supplementation on ewe performance and postnatal lamb growth and internal organ mass. J. Anim. Sci. 96:3471–3481. doi: 10.1093/jas/sky221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagaricano F, Souza A.H., Carvalho P.D., Driver A.M., Gambra R., Kropp J., Hackbart K.S., Luchini D., Shaver R.D., Wiltbank M.C., . et al. 2013. Effect of maternal methionine supplementation on the transcriptome of bovine preimplantation embryos. PLoS One 8:e72302. doi: 10.1371/journal.pone.0072302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezotto L D, Camacho L.E., Lemley C.O., Keomanivong F.E., Caton J.S., Vonnahme K.A., and Swanson K.C.. 2016. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 10:829–837. doi: 10.1017/S1751731115002645 [DOI] [PubMed] [Google Scholar]
- Prezotto L D, Lemley C.O., Camacho L.E., Doscher F.E., Meyer A.M., Caton J.S., Awda B.J., Vonnahme K.A., and Swanson K. C.. 2014. Effects of nutrient restriction and melatonin supplementation on maternal and foetal hepatic and small intestinal energy utilization. J. Anim. Physiol. Anim. Nutr. (Berl). 98:797–807. doi: 10.1111/jpn.12142 [DOI] [PubMed] [Google Scholar]
- Prezotto L D, Thorson J.F., Borowicz P.P., Peine J.L., Bedenbaugh M., Hileman S.M., Lents C.A., Caton J.S., and Swanson K.C.. 2018. Influences of maternal nutrient restriction and arginine supplementation on visceral metabolism and hypothalamic circuitry of offspring. Domest. Anim. Endocrinol. 65:71–79. doi: 10.1016/j.domaniend.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Radunz A E, Fluharty F.L., Relling A.E., Felix T.L., Shoup L.M., Zerby H.N., and Loerch S.C.. 2012. Prepartum dietary energy source fed to beef cows: II. Effects on progeny postnatal growth, glucose tolerance, and carcass composition. J. Anim. Sci. 90:4962–4974. doi: 10.2527/jas.2012-5098 [DOI] [PubMed] [Google Scholar]
- Reed S A, and Govoni K.E.. 2017. How mom’s diet affects offspring growth and health through modified stem cell function. Anim. Front. 7:25–31. doi: 10.2527/af.2017-0125 [DOI] [Google Scholar]
- Reed J J, Ward M.A., Vonnahme K.A., Neville T.L., Julius S.L., Borowicz P.P., Taylor J.B., Redmer D.A., Grazul-Bilska A.T., Reynolds L.P., . et al. 2007. Effects of selenium supply and dietary restriction on maternal and fetal body weight, visceral organ mass and cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J. Anim. Sci. 85:2721–2733. doi: 10.2527/jas.2006-785. [DOI] [PubMed] [Google Scholar]
- Rehfeldt C, Fiedler I., and Stickland N.C.. 2004. Number and size of muscle fibers in relation to meat production. In: te Pas M F W, Everts M.E., and Haagsman H P, editors. Muscle development of livestock animals: physiology, genetics, and meat quality. Cambridge (MA): CABI Publishing; p. 1–38. [Google Scholar]
- Reynolds L P, Borowicz P.P., Caton J.S., Vonnahme K.A., Luther J.S., Hammer C.J., Maddock Carlin K.R., Grazul-Bilska A.T., and Redmer D.A.. 2010. Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 88 (13 Suppl):E61–E72. doi: 10.2527/jas.2009-2359 [DOI] [PubMed] [Google Scholar]
- Reynolds L P, Borowicz P.P., Vonnahme K.A., Johnson M.L., Grazul-Bilska A.T., Wallace J.M., Caton J.S., and Redmer D.A.. 2005. Animal models of placental angiogenesis. Placenta 26:689–708. doi: 10.1016/j.placenta.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Reynolds L P, and Caton J.S.. 2012. Role of the pre- and post-natal environment in developmental programming of health and productivity. Mol. Cell. Endocrinol. 354:54–59. doi: 10.1016/j.mce.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L P, Grazul-Bilska A T, & Borowicz P P. 2018. Placental angiogenesis. In: Skinner M K, editor. Encyclopedia of reproduction, vol. 2 Cambridge, MA; Amsterdam, Netherlands: Academic Press, Elsevier; p. 521–529. doi: 10.1016/B978-0-12-801238-3.64681-0 [DOI] [Google Scholar]
- Reynolds C K, Tyrrell H.F., and Reynolds P.J.. 1991. Effects of diet forage-to-concentrate ratio and intake on energy metabolism in growing beef heifers: whole body energy and nitrogen balance and visceral heat production. J. Nutr. 121:994–1003. doi: 10.1093/jn/121.7.994 [DOI] [PubMed] [Google Scholar]
- Reynolds L P, and Vonnahme K.A.. 2016. Triennial reproduction symposium: developmental programming of fertility. J. Anim. Sci. 94:2699–2704. doi: 10.2527/jas.2015-0131 [DOI] [PubMed] [Google Scholar]
- Reynolds L P, and Vonnahme K.A.. 2017. Livestock as models for developmental programming. Anim. Front. 7:12–17. doi: 10.2527/af.2017-0123 [DOI] [Google Scholar]
- Reynolds L P, Vonnahme K.A., Lemley C.O., Redmer D.A., Grazul-Bilska A.T., Borowicz P.P., and Caton J.S.. 2013. Maternal stress and placental vascular function and remodeling. Curr. Vasc. Pharmacol. 11:564–593. doi:10.2174/1570161111311050003 [DOI] [PubMed] [Google Scholar]
- Reynolds L P, Ward A.K., and Caton J.S.. 2017. Epigenetics and developmental programming in ruminants – long-term impacts on growth and development. In: Scanes C F, and Hill R, editors. Biology of domestic animals. Milton Park (UK): CRC Press/Taylor & Francis Group. [Google Scholar]
- Reynolds L P, Wulster-Radcliffe M.C., Aaron D.K., and Davis T.A.. 2015. Importance of animals in agricultural sustainability and food security. J. Nutr. 145:1377–1379. doi: 10.3945/jn.115.212217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind S M, Rae M.T., and Brooks A.N.. 2001. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 122:205–214. doi:10.1530/rep.0.1220205 [DOI] [PubMed] [Google Scholar]
- Robinson J J. 1977. The influence of maternal nutrition on ovine foetal growth. Proc. Nutr. Soc. 36:9–16. doi: 10.1079/PNS19770003 [DOI] [PubMed] [Google Scholar]
- Robinson D L, Cafe L.M., and Greenwood P.L.. 2013. Meat science and muscle biology symposium: developmental programming in cattle: consequences for growth, efficiency, carcass, muscle, and beef quality characteristics. J. Anim. Sci. 91:1428–1442. doi: 10.2527/jas.2012-5799 [DOI] [PubMed] [Google Scholar]
- Robinson J J, Sinclair K.D., and McEvoy T.G.. 1999. Nutritional effects on foetal growth. Anim. Sci. 68:315–331. doi: 10.1017/S1357729800050323 [DOI] [Google Scholar]
- Russell R G, and Oteruelo F.T.. 1981. An ultrastructural study of the differentiation of skeletal muscle in the bovine fetus. Anat. Embryol. (Berl). 162:403–417. doi: 10.1007/BF00301866 [DOI] [PubMed] [Google Scholar]
- Schmidt K L, Macdougall-Shackleton E.A., Soma K.K., and Macdougall-Shackleton S.A.. 2014. Developmental programming of the HPA and HPG axes by early-life stress in male and female song sparrows. Gen. Comp. Endocrinol. 196:72–80. doi: 10.1016/j.ygcen.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Shankar K, Zhong Y., Kang P., Lau F., Blackburn M.L., Chen J.R., Borengasser S.J., Ronis M.J., and Badger T.M.. 2011. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 152:4158–4170. doi: 10.1210/en.2010-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B C, and West S.A.. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulates. Am. Nat. 163:40–54. doi: 10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Short R E, Bellows R.A., Staigmiller R.B., Berardinelli J.G., and Custer E.E.. 1990. Physiological mechanisms controlling anestrus and infertility in postpartum beef cattle. J. Anim. Sci. 68:799–816. doi: 10.2527/1990.683799x [DOI] [PubMed] [Google Scholar]
- Sletmoen-Olson K E, Caton J.S., Reynolds L.P., and Olson K.C.. 2000. Undegraded intake protein supplementation: I. Effects on forage utilization and performance of periparturient beef cows fed low-quality hay during gestation and lactation. J. Anim Sci. 78:449–455. doi: 10.2527/2000.782449x [DOI] [PubMed] [Google Scholar]
- Spencer T E, Dunlap K.A., and Filant J.. 2012. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Mol. Cell. Endocrinol. 354:34–53. doi: 10.1016/j.mce.2011.09.035 [DOI] [PubMed] [Google Scholar]
- Spitzer J C, Morrison D.G., Wettemann R.P., and Faulkner L.C.. 1995. Reproductive responses and calf birth and weaning weights as affected by body condition at parturition and postpartum weight gain in primiparous beef cows. J. Anim. Sci. 73:1251–1257. doi: 10.2527/1995.7351251x [DOI] [PubMed] [Google Scholar]
- Stalker L A, Adams D.C., Klopfenstein T.J., Feuz D.M., and Funston R.N.. 2006. Effects of pre- and postpartum nutrition on reproduction in spring calving cows and calf feedlot performance. J. Anim. Sci. 84:2582–2589. doi: 10.2527/jas.2005-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker L A, Ciminski L.A., Adams D.C., Klopfenstein T.J., and Clark R.T.. 2007. Effects of weaning date and prepartum protein supplementation on cow performance and calf growth. Rangeland Ecol. Manage. 60:578–587. doi: 10.2111/06-082R1.1 [DOI] [Google Scholar]
- Stickland N C, Bayol S., Ashton C., and Rehfeldt C.. 2004. Manipulation of muscle fiber number during prenatal development. In: te Pas M F W, Everts M.E., and Haagsman H P, editors. Muscle development of livestock animals: physiology, genetics, and meat quality. Cambridge (MA): CABI Publishing; p. 69–82. [Google Scholar]
- Swanson T J, Hammer C.J., Luther J.S., Carlson D.B., Taylor J.B., Redmer D.A., Neville T.L., Reed J.J., Reynolds L.P., Caton J.S., . et al. 2008. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J. Anim. Sci. 86:2415–2423. doi: 10.2527/jas.2008-0996 [DOI] [PubMed] [Google Scholar]
- Symonds M E, Pope M., Sharkey D., and Budge H.. 2012. Adipose tissue and fetal programming. Diabetologia 55:1597–1606. doi: 10.1007/s00125-012-2505-5 [DOI] [PubMed] [Google Scholar]
- Underwood K R, Tong J.F., Price P.L., Roberts A.J., Grings E.E., Hess B.W., Means W.J., and Du M.. 2010. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Sci. 86:588–593. doi: 10.1016/j.meatsci.2010.04.008 [DOI] [PubMed] [Google Scholar]
- United Nations News Centre 2015. World population projected to reach 9.6 billion by 2050 Available from http://www.un.org/en/development/desa/news/population/un-report-world-population-projected-to-reach-9-6-billion-by-2050.html Accessed December 28, 2015.
- Vonnahme K A, and Lemley C.O.. 2012. Programming the offspring through altered uteroplacental hemodynamics: how maternal environment impacts uterine and umbilical blood flow in cattle, sheep and pigs. Reprod. Fertil. Develop. 24:97–104. doi: 10.1071/RD11910 [DOI] [PubMed] [Google Scholar]
- Vonnahme K A, Lemley C.O., Caton J.S., and Meyer A.M.. 2015. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 7:3497–3523. doi: 10.3390/nu7053497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme K A, Luther J.S., Reynolds L.P., Hammer C.J., Carlson D.B., Redmer D.A., and Caton J.S.. 2010. Impacts of maternal selenium and nutritional level on growth, adiposity, and glucose tolerance in female offspring in sheep. Domest. Anim. Endocrinol. 39:240–248. doi: 10.1016/j.domaniend.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Vonnahme K A, Zhu M.J., Borowicz P.P., Geary T.W., Hess B.W., Reynolds L.P., Caton J.S., Means W.J., and Ford S.P.. 2007. Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J. Anim. Sci. 85:2464–2472. doi: 10.2527/jas.2006-805 [DOI] [PubMed] [Google Scholar]
- Wallace L R. 1948. The growth of lambs before and after birth in relation to the level of nutrition. J. Agric. Sci. (Camb.) 38:243–300. doi: 10.1017/S0021859600006079 [DOI] [Google Scholar]
- Wallace J M, Bourke D.A., and Aitken R.P.. 1999. Nutrition and fetal growth: paradoxical effects in the overnourished adolescent sheep. J. Reprod. Fertil. Suppl. 54:385–399. [PubMed] [Google Scholar]
- Walton A, and Hammond J.. 1938. The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proc. R. Soc. Lond., B, Biol. Sci. 125:311–335. doi: 10.1098/rspb.1938.0029 [DOI] [Google Scholar]
- Wang X, Lan X., Radunz A.E., and Khatib H.. 2015. Maternal nutrition during pregnancy is associated with differential expression of imprinted genes and DNA methyltranfereases in muscle of beef cattle offspring. J. Anim. Sci. 93:35–40. doi: 10.2527/jas.2014-8148 [DOI] [PubMed] [Google Scholar]
- Ward A K, Crouse M.S., Cushman R.A., McLean K.J., Dahlen C.R., Borowicz P.P., Reynolds L.P., and Caton J.S.. 2017. Maternal nutrient restriction in early gestation upregulates myogenic genes in cattle fetal muscle tissue. 36th International Society of Animal Genetics Conference; July 16 to 21, 2017; Dublin, Ireland p. 177. [Google Scholar]
- Ward M A, Neville T.L., Reed J.J., Taylor J.B., Hallford D.M., Soto-Navarro S.A., Vonnahme K. A., Redmer D.A., Reynolds L.P., and Caton J.S.. 2008. Effects of selenium supply and dietary restriction on maternal and fetal metabolic hormones in pregnant ewe lambs. J. Anim. Sci. 86:1254–1262. doi: 10.2527/jas.2007-0509 [DOI] [PubMed] [Google Scholar]
- Ward S S, and Stickland N.C.. 1991. Why are slow and fast muscles differentially affected during prenatal undernutrition? Muscle Nerve 14:259–267. doi: 10.1002/mus.880140310 [DOI] [PubMed] [Google Scholar]
- Wilson S J, Ross J.J., and Harris A.J.. 1988. A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development 102:815–821. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer F.W., Wallace J.M., and Spencer T.E.. 2006. Intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84:2316–2337. doi: 10.2527/jas.2006-156 [DOI] [PubMed] [Google Scholar]
- Xiong F, and Zhang L.. 2013. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 34:27–46. doi: 10.1016/j.yfrne.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova R, Neville T.L., Vonnahme K.A., Hammer C.J., Reed J.J., Taylor J.B., Redmer D.A., Reynolds L.P., and Caton J.S.. 2013. Impacts of maternal selenium supply and nutritional plane on visceral tissues and intestinal biology in offspring. J. Anim. Sci. 91:2229–2242. doi: 10.2527/jas.2012-5134 [DOI] [PubMed] [Google Scholar]
- Zambrano E, Guzmán C., Rodríguez-González G.L., Durand-Carbajal M., and Nathanielsz P.W.. 2014. Fetal programming of sexual development and reproductive function. Mol. Cell. Endocrinol. 382:538–549. doi: 10.1016/j.mce.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Zhang S T, Reguault R.H., Barker P.L., Botting K.J., McMillen I.C., McMillan C.M., Roberts C.T., and Morrison J.L.. 2015. Placental adaptations in growth restriction. Nutrients. 7:360–389. doi: 10.3390/nu7010360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M J, Ford S.P., Means W.J., Hess B.W., Nathanielsz P.W., and Du M.. 2006. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 575(Pt 1):241–250. doi: 10.1113/jphysiol.2006.112110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M J, Ford S.P., Nathanielsz P.W., and Du M.. 2004. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol. Reprod. 71:1968–1973. doi: 10.1095/biolreprod.104.034561 [DOI] [PubMed] [Google Scholar]