Abstract
Astrocytes are the major glia cells in the central nervous system (CNS). Increasing evidence indicates that more than to be safe-guard and supporting cells for neurons, astrocytes play a broad spectrum of neuroprotective and pathological functions. Thus, they are compelling models to decipher mechanistic insights of glia cells to CNS insults and for the development of drugs. Edaravone is a free radical scavenger with the capacity to eliminate hydroxyl radicals and lipid peroxides. In this study, we examined the neuroprotective effects of edaravone in rat astrocytes challenged by hydrogen peroxide (H2O2) or bacterial lipopolysaccharides (LPS), respectively. We discovered that edaravone attenuated H2O2-induced oxidative stress by reactivating the Akt signaling axis and antagonistically restoring the expression of apoptosis associated regulators such as Bcl-2 and Caspase-3. Consistently, inhibition of Akt signaling by LY294002 attenuated the anti-oxidative activity of edaravone. In addition, edaravone mitigated LPS-induced morphological changes in astrocytes and alleviated the inflammatory activation and expression of TNF-α, IL-1β, IL-6 and NOS2. In summary, our data suggested that edavarone effectively protects astrocytes from oxidative stress or infectious insults, which may pave a new avenue for its application in preclinical research and human disease therapeutics.
Abbreviations: ALS, amyotrophic lateral sclerosis; C1q, complement component 1q; CNS, central nervous system; GFAP, glial fibrillary acidic protein; H2O2, hydrogen peroxide; LPS, lipopolysaccharides; IL-1α, interleukin 1 alpha; IL-1β, interleukin 1beta; IL-6, interleukin 6; NOS2, nitric oxide synthase 2; TNF-α, tumor necrosis factor alpha; TLRs, Toll-like receptors
Keywords: edaravone, free radical scavenger, GFAP, oxidative stress, pro-inflammatory factors, TNF-α
Introduction
Astrocytes are the most abundant glial cell population in central nervous system (CNS) and are essential for brain homeostasis and neuronal survival (Almad and Maragakis, 2018; Sofroniew, 2015). Apart from microglia as the well-established immune sensor of CNS insults, astrocytes are compelling cells afford either pro-inflammatory functions or anti-inflammatory roles in CNS inflammation (Farina et al., 2007), owing to their different status and functions after activation (Liddelow and Barres, 2017). Increasing evidence implicates astrocytes as critical regulators in brain injury and ischemia, autoimmune and neurodegenerative disorders. They express an array of receptors such as Toll-like receptors (TLRs), scavenger receptors, and complement system to mediate immune signaling transduction in which TLR4 is response to bacterial lipopolysaccharides (LPS). LPS administration (signify bacterial infection) is extensively applied in research to activate astrocyte and evoke inflammation. Cytokines IL-1β, IL-6, TNF-α and small molecule effector nitric oxide (NO) are astrocyte-produced molecules that regulate pro-inflammatory response (Sofroniew, 2015). It is conceived that mice lack of TNF-α, or IL-1α live for a prolonged time (Marino et al., 1997). Thus, the elimination of neurotoxic inflammation is considered to afford neuroprotective functions and can be a promising therapeutic strategy.
Edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolin-5-one) is a free radical scavenger originally developed by Mitsubishi Chemical Industries Ltd. to treat neuropathy caused by cerebral infarction (Abe et al., 1988). It was approved in 2017 in the US with trade name Radicava to treat amyotrophic lateral sclerosis (ALS), owing to its capacity to mitigate oxidative injury in neurons and slow the decline of physical function (Kapoor, 2013; Rothstein, 2017; Valko and Ciesla, 2019). Increasing experimental and clinical evidence has well-established the neuroprotective effects of edaravone as antioxidants (Li et al., 2019; Watanabe et al., 2008) and antidepressants (Qin et al., 2014) in multiple lines of models from the bench to clinical trials. Especially in astrocyte, compelling evidence establishes the protective effects of edaravone in attenuating manganese, NO, MPP+, and hypoxia -induced cytotoxicity, apoptosis and cell death (Chen et al., 2008; Evren et al., 2015; Kawasaki et al., 2007; Yoshida et al., 2011). Therefore, edaravone is a potential antioxidant to cure neurological diseases.
In this study, H2O2-induced oxidative stress and LPS-induced activation and pro-inflammatory models in cultured rat astrocytes were established, respectively. On this basis, we examined the neuroprotective effects of edaravone to attenuate oxidative stress and diminish inflammation in astrocyte.
Experimental procedures
Animals
New born Sprague-Dawley (SD) rat was provided by the Experimental Animal Center of Peking University Health Science Center, Beijing, China, with a license of No. SCXK-2017-0005. Animal experiments were performed according to the Guidelines for Care and Use of Laboratory Animals of Beijing Municipality and approved by the Animal Care and Use Committee of Minzu University of China.
Chemicals
Eadravone injection (#H20050280, 1.5 mg/mL in 0.9% NaCl, purity: 98.5%) was provided by Nanjing Simcere Dongyuan Pharmaceutical Co., Ltd. Lipopolysaccharides (#L2630) were provided by Sigma-Aldrich, LLC.
Culture of rat astrocytes
Isolation and culture of rat astrocytes were performed as previously described (Souza et al., 2013; Tarassishin et al., 2014). In brief, rat cerebral cortex (near the prefrontal lobe) was aseptically dissected, mechanically dissociated, and digested in 0.25% trypsin (#15050065, Gibco) for 25 min at 37 °C. The digestion was terminated via the addition of 10% fetal bovine serum in DMEM (#11960044, Gibco). After filtration through 70 μm nylon-mesh strainer (#352350, BD Biosciences), the cells were collected by centrifugation (1,100 rpm x3 min) and resuspended in DMEM supplemented with 10% FBS, 2 g/L HEPES, penicillin-streptomycin (#15070063, Gibco). Cells were seeded on the culture flask and cultured in a 37 °C with 5% CO2 in air for 9 days to form a confluent monolayer. After the removal of low adhered cells by shaking (200 rpm) for 15 h, astrocytes were trypsin-digested, resuspended, and seeded on 6-well plates at a density of 105 cells/mL. The culture purity of astrocytes (is >95%) were identified by immunoflurocenses using an antibody against GFAP (glial fibrillary acidic protein).
WST-1 cell viability assay and LDH cytotoxicity assay
Cell viability was colorimetric measured by WST-1 cell proliferation and viability assay kit (#C0035, Beyotime Biotechnology), which are based on the cleavage of the tetrazolium salt (WST-1) to formazan by mitochondrial succinate-tetrazolium reductase. The absorbance of the produced formazan was measured at 450 nm by Epoch 2 microplate spectrophotometer (BioTek Instruments, Inc.) and is in direct proportion to the number of viable cells.
The cytotoxicity was assayed using the CytoTox96 Non-Radioactive cytotoxicity assay kit (#G1780, Promega). It measures lactate dehydrogenase (LDH) released from damaged cells upon environmental insults through a 30-min coupled enzymatic assay, which results in the conversion of tetrazolium salt (iodonitrotetrazolium violet) into a red formazan product. The absorbance signal was recorded at 490 nm by Epoch 2 microplate spectrophotometer (BioTek Instruments, Inc.).
Western blotting
Total protein of astrocytes was extracted using RIPA lysis buffer (#P0013C, Beyotime Biotechnology) supplemented with cOmplete protease inhibitor cocktail (#11697498001, Roche), followed by centrifuging at 4 °C, 13,000 rpm, 15 min. Protein concentrations were assayed by BCA protein assay kit (#PC0020, Solarbio). Equal protein was loaded and separated by 10% SDS-PAGE, followed by transblotting to nitrocellulose membrane. Target proteins were probed using primary antibodies as follows. The primary antibody anti phospho-Akt (Ser473) (#4060, dilution 1:1000), Bcl-2 (#3498, dilution 1:1000), β-actin (#4970, dilution 1:2000) and cleaved-Caspase-3 (#9662, dilution 1:1000) was provided by Cell Signaling Technology, Inc. Western blot results were scanned by the Odyssey CLx infrared fluorescence imaging system (LI-COR Biosciences). The relative protein expression levels were normalized to β-actin according to the optical densities of blot bands that were quantified by Image J software.
Immunofluorescence
The astrocytes seeded on coverslips in 24-well plates were used for immunofluorescence following the experimental treatments. Cells were fixed in 4% paraformaldehyde at room temperature for 20 min, rinsed in PBS 3 times, and incubated with 0.2% Triton X-100 at room temperature for 10 min. After blocking with 10% goat serum in PBS for 1 h, cells were immuno-labeled with antibody against GFAP (dilution 1:500, #3670, Cell Signaling Technology, Inc.) at 4 °C overnight and followed by incubation with highly cross-adsorbed secondary antibody, Alexa Fluor 488 (#A-11029, Invitrogen, dilution 1:5000). The nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Cell images were acquired using a Leica TCS SP8 confocal microscope (Leica Microsystems, Germany).
Enzyme-linked immunosorbent assays (ELISA)
Expression of pro-inflammatory factors TNF-α and IL-6 was determined by Rat TNF-α ELISA kit (#EK0526) and Rat IL-6 ELISA kit (#EK0412) respectively, according to the manufactory’s protocol (Boster Biological Technology Co., Ltd.)
Real time quantitative PCR (RT-PCR)
Total RNA was extracted from astrocytes using TRIzol reagent (#15596026, Invitrogen) and transcribed to cDNA using the RevertAid First Strand cDNA Synthesis kit (#K1622, Thermo Scientific). Primers used for qPCR are as follows. TNF-α, sense: 5′-TGACCCCCATTACTCTGACC-3′, antisense: 5′-GGCCACTACTTCAGCGTCTC-3′; IL-6, sense: 5′-ATTCTGTCTCGAGCCCACCA-3′, antisense: 5′-AGGCAACTGGCTGGAAGTCT-3′; IL-1β, sense: 5′-AATGCCTCGTGCTGTCTGA-3′, antisense: 5′-TGTCGTTGCTTGTCTCTCCT-3′. NOS2, sense: 5′-AAGAGACGCACAGGCAGAG-3′, antisense: 5′-CAGGCACACGCAATGATGG-3′; β-actin, sense: 5′-TGTCCACCTTCCAGCAGATG-3′, antisense: 5′-GCTCAGTAACAGTCCGCCTA-3′. The relative gene expression level was calculated using 2−△△Ct taking β-actin as a reference.
Statistical analysis
Results were analyzed using GraphPad Prism 7.0 software. Data were presented as mean ± S.E.M. and analyzed by one-way ANOVA with treatment as the independent factor and followed by Bonferroni’s multiple comparisons test to correct p value in multiple intergroup comparisons. Unpaired t test with Welch's correction was performed in comparisons of two groups. The results of repeated trials are displayed by points in the scatter dot plot.
Results
Edaravone effectively attenuates H2O2-induced oxidative stress in rat astrocyte.
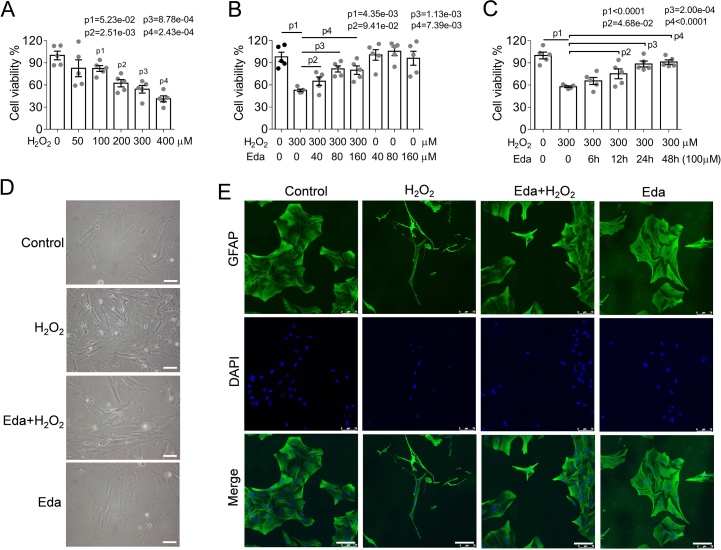
To investigate the anti-oxidative effects of edaravone, astrocytes were isolated from newborn SD rats and challenged with H2O2 in concentration dependent manner. Astrocytes were administrated with a set of concentrations (50, 100, 200, 300, 400 μM) of H2O2 and cultured for 24 h, followed by WST-1 cell viability assay. We found that H2O2-treatment caused a significant decline in cell viability, in which 300 μM of H2O2 caused a decrease of 45.81% (from 100% to 54.19 ± 2.33%) compared with that of healthy control (0 μM, the cell viability set as 100%). In other words, 300 μM of H2O2 treatment nearly inhibited half of the cell viability, thus this concentration was used in subsequent experiments (Fig. 1A). In the followed experiments, astrocytes were pre-treated with edaravone (40, 80, 160 μM) and then challenged with 300 μM H2O2 (Fig. 1B). Edaravone effectively reversed the decrease in cell viability upon H2O2-induced oxidative stress, in which the maximal cytoprotective effect was observed at 80 μM for Edaravone (Fig. 1B). In addition, the anti-oxidative effects of Edaravone persisted in a time-dependent manner in 48 h (Fig. 1C). In light of morphological observation, edaravone also effectively restored H2O2-induced morphological collapse in astrocytes, such as loss of outgrowth and shrink of the cell body (Fig. 1D). Immunofluorescence performed using a specific antibody against astrocyte specific intermediate filament protein GFAP (glial fibrillary acidic protein) also consistently supported the morphological maintenance effects of Eda (Fig. 1E). From the results aforementioned, Eda is an effective anti-oxidant to protect astrocytes.
Fig. 1.
Edaravone effectively attenuates H2O2-induced oxidative stress in rat astrocyte.
(A) H2O2 treatment introduced a significant decrease in cell viability to 84.67 ± 5.83%, 82.41 ± 1.77%, 62.34 ± 2.19%, 54.19 ± 2.33%, and 41.20 ± 1.83% at concentrations of 50, 100, 200, 300 and 400 μM, respectively. (B) Administration of edaravone (Eda) remarkably antagonized H2O2-induced cytotoxicity. (C) Eda afforded anti-oxidative effects in a time-dependent way. (A-C) Data represent the mean ± S.E.M. of five independent experiments indicated by points. Statistical significance of intergroup differences was examined by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (D) Images of astrocytes treated with H2O2 (300 μM), H2O2 (300 μM)+Eda(80 μM), Eda (80 μM). Scale bar, 100 μm. (E) Immunofluorescence of astrocytes using an antibody against GFAP (marker protein of astrocyte). Edaravone preserved the morphology of astrocyte with slender protrusions and spreading cellular body from H2O2-induced oxidative stress. Scale bar, 100 μm.
Edaravone exerts anti-oxidative effects by restoring Akt/Bcl-2/Caspase-3 signaling axis.
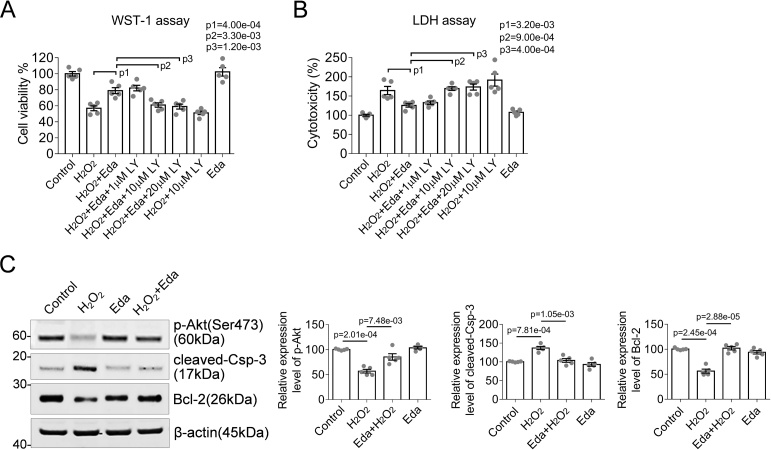
PI3K/Akt is a typical signaling pathway responding to various CNS insults. The activity of the pathway and its healthy response to stimuli is critical for neuronal survival (Brunet et al., 2001). Therefore, we hypothesized that inhibition of PI3K/Akt signaling pathway would attenuate the neuroprotective effects of edaravone. LY294002 is a small chemical well-established for its inhibitory effect on PI3K/Akt signaling axis. In astrocytes, the cells were pre-incubated with edavarone and then medicated with LY294002 (10 μM) and 300 μM of H2O2 for 24 h. Then the WST-1 cell viability assay and LDH cytotoxicity assay was performed as described in the Experimental procedures. As expected, we found that the administration of LY294002 antagonized the neuroprotective effects of edaravone (Fig. 2A and 2B). Therefore, PI3K/Akt signaling axis is essential for astrocyte viability, especially in free radical scavenger mediated anti-oxidative process (Fig. 2). Accordingly, the active status of Akt (phosphorylation on serine 473) and the expression of apoptosis associated proteins Bcl-2 and cleaved-Caspase-3 was examined using specific antibodies. H2O2 mediated oxidative stress attenuated the activation of Akt and downregulated the expression of anti-apoptotic protein Bcl-2, but upregulated the expression of apoptosis promoting enzyme--cleaved-Caspase-3. In contrary, medication of edaravone not only reactivated Akt signaling axis but also antagonistically restored the expression of Bcl-2 and cleaved-Caspase-3, resulting in the maintenance of cell viability and inhibition of apoptosis. From the results aforementioned, we concluded that edaravone effectively protected astrocytes from H2O2-induced oxidative stress, possibly by preserving the activity of PI3K/Akt signaling axis.
Fig. 2.
Akt/Bcl-2 signaling axis is required for Edaravone-mediated anti-oxidant properties.
(A) and (B) Edaravone protected astrocytes from H2O2-induced apoptosis and cell death that examined by WST-1 cell viability assay or LDH cytotoxicity assay. Inhibition of PI3K/Akt signaling axis by a small inhibitor LY294002 alleviated the anti-oxidative effect of edaravone. (C) Representative Western blots showed that edaravone rescued the activity of Akt, and antagonistically restored the expression of anti-apoptosis protein Bcl-2 and apoptosis promoting enzyme cleaved-Caspase-3 (Csp-3). The protein expression levels were normalized to β-actin. Intergroup significance of difference was determined by one-way ANOVA followed by Bonferroni’s multiple comparisons test.
Edaravone attenuates lipopolysaccharide-induced astrocyte activation and inflammation.
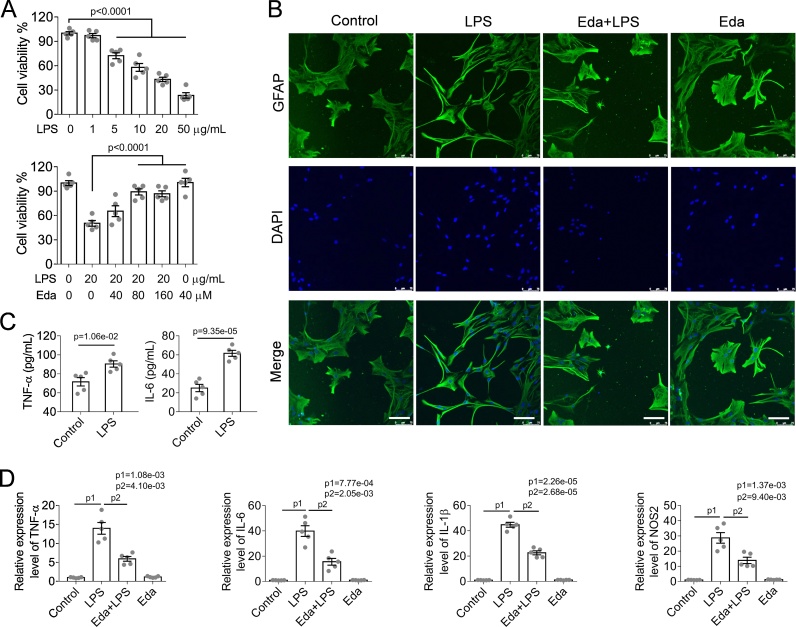
To investigate the anti-inflammatory effects of edaravone, astrocytes were first challenged with lipopolysaccharides (LPS, 20 μg/mL) for 48 h to establish astrocyte inflammatory models. We found that LPS-treated astrocytes remarkably showed inhibition of cell viability in concentration dependent manner (Fig. 3A). However, this inhibition can be recused by medication of edaravone (Fig. 3A). Besides, immunofluorescences were performed to examine the morphology of astrocytes. In the experiments, a moderate enhancement in cell proliferation (indicated by increased cell nuclei visualized by DAPI staining) was observed in LPS-treated astrocytes, where they showed reduced size in cell body and increased numbers of slender outgrow and dense branches (revealed by GFAP staining). In contrast, the medication of edaravone prevented astrocytes from LPS-induced morphological changes and maintained cell proliferation unchanged (Fig. 2B). Consistently, LPS-induced expression of pro-inflammatory factors TNF-α and IL-6 were examined by ELISA (Fig. 2C). We found that TNF-α and IL-6 obviously increased from 71.60 ± 1.98 pg/mL to 90.40 ± 1.50 pg/mL (for TNF-α), or 25.00 ± 1.71 pg/mL to 61.60 ± 1.40 pg/mL (for IL-6), respectively. Then edaravone (80 μM) was administrated to the inflammatory astrocytes for 24 h. The gene expression levels of pro-inflammatory factors such as TNF-α, IL-6, IL-1β and NOS2 were determined by real time quantitative PCR (RT-PCR). Consistent with the activation by LPS treatment, astrocytes showed dozens of times of increase in the expression of TNF-α, IL-6, IL-1β and NOS2 mRNA (Fig. 2D, LPS treated). However, edaravone treatment effectively alleviated LPS-induced astrocyte inflammation, for the expression of the above factors was significantly inhibited. In summary, edaravone is efficient to attenuate LPS-induced astrocyte activation and pro-inflammatory response.
Fig. 3.
Edaravone attenuates lipopolysaccharide-induced astrocyte activation and inflammation.
(A) LPS inhibited cell viability in a concentration-dependent manner. Eda preserved cell viability from LPS-induced cytotoxicity. (B) Eda effectively mitigated LPS-induced astrocyte activation and inflammation, suggesting by immuno-labeling of GFAP. Scale bar, 100 μm. LPS (20 μM)-treated astrocytes showed shrunk nuclei (visualized by DAPI staining of DNA) and increase in cell numbers with slender outgrowth and dense branching compared with their normal counterparts. However, Eda administration (80 μM) alleviated LPS-induced morphological changes in astrocytes. (C) Increased expression of pro-inflammatory factors TNF-α and IL-6 that determined by ELISA confirmed the induction of astrocyte activation and inflammation by LPS (20 μg/mL). Unpaired t-test with Welch's correction was performed to calculate the significance of intergroup difference, n = 5. (D) Eda (80 μM) alleviated LPS-induced astrocyte activation and inflammation by alleviating the expression of pro-inflammatory factors TNF-α, IL-6, IL-1β and NOS2. The relative mRNA expression level of the gene was normalized to β-actin.
Discussion
Astrocytes and microglia are two major CNS-resident cells in immune responses. Elimination of CNS insults such as free radicals, trauma, infection and neurodegenerative pathology are privileged events to maintain brain homeostasis and neuronal functions (Almad and Maragakis, 2018; Farina et al., 2007; Sofroniew, 2015). More than well-established functions to form the glia limitans and to nourish neurons, astrocytes are increasingly recognized as a key regulator in CNS homeostasis under normal conditions and in neurological diseases. Oxidative stress, infectious, ischemia and brain injury are common CNS insults cause cytotoxicity of neurons and cell death, resulting in neurological dysfunctions. For preclinical research and drug exploration, astrocytes are extensively established model to study the mechanistic insights of astrocytes functions and astrocytopathies. H2O2, Aβ or glutamate is widely used agents to induce neurotoxicity and apoptosis (Pan et al., 2017; Qin et al., 2010). LPS is administrated to activate astrocyte and evoke pro-inflammation (Tarassishin et al., 2014). Although normal onset of immune response is required for safe-guard brain functions against CNS insults, neurotoxic inflammation should be avoided. Ultimately, culture systems along with animal models both provide powerful tools to resolve the functions of astrocytes in normal conditions and diseases.
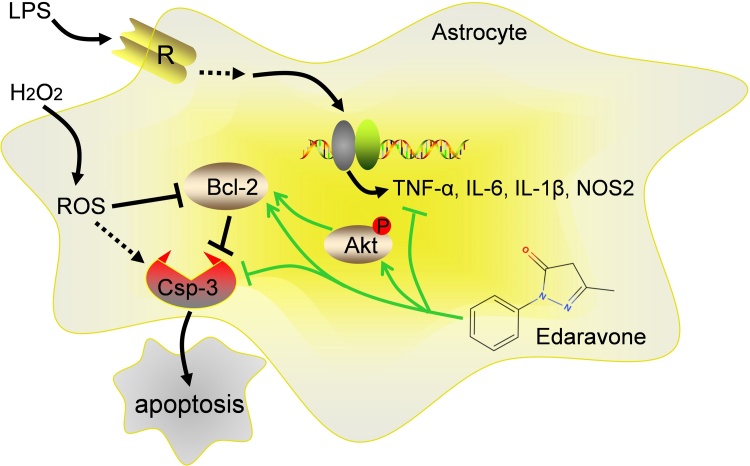
Edaravone is an antioxidant with the capability to clear free radicals, which have been widely used to treat acute ischemic stroke (Abe et al., 1988). It is a new drug approved for amyotrophic lateral sclerosis (ALS) (Rothstein, 2017; Valko and Ciesla, 2019), a neurodegenerative disease leading to death of motor neurons in the brain and spinal cord, resulting in the inability of muscle movements. Edaravone is considered to slow the decline of physical functions. In preclinical research, edaravone has been extensively reported as antioxidants in hypoxia, ischemia or cerebral infarction models to afford neuroprotective effects (Kawasaki et al., 2007; Wang et al., 2011; Yoshida et al., 2006). In addition, edaravone is reported to protect neurons against cognitive impairments caused by traumatic injury or Alzheimer’s disease in rodent models (Jiao et al., 2015; Ohta et al., 2013; Qin et al., 2014; Yang et al., 2015). Especially, edaravone combined with borneol was reported to alleviate LPS-induced acute lung injury in mice. Accordingly, LPS-induced elevation of IL-6 and cyclooxygenase-2 (COX-2) was restored by edaravone treatment in RAW264.7 cells (Zhang et al., 2017). Thus, edaravone might be a powerful agent for improving neurological functions in disease conditions. In this study, we took use of cultured rat astrocytes to investigate the neuroprotective role of edaravone to cope with H2O2-induced oxidative stress and LPS-medicated pro-inflammatory response. We found that edaravone attenuates astrocytes apoptosis or cell death upon oxidative stress through reactivating PI3K/Akt signaling and inhibiting apoptosis signaling, for the biochemistry of astrocytes were remarkably ameliorated by edaravone (Fig. 4). Consistently, in LPS-mediated model of astrocyte activation and pro-inflammatory response, edaravone blocks the morphological changes of astrocytes induced by LPS treatment, and right sets the expression of pro-inflammatory factors such as TNF-α, IL-1β, IL-6. In summary, edaravone is an effective free radicals scavenger to protect astrocytes. It can serve as an effective agent to cope with oxidative stress and to attenuate neurotoxic inflammation. Further research in animal models or clinical trials are needed to resolve the effects of edaravone in anti-oxidation or anti-neurotoxic inflammation in vivo may greatly deepen the mechanistic insights of edaravone medication, especially in the treatment of progressive and age-related neurodegenerative diseases.
Fig. 4.
A schematic model illustrates the anti-oxidative and anti-inflammatory effects of edaravone in response to H2O2-mediated oxidative stress or LPS-induced neurotoxic inflammatory insults. In astrocyte, H2O2-introduced oxidative stress induced the inhibition of Akt and Bcl-2 and activated apoptosis promoting enzyme Caspase-3, resulting in morphological collapse and cell apoptosis. Similarly, LPS induced pro-inflammatory response and the production of pro-inflammatory factors TNF-α, IL-6, IL-1β and NOS2 and inhibition of cell viability. Edaravone is an antioxidant and radical scavenger. Its medication reactivates Akt signaling axis, rescues the expression of anti-apoptotic protein Bcl-2, and inhibits Caspase-3, finally affords neuroprotective functions.
Conclusions
Edaravone attenuates H2O2-induced oxidative stress in rat astrocytes by reactivating the Akt signaling axis and antagonistically restoring the expression of apoptosis associated signaling regulators such as Bcl-2 and Caspase-3. Edaravone attenuates LPS-induced astrocytes activation and inhibits the expression of pro-inflammatory factors TNF-α, IL-1β, IL-6, and preserves the morphology of astrocyte.
Conflicts of interest
The authors have no conflicts of interest.
Author contributions
Guo Z.: performed the experiments and data analysis. Wu H.T.: performed the experiments, plotted the data and wrote the draft. Li X.X.: performed western blotting and statistical analysis. Yu Y.: astrocytes isolation and culture. Gu R.Z.: RT-PCR and data analysis. Qin X.Y: designed the experiments, and approved the manuscript, funding acquisition. Lan R.F.: wrote and approved the manuscript, funding acquisition.
Acknowledgments
We are grateful to the grants from the National Natural Science Foundation of China (81873088, 21778038), Shenzhen science and technology innovation committee program (JCYJ20180305163349116) and the MUC 111 project (URTP2017110030).
Contributor Information
Rongfeng Lan, Email: lan@szu.edu.cn.
Xiao-Yan Qin, Email: bjqinxiaoyan@muc.edu.cn.
References
- Abe K., Yuki S., Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke. 1988;19:480–485. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- Almad A., Maragakis N.J. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 2018;14:351–362. doi: 10.1038/s41582-018-0010-2. [DOI] [PubMed] [Google Scholar]
- Brunet A., Datta S.R., Greenberg M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Chen H., Wang S., Ding J.H., Hu G. Edaravone protects against MPP+ -induced cytotoxicity in rat primary cultured astrocytes via inhibition of mitochondrial apoptotic pathway. J Neurochem. 2008;106:2345–2352. doi: 10.1111/j.1471-4159.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- Evren V., Apaydin M., Khalilnezhad A., Erbas O., Taskiran D. Protective effect of edaravone against manganese-induced toxicity in cultured rat astrocytes. Environ Toxicol Pharmacol. 2015;40:563–567. doi: 10.1016/j.etap.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Jiao S.S., Yao X.Q., Liu Y.H., Wang Q.H., Zeng F., Lu J.J., Liu J., Zhu C. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A. 2015;112:5225–5230. doi: 10.1073/pnas.1422998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor S. Neuroprotective effects of edaravone: recent insights. J Neurol Sci. 2013;331:177. doi: 10.1016/j.jns.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kitao T., Nakagawa K., Fujisaki H., Takegawa Y., Koda K., Ago Y., Baba A. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- Li Q., Qiu Z., Lu Y., Lu P., Wen J., Wang K., Zhao X., Li R. Edaravone protects primary-cultured rat cortical neurons from ketamine-induced apoptosis via reducing oxidative stress and activating PI3K/Akt signal pathway. Mol Cell Neurosci. 2019;100 doi: 10.1016/j.mcn.2019.103399. [DOI] [PubMed] [Google Scholar]
- Liddelow S.A., Barres B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Marino M.W., Dunn A., Grail D., Inglese M., Noguchi Y., Richards E., Jungbluth A., Wada H. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Higashi Y., Yawata T., Kitahara M., Nobumoto A., Ishida E., Tsuda M., Fujimoto Y. Attenuation of axonal injury and oxidative stress by edaravone protects against cognitive impairments after traumatic brain injury. Brain Res. 2013;1490:184–192. doi: 10.1016/j.brainres.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Pan R.Y., Ma J., Wu H.T., Liu Q.S., Qin X.Y., Cheng Y. Neuroprotective effects of a Coeloglossum viride var. Bracteatum extract in vitro and in vivo. Sci Rep. 2017;7:9209. doi: 10.1038/s41598-017-08957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Zhang R.X., Li J.L., Wang J.X., Hou J., Yang X., Zhu W.L., Shi J. cRGD mediated liposomes enhanced antidepressant-like effects of edaravone in rats. Eur J Pharm Sci. 2014;58:63–71. doi: 10.1016/j.ejps.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Qin X.Y., Cheng Y., Yu L.C. Potential protection of curcumin against intracellular amyloid beta-induced toxicity in cultured rat prefrontal cortical neurons. Neurosci Lett. 2010;480:21–24. doi: 10.1016/j.neulet.2010.05.062. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D. Edaravone: A new drug approved for ALS. Cell. 2017;171:725. doi: 10.1016/j.cell.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza D.G., Bellaver B., Souza D.G.O., Quincozes-Santos A. Characterization of adult rat astrocyte cultures. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L., Suh H.S., Lee S.C. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. 2014;62:999–1013. doi: 10.1002/glia.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko K., Ciesla L. Amyotrophic lateral sclerosis. Prog Med Chem. 2019;58:63–117. doi: 10.1016/bs.pmch.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Wang G.H., Jiang Z.L., Li Y.C., Li X., Shi H., Gao Y.Q., Vosler P.S., Chen J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28:2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Tahara M., Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26:101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- Yang R., Wang Q., Li F., Li J., Liu X. Edaravone injection ameliorates cognitive deficits in rat model of Alzheimer’s disease. Neurol Sci. 2015;36:2067–2072. doi: 10.1007/s10072-015-2314-y. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Mimura J., Imaizumi T., Matsumiya T., Ishikawa A., Metoki N., Tanji K., Ota K. Edaravone and carnosic acid synergistically enhance the expression of nerve growth factor in human astrocytes under hypoxia/reoxygenation. Neurosci Res. 2011;69:291–298. doi: 10.1016/j.neures.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Yanai H., Namiki Y., Fukatsu-Sasaki K., Furutani N., Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Luo Z., Bi A., Yang W., An W., Dong X., Chen R., Yang S. Compound edaravone alleviates lipopolysaccharide (LPS)-induced acute lung injury in mice. Eur J Pharmacol. 2017;811:1–11. doi: 10.1016/j.ejphar.2017.05.047. [DOI] [PubMed] [Google Scholar]