Abstract
The use of milk leukocyte differential (MLD) test has been proposed as a complement to somatic cell count (SCC) to assess the presence and the severity of intramammary infection. However, detailed information regarding the behavior of MLD under different physiological or pathological stages of the cow is nonexistent. The objective was to analyze the association between milk leukocyte proportions provided by a commercial automated MLD test and multiple cow and quarter-level variables. The study population consisted of 104 Holstein cows (32 primiparous and 72 multiparous) in one farm. Cows were categorized by days in milk as early (<50 DIM; n=29), middle (50–250 DIM; n=25), and late lactation (>250 DIM; n = 50). Milk from 416 quarters was collected and analyzed for lymphocytes (LYM), neutrophils (NEU), and macrophages (MAC) counts using an automated milk fluorescence microscopy system. Concurrently, a sterile composite milk sample was collected from each cow for pathogen identification through microbiological culture. Culture results were classified as no growth (NOG), gram-negative (GN), gram-positive (GP), or other (OTH). Milk leukocyte proportions varied depending on the level of total leukocyte counts (TLC; P < 0.001). Similarly, leukocyte ratios (NEU:LYM, NEU:MAC, and phagocyte:LYM) were different for multiple TLC categories (P < 0.05). There was no association between parity number and MLD; however, cows in early lactation had the greatest proportions of NEU and LYM. Leukocyte ratios varied depending on parity number and stage of lactation. Cows in the medium milk-yield category had the smallest proportions of NEU and LYM, and there was significant variation in leukocyte ratios, depending on the level of milk yield. In healthy quarters, MLD were not associated with quarter position; however, the NEU:MAC ratio was greater in rear quarters than in front quarters. In quarters with TLC >100,000, NEU% was greater in rear quarters than in front quarters (P = 0.03). For quarters with pathogen growth, TLC was greatest for GN followed by OTH and GP (P < 0.001). Milk LD depended on the isolated pathogen group, although the magnitudes of the differences were small. Although the changes in the proportions of leukocytes in milk were associated with categories of TLC, levels of milk yield, and mastitis-causing pathogen groups, the deviations were small in magnitude. Additional research is necessary to determine the potential applications for this methodology.
Keywords: differential leukocyte count, mastitis, quarters
INTRODUCTION
Mastitis remains one of the most prevalent and costly diseases in dairy systems (Damm et al., 2017). The use of somatic cell count (SCC) for the diagnosis of intramammary infection (IMI) is widely accepted and is considered a reliable procedure for the detection of subclinical mastitis (SCM). Although SCC is a robust methodology, it does not provide a differentiation of cell types (Damm et al., 2017). The use of a milk leukocyte differential (MLD) test, providing the proportions of specific leukocytes in milk, has been proposed as a complement to SCC to assess the presence and the severity of IMI (Dohoo et al., 1981; Leitner et al., 2003; Godden et al., 2017). Moreover, in recent studies, MLD has been suggested as a screening option for IMI in selective dry cow therapy and fresh cow assessment (Godden et al., 2017; Gonçalves et al., 2017). According to a recent study (Godden et al., 2017), costs for the MLD test are approximately $18,000 for the reader, $5.00 per cow for the cassette, in addition to the cost of labor for the sampling and the analysis procedures.
The proportions of leukocytes in milk have been shown to change depending on the degree of IMI, suggesting that different frequencies of white cell types may be an indication of the diverse stages of progression of infection (Sarikaya et al., 2006). In addition, it is plausible to speculate that these fluctuations in cell proportions in milk from inflamed mammary glands may exhibit characteristic patterns, depending on the presence of specific groups of pathogens.
Microbiological culture of milk is the accepted gold standard for determining IMI status in both, clinical or SCM. In addition, this information is of significant value when selecting for the appropriate treatment options. However, the cost and time requirements associated with milk culture, together with the risk for contamination and mishandling of samples, limit this approach as a routine diagnostic procedure in commercial farms (Godden et al., 2017). Consequently, a rapid, cow-side test to determine IMI, providing an indication of the potential groups of pathogens involved, would be a beneficial tool in the control of mastitis.
As the application of the differential cell count in milk develops, more details on this parameter behavior under different physiological or pathological stages of the cow are required. The study hypothesis was that specific white cell proportions in milk will deviate depending upon multiple cows and quarter-level variables, including the presence of IMI and the infectious pathogens. Therefore, the study objective was to analyze the association between milk leukocyte proportions provided by a commercial automated MLD test and multiple cows and quarter-level variables, including SCC, parity number, stage of lactation, level of milk production, and presence of mastitis-causing pathogens.
MATERIALS AND METHODS
Study Population
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Colorado State University (protocol ID: 16-6775A).
The research was conducted in a commercial dairy herd in West Texas (Plainview, TX). Cows were housed in a freestall barn and milked three times daily in a rotary milking parlor (Afimilk, Kibbutz Afikim, Israel). Cows were not vaccinated against any mastitis pathogen. A total of 104 clinically healthy Holstein cows were randomly selected, among which 32 animals were primiparous, and 72 were multiparous cows (26, 20, and 26 cows in 2nd, 3rd, and ≥3rd lactation).
MLD Test and Bacteriological Cultures
Milk samples obtained from 416 quarters were evaluated for MLD using Qscout (Advanced Animal Diagnostics, Durham, NC). Milk was collected following standard aseptic procedures (National Mastitis Council, 1999) that included teat-end disinfection with alcohol and disposal of the first streams of milk. The device reader has programmable threshold levels on a scale of 1 to 18 that may be selected by the user, and different thresholds would result in higher sensitivity or higher specificity (Godden et al., 2017; Gonçalves et al., 2017). The reported sensitivity and specificity for this technology at threshold level 7, using microbiological culture as a gold standard, were 65.4% and 79.3%, respectively (Gonçalves et al., 2017).
Milk from each quarter was applied into a plastic container connected to one of four quadrants of a single-use cassette. The cassette was immediately loaded into the automated reading device, using the research mode (threshold level 6) that required about 15 min per cassette and offered increased accuracy for the differentials. The device uses a fluorescence microscopy imaging system taking images to identify and count lymphocytes (LYM), neutrophils (NEU), and macrophages (MAC; Godden et al., 2017; Gonçalves et al., 2017). Results are provided as total leukocyte counts (TLC) and as absolute values for each cell type (NEU, LYM, and MAC). In addition, the percentages of NEU, LYM, and MAC for each sample were directly obtained from the device for statistical analyses. Finally, absolute cell counts from MLD were used for determination of NEU:LYM, NEU:MAC, and phagocyte (PHAG):LYM (indicated by NEU + MAC) ratios.
Right before collection for MLD, a composite milk sample from the four quarters was collected from each study cow in a sterile tube using aseptic technique (National Mastitis Council, 1999). Briefly, the teats were cleaned and disinfected using 70% alcohol in a cotton gauze. The first few streams of milk were discarded, and 10 mL of foremilk was aseptically collected in a sterile tube as a composite sample from each of the four quarters. Samples were immediately frozen before lab submission for bacteriological culture.
All milk samples were submitted to and received at The Dairy Authority, LLC (Greely, CO). Approximately, 0.01 mL of each milk sample was inoculated using a disposable inoculation loop (Hardy Diagnostics, Santa Maria, CA) onto blood agar plates containing 4% washed bovine blood (Quad Five, Ryegate, MT) and 0.1% esculin (Sigma–Aldrich, St. Louis, MO) and MacConkey agar plates (Oxoid, ThermoFisher Scientific, Waltham, MA) and incubated aerobically at 37 °C. Bacterial growth was identified after 24 and 48 h of incubation according to National Mastitis Council standards. Briefly, Staphylococcus aureus and Staphylococcus spp. were identified by hemolytic pattern and tube coagulase test. Streptococcus spp. was identified by a catalase test (Hydrogen Peroxide) and gram stain (JorVet gram stain kit, Sigma–Aldrich). Escherichia coli and Klebsiella spp. were identified using morphologic characteristics of colonies on MacConkey agar, production of indole, motility, and utilization of citrate. Approximately, 0.1 mL of each milk sample was also inoculated onto a Modified Eaton’s Mycoplasma Agar (The Dairy Authority Labs) with a polyester swab (Hardy Diagnostics). Mycoplasma agar plates were incubated in an 8–10% CO2 incubator at 37 °C for 10 d. Mycoplasma agar plates were examined under a dissecting microscope at 3, 7, and 10 d.
Cow and Quarter-level Variable Categorization
Eight categories were created for quarter TLC (≤100,000; 101,000–200,000; 201,000–400,000; 401,000–700,000; 701,000–1,000,000; 1,001,000–3,000,000; 3,001,000–10,000,000; and >10,000,000 cells/mL). Results from bacterial culture (cow-level composite milk sample) were classified as gram-negative (GN) pathogens (Acinetobacter spp., E. coli, Pasteurella spp., and Pseudomonas spp.); gram-positive (GP; CN Staphylococcus, Corynebacterium spp., S. aureus, Streptococcus spp., and Trueperella pyogenes); other (OTH; Mycoplasma spp. and Prototheca spp.); and culture negative (NOG). Other variables at the cow level included parity number (1; 2; 3; >3) and stage of lactation (early [<50 DIM], middle [50–250 DIM], and late [>250 DIM]). Individual milk yield provided by in-line milk meters (Afimilk, Kibbutz Afikim, Israel) at the time of sampling was available. The average (SD) milk yield per day for the study cows was 32.6 (10.9) kg, with a range from 6.7 to 52.1 kg. Milk yield at the day of sampling was categorized into quartiles (Q1 <20 kg; Q2–Q3 = 20.1–29.0 kg; and Q4 >29.0 kg). To evaluate the effect of quarter position, quarters were grouped into front and rear quarters and were subsequently categorized as healthy if TLC ≤100,000 and as affected if TLC >100,000.
Statistical Analyses
Cow and quarter-level data were entered into spreadsheets (Excel 2016, Microsoft, Redmond, WA) and analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Data were checked for normal distribution, and subsequently, MLD values were arcsine transformed, while TLC data were reciprocally transformed, where they did not show a normal distribution. After completion of the analyses, the results were back-transformed to be reported in the original scales.
Pathogen data originated from bacteriological cultures from composite samples (cow-level data). Therefore, in cows with positive cultures, only the quarter with the greatest TLC was considered for the analyses testing associations between MLD and mastitis-causing pathogen groups. For cows with negative cultures, the average for the four quarters was considered. Statistical models were tested using PROC MIXED in SAS and included pathogen category, parity number, stage of lactation, milk-yield category at the time of sampling, and quarter position.
In general, depending on the analysis, the models were defined as follows:
Where:
Yijklm = dependent variable (TLC, quarter-level MLD, or leukocyte ratios)
µ = overall population mean
Pathi = effect of pathogen category (NOG, GN, GP, or OTH)
Parj = effect of parity number (1, 2, 3, or >3)
Stk = effect of stage of lactation (early, mid, or late)
Mlkl = effect of milk yield (low, medium, or high)
Qpm = effect of quarter position (front or rear)
eijklm = error term
For all the analyses, statistical significance was defined at P value <0.05.
RESULTS
Overall, milk leukocyte proportions from 411 quarters were available. Samples from five quarters did not return MLD results from Qscout and were removed from the analysis. Composite milk samples from 104 cows were submitted for bacteriologic testing. Cultures indicated no growth (NOG) for 44 samples, and 5 samples registered multiple pathogens growth (>2 different bacteria) and were considered contaminated and removed from the subsequent analyses. Main categories of reported pathogens were Coagulase Negative S. aureus (n = 30), Streptococcus spp. (8), Corynebacterium spp. (5), Mycoplasma spp. (4), E. coli (2), S. aureus (2), T. pyogenes (2), Prototheca spp. (1), and Pasteurella spp. (1).
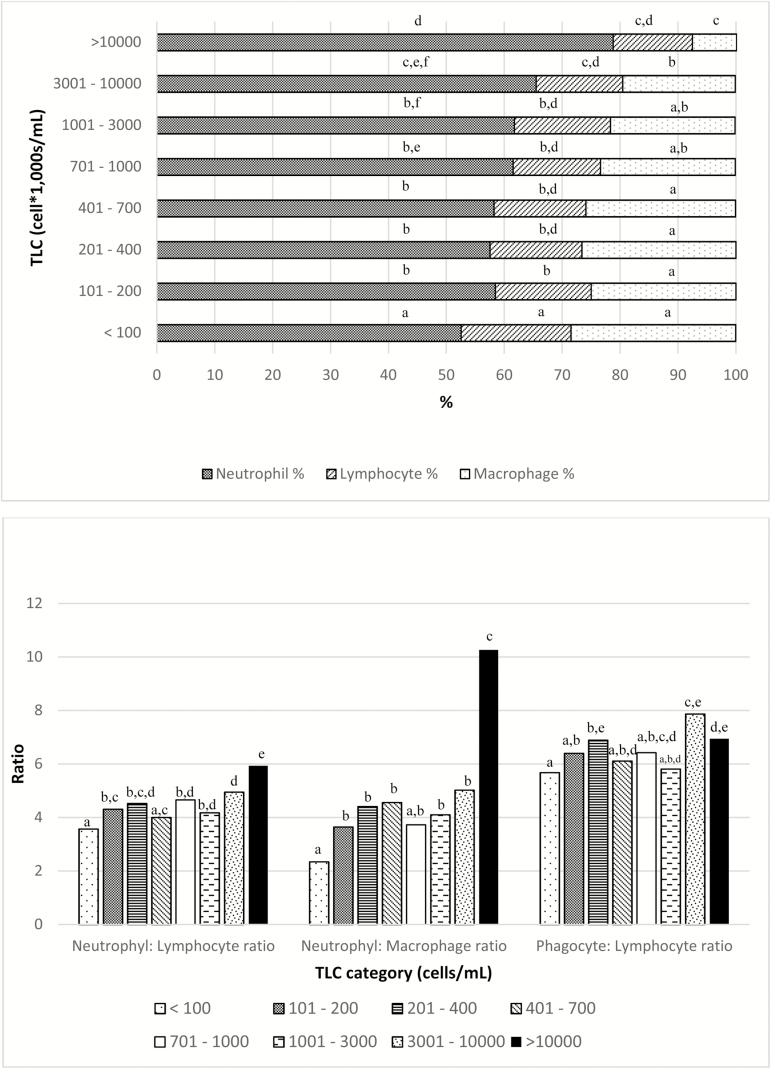
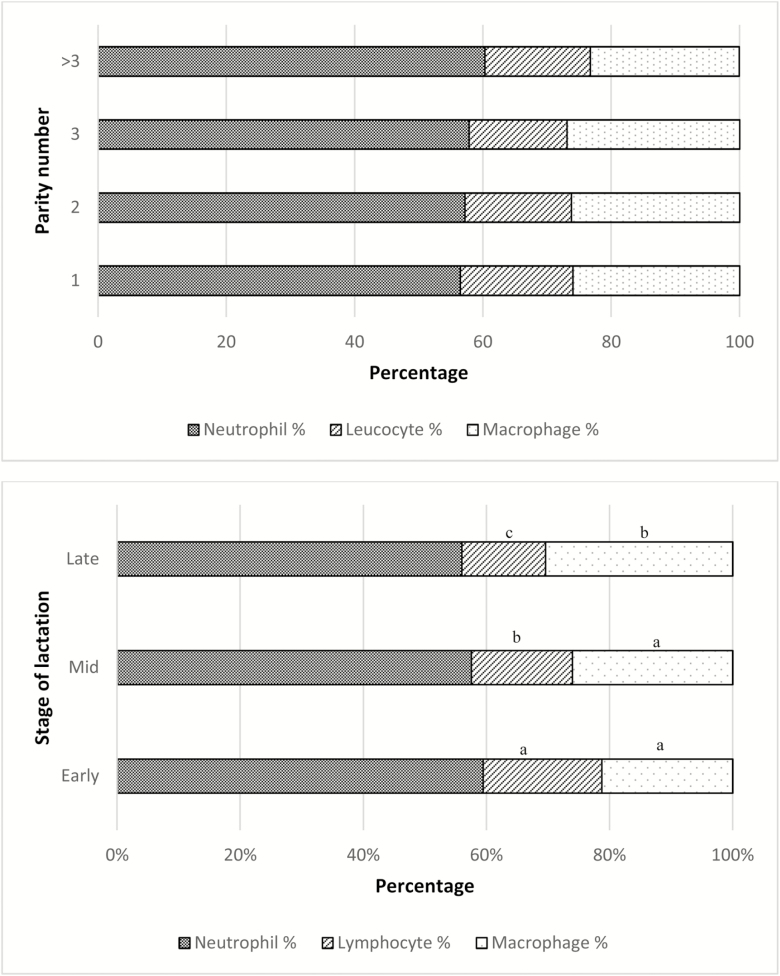
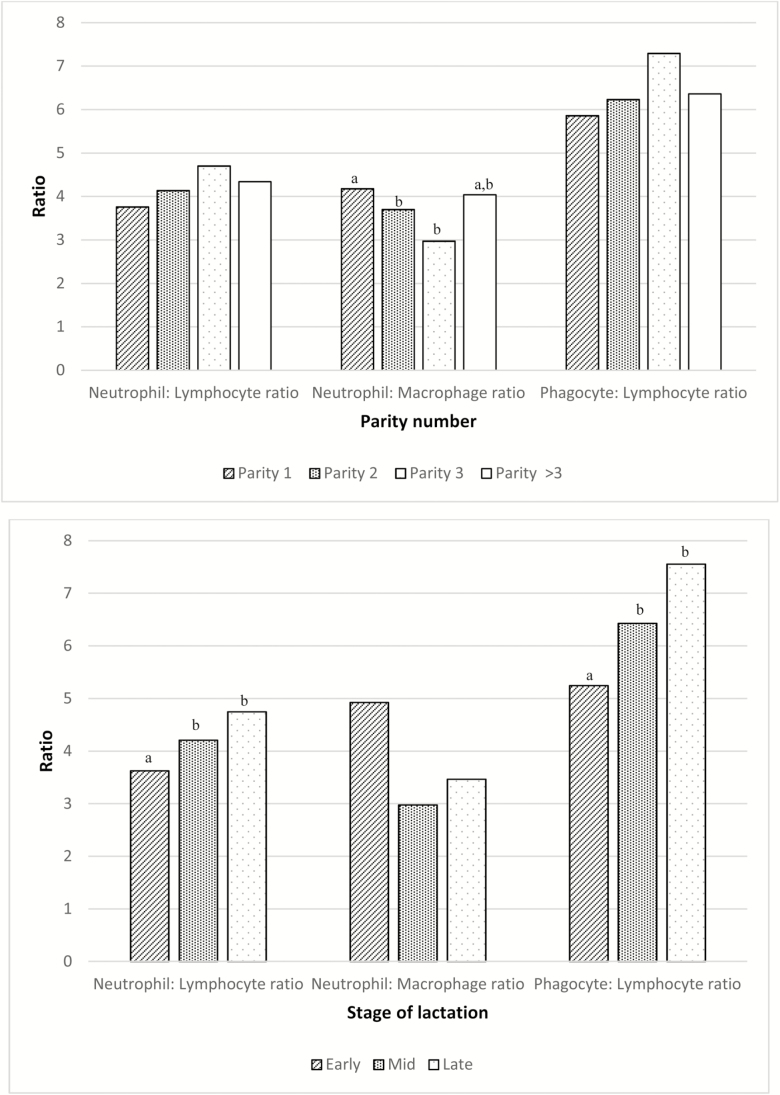
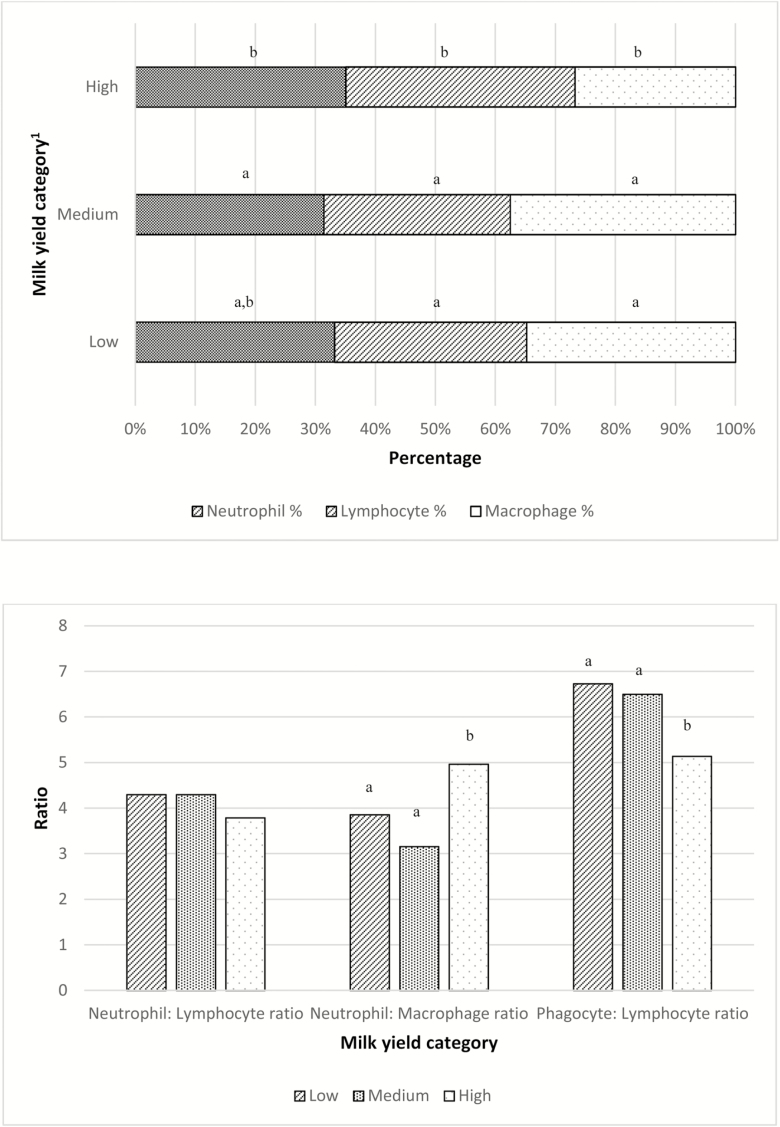
Mean (median) for quarter TLC was 1,553,000 (209,000) cells/mL. Milk leukocyte proportions varied depending on the category of TLC (P < 0.001; Figure 1). NEU% consistently increased as categories of TLC augmented (P < 0.001). This response was opposite for MAC%, which were larger for small TLC categories (P < 0.001). LYM% also varied by category of TLC (P < 0.0001) but in a smaller magnitude. Similarly, the three presented leukocyte ratios (NEU:LYM, NEU:MAC, and PHAG:LYM) were different depending on the TLC category (P < 0.001, P < 0.001, and P = 0.01, respectively; Figure 1). There was no association between parity number and MLD; however, LYM% were greatest in early lactation (P < 0.001). Contrarily, the greatest value for MAC% was determined in late lactation (P = 0.009; Figure 2). Only the NEU:MAC ratio was associated with parity, with the greatest value in parity 1 (P = 0.01). Conversely, the NEU:LYM ratio and the PHAG:LYM ratio were associated with time after calving and were smallest during early lactation (P < 0.001 and P < 0.001, respectively; Figure 3). Leukocyte proportions and ratios were both associated with milk-yield category (P < 0.05). NEU% was greater in the high milk-yield category compared with the medium-yield category (P = 0.01). LYM% was greater in the high milk-yield level than in both the medium- and the low-yield categories (P < 0.005). Finally, MAC% was greater in the low and medium categories (P < 0.001), and the NEU:MAC and the PHAG:LYM ratios were both greater in the low and medium milk-yield categories than in the high-yield category (P = 0.002 and P = 0.02, respectively; Figure 4)
Figure 1.
Milk leukocyte proportions (a) and milk leukocyte ratios (b) by category of TLC. Categories within same group (leukocyte type or leukocyte ratio) with different letters indicate statistically significant difference at P < 0.05.
Figure 2.
Milk leukocyte proportions by (a) parity number (1; 2; 3; >3) and (b) stage of lactation (early [<50 DIM], middle [50–250 DIM], and late [>250 DIM]). Categories within same leukocyte type with different letters indicate statistically significant difference at P < 0.05.
Figure 3.
Milk leukocyte ratios by (a) parity number (1; 2; 3; >3) and (b) stage of lactation (early [<50 DIM], middle [50–250 DIM], and late [>250 DIM]). Categories within same leukocyte ratio with different letters indicate statistically significant difference at P < 0.05.
Figure 4.
Milk leukocyte proportions (a) and milk leukocyte ratios (b) by milk-yield category. Categories within same group (leukocyte type or leukocyte ratio) with different letters indicate statistically significant differences at P < 0.05. 1Milk-yield categories: Low = <20 kg, medium = 20.1–29.0 kg, and High = >29.0 kg).
No associations between quarter position and NEU, LYM, and MAC% were determined in healthy quarters (TLC ≤ 100,000 cells/mL). Similarly, no associations were found for the NEU:LYM and the PHAG:LYM ratios. Interestingly, the PHAG:LYM ratio was greater in rear quarters than in front quarters (P = 0.01; Table 1). In high TLC quarters, only NEU (%) was greater in the rear quarters than in front quarters (P = 0.03).
Table 1.
Milk leukocyte proportions (mean ± SE) and milk leukocyte ratios by quarter position (front vs. rear) in healthy (TLC ≤ 100,000) and affected quarters (n = 411 quarters)
| Parameter | Front quarters | Rear quarters | P value |
|---|---|---|---|
| TLC ≤100,000 (n = 151) | |||
| NEU (%) | 51.4 ± 0.02 | 52.5 ± 0.02 | 0.50 |
| LYM (%) | 17.4 ± 0.02 | 19.1 ± 0.02 | 0.21 |
| MAC (%) | 30.1 ± 0.04 | 26.7 ± 0.03 | 0.11 |
| NEU:LYM ratio | 3.47 ± 0.28 | 3.48 ± 0.27 | 0.99 |
| NEU:MAC ratio | 1.84 ± 0.25 | 2.64 ± 0.24 | 0.01 |
| PHAG:LYM ratio | 5.78 ± 0.48 | 5.58 ± 0.46 | 0.75 |
| TLC >100,000 (n = 260) | |||
| NEU (%) | 58.4 ± 0.017 | 62.4 ± 0.02 | 0.03 |
| LYM (%) | 14.6 ± 0.005 | 14.6 ± 0.01 | 0.99 |
| MAC (%) | 25.0 ± 0.02 | 21.5 ± 0.03 | 0.06 |
| NEU:LYM ratio | 4.50 ± 0.17 | 4.62 ± 0.18 | 0.61 |
| NEU:MAC ratio | 4.31 ± 0.35 | 4.50 ± 0.35 | 0.71 |
| PHAG:LYM ratio | 7.12 ± 0.33 | 6.59 ± 0.34 | 0.26 |
Values for TLC varied by pathogen group (P < 0.001) and were largest in GN, followed by OTH and GP pathogens (Table 2). However, TLC were not different for GP and GN groups.
Table 2.
Least square means (±SE) for total milk leukocyte counts, milk leukocyte proportions, and milk leukocyte ratios by group of mastitis-causing pathogen (n = 99 quarters)
| Parameter | NOG | GN | GP | OTH | Group P value | GP vs. GN |
|---|---|---|---|---|---|---|
| P value | ||||||
| TLC (cells × 1000/mL) | 263 ± 11.2 | 39,174 ± 1,245 | 1,290 ± 110 | 2,585 ± 481 | <0.001 | 0.16 |
| NEU (%) | 55.4 ± 0.05 | 52.7 ± 1.02 | 66.9 ± 0.05 | 69.0 ± 0.42 | 0.001 | 0.16 |
| LYM (%) | 16.5 ± 0.11 | 6.54 ± 2.18 | 13.4 ± 0.01 | 11.6 ± 0.08 | <0.001 | 0.01 |
| MAC (%) | 26.9 ± 0.08 | 35.5 ± 1.85 | 18.3 ± 0.77 | 17.7 ± 0.62 | 0.03 | 0.12 |
| NEU:LYM ratio | 3.68 ± 0.28 | 7.16 ± 1.25 | 5.16 ± 0.28 | 6.08 ± 0.80 | <0.001 | 0.12 |
| NEU:MAC ratio | 3.57 ± 0.66 | 6.86 ± 2.95 | 4.97 ± 0.66 | 7.68 ± 1.88 | 0.11 | 0.53 |
| PHAG:LYM ratio | 5.79 ± 0.59 | 23.6 ± 2.64 | 7.40 ± 0.59 | 7.93 ± 1.68 | <0.001 | <0.001 |
Milk leukocyte proportions also varied according to the pathogen group involved. NEU% were highest in the OTH and GP groups, with the lowest level in GN and NOG (P < 0.001). However, no significant difference was established for GP vs. GN groups. On the other hand, LYM% were reduced in all GN, GP, and OTH pathogens as compared with the NOG group (P < 0.001). Furthermore, LYM% was greater for the GN compared with the GP group (P < 0.01). Finally, MAC% was increased in the GN group of pathogens and decreased in the OTH and the GP groups (P = 0.03), as compared with the NOG group (Table 2).
The smallest NEU:LYM ratio (mean ± SE) was for the NOG group (3.68 ± 0.27), and values increased to 5.16 (±0.28) in the GP group, 6.08 (± 0.78) in the OTH group, and 7.16 (± 1.25) in the GN group (P< 0.001). No difference among pathogen groups was observed for the NEU:MAC ratio. The PHAG:LYM ratio varied by pathogen group with 5.79 (±0.59) in the NO group, 23.5 (±2.64) in the GN group, and 7.93 (±1.68) in the OTH, followed by 7.40 (±0.59) in the GP group (P < 0.001). The ratio was greater in the GN group, compared with the GP group (P = 0.001).
DISCUSSION
We analyzed the association between milk leukocyte proportions provided by a commercial automated MLD test and multiple cow and quarter-level variables. Values for milk leukocyte proportions in normal milk reported in previous studies are widely variable (Koess and Hamann, 2008; Schwarz et al., 2011b). According to some researchers, MAC are the predominant cell type (Leitner et al., 2000; Riollet et al., 2001; Lindmark-Mansson et al., 2006), whereas others have indicated that LYM are the major population (Park et al., 1992; Schwarz et al. 2011a, 2011b).
Mielke and Koblenz (1981) reported that milk from cows with no evidence of clinical mastitis had 37–38% NEU, 13–20% LYM, and 17–20% MAC. In more recent reports, NEU proportions ranged between 6 and 50%, LYM proportions between 14 and 80%, and MAC proportions between 12 and 46% (Rivas et al., 2001; Merle et al., 2007; Koess and Hamann, 2008; Schwarz et al., 2011b). Interestingly, our observed distribution of white cells in milk from healthy quarters was similar to that recently described by Gonçalves et al. (2017). The main cell type for healthy quarters in our study was NEU (51.4%), followed by MAC (30.1%) and LYM (17.4%). In agreement, Gonçalves et al. (2017) reported NEU, MAC, and LYM percentages in milk of culture negative udder quarters of 49.4, 28.9, and 17.7%, respectively.
As reported previously (Leitner et al., 2000; Pillai et al., 2001; Dosogne et al., 2003; Pilla et al., 2013), stage of lactation was a significant factor affecting milk leukocyte proportions in our study. Dosogne et al. (2003) found that in early lactation, the percentage of LYM was greater and the percentages of mature MAC and NEU were lower than in the other stages of lactation. In another study (Pilla et al., 2013), percentages of mature MAC and NEU in early lactation were only about half of the values found in mid and late lactation. These findings have not been studied in detail, but a possible explanation may be that specific pathogens prevail in IMI at different stages of lactation, and each pathogen will recruit different proportions of white cell populations (Leitner et al., 2000). Moreover, parity number was another factor influencing the proportions of white cells in healthy milk.
In healthy quarters, the position of quarters did not affect the MLD, as well as milk white cells ratios; however, there were significant differences in the NEU:MAC ratios when quarters were categorized as front vs. rear. This difference in front and rear quarters may be related to greater volume of milk (about 60 vs. 40%) secreted from the rear quarter than from the front quarter (Tancin et al., 2006). Nevertheless, this difference is not observed in affected quarters, which may be a result of inflammation during mastitis. Furthermore, in the affected quarters, NEU% were higher in rear quarters, which may also be attributed to disproportionate milk production.
With increasing TLC levels, we observed increasing NEU% and decreasing MAC%. This phenomenon illustrates that MLD may be dependent on the severity of the inflammation, as determined here across the various levels of TLC, and thus can be used to indicate a specific level of inflammation in the quarter. However, in the case of leukocyte ratios, the patterns illustrated that the ratios can only be used to differentiate extreme low and high categories. Nonetheless, it should be noted that the accuracy of cell differentiation may be affected when TLC are greater and cell overlapping results on conflicting image interpretation.
The association of MLD and leukocyte ratios for milk-yield categories, as observed in this study, suggests an effect for different milk-yield categories. The MLD distribution in high-yield category is different from medium- and low-yield categories. Thus, any mastitis detection algorithm based on MLD should consider milk yield in the model to best identify the alterations of MLD related to infections.
As reviewed by Schwarz et al. (2011b), LYM, MAC, and NEU play specific roles in inflammatory responses within the mammary gland. LYM regulate immune responses recognizing specific antigens through membrane receptors. MAC are active-phagocytic cells, ingesting bacteria, and cellular debris. In addition, the release of chemoattractants from MAC induces the recruitment of NEU that will act against bacteria at the beginning of an acute inflammatory process. In consequence, the distribution of leukocyte numbers is important for the success of intramammary defenses against invading pathogens (Leitner et al., 2003), making plausible the idea that cell proportions would change depending on the type of pathogen and the severity and chronicity of infection. In fact, different cell patterns have been documented in the presence of different pathogens and during the course of infection. In acute mastitis, NEU are the predominant cell type, whereas in chronic infections, the MAC cell type is more prevalent (Leitner et al., 2003).
Our results evidenced significant differences in white cell proportions between specific pathogen groups, but the magnitude of the changes was small. In agreement with our findings, Damm et al. (2017) found increased proportions of NEU and reduced proportions of MAC in quarters with elevated SCC. However, LYM remained fairly constant, which was termed as antidromic trend of NEU and MAC. In a report by Schwarz et al. (2011b), the proportion of LYM decreased from >60% at SCC values <10,000 cells/mL to 18.7% when SCC was 1,394,000 cells/mL. Conversely, NEU increased from <30% within the SCC range of <10,000 cells/mL to 63.65% at SCC of 1,394,000 cells/mL.
Results from our study are similar to those observed by Gonçalves et al. (2017), who also reported higher MAC% in healthy quarters than in quarters infected by any pathogen. The proportional decrease of MAC% with increases in NEU% was evident in both studies. More in detail, Gonçalves et al. (2017) indicated that the NEU% were greater in specific-SCM (culture positive) cases (65.7%) than in nonspecific-SCM (culture negative) cases (55.2%), latent-SCM (cases with culture positive but low TLC; 55.0%), and healthy quarters (49.4%). The MAC% were lesser in quarters with specific-SCM (12.3%), nonspecific-SCM (17.3%), and latent-SCM TLC (23.0%), when compared with healthy quarters (28.9%). The LYM and PHAG% were similar among tested groups, but mammary quarters with specific-SCM, nonspecific-SCM, and latent-SCM had greater mean value of absolute number of LYM and PHAG than healthy quarters.
An interesting finding in our study is that the change in relative proportions of leukocytes partially depended on the mastitis pathogen group isolated from milk. In a study presented by Emanuelson et al. (1989), a different classification system considering minor and major pathogen groups resulted on differential SCC classifying 96 and 38% of infections due to minor and major pathogens correctly. However, in our study, when milk with GP and GN isolates were compared, LYM were the only population that indicated significant differences. This inability for differentiating GP from GN infections, probably due to a smaller sample size, limits the practical use of this analysis, as one the most relevant applications would be to direct the therapeutic approach for these groups of infections.
All the presented cell type ratios were moderately elevated in affected quarters when compared with those from healthy cows. In the three ratios, affected quarters showing NOG had slight increase while the greatest changes occurred in the GN group. Our results are in partial agreement with those from Gonçalves et al. (2017) where the cell ratio Log10 (NEU:LYM) was significantly higher in quarters infected by miscellaneous (0.62), contagious (0.57), environmental (0.53), and minor pathogens (0.52) than in healthy contralateral quarters (0.47). On the other hand, there was no difference in the cell ratio Log10 (PHAG:LYM) between healthy quarters (0.67) and quarters infected with miscellaneous (0.69), contagious (0.67), environmental (0.66), and minor pathogens (0.65).
Pathogens included in the GN group would normally produce acute inflammation (Wenz et al., 2001), thus resulting in high NEU percentages and very high NEU:LYM and PHAG:LYM ratios. However, in our study, low percentages of LYM can be attributed to this scenario and may be regarded as characteristic feature of this type of pathogen. On the other hand, the GP group had higher proportions of NEU and low MAC values, compared with the healthy quarters. Pathogens in the OTH group normally result in chronic infections, and therefore, moderate higher levels of all the leukocyte are expected (Oviedo-Boyso et al., 2007). This peculiar behavior in GN, OTH, and GP pathogens may be a useful tool for orientating toward specific pathogen groups.
It is important to notice that we had a significant proportion of culture negative samples in our study. Shedding of too low numbers of pathogens or ceased growth may be reasons for negative bacteriological results. Although epithelial cells are also detected from fluorescence microscopy, their immunological function is not well established; therefore, we did not consider them for analysis. The number of cell fractions sampled also depends on milk sampled because cisternal milk shows a reduced NEU counts, compared with alveolar milk (Pilla et al., 2012). We used squirts of milk after discarding first few streams of the milk from the teats, and this could have altered our results.
Another limitation of the study was the use of pooled milk samples for microbiological culture. This is a cost-effective method that does not allow for quarter-level discrimination. To partially address this problem, we considered using the MLD values corresponding to greatest TLC quarter for culture positive animals and an average MLD of all four quarters for the culture negative animals. Although the procedure reduced the number of quarters used for the analyses, we are more confident about the values for each category.
CONCLUSION
Our results demonstrated significant differences in white cell proportions in different physiological and pathological stages of the lactating dairy cow, although the magnitudes of the differences were small. Altered MLD in multiple categories of TLC suggests its value in detecting mastitis of varying severity. Differentiable patterns in the changes in cell proportions and leukocyte ratios observed in the presence of specific pathogen groups may provide useful information for the identification of specific causal agent groups in mastitis cases. Additional research is necessary to determine the potential applications for this methodology.
LITERATURE CITED
- Damm M., Holm C., Blaabjerg M., Bro M. N., and Schwarz D.. 2017. Differential somatic cell count—a novel method for routine mastitis screening in the frame of dairy herd improvement testing programs. J. Dairy Sci. 100:4926–4940. doi: 10.3168/jds.2016-12409 [DOI] [PubMed] [Google Scholar]
- Dohoo I. R., Meek A. H., Martin S. W., and Barnum D. A.. 1981. Use of total and differential somatic cell counts from composite milk samples to detect mastitis in individual cows. Can. J. Comp. Med. 45:8–14. [PMC free article] [PubMed] [Google Scholar]
- Dosogne H., Vangroenweghe F., Mehrzad J., Massart-Leën A. M., and Burvenich C.. 2003. Differential leukocyte count method for bovine low somatic cell count milk. J. Dairy Sci. 86:828–834. doi: 10.3168/jds.S0022-0302(03)73665-0 [DOI] [PubMed] [Google Scholar]
- Emanuelson U., and Wever P.. 1989. Potential of differential somatic cell counts as indicators of mastitis in quarter milk samples from dairy cows. Acta Vet. Scand. 30:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden S. M., Royster E., Timmerman J., Rapnicki P., and Green H.. 2017. Evaluation of an automated milk leukocyte differential test and the California mastitis test for detecting intramammary infection in early- and late-lactation quarters and cows. J. Dairy Sci. 100:6527–6544. doi: 10.3168/jds.2017-12548 [DOI] [PubMed] [Google Scholar]
- Gonçalves J. L., Lyman R. L., Hockett M., Rodriguez R., Dos Santos M. V., and Anderson K. L.. 2017. Using milk leukocyte differentials for diagnosis of subclinical bovine mastitis. J. Dairy Res. 84:496–497. doi: 10.1017/S0022029917000577 [DOI] [PubMed] [Google Scholar]
- Koess C., and Hamann J.. 2008. Detection of mastitis in the bovine mammary gland by flow cytometry at early stages. J. Dairy Res. 75:225–232. doi: 10.1017/S0022029908003245 [DOI] [PubMed] [Google Scholar]
- Leitner G., Eligulashvily R., Krifucks O., Perl S., and Saran A.. 2003. Immune cell differentiation in mammary gland tissues and milk of cows chronically infected with Staphylococcus aureus. J. Vet. Med. B. Infect. Dis. Vet. Public Health 50:45–52. doi: 10.1046/j.1439-0450.2003.00602.x [DOI] [PubMed] [Google Scholar]
- Leitner G., Shoshani E., Krifucks O., Chaffer M., and Saran A.. 2000. Milk leucocyte population patterns in bovine udder infection of different aetiology. J. Vet. Med. B. Infect. Dis. Vet. Public Health 47:581–589. doi: 10.1046/j.1439-0450.2000.00388.x [DOI] [PubMed] [Google Scholar]
- Lindmark-Mansson H., Branning C., Alden G., and Paulsson M.. 2006. Relationship between somatic cell count, individual leukocyte populations and milk components in bovine udder quarter milk. Int. Dairy J. 16:717–727. doi: 10.1016/j.idairyj.2005.07.003 [DOI] [Google Scholar]
- Merle R., Schröder A., and Hamann J.. 2007. Cell function in the bovine mammary gland: a preliminary study on interdependence of healthy and infected udder quarters. J. Dairy Res. 74:174–179. doi: 10.1017/S002202990600238X [DOI] [PubMed] [Google Scholar]
- Mielke H., and Koblenz C.. 1981. Origin and behavior of macrophages in the milk of cows with healthy and pathological udders. Arch. Exp. Veterinarmed. 35:1–18. [PubMed] [Google Scholar]
- National Mastitis Council 1999. Laboratory handbook on bovine mastitis. Madison (WI): National Mastitis Council; p. 1–30. [Google Scholar]
- Oviedo-Boyso J., Valdez-Alarcón J. J., Cajero-Juárez M., Ochoa-Zarzosa A., López-Meza J. E., Bravo-Patiño A., and Baizabal-Aguirre V. M.. 2007. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infect. 54:399–409. doi: 10.1016/j.jinf.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Park Y. H., Fox L. K., Hamilton M. J., and Davis W. C.. 1992. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J. Dairy Sci. 75:998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4 [DOI] [PubMed] [Google Scholar]
- Pillai S. R., Kunze E., Sordillo L. M., and Jayarao B. M.. 2001. Application of differential inflammatory cell count as a tool to monitor udder health. J. Dairy Sci. 84:1413–1420. doi: 10.3168/jds.S0022-0302(01)70173-7 [DOI] [PubMed] [Google Scholar]
- Pilla R., Malvisi M., Snel G. G., Schwarz D., König S., Czerny C. P., and Piccinini R.. 2013. Differential cell count as an alternative method to diagnose dairy cow mastitis. J. Dairy Sci. 96:1653–1660. doi: 10.3168/jds.2012-6298 [DOI] [PubMed] [Google Scholar]
- Pilla R., Schwarz D., König S., and Piccinini R.. 2012. Microscopic differential cell counting to identify inflammatory reactions in dairy cow quarter milk samples. J. Dairy Sci. 95:4410–4420. doi: 10.3168/jds.2012-5331 [DOI] [PubMed] [Google Scholar]
- Riollet C., Rainard P., and Poutrel B.. 2001. Cell subpopulations and cytokine expression in cow milk in response to chronic Staphylococcus aureus infection. J. Dairy Sci. 84:1077–1084. doi: 10.3168/jds.S0022-0302(01)74568-7 [DOI] [PubMed] [Google Scholar]
- Rivas A. L., Quimby F. W., Blue J., and Coksaygan O.. 2001. Longitudinal evaluation of bovine mammary gland health status by somatic cell counting, flow cytometry, and cytology. J. Vet. Diagn. Invest. 13:399–407. doi: 10.1177/104063870101300506 [DOI] [PubMed] [Google Scholar]
- Sarikaya H., Schlamberger G., Meyer H. H. D., and Bruckmaier R. M.. 2006. Leukocyte populations and mRNA expression of inflammatory factors in quarter milk fractions at different somatic cell score levels in dairy cows. J. Dairy Sci. 89:2479–2486. doi: 10.3168/jds.s0022-0302(06)72322-0 [DOI] [PubMed] [Google Scholar]
- Schwarz D., Diesterbeck U. S., König S., Brügemann K., Schlez K., Zschöck M., Wolter W., and Czerny C. P.. 2011a. Microscopic differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J. Dairy Res. 78:448–455. doi: 10.1017/S0022029911000574 [DOI] [PubMed] [Google Scholar]
- Schwarz D., Diesterbeck U. S., König S., Brügemann K., Schlez K., Zschöck M., Wolter W., and Czerny C. P.. 2011b. Flow cytometric differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J. Dairy Sci. 94:5033–5044. doi: 10.3168/jds.2011-4348 [DOI] [PubMed] [Google Scholar]
- Tancin V., Ipema B., Hogewerf P., and Macuhova J.. 2006. Sources of variation in milk flow characteristics at udder and quarter levels. J. Dairy Sci. 89:978–988. doi: 10.3168/jds.S0022-0302(06)72163-4 [DOI] [PubMed] [Google Scholar]
- Wenz J. R., Barrington G. M., Garry F. B., McSweeney K. D., Dinsmore R. P., Goodell G., and Callan R. J.. 2001. Bacteremia associated with naturally occurring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. 219:976–981. doi: 10.1292/jvms.13-0610 [DOI] [PubMed] [Google Scholar]