Abstract
Two experiments were conducted to evaluate the use of supplemental betaine in commercially available semen extenders. In experiment 1 (Exp1), semen was collected from six mature boars once weekly for 6 wk (3 wk in summer and 3 wk in winter) and diluted into a commercial extender with the following betaine concentrations: 0, 51, 102, and 205 mM. Semen samples were analyzed on the day of collection (D0) and after 72 h of storage (D3). In experiment 2 (Exp2), semen was collected from four mature boars for 3 wk and was diluted into three commercially available semen extenders (short term, ST; long term with bovine serum albumin, BSA; and long term without BSA, LT), with and without supplemental betaine (0 and 70 mM), and analyzed on D0 and D3. Semen was analyzed using computer-assisted sperm assessment (Ceros II, IMV) and morphology using phase contrast microscopy. In Exp1, total motility on D0 was less for 0 mM than that for 102 mM (P = 0.038) and was substantially reduced for 205 mM compared with 102 mM (P < 0.001). Supplementation with 205 mM betaine resulted in a significant reduction in the percentage of morphologically normal sperm (P < 0.001). In Exp2, 70 mM betaine reduced the total motility compared with 0 mM (P = 0.010) but did not impact percentage of normal sperm (P = 0.942). The use of supplemental betaine may partially alleviate the dilution effect on sperm, though boar genetics may impact its efficacy. Further research is needed to make a definitive conclusion.
Keywords: betaine, boar, semen extender, sperm motility, swine
INTRODUCTION
Greater than 90% of the swine industry utilizes artificial insemination for breeding sows. To accomplish this, semen is collected from boars, diluted into a commercially available semen extender, chilled to 17 °C, and transported to the sow farms from the boar studs, all of which is stressful to the sperm (Johnson et al., 2000). This stressful event is known as the “dilution effect,” which results in an initial increase in sperm motility, followed by a sustained decrease in motility (Johnson et al., 2000). At the sow farm, the liquid semen must be stored at 17 °C until it is used for insemination of the sow. Over time in storage, quality of the semen will diminish resulting in reductions in fertilizing abilities of the sperm cells. Various commercial semen extenders are available and are classified as short term (storage for 3 to 5 d) or long term (storage 5 to 10 d) based on manufacturer’s recommendations for how long the semen can be stored prior to insemination. Improving the storability of liquid semen in both short- and long-term extenders would be valuable to the swine industry.
Betaine is a natural methylamine in the methionine cycle in mammals, which acts as a methyl donor to convert the harmful homocysteine into methionine (Kidd et al., 1997). It has also been shown to act as an osmolyte or osmoprotectant in many plant and bacteria species by preserving cell volume via fluid balance during times of osmotic stress (Hayashi et al., 1997; Record et al., 1998; Xing and Rajashekar, 2001). Betaine supplemented to diluents has been investigated in many studies evaluating its impact on semen quality for cooled and frozen semen in a variety species (Sanchez-Partida et al., 1992; Pena et al., 1998; Trimeche et al., 1999; Zhang et al., 2001). Most studies investigating betaine in semen diluents have yielded varying results and have focused on the cryoprotective characteristics of betaine and its potential role to improve sperm motility in frozen-thawed semen for artificial insemination. However, little research has been conducted on the use of betaine in fresh, extended semen that undergoes a dilution stress. Therefore, the objectives of our studies were to investigate the use of betaine supplemented in commercially available extenders and to determine its impacts on semen quality and storability.
MATERIALS AND METHODS
Experimental Design
The Purdue Animal Care and Use Committee approved all procedures performed prior to conducting this trial. Semen was collected from intact male pigs one time per week for a minimum of 6 wk prior to initiation of the study and continued through the entire length of the experiment. Semen collection was performed using the double-gloved hand technique. In experiment 1 (Exp1), six mature, terminal crossbred boars (415.2 ± 1.0 d of age; mean ± SD) were utilized in a multifactorial study design, which was repeated in two seasons (summer and winter; 3 wk per season) with the same boars used in both seasons. Collected semen from each boar was aliquoted, diluted into a commercially available semen extender containing four concentrations of supplemental betaine (TRT; 0 mM, B0; 51 mM, B51; 102 mM, B102; and 205 mM, B205), and analyzed twice, on the day of collection (D0) and after 72 h of storage at 17 °C (day 3, D3). These concentrations of betaine were chosen based on previous work with betaine used in semen diluents in other species (Renard et al., 1996; Pena et al., 1998; Sanchez-Partida et al., 1999; Trimeche et al., 1999; Zhang, et al., 2001), as well as preliminary studies in semen diluents for fresh boar ejaculates (data not shown). A regression analysis was performed on the motility results of the summer replicate of Exp1, resulting in an optimal supplemental betaine concentration of 70 mM (data not shown), which was then used in experiment 2 (Exp2). Exp2 used four mature, maternal crossbred boars (421.5 ± 1.9 d of age; mean ± SD) in a factorial study design, repeated over 3 wk in the winter season after completion of Exp1. Semen was collected once weekly, aliquoted, diluted into three commercially available semen extenders (EXT: short-term, ST; long-term containing bovine serum albumin, BSA; or long-term not containing bovine serum albumin, LT), and were supplemented with two concentrations of betaine (BET; 0 and 70 mM). This resulted in six treatment groups for each ejaculate from each of the four boars (STO, ST70, BSA0, BSA70, LT0, and LT70). Treatment extenders were allowed to incubate for 30 min at 37 °C, followed by analysis for pH using a portable pH meter (EL2; Mettler Toledo, Columbus, OH), and for refractive index using a digital refractometer (PA203; Misco Palm Abbe, Solon, OH). The refractive index was used as a measure of solute concentration in the extender and has been previously correlated to osmolality (Schulze et al., 2015). Diluted semen was analyzed twice (D0 and D3) for semen quality parameters, similarly as in Exp1.
Semen Analysis
Semen was analyzed at collection prior to dilution into treatment extenders for sperm concentration using a spectrophotometer (SpermaQ-II, MOFA Global, Verona, WI), semen volume using a gram scale, and relative motility (subjective evaluation by bright-field microscopy), and diluted to a final concentration of 37.5 × 106 sperm/mL into each treatment extender. Relative motility was used only to confirm that the ejaculates were motile prior to dilution and was not statistically analyzed in either study. Semen volume and concentration were measured to dilute the samples to the appropriate final concentration and were not analyzed statistically. Diluted semen was allowed to cool to 17 °C for approximately 1 h and was then transported to a university laboratory 11.4 miles away. Samples were further analyzed for mobility and motility parameter estimates by computer-assisted sperm assessment (CASA; Ceros II; IMV Technologies, Maple Grove, MN) and for morphological assessment using phase contrast microscopy. Prior to CASA evaluation and morphological assessment, semen samples were warmed to 37 °C for 30 min. After CASA, 10 µL of formalin was added to 1 mL of the samples to preserve the samples for morphological assessment. Morphological assessment consisted of counting a minimum of 200 sperm cells, which were categorized as normal, or containing a proximal droplet, distal droplet, distal mid-piece retroflection (DMR), or tail abnormality.
Statistical Analysis
All statistical analyses were performed using the mixed procedure of SAS (version 9.4; SAS Institute Inc., Cary, NC). All parameters measured were analyzed using repeated measures with a compound symmetry covariance structure to minimize Akaike Information Criterion. Tukey-Kramer adjustments were used for least square (LS) means separation tests for all parameters when appropriate. Statistical significance was defined as P ≤ 0.05.
For Exp1’s pH and refractive index measures, TRT and season were main effects with their interactions. For sperm quality parameters, TRT, season, and day of analysis were used as main effects, with their appropriate interactions. Extender pH prior to diluting semen was used as a covariate for all semen quality parameters. For morphology assessment and CASA parameters, laboratory technician was used as a random effect. Quadratic regression analyses were performed for total motility and progressive motility after the summer season (to determine the concentration used for Exp2) and again at the end of the study. A regression was performed for the overall effects and by day of analysis for total motility and progressive motility, with TRT and the intercept as random effects in an unstructured covariance structure.
For Exp2’s pH and osmolality measures, BET and EXT were used as main effects with their interactions. For morphology and CASA parameters, BET, EXT, and day of analysis were used as main effects with their appropriate interactions. Laboratory technician was used as a random effect for morphology and CASA parameters. Extender pH prior to dilution of the ejaculate was used as a covariate for all semen quality parameters.
RESULTS AND DISCUSSION
Extender Osmolality and pH Analysis
Refractive index and pH data for Exp1 and Exp2 are summarized in Tables 1 and 2, respectively. In Exp1, a treatment by season interaction for pH was observed (P = 0.009). There were slight differences in pH in the different treatment season combinations; however, the accuracy of the thermometer used was 0.1 ℃, and all treatment by season combinations had a pH of 6.7 ± 0.01, except for B205 in the summer which had a pH of 6.8 ± 0.01 and was significantly higher than B0 in the winter, B51 in the winter, and B205 in the winter (P ≤ 0.001). Although pH values differed statistically, the overall effect is likely biologically insignificant with a pH range of 6.7 to 6.8 and commercial semen extenders typically ranging from 6.70 to 7.15 (Newth and Levis, 1999). Refractive index in Exp1 had a significant treatment effect (P < 0.001), where B0 was less than B51, which was less than B102, which was less than B205 (P < 0.001). This was expected as the addition of supplemental betaine to the extender would cause an increase in the solute content of the extender. In Exp2, an EXT by BET interaction effect was observed (P = 0.024), where all the pH of all extenders differed (P ≤ 0.012), except for BSA0 and BSA70, and LT0 and LT70 (P = 1.000). Extender had the greatest impact on pH where BSA had a pH of 6.7 ± 0.005, ST had a pH of 7.1 ± 0.005, and LT had a pH of 6.9 ± 0.005, all of which were within the normal range of semen extenders and were not considered biologically relevant (Newth and Levis, 1999). In Exp2, there were EXT and BET effects for refractive index (P < 0.001). The LT extender had a lower brix score compared with BSA and ST (P < 0.001) but did not differ between BSA and ST (P = 0.129). The supplementation of 70 mM of betaine to the extender resulted in a higher brix score compared with the nonsupplemented control (0 mM; P < 0.001). This was expected as commercial extenders utilize different ingredients and often have different osmolalities (Johnson et al., 2000). Additionally, it is intuitive that the addition of supplemental betaine to the extenders would result in an increase in the solute concentration of the extender, as indicated by the brix score. In Exp2, statistically significant effects were seen for pH, which may be due to the incubation time prior to analysis of pH for each extender. The three extender types utilized in the present study had different manufacturer-suggested incubation times; however, the present study used a common 30-min incubation. The manufacturer instructions for incubation time for the extenders used were as follows: BSA 30 min, ST 60 min, and LT 20 min. These differences may have accounted for some of the variation in the pH values observed in the present study. Newth and Levis (1999) tested various semen extenders, tracking the pH changes in those extenders, and observed that some extenders take up to 60 min of incubation at 35 ℃ before the pH equilibrates. While the pH in the present study may not have been in equilibrium, the values seen in the present study were well within the normal range of extenders. The refractive index in the present study was a measure of the solute concentration in the extender and has been correlated to osmolality for Beltsville Thawing Solution in a previous study (Schulze et al., 2015). In this study, researchers investigated the effects of mixing extenders at different concentrations and used refractometry and osmolality to determine the effects on semen quality, and showed that extender with a brix score of 8.6 caused significant damage to the sperm, but a brix score of 6.5 was no different than the control (normal proportion of extender:water ratio) that was at a brix score of 4.6. In the present study, the brix score was between 4.4 and 6.7; however, different extenders were used.
Table 1.
Refractive index and pH LS means for Exp11
| Betaine concentration2 | SE | P values | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 51 mM | 102 mM | 205 mM | TRT | Season | TRT × Season | ||
| Refractive index, brix score | 4.5a | 5.1b | 5.7c | 6.7d | 0.03 | <0.001 | 0.014 | 0.201 |
| pH | 6.7a | 6.7ab | 6.7b | 6.7ab | 0.004 | 0.010 | 0.001 | 0.009 |
TRT = treatment concentration of betaine; Season = season study was conducted in (summer vs. winter).
1 Columns within rows separated by differing letters are different, P < 0.05.
2 Concentration of betaine supplemented to commercial semen extender.
Table 2.
Refractive index and pH LS means for Exp21
| Treatment extender2 | SE | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST0 | ST70 | BSA0 | BSA70 | LT0 | LT70 | BET | EXT | BET × EXT | ||
| Refractive index, brix score | 4.6 | 5.3 | 4.6 | 5.5 | 4.1 | 4.9 | 0.04 | <0.0013 | <0.001 | 0.661 |
| pH | 7.1a | 7.1b | 6.7c | 6.7c | 6.9d | 6.9d | 0.01 | 0.052 | <0.001 | 0.024 |
BET = betaine concentration; EXT = extender type.
1 Columns within rows separated by differing letters are different, P < 0.05.
2 Different commercial semen extenders with supplemental betaine; short-term 0 mM, ST0; short-term 70 mM, ST82; long-term containing bovine serum albumin 0 mM, BSA0; long-term containing bovine serum albumin 70 mM, BSA70; long-term not containing bovine serum albumin 0 mM, LT0; long-term not containing bovine serum albumin 70 mM, LT70.
3 Refractive index was greater for 70 mM compared with 0 mM of betaine (5.4 vs. 4.4 ± 0.05).
Sperm Mobility Analysis
Sperm mobility parameter estimates were measured on D0 and D3 using CASA and are summarized for Exp1 and Exp2 in Tables 3 and 4, respectively. Curvilinear velocity (VCL) and beat cross frequency (BCF) combined have been shown to account for up to 9% of variability in farrowing rate, where there was a negative correlation between BCF and VCL at collection with farrowing rate (Broekhuijse et al., 2012). This finding means that lower BCF and VCL at the time of semen collection are mildly correlated to increased farrowing rates (Broekhuijse et al., 2012). For this reason, these two parameters will be discussed herein; the remainder of the mobility parameters’ estimates are summarized in Tables 3 and 4. In Exp1, there was a treatment by day interaction for BCF (P < 0.001), where in general the addition of betaine reduced the BCF and storage for 72 h reduced it further. There was a treatment by season interaction for BCF (P < 0.001), where in general the addition of betaine caused a reduction in BCF, and B51 was different in the summer and winter replicates (P = 0.044). There was a significant treatment by day of analysis interaction for VCL (P < 0.001), where in general the addition of betaine caused an increase in VCL, except for B205 on D3, which was significantly reduced compared with all other treatment by day combinations (P < 0.001). There were no differences within betaine treatment for D0 compared with D3 for VCL, except for B205 where the 72 h storage resulted in a significantly reduced VCL on D3 (P < 0.001). There was a significant treatment by season interaction for VCL (P < 0.001), where in general the addition of betaine in the winter replicate caused an increase in VCL up to B205, which resulted in a significant decrease in VCL compared with all other treatments in that replicate (P ≤ 0.008). During the summer replicate, there were no treatment differences (P ≥ 0.374). In Exp2, there was a BET by day of analysis interaction for BCF (P = 0.004), where in general the addition of 70 mM of betaine caused a reduction in the BCF. There was an EXT by day of analysis interaction for VCL (P = 0.020); however, no discernable trends were detected. There was also an effect of EXT (P = 0.003), but no discernable trend was observed. These parameter estimates are good descriptors of how the sperm are swimming but have only weak associations with predicting fertility. A high VCL may be indicative of capacitation and hyperactivation, causing high-velocity, asymmetrical movements of the flagella (Kay and Robertson, 1998; Suarez, 2008). These sperm that undergo capacitation shortly after collection will likely not survive long enough to fertilize oocytes, due to limited energy remaining in the sperm. In the present studies, the addition of supplemental betaine reduced the BCF but, in Exp1, resulted in an increased VCL, except for B205 that had a reduced VCL in Exp1. Exp2 did not indicate any treatment differences for VCL. This reduction of BCF caused by betaine may be due to the preservation of their plasma membranes and thus maintaining ionic balance and preventing capacitation and capacitation-associated, hyper-activated motility, although membrane integrity was not examined in the present study.
Table 3.
Computer-assisted sperm assessment LS means for Exp11
| Treatment extender | SE | P values | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 51 mM | 102 mM | 205 mM | TRT | TRT × Day | TRT × Season | ||
| Mobility parameter | ||||||||
| ALH, µm | 5.2a | 5.6ab | 5.9bc | 5.9c | 0.2 | <0.001 | <0.001 | 0.011 |
| BCF, µm/s | 34.6a | 33.1b | 31.5c | 23.1d | 0.4 | <0.001 | <0.001 | <0.001 |
| DAP, µm | 26.9a | 28.8b | 30.8c | 21.9d | 0.9 | <0.001 | <0.001 | <0.001 |
| DCL, µm | 45.8a | 47.9a | 50.8b | 42.5c | 1.3 | <0.001 | <0.001 | 0.858 |
| DSL, µm | 22.5a | 23.9a | 25.3b | 16.1c | 0.9 | <0.001 | <0.001 | <0.001 |
| LIN, % | 51.0a | 51.8a | 51.6a | 40.8b | 2.2 | <0.001 | 0.832 | 0.049 |
| STR, % | 83.4a | 82.5ab | 81.6b | 72.9c | 1.5 | <0.001 | <0.001 | 0.015 |
| VAP, µm/s | 64.9a | 70.9b | 77.1c | 53.2d | 2.2 | <0.001 | <0.001 | <0.001 |
| VCL, µm/s | 111.4a | 118.5a | 127.5b | 102.6c | 4.7 | <0.001 | <0.001 | <0.001 |
| VSL, µm/s | 54.3a | 58.8b | 63.5c | 39.1d | 2.1 | <0.001 | <0.001 | <0.001 |
| WOB, % | 60.1a | 61.6b | 62.1b | 54.7c | 1.5 | <0.001 | 0.001 | 0.011 |
| Motility parameter | ||||||||
| Total motility, % | 74.7a | 78.5ab | 80.7b | 55.5c | 4.7 | <0.001 | <0.001 | 0.111 |
| Progressive motility, % | 68.9a | 71.5a | 73.1a | 46.3b | 4.2 | <0.001 | <0.001 | 0.052 |
TRT= betaine concentration in extender; Day = day of semen analysis; Season = season study was conducted in (summer vs. winter); ALH = anterior lateral head displacement; BCF = beat cross frequency; DSL = straight-line distance; DAP = average path distance; DCL = curvilinear distance; LIN = linearity; STR = straightness; VSL = straight-line velocity; VAP = average path velocity; VCL = curvilinear velocity; WOB = wobble.
1 Columns within rows separated by differing letters are different (P < 0.05).
Table 4.
Computer-assisted sperm assessment LS means for Exp21
| Treatment extender | SE | P Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST0 | ST70 | BSA0 | BSA70 | LT0 | LT70 | BET | BET × EXT | BET × Day | ||
| Mobility parameters | ||||||||||
| ALH, µm | 4.0 | 4.5 | 5.2 | 5.4 | 5.0 | 5.4 | 0.7 | 0.009 | 0.639 | 0.325 |
| BCF, µm/s | 35.7 | 33.3 | 35.9 | 34.3 | 35.7 | 33.5 | 1.4 | <0.001 | 0.451 | 0.004 |
| DAP, µm | 25.3 | 26.8 | 28.5 | 28.9 | 30.0 | 30.7 | 3.1 | 0.239 | 0.778 | 0.125 |
| DCL, µm | 39.1 | 42.8 | 47.8 | 48.3 | 48.7 | 50.1 | 5.8 | 0.152 | 0.624 | 0.264 |
| DSL, µm | 21.0 | 21.9 | 24.4 | 24.2 | 25.0 | 25.3 | 2.3 | 0.528 | 0.776 | 0.169 |
| LIN, % | 57.2 | 55.1 | 51.4 | 51.2 | 53.6 | 52.8 | 3.3 | 0.217 | 0.639 | 0.803 |
| STR, % | 83.7 | 81.9 | 84.9 | 83.7 | 83.5 | 82.6 | 1.8 | 0.009 | 0.738 | 0.275 |
| VAP, µm/s | 64.3 | 69.3 | 66.7 | 68.2 | 74.4 | 76.4 | 8.3 | 0.150 | 0.744 | 0.081 |
| VCL, µm/s | 99.7 | 110.4 | 110.9 | 113.5 | 120.2 | 124.3 | 15.8 | 0.115 | 0.635 | 0.185 |
| VSL, µm/s | 53.9 | 57.1 | 57.1 | 57.4 | 62.3 | 63.6 | 6.3 | 0.302 | 0.750 | 0.088 |
| WOB, % | 66.2 | 64.8 | 60.3 | 60.8 | 63.2 | 62.7 | 2.6 | 0.564 | 0.585 | 0.822 |
| Motility parameters | ||||||||||
| Total motility, % | 75.9 | 70.9 | 84.2 | 81.8 | 86.7 | 84.0 | 4.5 | 0.010 | 0.668 | 0.174 |
| Progressive motility, % | 69.6 | 64.1 | 79.9 | 76.8 | 80.2 | 77.3 | 3.8 | 0.001 | 0.567 | 0.246 |
ST0 = short-term 0 mM; ST70 = short-term 70 mM; BSA0 = long term with bovine serum albumin 0 mM; BSA70 = long term with bovine serum albumin 70 mM; LT0 = long-term 0 mM; LT70 = long-term 70 mM; BET = betaine concentration in extender; EXT = extender type; Day = day of semen analysis; ALH = anterior lateral head displacement; BCF = beat cross frequency; DSL = straight-line distance; DAP = average path distance; DCL = curvilinear distance; LIN = linearity; STR = straightness; VSL = straight-line velocity; VAP = average path velocity; VCL = curvilinear velocity; WOB = wobble.
1 Columns within rows separated by differing letters are different (P < 0.05) for BET × EXT effect.
Sperm Motility Analysis
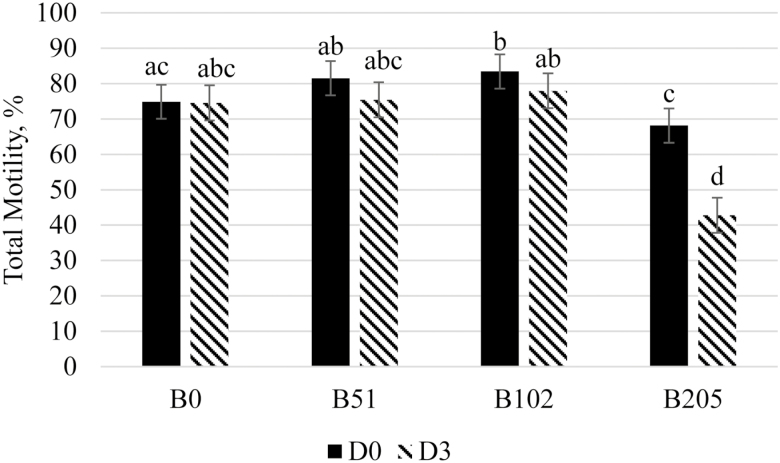
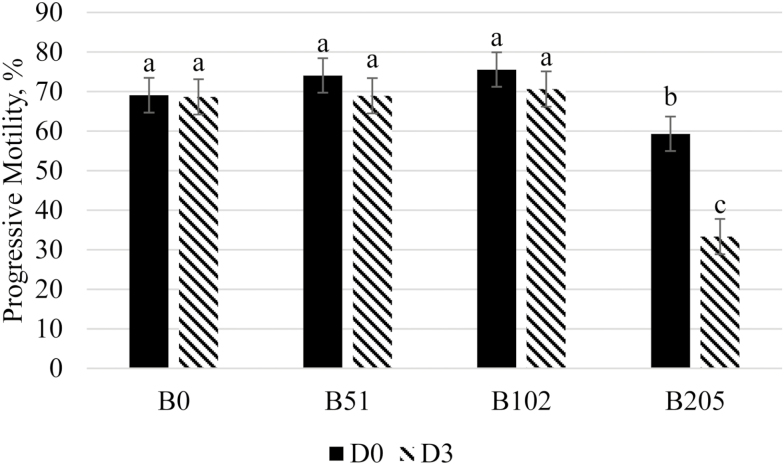
Total motility and progressive motility data for Exp1 and Exp2 are summarized in Tables 3 and 4, respectively. In Exp1, total motility had a treatment by day of analysis interaction (P < 0.001; summarized in Figure 1). There were no differences between B51 and B102 on either day of analysis and B0 on D3 (P ≥ 0.085); between B0 and B51 on either day of analysis (P ≥ 0.119); and between B0 on either day of analysis, B51 on D3 and B205 on D0 (P ≥ 0.116). Total motility was greater for B51 on D0 and B102 on D0 and D3 compared with B205 on either day of analysis (P < 0.001); and it was greater for B102 on D0 compared with B0 on D0 (P = 0.014). After 72 h of storage, B205 had a significantly lower total motility compared with all other treatments and day combinations (P < 0.001). There was no impact of season on total motility (P = 0.649). A significant treatment by day of analysis was observed for progressive motility (P < 0.001; summarized in Figure 2), where B0, B51, and B102 did not differ on either day of analysis (P ≥ 0.063); B205 had a lower progressive motility compared with B0, B51, and B102 on either day of analysis (P ≤ 0.011); and B205 on D3 had a lower progressive motility compared with B205 on D0 (P < 0.001). Overall, D0 total motility was increased for B102 compared with B0, and B205 caused a reduction in total and progressive motility. In Exp2, there was an EXT by day of analysis interaction for total motility (P < 0.001), where BSA was greater on D0 than on D3 (P < 0.001), LT on D0 was greater than ST on D3 (P = 0.017), and no other differences were detected (P ≥ 0.071). There was also an effect of BET on total motility (P = 0.010), where the 70 mM betaine treatment had a reduced total motility compared with 0 mM betaine treatment (78.9 ± 1.5% and 82.2 ± 1.5%, respectively). Similarly, a BET effect was observed for progressive motility (P = 0.038), where the control (0 mM betaine) had a greater progressive motility than the 70 mM betaine treatment (76.5 ± 1.1% and 72.7 ± 1.1%, respectively). There was also an EXT by day of analysis interaction for progressive motility (P = 0.025), where the BSA D0 had a greater progressive motility compared with the BSA D3 (P = 0.004) and the LT D0 had a greater motility than the ST D3 (P =0.044); however, no other differences were detected (P ≥ 0.126). In general, 205 mM had a lower total motility compared with 0, 51, and 102 mM throughout the study, and 102 mM had a greater total motility than 0 mM in Exp1, but 70 mM had a lower total and progressive motility compared with 0 mM in Exp2. The differences in these two studies are likely due to the naturally present betaine in the boar’s seminal plasma. Cabezon et al. (2016) showed that not only is betaine present and variable in boar seminal plasma, but also that supplementing betaine into the diets of boars increases the betaine concentration in the seminal plasma. The boars used in Exp1 had differing genetics and were 6 mo older than those in Exp2. These differences would result in differing metabolisms and in turn resulting in inherent differences in seminal plasma betaine.
Figure 1.
Treatment by day of semen analysis effect on total motility for Exp1. Semen was collected from six boars and diluted into a commercial extender that was supplemented with the following: 0 mM of betaine (B0), 51 mM of betaine (B51), 102 mM of betaine (B102), and 205 mM of betaine (B205). Semen was analyzed on day 0 (D0) and after 72 h of storage (D3) and analyzed using a CASA system. This figure shows that 102 mM of betaine improved D0 total motility compared with the 0 mM control (P = 0.014), but in general 205 mM of betaine reduced the total motility, especially after storage for 72 h.
Figure 2.
Treatment by day of semen analysis effect on total motility for Exp1. Semen was collected from six boars and diluted into a commercial extender that was supplemented with the following: 0 mM of betaine (B0), 51 mM of betaine (B51), 102 mM of betaine (B102), and 205 mM of betaine (B205). Semen was analyzed on day 0 (D0) and after 72 h of storage (D3) and analyzed using a CASA system. This figure illustrates that betaine supplemented into semen extender did not impact progressive motility up to 102 mM of betaine (P ≥ 0.063). Progressive motility was significantly reduced with 205 mM of supplemented betaine at D0 and D3 of analysis (P ≤ 0.011), where progressive motility was more severely reduced after storage with B205.
In a post-hoc analysis of commercial fertility data by Broekhuijse et al. (2012), total motility was positively correlated to and explained 10% of the variation in total number of piglets born, whereas progressive motility correlated positively with farrowing rate, explaining 9% of the variation. It is intuitive that if progressive motility is increased, so is total motility, as total motility encompasses all forms of motility, including progressive. Thus, the conclusion of the Broekhuijse study is that by increasing progressive motility, more pigs are born per litter with no impact on prenatal mortality. Therefore, research should target technologies that aim to increase total and progressive motility. To the authors’ knowledge, betaine supplementation has never been used in the semen extenders for liquid, boar semen dilution. It has, however, been used in other species to improve the post-thaw total motility and progressive motility of ram (Sanchez-Partida et al., 1992; Ollero et al., 1998; Sanchez-Partida et al., 1998, 1999) and stallion (Koskinen et al., 1989) semen. Other studies have shown that betaine did not improve post-thaw total motility in stallions (Trimeche et al., 1999) or dogs (Pena et al., 1998). Liquid bull semen diluted with supplemental betaine has been shown to have improved total motility between 0 and 30 °C, but not below 0 °C (Zhang et al., 2001). The present studies also present conflicting results, where in Exp1, 102 mM betaine supplementation improved total motility, but not progressive motility, and 205 mM caused a significant reduction in total and progressive motility. However, in Exp2, 70 mM betaine tended to reduce total motility and progressive motility. The current studies utilized different genetic lines of boars. Exp1 used a terminal genetic line, where a maternal genetic line was used in Exp2, and Exp2 boars had an intrinsically higher total motility and progressive motility than the Exp1 boars (82.3 ± 1.5% vs. 74.7 ± 4.7% for total motility, respectively; 76.5 ± 1.1% vs. 68.9 ± 4.2% for progressive motility, respectively). The boars in Exp2 may have had a higher concentration of betaine in their seminal plasma, which may partially explain their inherently better motility, although betaine concentration was not measured in the present study.
Schulze et al. (2015) investigated the effect of refractive index and osmolality on semen, when the diluent was prepared at differing concentrations. In this study, the researchers demonstrated that when the extender was mixed at 200% (i.e., twice as much extender powder when prepared), the extender was at a brix score of 8.6, which caused a significant reduction in total motility on the day of collection and after 4 d of storage. Additionally, they showed that 150% extender had a brix score of 6.5, but total motility did not differ from the control (100%). This demonstrated that there is a threshold refractive index score in relation to motility, where the optimal motility for the extender, which was used in their study, had a brix score of 4.6. While the present study utilized different extenders, it also demonstrated that when the solute concentration of the extender is too high, motility is reduced, though in the present study, the extender with the highest refractive index had a brix score of 6.7. Exp1 demonstrates that betaine supplementation may alleviate some of the dilution stress on the sperm, as motility was not significantly reduced until the brix score was at 6.7 and was increased when the brix score was at 5.7. Diet supplementation of betaine to boars has been shown to increase seminal plasma concentration of betaine, though no significant differences were detected in total motility or progressive motility (Cabezon et al., 2016).
Sperm Motility Regression Analysis
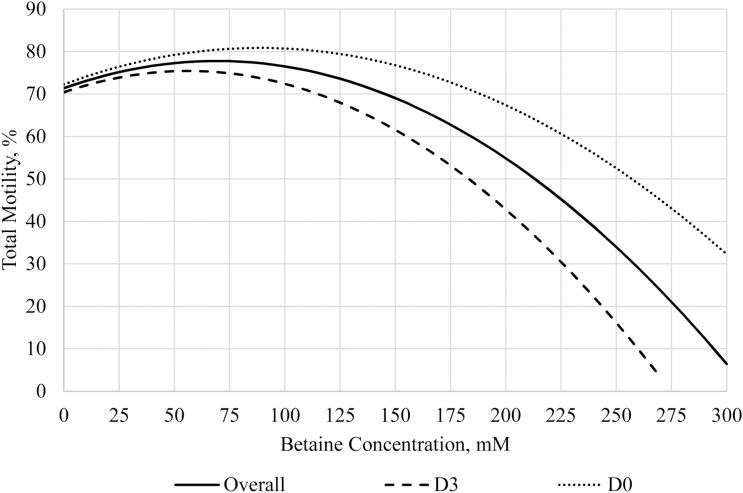
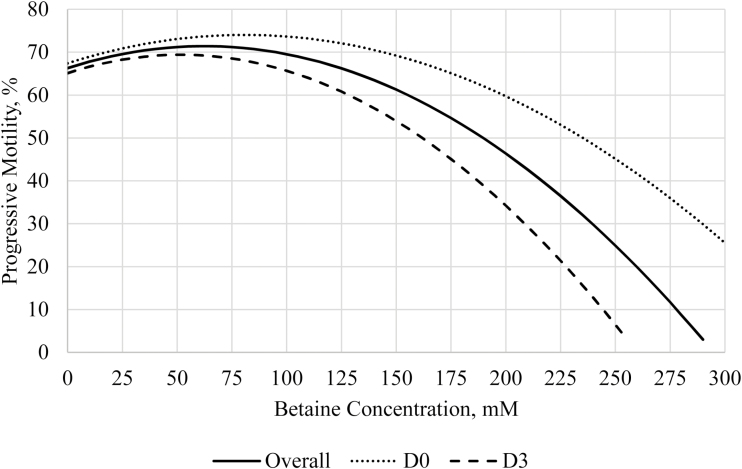
Three regression models were analyzed on the total motility and progressive motility data from Exp1, which were an overall regression, D0 regression, and D3 regression. Total motility regression analyses are summarized in Figure 3 and progressive motility in Figure 4. The overall regression for total motility was quadratic with a y intercept at 71.3%, a maximum at 77.8% when betaine was 70 mM, and an R2 = 0.515. Day 0 was quadratic with a y intercept at 72.2%, a maximum at 80.8% when betaine was 90 mM, and an R2 = 0.545. Day 3 was quadratic with a y intercept at 70.4%, a maximum at 75.4% when betaine was 55 mM, and an R2 = 0.711. These data suggest that supplementing semen extender with 90 mM betaine can improve total motility by 8.6% in fresh semen, and supplementing with 55 mM can improve total motility by 5% in stored semen. The overall regression for progressive motility was quadratic and had a y intercept at 66.3%, a maximum at 71.4% when betaine was 60 mM, and an R2 = 0.577. Day 0 was quadratic with a y intercept at 67.4%, a maximum at 74% when betaine was 80 mM, and an R2 = 0.638. Day 3 was quadratic with a y intercept at 65.1%, a maximum at 69.4% when betaine was 50 mM, and an R2 = 0.766. These data suggest that supplementation with 80 mM betaine in semen extenders can improve progressive motility by 6.6% in fresh semen, and supplementing with 50 mM betaine can improve progressive motility 4.3% in stored semen. This analysis used the total motility and progressive motility data from Exp1, which had conflicting results with that of Exp2; thus, the optimal concentrations presented here may not apply to all boars and genetic lines. Furthermore, progressive motility was not statistically improved by betaine supplementation in Exp1, and thus, more research is needed to confirm these results.
Figure 3.
Regression analyses for total motility in Exp1. Semen was collected from six boars and diluted into four treatment extenders that were supplemented with 0, 51, 102, and 205 mM of betaine and analyzed on day of collection (D0) and after 72 h of storage (D3). The mixed procedure of SAS 9.4 was used to perform the regression analyses, where three regressions were performed: overall total motility, D0 total motility, and D3 total motility. All regressions were quadratic. For the overall total motility regression, the following equation was determined to best fit the data: y = 71.3 + 0.19x – 0.0013x2, where x = mM betaine; R2 = 0.52; maximum total motility = 77.8% when x = 70 mM. For fresh semen (D0), the following equation best fits the data: y = 72.2 + 0.19x – 0.0011x2, where x = mM betaine; R2 = 0.55; maximum total motility = 80.8 when x = 90 mM. For stored semen (D3), the following equation best fits the data: y = 70.4 + 0.18x – 0.0016x2, where x = mM betaine; R2 = 0.71; maximum total motility = 75.4 when x = 55 mM.
Figure 4.
Regression analyses for progressive motility for Exp1. Semen was collected from six boars and diluted into four treatment extenders that were supplemented with 0, 51, 102, and 205 mM of betaine and analyzed on day of collection (D0) and after 72 h of storage (D3). The mixed procedure of SAS 9.4 was used to perform the regression analyses, where three regressions were performed: overall progressive motility, D0 progressive motility, and D3 progressive motility. All regressions were quadratic. For overall progressive motility, the following equation was determined to best fit the data: y = 66.3 + 0.16x – 0.0013x2, where x = mM betaine; R2 = 0.58; maximum progressive motility = 71.4 when x = 60 mM. For fresh semen (D0), the following equation best fits the data: y = 67.4 + 0.03x – 0.0010x2, where x = mM betaine; R2 = 0.64; maximum progressive motility = 74, when x = 80 mM. For stored semen (D3), the following equation best fits the data: y = 65.1 + 0.17x – 0.0016x2, where x = mM betaine; R2 = 0.77; maximum progressive motility = 69.4 when x = 50 mM.
Sperm Morphology Analysis
Morphology data for Exp1 and Exp2 are summarized in Tables 5 and 6, respectively. In Exp1, there was a treatment by day interaction for percent normal sperm morphology (P < 0.023), where, in general, B205 D3 had a reduced percent normal sperm morphology. Proximal droplets had a season by day of analysis effect (P = 0.038), where D0 in the summer was greater than D3 in summer and D3 in winter (P = 0.004 and P = 0.026, respectively). There was no effect of betaine on incidence of proximal droplets (P = 0.295). There were day of analysis and season effects for incidence of distal droplets (P = 0.026 and P < 0.001), where D0 was greater than D3 and summer was greater than winter. There was no effect of betaine on incidence of distal droplets or DMR (P = 0.156 and P = 0.085, respectively). Tail abnormalities had a treatment by day of analysis by season interaction effect (P = 0.006), where in general B205 caused an increased incidence of tail abnormalities. Overall, the present study showed that 205 mM betaine supplementation in semen extenders induced tail abnormalities, also seen as a reduction in the percentage of normal sperm. In Exp2, there was an EXT effect for percentage of normal sperm (P = 0.022), where BSA had a greater percent normal than LT (P = 0.031). There was a day of analysis effect for distal droplets and DMR (P = 0.032 and P = 0.008, respectively), where D0 was greater than D3. There was no effect of betaine on percentage of normal sperm (P = 0.942), proximal droplets (P = 0.723), distal droplets (P = 0.745), DMR (P = 0.817), and tail abnormalities (P = 0.745). Supplementation of semen extenders with betaine was not expected to impact proximal and distal droplets and DMR because the abnormalities are generated prior to ejaculation. Tail abnormalities increased with high levels (205 mM) of betaine supplementation in the present study. Cabezon et al. (2016) demonstrated that boars fed diets containing 1.26% betaine had a higher incidence of DMRs compared with 0.63% betaine but were not different from the nonsupplemented controls. The induced tail abnormalities and reduced motility seen in Exp1 with 205 mM betaine supplementation were likely caused by the high solute content of the extender, which likely caused alterations in cell volume, leading to membrane damage (Du et al., 1994; Gilmore et al., 1996). Betaine at 51, 70, and 102 mM may have prevented the changes in cell volume due to betaine’s osmolyte characteristics and thus preserved the cell volume of the sperm. Schulze et al. (2015) demonstrated with a short-term commercial extender that extenders with a high refractive index (brix score of 8.6) had a lower percentage of normal sperm and total motility than at lower refractive indices (brix scores 4.6 to 6.5). The results of the present study showed that when the brix score was at 6.7, tail abnormalities were increased. The commercial semen extenders used in the present study were different than for Schulze et al. This may in part explain why the present study saw differences at a brix score of 6.7, whereas Schulze et al. saw no differences at a brix score of 6.5.
Table 5.
Morphological assessment LS means for Exp11
| Treatment extender | SE | P values | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 51 mM | 102 mM | 205 mM | TRT | TRT × Day | TRT × Season | ||
| Normal, % | 79.6a | 79.8a | 79.8a | 70.6b | 3.3 | <0.001 | <0.001 | 0.077 |
| Proximal droplet, % | 4.0 | 4.0 | 3.5 | 3.4 | 0.3 | 0.295 | 0.071 | 0.038 |
| Distal droplet, % | 5.7 | 6.2 | 6.8 | 6.5 | 1.3 | 0.156 | 0.827 | 0.143 |
| DMR, % | 5.9 | 5.0 | 4.9 | 4.6 | 1.8 | 0.090 | 0.778 | 0.642 |
| Tail abnormality, % | 4.8a | 5.0a | 4.9a | 14.9b | 1.7 | <0.001 | <0.001 | 0.372 |
TRT = betaine concentration in extender; Day = day of semen analysis; Season = season study was conducted in (summer vs. winter); DMR = distal mid-piece retroflection.
1 Columns within rows separated by differing letters are different (P < 0.05) for TRT effect.
Table 6.
Morphological assessment LS means for Exp21
| Treatment extender | SE | P values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST0 | ST70 | BSA0 | BSA70 | LT0 | LT70 | BET | BET × EXT | BET × Day | ||
| Normal, % | 84.6 | 83.9 | 91.1 | 91.1 | 86.5 | 87.4 | 2.8 | 0.942 | 0.487 | 0.178 |
| Proximal droplet, % | 5.0 | 4.7 | 1.0 | 0.9 | 2.8 | 2.9 | 1.2 | 0.723 | 0.898 | 0.687 |
| Distal droplet, % | 4.8 | 5.3 | 4.0 | 3.7 | 5.1 | 4.6 | 1.4 | 0.745 | 0.329 | 0.755 |
| DMR, % | 1.2 | 1.1 | 0.6 | 0.8 | 1.1 | 1.1 | 0.7 | 0.817 | 0.661 | 0.929 |
| Tail, % | 4.6 | 5.1 | 3.2 | 3.4 | 4.4 | 4.1 | 1.7 | 0.745 | 0.713 | 0.207 |
ST0 = short-term 0 mM; ST70 = short-term 70 mM; BSA0 = long term with bovine serum albumin 0 mM; BSA70 = long term with bovine serum albumin 70 mM; LT0 = long-term 0 mM; LT70 = long-term 70 mM; BET = betaine concentration in extender; EXT = extender type; Day = day of semen analysis; DMR = distal mid-piece retroflection.
1 Columns within rows separated by differing letters are different within Ext (ST, LA, LB; P < 0.05) for Bet × Ext effect.
The present studies have evaluated the use of betaine supplementation in commercial semen extenders to prevent the dilution effect when semen is diluted into commercial semen extenders, thereby improving semen quality. The results have shown that supplemental betaine up to 102 mM may alleviate this effect as determined by improved motility and mobility parameters. However, the present studies also had conflicting motility results when different genetic lines of boars were used, suggesting that genetics and perhaps seminal plasma betaine concentration may play a role in supplemental betaine’s efficacy. Further research should investigate the use of added betaine to commercial semen extenders and compare seminal plasma concentrations of betaine, as well as to determine different responses to supplemental betaine of different genetic lines.
Drew Lugar is an Iowa native that received his bachelors degree from Iowa State and his Master’s degree from Virginia Tech. He is currently a Ph.D. candidate at Purdue University.
Wyatt Krom and Paige Mings are undergraduate students at Purdue University.
Dr. Kara Stewart is a Reproductive Physiologist at Purdue University specializing in swine.
LITERATURE CITED
- Broekhuijse M.L., E. Šoštarić, Feitsma H., and Gadella B.M.. 2012. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 90:779–789. doi:10.2527/jas.2011-4311. [DOI] [PubMed] [Google Scholar]
- Cabezón F.A., Stewart K.R., Schinckel A.P., Barnes W., Boyd R.D., Wilcock P., and Woodliff J.. 2016. Effect of natural betaine on estimates of semen quality in mature ai boars during summer heat stress. Anim. Reprod. Sci. 170:25–37. doi:10.1016/j.anireprosci.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Du J., Tao J., Kleinhans F.W., Peter A.T., and Critser J.K.. 1994. Determination of boar spermatozoa water volume and osmotic response. Theriogenology 42:1183–1191. doi:10.1016/0093-691X(94)90867-2 [DOI] [PubMed] [Google Scholar]
- Gilmore J.A., Du J., Tao J., Peter A.T., and Critser J.K.. 1996. Osmotic properties of boar spermatozoa and their relevance to cryopreservation. J. Reprod. Fertil. 107:87–95. doi:10.1530/jrf.0.1070087 [DOI] [PubMed] [Google Scholar]
- Hayashi H. Alia L. Mustardy P. Deshnium M. Ida, and Murata N.. 1997. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 12:133–142. doi:10.1046/j.1365-313X.1997.12010133.x [DOI] [PubMed] [Google Scholar]
- Johnson L.A., Weitze K.F., Fiser P., and Maxwell W.M.. 2000. Storage of boar semen. Anim. Reprod. Sci. 62:143–172. doi:10.1016/S0378-4320(00)00157-3 [DOI] [PubMed] [Google Scholar]
- Kay V.J. and Robertson L.. 1998. Hyperactivated motility of human spermatozoa: a review of physiological function and application in assisted reproduction. Hum. Reprod. Update 4:776–786. doi:10.1093/humupd/4.6.776 [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Ferket P.R., and Garlich J.D.. 1997. Nutritional and osmoregulatory functions of betaine. World Poultry Sci. J. 53:125–139. doi:10.1079/WPS19970013 [Google Scholar]
- Koskinen E., Junnila M., Katila T., and Soini H.. 1989. a preliminary study on the use of betaine as a cryoprotective agent in deep freezing of stallion semen. Zentralbl. Veterinarmed. A 36:110–114. doi:10.1111/j.1439-0442.1989.tb00710.x [DOI] [PubMed] [Google Scholar]
- Newth M.S., and Levis D.G.. 1999. Changes in pH of boar semen extenders. 1999 Nebraska Swine Report: 3–6. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1133&context=coopext_swine [Google Scholar]
- Ollero M., Perez-Pe R., Muiño-Blanco T., and Cebrian-Perez J.A.. 1998. Improvement of ram sperm cryopreservation protocols assessed by sperm quality parameters and heterogeneity analysis. Cryobiology 37:1–12. doi:10.1006/cryo.1998.2092. [DOI] [PubMed] [Google Scholar]
- Pena A.I., Barrio F., Quintela L.A., and Herradon P.G.. 1998. Proline and glycine betaine in a diluent for freezing canine spermatozoa. Reprod. Domest. Anim. 33:5–9. doi:10.1111/j.1439-0531.1998.tb01307.x [Google Scholar]
- Record M.T. Jr, Courtenay E.S., Cayley S., and Guttman H.J.. 1998. Biophysical compensation mechanisms buffering e. coli protein-nucleic acid interactions against changing environments. Trends Biochem. Sci. 23:190–194. doi:10.1016/S0968-0004(98)01207-9 [DOI] [PubMed] [Google Scholar]
- Renard P., Grizard G., Griveau J.F., Sion B., Boucher D., and Le Lannou D.. 1996. Improvement of motility and fertilization potential of postthaw human sperm using glutamine. Cryobiology 33:311–319. doi:10.1006/cryo.1996.0031. [DOI] [PubMed] [Google Scholar]
- Sánchez-Partida L.G., Maxwell W.M., Paleg L.G., and Setchell B.P.. 1992. Proline and glycine betaine in cryoprotective diluents for ram spermatozoa. Reprod. Fertil. Dev. 4:113–118. [DOI] [PubMed] [Google Scholar]
- Sánchez-Partida L.G., Setchell B.P., and Maxwell W.M.. 1998. Effect of compatible solutes and diluent composition on the post-thaw motility of ram sperm. Reprod. Fertil. Dev. 10:347–357. doi:10.1071/R98053 [DOI] [PubMed] [Google Scholar]
- Sánchez-Partida L.G., Windsor D.P., Eppleston J., Setchell B.P., and Maxwell W.M.. 1999. Fertility and its relationship to motility characteristics of spermatozoa in ewes after cervical, transcervical, and intrauterine insemination with frozen-thawed ram semen. J. Androl. 20:280–288. doi:10.1002/j.1939-4640.1999.tb02519.x [PubMed] [Google Scholar]
- Schulze M., Rüdiger K., Jung M., and Grossfeld R.. 2015. Use of refractometry as a new management tool in ai boar centers for quality assurance of extender preparations. Anim. Reprod. Sci. 152:77–82. doi:10.1016/j.anireprosci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Suarez S.S. 2008. Control of hyperactivation in sperm. Hum. Reprod. Update 14:647–657. doi:10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- Trimeche A., Yvon J.M., Vidament M., Palmer E., and Magistrini M.. 1999. Effects of glutamine, proline, histidine and betaine on post-thaw motility of stallion spermatozoa. Theriogenology 52:181–191. doi:10.1016/S0093-691X(99)00120-X [DOI] [PubMed] [Google Scholar]
- Xing W., and Rajashekar C.B.. 2001. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 46:21–28. doi:10.1016/S0098-8472(01)00078-8 [DOI] [PubMed] [Google Scholar]
- Zhang B.R., Buhr M., Kroetschd T., and Leibo S.P.. 2001. Glycine betaine improves survival of fresh bovine spermatozoa. Reprod. Fertil. Dev. 13:187–192. doi:10.1071/RD01006 [DOI] [PubMed] [Google Scholar]