Abstract
Two treatments were evaluated in heifers to determine the effects of a yeast supplement on immune and metabolic responses to a combined (tandem viral-bacterial) respiratory disease challenge. Thirty-two beef heifers (325 ± 20.1 kg BW) were selected from a larger population previously assigned to one of two treatments: Control (CON), receiving no yeast supplement in the diet, or yeast (YST), CON diet plus a combination live yeast (2.5 g·heifer−1·d−1) and yeast cell wall (2.5 g·heifer−1·d−1) supplement (Phileo Lesaffre Animal Care, Milwaukee, WI). Heifers were maintained on treatments for 31 d prior to the challenge. On day −3 all heifers were fitted with an indwelling vaginal temperature recording device, received an intranasal challenge with 2 × 108 plaque-forming units of bovine herpesvirus-1 (BHV-1), and placed in outdoor pens. On day 0, all heifers were fitted with an indwelling jugular catheter, challenged intratracheally with an average dose of 3.0 × 107 colony-forming units (cfu) of Mannheimia haemolytica in 100 mL media, and were transferred into individual stanchions in an enclosed, environmentally controlled barn. Whole blood samples were collected at −72 h and at 0, 2, 4, 6, 8, 12, 24, 36, 48, 60, and 72 h (hematology) and at 0, 1, 2, 3, 4, 5, 6, 7, 8, 12, 24, 36, 48, 60, and 72 h (serum isolation) relative to M. haemolytica challenge (0 h). Data were analyzed using the MIXED procedure of SAS specific for repeated measures with fixed effects of treatment, time, and the treatment × time interaction. Vaginal temperature and cortisol concentrations were similar between treatments (P ≥ 0.39). Although total leukocyte count following BHV-1 challenge was similar between treatments (P = 0.21), there was a tendency (P = 0.07) for CON heifers to have greater neutrophil counts than YST heifers. Serum haptoglobin concentration was similar between treatments (P = 0.13). Heifers in the YST treatment had similar serum glucose concentrations (P = 0.25) and decreased serum concentrations of urea nitrogen compared to CON (P = 0.03). Dietary treatment did not affect serum nonesterified fatty acid concentrations (P = 0.37). Nasal lesion score severity (0–4) tended (P = 0.07) to be decreased in YST compared to CON (2.5 vs. 3.19 ± 0.26), while water intake tended to be increased in YST (P = 0.06). Feeding a yeast supplement had little effect on the acute-phase response but improved metabolic outcomes in heifers during a respiratory disease challenge.
Keywords: feedlot health, immune response, metabolic response, respiratory disease challenge, yeast
INTRODUCTION
Bovine respiratory disease (BRD) is the leading cause of morbidity and mortality in U.S. feedlots (USDA, 2013a), with 16% of cattle requiring BRD treatment during the feeding period (USDA, 2013b). Losses in live performance or carcass value may be experience by cattle diagnosed with BRD even after clinical signs subside (Duff and Galyean, 2007). While BRD is a multifaceted disease, several known viral and bacterial pathogens have been reported to be causal factors, including bovine herpesvirus-1 (BHV-1) and Mannheimia haemolytica (Duff and Galyean, 2007). Currently, 59% of feedlots treat at least some cattle with metaphylaxis to control BRD (USDA APHIS, 2013b), a process that is under scrutiny for the judicious use of antimicrobials (Dennis et al., 2018). Methods to prevent or control BRD are therefore needed.
Yeast supplementation, specifically Saccharomyces cerevisiae supplementation, may serve as a means of altering beef cattle response to BRD during the feeding period (Finck et al., 2014). Yeast cell wall contains β-glucans that have been reported to activate macrophage and neutrophil function in mice (Akramiene et al., 2007). These β-glucans are associated with improved immune function (Broadway et al., 2015) and therefore health of newly received feedlot cattle (Keyser et al., 2007). Live yeast and yeast cell wall supplements may each bind pathogenic microorganisms such as Salmonella or Escherichia coli and prevent colonization of these bacteria in the gastrointestinal tract (Perez-Sotelo et al., 2005; Posadas et al., 2017). While the mechanism of action is not completely understood, yeast supplements have altered morbidity and mortality in feedlot cattle (Keyser et al., 2007; Finck et al., 2014), and may reduce immune response severity and negative metabolic outcomes (Burdick Sanchez et al., 2013, 2014). Therefore, the objective of the current study was to evaluate the effects of a supplement containing live S. cerevisiae and S. cerevisiae cell wall components on the immune and metabolic outcomes of feedlot heifers administered a combined viral and bacterial BRD challenge. The experimental hypothesis was that supplementing heifers with yeast for 31 d prior to the BRD challenge would improve immune and metabolic responses to a BRD challenge.
MATERIALS AND METHODS
All experimental procedures were in compliance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committee of the Livestock Issues Research Unit (LIRU IACUC #1605F).
Yeast additive was a combination yeast cell wall and live yeast product developed from a primary S. cerevisiae yeast strain. Thirty-two British crossbred heifers were obtained from a commercial feedlot in the Texas panhandle and transported approximately 112 km to the USDA-ARS Livestock Issues Research Unit’s Research Complex in New Deal, TX. Two treatments were evaluated: 1) Control (CON) receiving no yeast additive in the diet, or 2) CON diet plus 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall, dosed to 8 g·heifer−1·d−1 with carrier (YST, Phileo Lesaffre Animal Care, Milwaukee, WI). The live yeast was fed to 25 × 109 colony-forming units (cfu)·heifer−1·d−1. Heifers were chosen from a larger herd of 560 heifers per treatment which had been received at a commercial feeding facility 35 d prior to transport to the USDA facilities. Processing at the commercial feeding facility included individual identification with uniquely numbered ear tags, treatment with an endectocide (Cydectin Injectable, Bayer Animal Health, Whippany, NJ), vaccination against viral diseases (Vista 5 SQ, Merck Animal Health, Summit, NJ) and clostridial diseases (Ultrabac 7, Zoetis, Florham Park, NJ), and administered an anabolic implant (Revalor-IH, Merck Animal Health). Heifers were selected for the current trial from the larger herd in order to select a sample with similar phenotype. Selection criteria included black hided cattle without visible Brahman influence, cattle with a similar temperament using the pen and chute scoring system described by King et al. (2006), uniformity of BW, and average serum antibody titer against BHV-1 of 16 for each treatment (Texas A&M Veterinary Diagnostic Laboratory, Amarillo, TX). Blood for serum was collected on a larger sample of cattle at the commercial facility and analyzed for serum antibody titer against BHV-1 prior to arrival at USDA facilities. The scoring system outlined by King et al. (2006) was used to assign cattle to subjective pen scores prior to being processed where 1 = walks slowly, can be approached slowly, not excited by humans; 2 = runs along fences, stands in corner if humans stay away; 3 = runs along fences, head up and will run if humans approach but stops before hitting gates and fences; 4 = runs, stays in back of group, head high and aware of humans, may run into fences and gates; 5 = excited, runs into fences or anything in path. Chute scores were assigned as cattle were processed where 1 = calm, no movement; 2 = slightly restless; 3 = squirming, occasionally shaking the chute; 4 = continuous, very rigorous movement and shaking of the chute; 5 = rearing, twisting of the body and struggling violently. Pen and chute score were then averaged to assign each heifer a temperament score, and animals were chosen based on an average score of less than 3. All heifers selected had not received treatment for clinical BRD during the feeding period. All heifers had been randomized to treatment group and maintained on respective treatments (n = 16 per treatment) for 31 d prior to arrival at the USDA facilities for a commercial research trial evaluating the same yeast product used in the current study. At the commercial facility, heifers were fed 100% RAMP (Cargill Corn Milling, Bovina, TX) and transitioned to the same diet fed at the USDA facilities (Table 1) by incremental substitution of the finish diet for RAMP at 15% intervals every 2 to 4 d. Feed was transported every 3 d from commercial facilities to USDA facilities after cattle were transported, and stored in a tarp-covered trailer inside a covered, temperature-controlled barn. Average initial BW of the cattle transported to USDA facilities was 325 ± 20.1 kg. Following arrival at the USDA facility, heifers were allowed to rest overnight in dirt pens (7.6 × 18.3 m) and were maintained on the respective treatment diets previously assigned at the commercial feeding location, with ad libitum access to water. On study day −3, heifers were weighed and fitted with a vaginal temperature recording device. Briefly, a temperature sensor (Star-ODDi DST micro-T; MeterMall USA, Marysville, OH) was secured into a blank controlled internal drug release (CIDR) device (Pfizer Animal Health, Sterling, CO) as described by Burdick et al. (2011), which was then placed using a CIDR applicator into the vagina of each heifer. Vaginal temperature was measured every 5 min from insertion until the completion of the study. A nasal lesion score was also recorded at this time where 0 = absence of lesions; 1 = less than 10% coverage of the visible naris; 2 = 11% to 25% coverage; 3 = 26% to 50% coverage; and 4 = 50% or greater coverage of the visible naris. All heifers were challenged intranasally at this time with approximately 2 × 108 plaque-forming units (pfu) of BHV-1. For the BHV-1 dose, a single vial of BHV-1 was diluted to a final concentration of 1 × 108 pfu/mL, and then 1 mL of diluted virus was administered per naris in each heifer using a syringe and a nasal atomization device. Whole blood was collected into a 4-mL tube containing 7.2 mg K2 EDTA (Vacutainer; Becton, Dickinson, and Company, Franklin Lakes, NJ) and blood for serum isolation collected into a 9-mL tube (Vacutainer; Becton, Dickinson, and Company, Franklin Lakes, NJ) containing no additive via jugular venipuncture prior to challenge. All heifers were then returned to the outdoor pens and were monitored daily for clinical symptoms of BRD. Indwelling vaginal temperature recording devices were removed on study day 3.
Table 1.
Ingredient formulation and analyzed composition of finish diets
| Finish diet formulations (% of DM) | ||
|---|---|---|
| Ingredient | ||
| Corn, steam flaked | 58.5 | |
| Corn distillers grain, wet | 17.1 | |
| Wet corn gluten feed1 | 16.1 | |
| Corn stalks, chopped | 7.2 | |
| Fat | 1.1 | |
| Additives | ||
| Vitamin A, IU/kg | 2,645 | |
| Vitamin D, IU/kg | 264.6 | |
| Melengesterol acetate, mg·heifer−1·d−1 | 0.40 | |
| Monensin, mg/kg2 | 46.85 | |
| Tylosin, mg/kg3 | 10.69 | |
| Analyzed composition4 (% of DM) | CON | YST5 |
| Dry matter | 60.3 | 60.8 |
| Crude protein | 15.1 | 15.3 |
| Equivalent crude protein | 1.0 | 1.0 |
| Neutral detergent fiber | 20.8 | 20.9 |
| Fat | 5.2 | 5.2 |
| Ca | 0.74 | 0.70 |
| P | 0.51 | 0.51 |
| Mg | 0.24 | 0.24 |
| K | 0.81 | 0.82 |
| S | 0.25 | 0.25 |
1Sweet Bran wet corn gluten feed (Cargill Corn Milling, Bovina, TX) with added calcium carbonate (4% as fed), salt (1.8%), urea (1.1%), and trace mineral premix (0.2%).
2Rumensin 90, Elanco Animal Health.
3Tylosin 100, Elanco Animal Health.
4Reported by Servi-Tech Laboratories, Amarillo, TX. Values are means (n = 7).
5Treatments were: 1) negative control (CON) fed a standard finishing diet, or 2) YST, fed the same finishing diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall (Phileo Lesaffre Animal Care, Milwaukee, WI).
The morning of study day 0, heifers were fitted with an indwelling jugular catheter. Whole blood and blood for serum was collected via the jugular catheter and heifers were challenged with logarithmic phase culture M. haemolytica in 100 mL media. Four batches of M. haemolytica were made and heifers received the challenge in blocks of eight heifers to ensure that each received log phase culture. Mannheimia haemolytica titers (cfu counts) were determined by serial dilution and plating immediately prior to the first animal receiving the intratracheal challenge and immediately following the eighth (final) animal challenged for each batch. Each batch was measured on a UV visible spectrophotometer at 590 nm to a transmittance between 62% and 68%. Based on this, the average dose of the four batches was 3.0 × 107 cfu of M. haemolytica. Each heifer was challenged with the prepared M. haemolytica via intratracheal injection using a 16 gauge, 25.4 mm needle and two 60 mL syringes. Heifers were then moved to individual stalls (2.28 m in length, 0.76 m in width, and 1.67 m in height). Heifers were fed a complete finishing diet (Table 1) once daily ad libitum such that each heifer was offered 4.5 kg of fresh feed each morning and orts were discarded (Table 1). Yeast supplement was hand mixed into the daily ration while heifers were in the stanchions to the appropriate treatments. Prior to the immunological challenge, no feed was discarded, although yeast treatment may have been included in feed that was refused by heifers during the immunological challenge and subsequently discarded. Individual water intake was measured from 0 to 72 h while heifers were housed in individual stanchions via a Suevia Cup (QC Supply Inc., Schuyler, NE). Whole blood was collected in 4-mL tube containing 7.2 mg K2 EDTA at 0, 2, 4, 6, 8, 12, 24, 36, 48, 60, and 72 h post-M. haemolytica challenge via the jugular catheter. Blood for serum was collected in 9-mL Sarstedt tubes containing no additive (Sarstedt, Inc., Newton, NC) every hour until hour 8, at 12 h, and then every 12 h until 72 h via the jugular catheter. Blood for serum was incubated at room temperature for 30 min and then centrifuged at 1,500 × g for 20 min at 4 °C and serum removed and frozen at −80 °C. Following the collection of the 72 h sample, heifers were weighed, jugular catheters were removed, and all heifers were treated with florfenicol (Nuflor Gold, Merck Animal Health, Madison, NJ) by subcutaneous injection at 40 mg florfenicol/kg body weight to treat the known bacterial (M. haemolytica) infection that had been administered to the animals.
Whole blood samples were analyzed for hematology, including: total leukocyte counts, leukocyte differential, hematocrit, and hemoglobin using an IDEXX Procyte Dx Hematology Analyzer (IDEXX Labs, Westbrook, ME) with bovine-specific algorithms. Whole blood was also collected in heparinized tubes at −72, 0, and 48 h relative to M. haemolytica challenge and analyzed to determine polymorphonuclear (PMN) leukocyte activity.
All serum analyses were performed in duplicate. Serum cortisol concentrations were determined using a commercially available enzyme immunoassay kit according to the manufacturer’s directions (Arbor Assays, Ann Arbor, MI) by comparison of unknowns to standard curves generated with known concentrations of cortisol. Minimum detectable cortisol concentration was 0.4 ng/mL, and the intra- and inter-assay coefficients of variation were 10.6% and 4.2%, respectively. Serum haptoglobin concentrations were also determined using a commercially available enzyme immunoassay kit according to the manufacturer’s directions (Immunology Consultants Laboratory, Inc., Portland, OR). Intra- and inter-assay coefficients of variation were 6.0% and 15.3%, respectively.
Glucose concentrations were determined by modification of the enzymatic Autokit glucose (Wako Pure Chemical Industries, Chuo-Ku, Osaka, Japan) to fit a 96-well format, where 300 µL of prepared working solution was added to 2 µL of serum or prepared standards in a 96-well plate. Plates were incubated at 3 °C for 5 min and then read using a plate reader at 505 nm. Concentration of glucose was determined by comparing unknown samples to a standard curve of known glucose concentrations. Minimum detectable concentration was 3.8 mg/dL and intra- and inter-assay coefficients of variation were 7.4% and 10.7%, respectively.
Serum nonesterified fatty acid (NEFA) concentrations were determined by modification of the enzymatic HR Series NEFA-HR (2) assay (Wako Diagnostics, Richmond, VA) to fit a 96-well format where 200 µL of the Color Reagent A were added to 5 µL of serum or prepared standards in a 96-well plate. Plates were incubated at 37 °C for 5 min and then the absorbance was read using a spectrophotometer at 550 nm. Next, 100 µL of Color Reagent B was added to all wells on the 96-well plate. Plates were incubated for an additional 5 min and read for a second time using a plate reader at 550 nm. A final absorbance was obtained by subtracting the first reading, which was multiplied by a factor of 0.67 to account for changes in volume, from the second reading. The final absorbance values were used for all calculations. Concentrations of NEFA were determined by comparing unknown samples to a standard curve of known NEFA concentrations. The minimum detectable concentration was 0.021 mmol/L and the intra- and inter-assay coefficients of variation were 8.8% and 10.5%, respectively.
Concentrations of serum urea nitrogen were determined by a colorimetric assay according to the manufacturer’s directions (K024-H1; Arbor Assays, Ann Arbor, MI) by comparison of unknowns to standard curves generated with known concentrations of urea nitrogen. The minimum detectable concentration was 0.093 mg/dL and intra- and inter-assay coefficients of variation were 12.8% and 8.7%, respectively.
Neutrophil functionality was determined by the phagocytic and oxidative burst capacity against an environmental E. coli as well as the surface expression of the adhesion molecule, CD62L (i.e., L-selectin), as described by Obeidat et al. (2013). Briefly, oxidative burst intensity and phagocytic activity was determined by incubating 200 µL of whole blood in an ice bath and then combining with 40 µL each of 100 µM dihydrorhodamine and heat-killed E. coli at 1 × 106 cells per mL. Samples were incubated in a 38.5 °C water bath for 10 min, then subsequently placed in an ice bath for 15 min to stop the reaction, hypotonically lysed, and then washed with ice cold phosphate-buffered saline (PBS). A BD Accuri flow cytometer was used to analyze leukocytes, and neutrophils were gated on the scatterplot of forward scatter × side scatter. Data are reported as the percentage of neutrophils that were positive for phagocytosis and oxidative burst as well as the mean fluorescence intensity of the positive populations. Negative controls included E. coli only, remained on ice throughout the dihydrorhodamine, and incubated with remaining samples. The surface expression of L-selectin was determined by incubating 50 µL of whole blood with anti-bovine CDL62L (monoclonal antibody IgG1-isotype in the mouse; VMRD Inc., Pullman, WA) at a final dilution of 5 µg/mL for 1 h in an ice bath, then hypotonically lysed and rinsed with PBS. The pellet was resuspended in fluorescein-labeled secondary antibody and incubated on ice for 1 h, then washed twice using PBS and analyzed using flow cytometry as described above. The negative control was secondary antibody only. Data are reported as the mean fluorescence intensity of each sample.
Vaginal temperature data were averaged into 1-h intervals for analysis. Hematological, metabolic, and temperature data were analyzed using the MIXED procedure of SAS specific for repeated measures (SAS Institute Inc., Cary, NC) with fixed effects of treatment, time, and the treatment × time interaction. The autoregressive (1) covariance structure for the within-subject measurement was used in all models except for vaginal temperature data, where the compound symmetry covariance structure was used. Covariance structures were chosen based on the smaller Bayesian Information Criterion. For serum glucose, change relative to the sample collected at 0 h was analyzed due to differences between treatment at 0 h. Nasal lesion scores were analyzed using the GLIMMIX procedure of SAS with fixed effects of treatment, time, and the treatment × time interaction. All data presented are as LSM ± SEM with P ≤ 0.05 considered significant and 0.05 < P ≤ 0.10 considered a tendency.
RESULTS
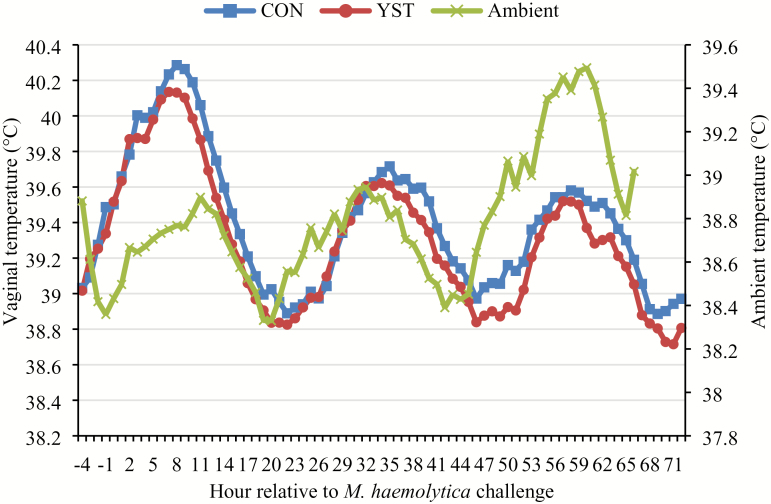
No treatment × time interaction occurred (P = 0.84) for vaginal temperature, and temperature was similar (P = 0.39) between CON and YST (Figure 1; 39.4 ± 0.08 vs. 39.3 ± 0.08 °C, respectively). Vaginal temperature increased (P < 0.001) for 10 h in both treatments following the BHV-1/M. haemolytica challenge with a peak of 40.2 °C occurring at 10 h. Water intake, and hematological measures are reported in Table 2. There was no treatment × time interaction (P = 0.52) for water intake, and total water intake tended to be greater (P = 0.06) in the YST treatment compared to CON (676 ± 32.3 vs. 588 ± 32.3 mL/h, respectively). Water intake fluctuated over time following viral challenge (P < 0.01).
Figure 1.
Effect of yeast supplementation on vaginal temperature response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. Data presented as least squares means. There was no treatment × time interaction (P = 0.84) and vaginal temperature was not affected by dietary treatment (P = 0.39), although temperature decreased over time (P < 0.01). Treatment means were 39.4 ± 0.08 for CON and 39.3 ± 0.08 °C for YST. SEM = 0.084.
Table 2.
Effect of yeast supplementation on vaginal temperature, water intake, nasal lesion score, serum and hematology variables, and neutrophil functionality in feedlot heifers administered a respiratory disease challenge
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | YST | SEM | P-value2 |
| Vaginal temperature, °C | 39.4 | 39.3 | 0.08 | 0.39 |
| Water intake, mL/h | 588 | 676 | 32.3 | 0.06 |
| Serum haptoglobin, µg/dL | 15,396 | 11,757 | 1,631.7 | 0.13 |
| Serum cortisol, ng/mL | 20.7 | 20.5 | 1.14 | 0.90 |
| Lymphocytes, 103/µL | 4.65 | 4.59 | 0.26 | 0.86 |
| Neutrophils, 103/µL | 6.39 | 5.37 | 0.39 | 0.07 |
| Neutrophil:Lymphocyte | 1.50 | 1.35 | 0.12 | 0.36 |
| Platelets, 103/µL | 438.8 | 399.3 | 30.5 | 0.36 |
| Red blood cells, 106/µL | 7.60 | 7.29 | 0.15 | 0.17 |
| Eosonophils, 103/µL | 0.18 | 0.17 | 0.03 | 0.72 |
| Hematocrit, % | 34.3 | 33.8 | 0.48 | 0.43 |
| Hemoglobin, g/dL | 11.2 | 11.0 | 0.13 | 0.16 |
| Monocytes, 103/µL | 1.60 | 1.64 | 0.073 | 0.65 |
| Neutrophil oxidative burst intensity, GMFI3 | 119,898 | 135,696 | 7,227.5 | 0.13 |
| PMN phagocytosis, GMFI | 47,013 | 45,867 | 2,650.8 | 0.76 |
| L-Selectin expression, GMFI | 98,030 | 103,914 | 3,966.3 | 0.30 |
1Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) 31 d prior to immunological challenge (YST). Whole blood was collected every 2 h from 0 to 8 h and every 12 h from 12 to 72 h for hematology, and every 1 h from 0 to 2 h and every 12 h from 12 to 72 h for serum analysis.
2Represents treatment P-value. All treatment × time interactions were P ≥ 0.19.
3Geometric mean florescence intensity (GMFI) for neutrophil oxidative burst intensity, phagocytosis, and L-selectin expression. Whole blood was collected −72, 0, and 48 h relative to Mannheimia haemolytica immunological challenge.
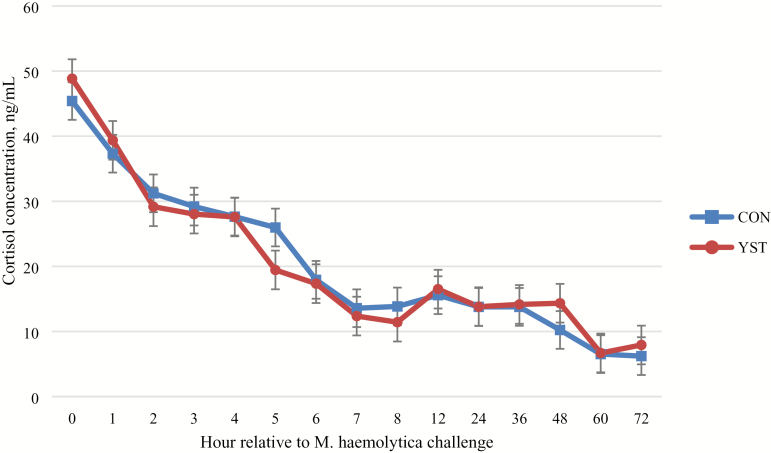
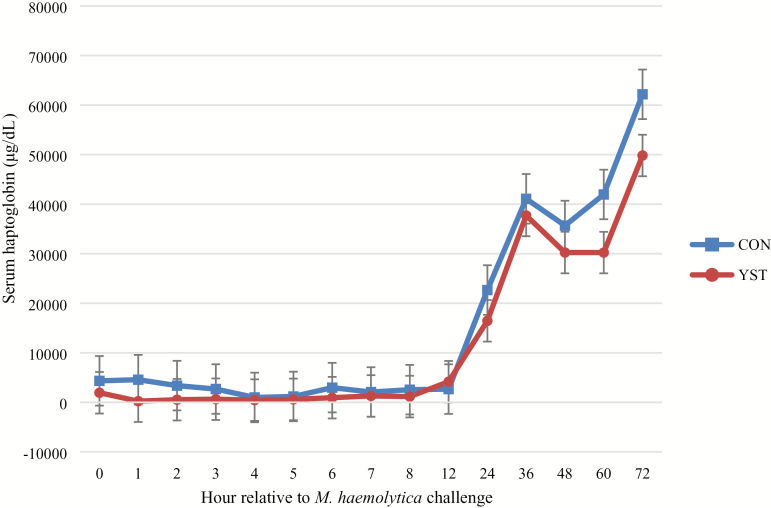
No treatment × time interaction occurred (P = 0.98) for serum cortisol concentration (Figure 2). Serum cortisol was similar (P = 0.90) between treatments (20.7 ± 1.14 vs. 20.5 ± 1.14 ng/mL for CON and YST, respectively). Serum cortisol concentration was greatest at time of M. haemolytica challenge and decreased over time (P < 0.01). No treatment × time interaction (P = 0.23) occurred for any hematological measure. Hematological measures, except for neutrophil concentrations, were not different (P ≥ 0.16) between treatments. Total neutrophil concentration tended (P = 0.07) to be greater in CON heifers (6.4 ± 0.39 vs. 5.4 ± 0.37 103 cells per µL, respectively). Number of neutrophils in circulation increased following live pathogen challenge until hour 8 and then decreased (P < 0.01). No treatment × time interaction occurred (P ≥ 0.47) for any measure of neutrophil functionality. Neutrophil phagocytosis, oxidative burst intensity, and expression of L-selectin were not different between treatments (P ≥ 0.13). Oxidative burst intensity and expression of L-selectin increased in both treatments over time (P < 0.001). There was no treatment × time interaction (P = 0.94) for serum haptoglobin concentration. Haptoglobin was not different (P = 0.13) between treatments (15,396.2 ± 1,631.7 vs. 11,757.3 ± 1,631.7 µg/dL; P = 0.13; Figure 3), although haptoglobin increased (time effect: P < 0.01) in both treatments and remained so through the final sampling time point at 72 h following the BHV-1/M. haemolytica challenge.
Figure 2.
Effect of yeast supplementation on serum cortisol response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. Data presented as least squares means ± SEM. There was no treatment × time interaction (P = 0.98) and serum cortisol was not affected by dietary treatment (P = 0.90), although serum cortisol decreased over time (P < 0.01) as cattle adapted to stanchions.
Figure 3.
Effect of yeast supplementation on serum haptoglobin response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. Data presented as least squares means ± SEM. There was no treatment × time interaction (P = 0.94) and serum haptoglobin was not affected by dietary treatment (P = 0.13), although serum haptoglobin increased over time (P < 0.01).
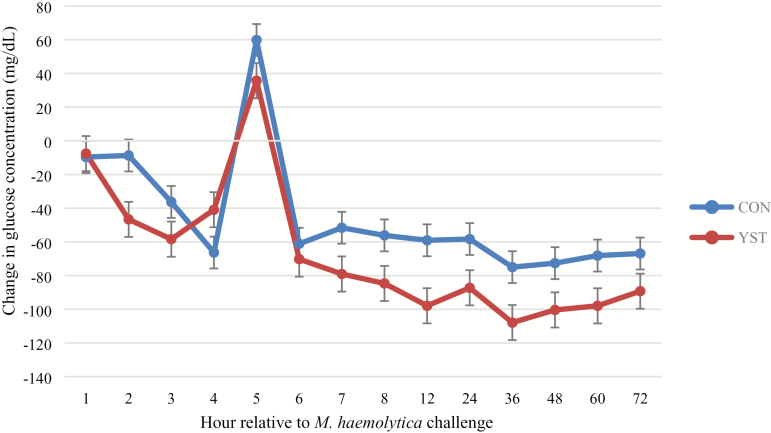
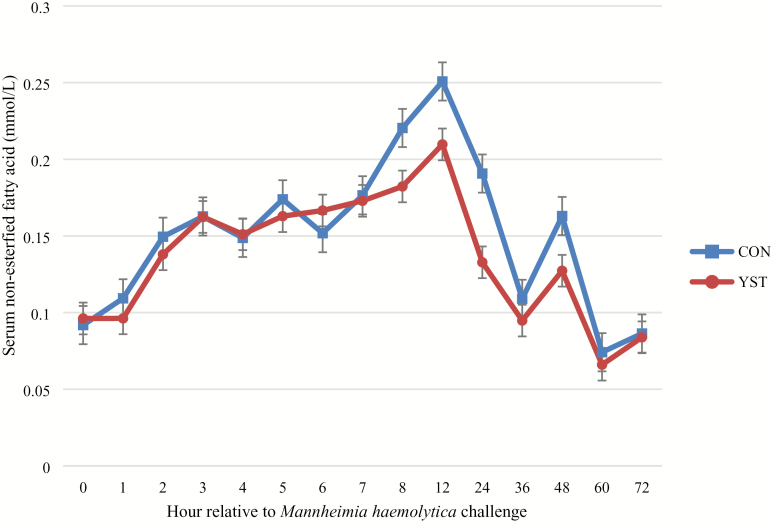
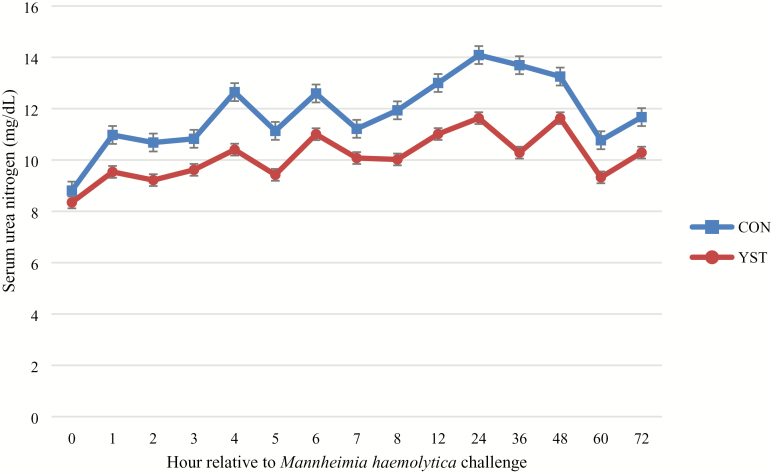
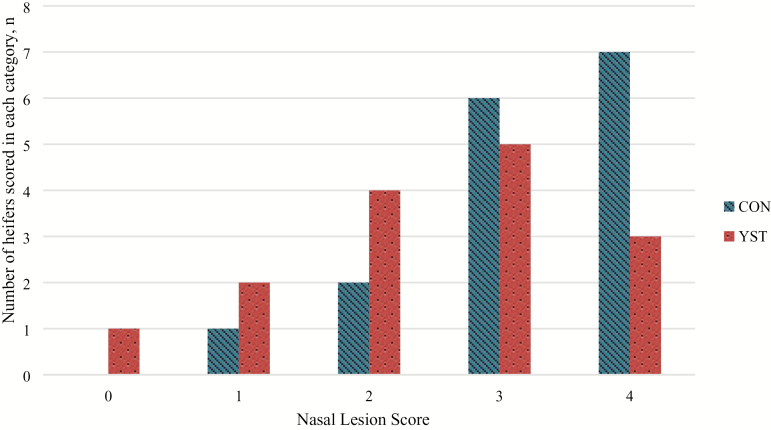
There was a trend (P = 0.04) for a treatment × time interaction for serum glucose where CON heifers had decreased serum glucose at 1 h and remained so until 6 h following BHV-1/M. haemolytica challenge, at which time the treatments were similar. Serum glucose concentration was similar between treatments (P = 0.25; Figure 4). There was no treatment × time interaction (P ≥ 0.65) for serum NEFA or urea N concentrations. Serum NEFA increased in both treatments from 0 to 12 h, and subsequently decreased (time P < 0.01), but was not different (P = 0.37) between treatments (0.15 ± 0.01 vs. 0.14 ± 0.01 mmol/L for CON and YST, respectively; Figure 5). Serum urea N was greater (P = 0.03) in the CON treatment compared to YST (11.8 ± 0.53 vs. 10.1 ± 0.53 mg/dL, respectively; Figure 6). Serum urea N generally increased over time following BHV-1/M. haemolytica challenge (P < 0.001). Nasal lesion scores recorded 6 d following BHV-1 challenge tended (P = 0.07) to be more severe in CON heifers compared to YST (Figure 7). However, heifers in both treatments presented with nasal lesions of varying severity.
Figure 4.
Effect of yeast supplementation on serum glucose response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). Data presented as least squares means ± SEM and 0 h was used as a covariate in the analysis. All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 (BHV-1) at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. There was a tendency for a treatment × time interaction (P = 0.04) such that CON heifers had decreased serum glucose at 1 h and remained so until 6 h following BHV-1/M. haemolytica. Serum glucose was similar between dietary treatments (P = 0.25).
Figure 5.
Effect of yeast supplementation on serum NEFA response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. Data presented as least squares means ± SEM. Serum NEFA was not affected by dietary treatment (P = 0.37) and there was no treatment × time interaction (P = 0.88).
Figure 6.
Effect of yeast supplementation on serum urea nitrogen response in beef heifers (n = 16/trt). Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h. Data presented as least squares means ± SEM. Serum urea nitrogen was decreased in YEAST compared to CON (P = 0.03) and there was no treatment × time interaction (P = 0.65).
Figure 7.
Effect of yeast supplementation on nasal lesion scores (0–4) in beef heifers (n = 16/trt) All heifers were challenged intranasally with 2 × 108 plaque-forming units bovine herpesvirus-1 (BHV-1) at −144 h and 3 × 107 colony-forming units Mannheimia haemolytica at 0 h and scores were collected 72 h following BHV-1 challenge. Heifers were fed one of two dietary treatments: 1) negative control (CON), fed a standard feedlot diet, or 2) the same standard feedlot diet with 2.5 g·heifer−1·d−1 live yeast and 2.5 g·heifer−1·d−1 yeast cell wall product (Phileo Lesaffre Animal Care, Milwaukee, WI) for 31 d prior to challenge (YST). Score was determined according to the following criteria: 0 = absence of lesions; 1 = less than 10% coverage of the visible naris; 2 = 11% to 25% coverage; 3 = 26% to 50% coverage; and 4 = 50% or greater coverage of the visible naris. Data presented as number of animals receiving a score within each category. Nasal lesion scores tended (P = 0.07) to be more severe in CON compared to YST.
DISCUSSION
Dietary treatment did not affect vaginal temperature in the current study. In contrast, Finck et al. (2014) reported decreased rectal temperature following an lipopolysaccharide (LPS) challenge in steers fed yeast cell wall or a combination live and yeast cell wall compared to a negative control. Vaginal temperature increased in all treatments when calves were experimentally challenged at 0 h. Peak temperatures (40.2 °C in both treatments) were similar to the threshold (40 °C) febrile response reported by Perino and Apley (1998) for BRD treatment in feedlots. Average temperature in each treatment group in this trial was near the threshold for clinical illness outlined by Duff and Galyean (2007), and is similar to a febrile response from which cellular immunological responses could be anticipated (Thomson et al., 1975). Although the current study collected vaginal temperature, rectal and vaginal temperatures have been reported to be highly correlated during immunological challenges (Burdick et al., 2011; Falkenberg et al., 2014). Jericho and Langford (1978) established an interaction between BHV-1 and M. haemolytica in BRD when they reported fibrous pneumonic lesions similar to lesions occurring in field BRD calves experimentally challenged with these pathogens, dependent on a 3- to 5-d interval between viral and bacterial inoculation similar to the interval used in the current trial. Vaginal temperature also fluctuated within each day, which was expected. Although heifers were housed in an environmentally controlled barn, daily ambient temperature fluctuations occurred, which paired with the circadian rhythm of vaginal temperature may have resulted in the daily fluctuations observed. Burdick et al. (2011) reported that the vaginal temperature devices used in the current trial are capable of measuring the circadian rhythm in vaginal temperature.
Water intake was greater in the YST treatment compared to CON. Previous literature has reported that frequency and duration of water intake was similar (Sowell et al., 1999) or increased (Buhman et al., 2000) in feedlot cattle that were clinically ill compared to healthy cattle. Water is required for multiple maintenance functions including regulation of body temperature, metabolism, and transportation of cells through the bloodstream, including immune cells. In terms of total daily intake, heifers in both treatment groups were not meeting outlined requirements for water intake in finishing cattle (NRC, 2000). However, hematocrit percentage and other hematological measurements were similar between treatments and were within the range of what is considered normal in beef cattle (Radostits et al., 2000) so water intake is not expected to have affected the interpretation of hematological or other measures reported in this trial.
Nasal lesion scores tended to be more severe in the CON treatment. Ulcerated nasal mucosa is a symptom of respiratory infection with BHV-1, and leads to the more severe “red nose” infection frequently associated with infectious bovine rhinotracheitis (Nandi et al., 2009). Heifers in both treatment groups exhibited nasal lesion scores ≥1 and had lesions of varying severity present; therefore, the BHV-1 challenge did successfully elicit clinical signs of an immune response in both treatment groups.
Dietary treatment did not affect serum cortisol concentration in the current study. This response is similar to the serum cortisol response observed by Burdick Sanchez et al. (2013), who reported no difference when beef heifers were supplemented with yeast cell wall compared to a negative control and challenged with LPS. In contrast, Finck et al. (2014) reported that feeding combination live yeast and yeast cell wall to beef steers decreased serum cortisol concentrations compared to a negative control following an LPS challenge. In the current study, increased cortisol production due to stress during processing and administration of the bacterial challenge marked a rapid increase in circulating cortisol in both treatments. However, serum cortisol concentrations in both treatments were greatest immediately following M. haemolytica challenge and decreased at each subsequent time point. Such a response suggests that heifers may have been stressed due to handling involved with catheterization and challenge, but serum cortisol concentrations decreased as heifers became adapted to the stanchions. Therefore, there appears to be no cortisol response directly to the viral-bacterial challenge.
A tendency for greater circulating neutrophil concentrations occurred in CON compared to YST in the current trial. While the mode of action of yeast products has not been clearly defined, cell wall of S. cerevisiae contains β-glucans that have also been reported to enhance phagocytic activity of neutrophils in vitro (Akramiene et al., 2007). Neutrophil concentration in circulation has also been reported to increase in the presence of glucocorticoids, as L-selectin expression and therefore chemotaxis of neutrophils into peripheral tissues decreases in the presence of glucocorticoids (Burton et al., 1995). However, these neutrophils may exhibit a “rebound effect” after circulating glucocorticoids decrease that allow chemotaxis into peripheral tissues, increasing phagocytic activity and thereby lung tissue damage in the case of BRD-affected calves (Burton et al., 2005). While increased circulating neutrophils may indicate greater probability for long-term tissue damage as neutrophils undergo apoptosis or migrate into peripheral tissue, expression of L-selectin was not different between treatments in the current trial. Therefore, the tendency for increased circulating neutrophils was not explained by decreased chemotaxis mediated by L-selectin. Results for both treatments agree with the literature as expression of L-selectin increased over time in conjunction with decreased circulating cortisol (Burton et al., 1995). In contrast to Akramiene et al. (2007), PMN phagocytosis and oxidative burst intensity in the current trial were not different between treatments but generally increased following M. haemolytica challenge as neutrophils became active in tissue and began phagocytizing and ultimately destroying bacterial pathogens.
Serum haptoglobin concentrations were similar between CON and YST treatments following the BHV-1/M. haemolytica challenge. Haptoglobin is an acute-phase protein secreted from hepatocytes during inflammation, is undetectable in healthy calves, and could take up to 4 to 8 d after live pathogen infection to be detectable in sick cattle (Ganheim et al., 2003). In the current study, data were only collected for 6 d following BHV-1 inoculation, which may not have been enough time to measure peak haptoglobin response. Future studies should collect serum haptoglobin concentrations for up to 14 d, as concentrations may begin to return to normal after 13 d (Ganheim et al., 2003). Acute-phase protein production from hepatocytes is stimulated by pro-inflammatory cytokines, specifically IL-6 (Naka et al., 2002). Burdick Sanchez et al. (2013) reported that serum IL-6 concentration was decreased in cattle supplemented with a similar yeast cell wall product when compared to a negative control following LPS challenge. In contrast, Zaworski et al. (2014) reported no difference in serum haptoglobin concentration postpartum in dairy cows supplemented with S. cerevisiae. However, these authors did report increased Serum Amyloid A concentration 1 d after calving in cows supplemented with S. cerevisiae, which they hypothesize may be beneficial after calving because acute-phase protein production creates a negative feedback loop that downregulates the production of pro-inflammatory cytokines.
Immune responses to an immunological insult require large amounts of energy and potentially change nutrient partitioning. A 1 °C increase in body temperature increases resting metabolic rate by 10% to 20% in mice (Buchanan et al., 2003), and further energy is required to produce acute-phase proteins and other mediators of inflammation and the immune response (Carroll and Forsberg, 2007). In the current study, there was a treatment effect for serum glucose concentrations where serum glucose was greater in the YST treatment compared to CON. The increase in glucose concentration in the YST group may imply that a greater amount of readily available glucose was being utilized in the CON treatment compared to the YST, potentially to meet increased metabolic requirements of the immune system. Cytokines were reported to stimulate hepatic gluconeogenesis while increasing overall utilization of glucose in mice and pigs (Lang and Spitzer, 1987; Webel et al., 1997), although this proposed mechanism cannot be confirmed as cytokines were not measured in the current study. When challenging with LPS and feeding a yeast cell wall, Burdick Sanchez et al. (2014) reported no difference in serum glucose concentration compared to a negative control, although insulin concentration was greatest in cattle supplemented with yeast cell wall, which is in contrast with results of the current trial. However, Zaworski et al. (2014) reported a tendency for increased serum glucose and serum urea nitrogen postpartum in dairy cows fed a S. cerevisiae strain, which the authors propose to be a faster transition out of a negative energy balance postpartum. Metabolic results of that study could be in agreement with results of the current study, where increased serum glucose concentration is considered desirable because cattle are not partitioning that energy elsewhere.
Immunological insults initially induce hyperglycemia, as has been demonstrated during the stress response, and subsequently cause hypoglycemia in response to increased metabolic demands for energy. Waggoner et al. (2009) reported that beef steers challenged with LPS initially increased above and subsequently decreased below the serum glucose concentration of an unchallenged cohort, suggesting an initial increase during the inflammatory response and a decrease as cattle use the energy to deal with the immunological challenge, which could explain the rapid increase at 5 h and subsequent decrease reported in the current trial. When testing for a metabolite that could aid in the prediction of BRD outcomes, Montgomery et al. (2009) reported a linear decrease in blood glucose concentration measured on-arrival as subsequent number of BRD treatments during the receiving period increased. The authors hypothesized cattle with decreased blood glucose on-arrival that were later treated for BRD may have been exposed to pathogens prior to arrival and experienced hypoglycemia on-arrival because they were already fighting disease. Serum glucose concentration in the current trial was decreased in the CON treatment from 1 to 5 h compared to the YST treatment, in line with the initial decrease that Montgomery et al. (2009) and Waggoner et al. (2009) have suggested to be indicative of disease risk and indicates that cattle were already responding to the BHV-1 challenge prior to the M. haemolytica challenge administered at 0 h.
Serum NEFA concentrations were similar between CON and YST treatments. In contrast, previous studies have reported both decreased IL-6 production and subsequent decreased serum NEFA concentration in cattle fed yeast cell wall compared to a negative control when each were challenged with LPS (Burdick Sanchez et al., 2013, 2014). Exposure to endotoxin induced hypertriglyceridemia by inhibiting lipoprotein lipase activity and inducing hepatic fatty acid synthesis in rats, which is stimulated in part by production of the pro-inflammatory cytokine IL-6 (Hardardottir et al., 1994). The authors explain benefits of these metabolic changes include meeting increased metabolic needs and the ability of lipoproteins to both bind endotoxin and inhibit viral replication. An increase in serum NEFA in all treatments following BHV-1/M. haemolytica challenge in the current trial could be the mixed result of cattle in both treatments needing to meet the energetic requirements of the immune system and altered need for antiviral mechanisms following the viral challenge.
Serum urea N was increased in the CON treatment compared to YST in the current study. Pro-inflammatory cytokines have been reported to increase catabolism of lean tissue in pigs (Hardardottir et al., 1994), potentially to provide amino acids required for synthesis of acute-phase proteins from hepatocytes (Reeds et al., 1994). Glucocorticoids have also been reported to have catabolic effects on tissues, increasing proteolysis and lipolysis, and increasing NEFA and urea N concentrations in blood (Brockman and Laarveld, 1986; Elsasser et al., 1997). However, previous research comparing cattle fed yeast cell wall products to a negative control reported no differences in urea N when cattle were challenged with LPS (Sanchez et al., 2014). Increased serum urea N concentrations in the CON treatment in the current study support the hypothesis that yeast supplementation may be modulating the immune system in a manner that requires decreased inflammatory response and thereby decreased lean tissue catabolism. However, this hypothesis is not supported by the alteration of any immune variables measured in the current study. Although a reduction in serum urea N could result in improved performance in cattle supplemented with yeast and exposed to an immunological insult, previous studies have reported mixed results in live performance when cattle are supplemented with yeast products. Specifically, yeast cell wall supplementation tended to increase average daily gain (ADG) over 14 d following an LPS challenge, although performance in the study was measured only on 24 animals (Burdick Sanchez et al., 2014). However, Finck et al. (2014) reported no difference in live feedlot performance when cattle were supplemented with a yeast cell wall product without an immunological challenge but with a trend toward decreased morbidity in cattle supplemented with yeast. It is therefore unknown if the change in metabolism in cattle supplemented with yeast in the current trial may carry over to live performance and clinical health differences when cattle are exposed to a natural immunological insult. Further research may help elucidate more exact performance differences and if yeast supplementation may be more beneficial in certain populations of cattle where increased morbidity is expected.
Results of this trial indicate that alterations to the immune response to a BRD challenge when cattle are supplemented with combination live yeast and yeast cell wall may be minimal, while some aspects of the metabolic response may be affected. Specifically, these data suggest that serum urea N could be reduced when cattle are supplemented with yeast and exposed to an immunological challenge. While nasal lesion scores tended to be less severe and neutrophil concentration tended to be decreased when cattle were supplemented with yeast, no alteration in vaginal temperature, serum haptoglobin, cortisol, NEFA, or neutrophil functionality was observed.
ACKNOWLEDGMENTS
The authors acknowledge the technical support of J. W. Dailey, J. R. Carroll, and R. Buchanan. Research was funded by Phileo Lesaffre Animal Care, Milwaukee, WI. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
Conflict of interest statement: None declared.
LITERATURE CITED
- Akramiene D., Kondrotas A., Didziapetriene J., and Kevelaitis E.. 2007. Effects of beta-glucans on the immune system. Medicina (Kaunas) 43:597–606. doi:10.3390/medicina43080076 [PubMed] [Google Scholar]
- Broadway P. R., Carroll J. A., and Sanchez N. C.. 2015. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: a review. Microorganisms 3:417–427. doi:10.3390/microorganisms3030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. P., and Laarveld B.. 1986. Hormonal regulation of metabolism in ruminants; a review. Livest. Prod. Sci. 14:313–334. doi:10.1016/0301-6226(86)90012-6 [Google Scholar]
- Buchanan J. B., Peloso E., and Satinoff E.. 2003. Thermoregulatory and metabolic changes during fever in young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285:R1165–R1169. doi:10.1152/ajpregu.00238.2003 [DOI] [PubMed] [Google Scholar]
- Buhman M. J., Perino L. J., Galyean M. L., Wittum T. E., Montgomery T. H., and Swingle R. S.. 2000. Association between changes in eating and drinking behaviors and respiratory tract disease in newly arrived calves at a feedlot. Am. J. Vet. Res. 61:1163–1168. doi:10.2460/ajvr.2000.61.1163 [DOI] [PubMed] [Google Scholar]
- Burdick N. C., Carroll J. A., Dailey J. W., Randel R. D., Falkenberg S. M., and Schmidt T. B.. 2011. Development of a self-contained, indwelling vaginal temperature probe for use in cattle research. J. Thermal. Biol. 37:339–343. doi:10.1016/j.jtherbio.2011.10.007 [Google Scholar]
- Burdick Sanchez N. C., Young T. R., Carroll J. A., Corley J. R., Rathmann R. J., and Johnson B. J.. 2013. Yeast cell wall supplementation alters aspects of the physiological and acute phase responses of crossbred heifers to an endotoxin challenge. Innate Immun. 19:411–419. doi:10.1177/1753425912469673 [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez N. C., Young T. R., Carroll J. A., Corley J. R., Rathmann R. J., and Johnson B. J.. 2014. Yeast cell wall supplementation alters the metabolic responses of crossbred heifers to an endotoxin challenge. Innate Immun. 20:104–112. doi:10.1177/1753425913482152 [DOI] [PubMed] [Google Scholar]
- Burton J. L., Kehrli M. E. Jr, Kapil S., and Horst R. L.. 1995. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 57:317–325. doi:10.1002/jlb.57.2.317 [DOI] [PubMed] [Google Scholar]
- Burton J. L., Madsen S. A., Chang L. C., Weber P. S., Buckham K. R., van Dorp R., Hickey M. C., and Earley B.. 2005. Gene expression signatures in neutrophils exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet. Immunol. Immunopathol. 105:197–219. doi:10.1016/j.vetimm.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., and Forsberg N. E.. 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am. Food Anim. Pract. 23:105–149. doi:10.1016/j.cvfa.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Dennis E. J., Schroeder T. C., Renter D. G., and Pendell D. L.. 2018. Value of arrival metaphylaxis in U.S. cattle industry. J. Agr. Resour. Econ. 43:233–250. [Google Scholar]
- Duff G. C., and Galyean M. L.. 2007. Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi:10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser T. H., Kahl S., Steele N. C., and Rumsey T. S.. 1997. Nutritional modulation of somatotropic axis-cytokine relationships in cattle: a brief review. Comp. Biochem. Physiol. A Physiol. 116:209–221. doi:10.1016/S0300-9629(96)00279-4 [DOI] [PubMed] [Google Scholar]
- Falkenberg S. M., Ridpath J., Vander Ley B., Bauermann F. V., Burdick Sanchez N. C., and Carroll J. A.. 2014. Comparison of temperature fluctuations at multiple anatomical locations in cattle during exposure to bovine viral diarrhea virus. Live. Sci. 164:159–167. doi:10.1016/j.livsci.2014.03.018 [Google Scholar]
- Finck D. N., Ribeiro F. R. B., Burdick N. C., Parr S. L., Carroll J. A., Young T. R., Bernhard B. C., Corley J. R., Estefan A. G., Rathmann R. J.,. et al. 2014. Yeast supplementation alters the performance and health status of receiving cattle. Prof. Anim. Sci. 30:333–341. doi:10.15232/S1080-7446(15)30125-X [Google Scholar]
- Ganheim C., Hultén C., Carlsson U., Kindahl H., Niskanen R., and Waller K. P.. 2003. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J. Vet. Med. B Infect. Dis. Vet. Public Health 50:183–190. doi:10.1046/j.1439-0450.2003.00658.x [DOI] [PubMed] [Google Scholar]
- Hardardottir I., Grünfeld C., and Feingold K. R.. 1994. Effects of endotoxin and cytokines on lipid metabolism. Curr. Opin. Lipidol. 5:207–215. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., and Langford E. V.. 1978. Pneumonia in calves produced with aerosols of bovine herpesvirus 1 and Pasteurella haemolytica. Can. J. Comp. Med. 42:269–277. [PMC free article] [PubMed] [Google Scholar]
- Keyser S. A., McMeniman J. P., Smith D. R., MacDonald J. C., and Galyean M. L.. 2007. Effects of Saccharomyces cerevisiae subspecies boulardii CNCM I-1079 on feed intake by healthy beef cattle treated with florfenicol and on health and performance of newly received beef heifers. J. Anim. Sci. 85:1264–1273. doi:10.2527/jas.2006-751 [DOI] [PubMed] [Google Scholar]
- King D. A., Schuehle Pfeiffer C. E., Randel R. D., Welsh T. H. Jr, Oliphint R. A., Baird B. E., Curley K. O. Jr, Vann R. C., Hale D. S., and Savell J. W.. 2006. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 74:546–556. doi:10.1016/j.meatsci.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Lang C. H., and Spitzer J. A.. 1987. Glucose kinetics and development of endotoxin tolerance during long-term continuous endotoxin infusion. Metabolism 36:469–474. doi:10.1016/0026-0495(87)90045-X [DOI] [PubMed] [Google Scholar]
- Montgomery S. P., Sindt J. J., Greenquist M. A., Miller W. F., Pike J. N., Loe E. R., Sulpizio M. J., and Drouillard J. S.. 2009. Plasma metabolites of receiving heifers and the relationship between apparent bovine respiratory disease, body weight gain, and carcass characteristics. J. Anim. Sci. 87:328–333. doi:10.2527/jas.2008-0969 [DOI] [PubMed] [Google Scholar]
- Naka T., Nishimoto N., and Kishimoto T.. 2002. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 4(Suppl. 3):S233–S242. doi:10.1186/ar565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S., Kumar M., Manohar M., and Chauhan R. S.. 2009. Bovine herpes virus infections in cattle. Anim. Health Res. Rev. 10:85–98. doi:10.1017/S1466252309990028 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th ed. Washington (DC): Natl. Acad. Press. [Google Scholar]
- Obeidat B. S., Cobb C. J., Sellers M. D., Pepper-Yowell A. R., Earleywine T. J., and Ballou M. A.. 2013. Plane of nutrition during the preweaning period but not the grower phase influences the neutrophil activity of Holstein calves. J. Dairy Sci. 96:7155–7166. doi:10.3168/jds.2013-6699 [DOI] [PubMed] [Google Scholar]
- Perez-Sotelo L. S., Talavera-Rojas M., Monroy-Salazar H. G., Lagunas-Bernabé S., Cuarón-Ibargüengoytia J. A., Jimenez R. M., and Vázquez-Chagoyán J. C.. 2005. In vitro evaluation of the binding capacity of Saccharomyces cerevisiae Sc47 to adhere to the wall of Salmonella spp. Rev. Latinoam. Microbiol. 47:70–75. [PubMed] [Google Scholar]
- Perino L. J., and Apley M. D.. 1998. Clinical trial design in feedlots. Vet. Clin. North Am. Food Anim. Pract. 14:343–365. doi:10.1016/S0749-0720(15)30258-9 [DOI] [PubMed] [Google Scholar]
- Posadas, G. A., P. R. Broadway, J. A. Thornton, J. A. Carroll, A. Lawrence, J. R. Corley, A. Thompson, and J. R. Donaldson. 2017. Yeast pro- and paraprobiotics have the capability to bind pathogenic bacteria associated with animal disease. Transl. Anim. Sci. 1(1):60–68. doi:10.2527/tas2016.0007. doi: 10.2527/tas2016.0007. [DOI] [PMC free article] [PubMed]
- Radostits O. M., Gay C. C., Blood D. C., and Hinchcliff K. W.. 2000. Veterinary medicine. 9th ed. London: W.B. Saunders. [Google Scholar]
- Reeds P. J., Fjeld C. R., and Jahoor F.. 1994. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states?J. Nutr. 124:906–910. doi:10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Sowell B. F., Branine M. E., Bowman J. G., Hubbert M. E., Sherwood H. E., and Quimby W.. 1999. Feeding and watering behavior of healthy and morbid steers in a commercial feedlot. J. Anim. Sci. 77:1105–1112. doi:10.2527/1999.7751105x [DOI] [PubMed] [Google Scholar]
- Thomson R. G., Chander S., Savan M., and Fox M. L.. 1975. Investigation of factors of probable significance in the pathogenesis of pneumonic pasteurellosis in cattle. Can. J. Comp. Med. 39:194–207. [PMC free article] [PubMed] [Google Scholar]
- USDA 2013a. Types and costs of respiratory disease treatments in U.S. feedlots Fort Collins (CO): USDA, Animal and Plant Health Inspection Service, Veterinary Services, Center for Epidemiology and Animal Health, National Animal Health Monitoring System; Available from https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_is_RespDis.pdf. Accessed August 2017. [Google Scholar]
- USDA 2013b. Feedlot 2011 part IV: health and health management on U.S. feedlots with a capacity of 1,000 or more head Fort Collins (CO): USDA, Animal and Plant Health Inspection Service, Veterinary Services, Center for Epidemiology and Animal Health, National Animal Health Monitoring System; Available from https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartIV.pdf. [Google Scholar]
- Waggoner J. W., Löest C. A., Turner J. L., Mathis C. P., and Hallford D. M.. 2009. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J. Anim. Sci. 87:3656–3668. doi:10.2527/jas.2009-2011 [DOI] [PubMed] [Google Scholar]
- Webel D. M., Finck B. N., Baker D. H., and Johnson R. W.. 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514–1520. doi:10.2527/1997.7561514x [DOI] [PubMed] [Google Scholar]
- Zaworski E. M., Shriver-Munsch C. M., Fadden N. A., Sanchez W. K., Yoon I., and Bobe G.. 2014. Effects of feeding various dosages of Saccharomyces cerevisiae fermentation product in transition dairy cows. J. Dairy Sci. 97:3081–3098. doi:10.3168/jds.2013-7692 [DOI] [PubMed] [Google Scholar]