Abstract
Although microalgae can be used as a source of energy and macronutrients in pig diets, there is limited information on the use of partially de-oiled microalgae coproducts in swine feeding programs. The objectives of this study were to evaluate the effects of a partially de-oiled microalgae extract (MAE) in nursery pig diets on growth performance and health status. A total of 300 pigs (initial BW = 6.3 ± 2.1 kg) were used in a 42-d experiment. Treatments included a standard corn-soybean meal control diet, and diets containing 1, 5, 10, or 20% MAE replacing primarily corn. The ME content of MAE was calculated from the chemical composition, and diets were formulated to meet or exceed nutrient requirements for nursery pigs. Pigs were stratified by weaning BW into 12 blocks in a randomized complete block design, with sex distributed evenly among blocks. Pens of pigs (5 pigs/pen) were assigned randomly within block to one of five dietary treatments. Pig BW and feed disappearance were recorded weekly. On day 42, 30 pigs were harvested and sections of the jejunum and ileum were collected for gut morphology analysis, and a liver sample was collected for metabolomic analysis using liquid chromatography-mass spectroscopy. Data were analyzed by ANOVA with diet as treatment effect, and contrasts were used to test linear or quadratic effects of dietary MAE inclusion level. Overall, pigs fed 1% and 5% MAE had the greatest (quadratic P < 0.05) ADG, resulting from greater (quadratic P < 0.05) ADFI. There was a tendency for a greater number of pigs requiring injectable treatments (P = 0.16) and a greater mortality (P = 0.14) in pigs fed the control diet than pigs in any of the diets with the MAE. Final BW increased (P < 0.05) for pigs fed 1% and 5% MAE diets. The improvements in ADG were not explained by differences in mucosa height or goblet cell count among dietary treatments. Pigs fed diets containing 1% or 5% MAE had relatively less concentration (P < 0.05) of ammonia in the liver and had changes in metabolites associated with the urea cycle. In conclusion, feeding MAE resulted in increased growth responses and may have beneficial health effects when fed to nursery pigs.
Keywords: de-oiled microalgae, growth performance, mortality, nursery pigs
INTRODUCTION
Microalgae are single-celled microorganisms with multiple industrial uses, such as biofuel production (Gatrell et al., 2014) and waste water remediation (Lu et al., 2015, 2016). Likewise, interest in using microalgae coproducts in animal-feeding programs is increasing because microalgae grow rapidly and can sequester and convert carbon dioxide into energy- and nutrient-rich biomass that can be later fed to livestock at lower carbon, water, or land footprint than common feed ingredients (Gatrell et al., 2014). Depending on the specific species, cell fraction, and processing methods used, microalgae contain variable concentrations of protein (10–70%), lipids (1–40%), and carbohydrates (8–30%), as well as vitamins and trace minerals in forms that appear to be highly bioavailable (Becker, 2004). Microalgae and their coproducts have also been shown to have several functional properties that include serving as antibacterial, antiviral, and antioxidant agents (Ma et al., 2015; De Jesus Raposo et al., 2016). Therefore, microalgae and their coproducts could be used not only as sources of energy and nutrients, but also as sources of nutraceuticals and prebiotic carbohydrates in animal feeds (Yang et al., 2014).
Unfortunately, there is limited information on the benefits and limitations of various feeding applications of microalgae coproducts in swine diets (Gatrell et al., 2014). Early studies showed that feeding some species of microalgae to pigs had no negative effects on growth performance (Hintz and Heitman, 1967; Fevrier and Seve, 1975), and replacing soy protein in weaned pig diets with a microalgae mixture resulted in no diarrhea or gastrointestinal lesions (Yap et al., 1982). More recent studies have shown that feeding either full-fat or defatted microalgae biomass to weaned pigs either had no effect on growth rate and health status (Isaacs et al., 2011) or reduced growth performance (Lum et al., 2013). The lack of adequate nutrient profile (content and digestibility) may explain the decrease in growth performance when new feed ingredients are evaluated. Likewise, microalgae biomass could have nutritional beneficial properties that are unknown. Therefore, the aim of this study was to evaluate the potential use of microalgae extract (MAE) as a feed ingredient in nursery pig diets.
MATERIALS AND METHODS
All experimental procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocol No. 1411-32072A).
Ingredient
The MAE coproduct used in this study was provided by Solazyme Inc. (TerraVia, San Francisco, CA). The MAE product was analyzed using standard procedures (AOAC, 2012) for moisture (Method 030.15), CP (Method 990.03), ether extract (EE; Method 920.39), and ash (Method 942.05) content, complete amino acid profile (Method 982.3; including sections a, b, and c for hydrolysis of cysteine, methionine, and tryptophan), and complete fatty acid profile (Method 996.06; Table 1) by the New Jersey Feed Laboratory, Inc. (Ewin Township, NJ).
Table 1.
Nutrient composition of the partially de-oiled microalgae extract (MAE) supplemented to nursery pig diets
| Nutrient | Value* |
|---|---|
| Proximate analysis, % as-fed | |
| Moisture | 3.99 |
| Crude protein | 5.72 |
| Ether extract | 7.63 |
| Ash | 6.20 |
| Carbohydrates by difference† | 76.62 |
| Total dietary fiber, % | 33.30 |
| Soluble dietary fiber | 13.60 |
| Insoluble dietary fiber | 19.70 |
| Neutral detergent fiber, % | 7.70 |
| Acid detergent fiber, % | 2.87 |
| Metabolizable energy content, kcal/kg‡ | 2,600 |
| Amino acid profile, % as-fed | 4.38 |
| Methionine | 0.08 |
| Cystine | 0.07 |
| Lysine | 0.05 |
| Phenylalanine | 0.21 |
| Leucine | 0.42 |
| Isoleucine | 0.19 |
| Threonine | 0.20 |
| Valine | 0.29 |
| Histidine | 0.10 |
| Arginine | 0.13 |
| Glycine | 0.28 |
| Aspartic acid | 0.45 |
| Serine | 0.27 |
| Glutamic acid | 0.84 |
| Proline | 0.26 |
| Hydroxyproline | 0.02 |
| Alanine | 0.34 |
| Tyrosine | 0.10 |
| Tryptophan | 0.05 |
| Taurine | 0.03 |
| Fatty acid profile, % as-fed | 7.19 |
| Capric | 0.02 |
| Lauric | 0.08 |
| Myristic | 0.07 |
| Palmitic | 0.41 |
| Palmitoleic | 0.01 |
| Heptadecanoic | 0.00 |
| Stearic | 0.20 |
| Oleic | 6.20 |
| Linoleic | 0.13 |
| Linolenic | 0.02 |
| Arachidic | 0.03 |
| Eicosanoic | 0.01 |
| Eicosapentaenoic (EPA) | 0.01 |
| Glycosyl composition of nonhydrolyzed MAE§ | |
| Arabinose | Not detected |
| Rhamnose | Not detected |
| Xylose | Not detected |
| Mannose | 11.2 |
| Galactose | 2.8 |
| Glucose | 86.0 |
| Glycosyl composition of hydrolyzed MAE# | |
| Arabinose | 2.3 |
| Rhamnose | Not detected |
| Fructose | Not detected |
| Xylose | 0.5 |
| Mannose | 10.8 |
| Galactose | 15.1 |
| Glucose | 71.3 |
*Values are the result of one analyzed sample.
†Calculated as 100 − (DM + CP + ether extract + ash).
‡Calculated (Noblet and Perez, 1993): ME (kcal/kg as-fed) = 4,194 − (9.2 × ash) + (1.0 × CP) + (4.1 × EE) − (3.5 × NDF), where CP, EE, and NDF composition data (g/kg) were used on an as-fed basis.
§Glycosyl composition (mol % as-fed) before hydrolysis with 2 M trifluoracetic acid.
#Glycosyl composition (mol % as-fed) after hydrolysis with 2 M trifluoracetic acid.
Glycosyl Composition
Carbohydrate composition of MAE was analyzed using several different assays (Table 1). Briefly, the glycosyl composition of the sample (1.5 mg) was analyzed before and after hydrolysis in 2 M trifluoroacetic acid (Sigma-Aldrich, St. Louis, MO) at the Complex Carbohydrate Research Center (Athens, GA). The sample (1.5 mg) was placed in a screw-cap tube with 80 µg of inositol as internal standard and hydrolyzed in 2 M trifluoroacetic for 2 h in sealed tubes at 121 °C, reduced with sodium borodeuteride (NaBD4), and acetylated using acetic anhydride/trifluoroacetic acid. A different sample (3.0 mg) was mixed with 160 µg of inositol as internal standard, directly reduced with NaBD4, and acetylated using acetic anhydride/trifluoroacetic acid. The resulting alditol acetates were analyzed on an Agilent 7890A GC (Minnetonka, MN) interfaced to a 5975C MSD (triple-axis) in electron impact ionization mode. Separation was performed on a 30-m Supelco 2330 bonded phase fused silica capillary column (Sigma-Aldrich). The concentration of carbohydrates presumed to be indigestible in the small intestine, but degradable during fermentation in the hindgut, were analyzed by the total dietary fiber assay following procedure 985.29 (AOAC, 2012) and procedure 993.19 for the analysis of insoluble fiber. Soluble dietary fiber was calculated as the difference between total dietary fiber and insoluble dietary fiber.
Dietary Treatments and Experimental Design
Dietary treatments included: 1) corn and soybean meal (CON), 2) CON with 1% MAE, 3) CON with 5% MAE, 4) CON with 10% MAE, and 5) CON with 20% MAE. Diets were formulated to meet the nutrient requirements of nursery pigs and fed using a 3-phase program, where each phase consisted of a 2-wk period (Tables 2–4). Diets for all phases were formulated with the MAE to partially replace corn and soybean meal. We adjusted diet ME by adding soybean oil to meet or exceed nutrient requirement recommendations for nursery pigs fed diets containing 3,400 kcal/kg of ME (NRC, 2012). The ME of MAE was estimated using a prediction equation based on chemical composition (Noblet and Perez, 1993):
Table 2.
Ingredient and nutrient composition of experimental diets (as-fed) fed during days 1 and 14 (phase 1)
| Ingredient | Control | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Microalgae extract | 0.00 | 1.00 | 5.00 | 10.00 | 20.00 |
| Corn | 33.37 | 32.17 | 27.36 | 21.35 | 9.32 |
| Lactose | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Soybean meal, 47.5% CP | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Soy protein concentrate | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Whey, dried | 5.50 | 5.50 | 5.50 | 5.50 | 5.50 |
| Fish meal, menhaden | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean oil | 3.06 | 3.26 | 4.05 | 5.04 | 7.01 |
| L-Lysine HCl | 0.18 | 0.19 | 0.20 | 0.21 | 0.24 |
| L-Threonine | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| DL-Methionine | 0.13 | 0.13 | 0.14 | 0.16 | 0.18 |
| Monocalcium phosphate | 1.23 | 1.23 | 1.23 | 1.23 | 1.22 |
| Limestone | 0.62 | 0.62 | 0.62 | 0.62 | 0.61 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin and trace mineral premix* | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Calculated nutrient composition | |||||
| ME, kcal/kg | 3,480 | 3,480 | 3,480 | 3,480 | 3,480 |
| CP, % | 23.59 | 23.55 | 23.39 | 23.19 | 22.78 |
| NDF, % | 5.87 | 6.14 | 7.19 | 8.51 | 11.14 |
| Ether extract, % | 5.78 | 6.01 | 6.91 | 8.04 | 10.30 |
| Linoleic acid, % | 2.39 | 2.46 | 2.78 | 3.18 | 3.97 |
| P % (total) | 0.77 | 0.77 | 0.77 | 0.77 | 0.77 |
| P % (STTD)† | 0.46 | 0.46 | 0.46 | 0.45 | 0.44 |
| Ca, % (total) | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 |
| Ca:P | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 |
| Total Lys % | 1.60 | 1.60 | 1.60 | 1.60 | 1.59 |
| Lactose % | 23.96 | 23.96 | 23.96 | 23.96 | 23.96 |
| Standardized ileal digestible | |||||
| Lys, % | 1.49 | 1.49 | 1.49 | 1.49 | 1.49 |
| Lys:ME (g/Mcal/kg) | 4.28 | 4.28 | 4.28 | 4.28 | 4.28 |
| Met/Cys % | 0.82 | 0.82 | 0.82 | 0.82 | 0.82 |
| Thr % | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 |
| Trp % | 0.27 | 0.27 | 0.27 | 0.27 | 0.26 |
| Analyzed composition | |||||
| GE, kcal/kg | 4,101 | 4,084 | 4,095 | 4,238 | 4,381 |
| CP, % | 22.66 | 24.08 | 22.43 | 21.65 | 20.73 |
| Ether extract, % | 4.35 | 4.51 | 7.12 | 7.80 | 10.52 |
| Ash, % | 6.10 | 5.67 | 5.51 | 6.27 | 6.55 |
| Moisture, % | 7.68 | 7.30 | 7.28 | 6.58 | 5.16 |
*Premix supplied the following nutrients per kilogram of diet: 11,023 IU of vitamin A as retinyl acetate; 2,756 IU of vitamin D3; 22 IU of vitamin E as dl-alpha tocopheryl acetate; 4.41 mg of vitamin K as menadione dimethylpyrimidinol bisulfite; 9.92 mg of riboflavin; 55.11 mg of niacin; 33.07 mg of pantothenic acid as d-calcium pantothenate; 992 mg of choline as choline chloride; 0.06 mg of vitamin B12; 14.3 mg of pyridoxine; 1.65 mg of folic acid; 2.20 mg of thiamine; 0.33 mg of biotin; 2.20 mg of iodine as ethylenediamine dihydroiodide; 0.30 mg of selenium as sodium selenite; 299 mg of zinc as zinc sulfate; 299 mg of iron as ferrous sulfate; 19.8 mg of copper as copper sulfate; and 17.6 mg of manganese as manganese oxide.
†Standardized total tract digestibility.
Table 3.
Ingredient and nutrient composition of experimental diets (as-fed) fed during days 15 to 28 (phase 2)
| Ingredient | Control | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Microalgae extract | 0.00 | 1.00 | 5.00 | 10.00 | 20.00 |
| Corn | 45.33 | 44.62 | 40.16 | 34.14 | 22.06 |
| Soybean meal, 47.5% CP | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Whey, Dried | 3.75 | 3.75 | 3.75 | 3.75 | 3.75 |
| Soy Protein Concentrate | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Lactose | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Fish Meal, menhaden | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Soybean Oil | 0.00 | 0.00 | 0.65 | 1.64 | 3.65 |
| L-Lysine HCl | 0.13 | 0.13 | 0.14 | 0.15 | 0.18 |
| DL-Methionine | 0.06 | 0.07 | 0.07 | 0.09 | 0.11 |
| Monocalcium phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.06 |
| Limestone | 1.32 | 1.04 | 0.84 | 0.83 | 0.80 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin and trace mineral premix* | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Calculated nutrient composition | |||||
| ME, kcal/kg | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 |
| CP, % | 23.41 | 23.41 | 23.27 | 23.07 | 22.65 |
| NDF, % | 7.47 | 7.78 | 8.86 | 10.18 | 12.80 |
| Ether extract, % | 3.09 | 3.13 | 3.91 | 5.05 | 7.33 |
| Linoleic acid, % | 1.08 | 1.07 | 1.32 | 1.72 | 2.52 |
| P % (total) | 0.69 | 0.69 | 0.69 | 0.69 | 0.71 |
| P % (STTD)† | 0.37 | 0.37 | 0.36 | 0.36 | 0.36 |
| Ca% (total) | 0.97 | 0.86 | 0.78 | 0.78 | 0.78 |
| Ca:P | 1.41 | 1.25 | 1.13 | 1.13 | 1.10 |
| Total Lys % | 1.49 | 1.49 | 1.49 | 1.48 | 1.48 |
| Lactose | 12.70 | 12.70 | 12.70 | 12.70 | 12.70 |
| Standardized ileal digestible | |||||
| Lys % | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 |
| Lys:ME (g/Mcal/kg) | 4.10 | 4.10 | 4.10 | 4.10 | 4.10 |
| Met/Cys % | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| Thr % | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Trp % | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Analyzed composition | |||||
| GE, kcal/kg | 3,916 | 3,831 | 3,955 | 4,005 | 4,171 |
| CP, % | 22.25 | 25.59 | 21.14 | 23.10 | 22.00 |
| Ether extract, % | 1.87 | 2.10 | 4.04 | 4.20 | 6.70 |
| Ash, % | 7.86 | 5.41 | 5.77 | 5.66 | 6.83 |
| Moisture, % | 10.40 | 11.38 | 9.54 | 9.46 | 7.68 |
*Premix supplied the following nutrients per kilogram of diet: 11,023 IU of vitamin A as retinyl acetate; 2,756 IU of vitamin D3; 22 IU of vitamin E as dl-alpha tocopheryl acetate; 4.41 mg of vitamin K as menadione dimethylpyrimidinol bisulfite; 9.92 mg of riboflavin; 55.11 mg of niacin; 33.07 mg of pantothenic acid as d-calcium pantothenate; 992 mg of choline as choline chloride; 0.06 mg of vitamin B12; 14.3 mg of pyridoxine; 1.65 mg of folic acid; 2.20 mg of thiamine; 0.33 mg of biotin; 2.20 mg of iodine as ethylenediamine dihydroiodide; 0.30 mg of selenium as sodium selenite; 299 mg of zinc as zinc sulfate; 299 mg of iron as ferrous sulfate; 19.8 mg of copper as copper sulfate; and 17.6 mg of manganese as manganese oxide.
†Standardized total tract digestibility.
Table 4.
Ingredient and nutrient composition of experimental diets fed during days 29 to 42 post-weaning (phase 3)
| Ingredient composition | Control | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Microalgae extract | 0.00 | 1.00 | 5.00 | 10.00 | 20.00 |
| Corn | 66.10 | 65.05 | 60.25 | 54.26 | 42.16 |
| Soybean meal, 47.5% CP | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Soybean Oil | 0.00 | 0.05 | 0.83 | 1.81 | 3.82 |
| L-Lysine HCl | 0.41 | 0.41 | 0.42 | 0.43 | 0.46 |
| DL-Methionine | 0.12 | 0.12 | 0.13 | 0.14 | 0.17 |
| Monocalcium phosphate | 1.36 | 1.36 | 1.38 | 1.41 | 1.47 |
| Limestone | 0.96 | 0.96 | 0.95 | 0.94 | 0.91 |
| Salt | 0.44 | 0.44 | 0.42 | 0.40 | 0.40 |
| Vitamin and trace mineral premix* | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| L-Threonine | 0.11 | 0.11 | 0.11 | 0.12 | 0.12 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Calculated nutrient composition | |||||
| ME, kcal/kg | 3,300 | 3,300 | 3,300 | 3,300 | 3,300 |
| CP, % | 20.28 | 20.24 | 20.08 | 19.88 | 19.47 |
| NDF, % | 9.02 | 9.29 | 10.35 | 11.66 | 14.29 |
| Fat, % | 3.48 | 3.56 | 4.46 | 5.58 | 7.87 |
| Linoleic acid, % | 1.45 | 1.45 | 1.77 | 2.16 | 2.96 |
| P % (total) | 0.68 | 0.68 | 0.69 | 0.69 | 0.70 |
| P % (STTD)† | 0.35 | 0.36 | 0.36 | 0.37 | 0.37 |
| Ca% (total) | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 |
| Ca: P | 1.06 | 1.06 | 1.04 | 1.04 | 1.03 |
| Total Lys % | 1.40 | 1.40 | 1.40 | 1.39 | 1.39 |
| Standardized ileal digestible | |||||
| Lys % | 1.27 | 1.27 | 1.27 | 1.27 | 1.27 |
| Lys:ME (g/Mcal/kg) | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 |
| Met/Cys % | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 |
| Thr % | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Trp % | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 |
| Analyzed composition | |||||
| GE, kcal/g | 3,885 | 3,853 | 3,897 | 3,954 | 4,039 |
| CP, % | 18.66 | 19.12 | 25.53 | 17.65 | 17.50 |
| Ether extract, % | 2.53 | 2.10 | 3.82 | 4.23 | 8.59 |
| Crude fiber, % | 3.37 | 2.28 | 2.59 | 2.89 | 3.15 |
| Ash, % | 5.09 | 5.43 | 6.15 | 5.50 | 5.70 |
| Moisture, % | 12.27 | 12.36 | 11.91 | 10.95 | 9.62 |
*Premix supplied the following nutrients per kilogram of diet: 11,023 IU of vitamin A as retinyl acetate; 2,756 IU of vitamin D3; 22 IU of vitamin E as dl-alpha tocopheryl acetate; 4.41 mg of vitamin K as menadione dimethylpyrimidinol bisulfite; 9.92 mg of riboflavin; 55.11 mg of niacin; 33.07 mg of pantothenic acid as d-calcium pantothenate; 992 mg of choline as choline chloride; 0.06 mg of vitamin B12; 14.3 mg of pyridoxine; 1.65 mg of folic acid; 2.20 mg of thiamine; 0.33 mg of biotin; 2.20 mg of iodine as ethylenediamine dihydroiodide; 0.30 mg of selenium as sodium selenite; 299 mg of zinc as zinc sulfate; 299 mg of iron as ferrous sulfate; 19.8 mg of copper as copper sulfate; and 17.6 mg of manganese as manganese oxide.
†Standardized total tract digestibility.
where CP, EE, and NDF composition data (g/kg) were used on an as-fed basis.
Samples of complete diets were obtained after mixing, frozen at −20 °C, and analyzed for nutrient composition at the Experiment Station Chemical Laboratories (University of Missouri, Columbia, MO) for DM, CP, EE, crude fiber, and ash following AOAC procedures (AOAC, 2012). Concentration of Ca and P were analyzed using inductively coupled plasma–optical emission spectroscopy (Method 985.01; AOAC, 2012). The concentration of amino acids (including Trp, Met, and Cys) were analyzed after appropriate hydrolysis [Method 982.30 including sections E (a, b, and c) in AOAC (2012)].
Diet flowability was determined by measuring the poured angle of repose (McGlinchey, 2009; Jiang and Rosentrater, 2015). Briefly, at the time of diet formulation, a sample of each diet (1 kg) was collected in an air-tight plastic bag and stored at −20 °C until analyzed. A modified Hele-Shaw cell was used to measure the angle between the horizontal base and the slope of the pile of feed that dropped from a height of 60 cm using a funnel, and the poured and drained angles of repose were calculated (Johnston et al., 2009). Measures of each diet were determined in triplicate and data are presented as means ± SD.
Animals, Housing, and Management
Weaned pigs (n = 300; 21 days of age; 6.27 ± 0.02 kg) were selected from a batch of 400 pigs and blocked by initial BW and allotted to 60 pens, with five pigs per pen in 12 blocks. Ratio of gilts and barrows was balanced evenly within blocks and treatments. Pens within blocks were assigned randomly to one of five dietary treatments. Pigs were housed in a temperature-controlled nursery facility at the University of Minnesota’s West Central Research and Outreach Center in Morris, MN, and were provided ad libitum access to feed and water throughout the entire 42-day experiment. Each pen (2.4 × 1.2 m) included plastic grated flooring, one cup drinker, and one 4-hole stainless steel self-feeder (Hog Slat Inc., Newton Grove, NC). Individual pigs in each pen were weighed once weekly to calculate pen ADG. All feed additions to feeders were weighed and remaining feed in the feeders was weighed the same day pigs were weighed and subtracted from feed added to determine feed disappearance and calculate ADFI and G:F. Pigs were monitored daily for signs of poor health, and appropriate medication treatments were administered as prescribed by an attending veterinarian as needed.
Growth Performance Data Collection and Statistical Analysis
Growth performance data were analyzed using the Mixed Procedure of SAS (v9.3; SAS Inst. Inc., Cary, NC), based on a randomized complete block design. Pen served as the experimental unit that was nested within block and diet and was used as the subject for repeated measures with autoregressive covariance structure. Growth performance data were analyzed using block as the random effect and treatment, week, and week × treatment interaction as fixed effects. Linear and quadratic contrasts were estimated using coefficients that were adjusted for the separation among dietary treatments. Iterations of models were tested modifying covariance structure and interactions using Bayesian information criterion to select the final model. The univariate test in SAS was used to evaluate the normality of residuals within the model, and to test for outliers and equal distribution in variance. Data are presented as the least squares means of each treatment in each week, and means were separated using the Tukey test adjusted for multiple comparisons.
Mortality and health treatment data were collected to assess health status by recording the number of pigs within each treatment group that received individual antibiotic. Treated pigs and pig mortality were calculated using the number of pigs that received individual treatments (0 vs. 1) or died (0 vs. 1), within each treatment, divided by the total number of pigs assigned to each dietary treatment (n = 60 pigs/treatment). Differences in treatment and mortality curves were tested using the Mantel-Cox Log–rank test in GraphPad Prism 7.03 (GraphPad Software, Inc., La Jolla, CA).
Tissue Collection and Analysis
Thirty pigs (six pigs/treatment) were euthanized on day 42 by captive bolt followed by exsanguination; within a pen, the pig with the BW closer to the pen average that did not receive additional antibiotics was selected. Samples of the jejunum (1 m distal to the pyloric sphincter) and ileum (15 cm proximal to the ileocecal valve) were collected and fixed in 4% buffered formalin for histological evaluation. Liver samples (500 mg) were collected from the left lateral lobe, snap frozen in liquid nitrogen, and stored at −80 °C for further processing.
Formalin-fixed intestinal samples were processed and embedded in paraffin following the standard protocols of the University of Minnesota’s Comparative Pathology Shared Resource (St. Paul, MN). Tissue blocks were sectioned at 5 µm thickness and stained with hematoxylin and eosin (HE). Total mucosal height was measured from the tip of the villi to the bottom of the crypt on the HE-stained sections at 100× amplification under a light microscope (Olympus BX53). Data reported are the average of measurements of five well-oriented fields (fields that allow observing villi in their axis) per pig.
Tissue sections of 5 µm were stained with periodic acid-Schiff with Alcian blue (PAS-AB, Newcomer Supply, Middleton, WI) following manufacturer’s instructions. The stained slides were analyzed at 100 × magnification under a light microscope (Olympus BX53) in five well-oriented fields. Within each field, the total area (µm2) of the mucosa was first measured, then the area (µm2) stained positive for PAS-AB (goblet cell area) was determined by color using a cell imaging software (CellSense, Olympus, Center Valley, NJ). Mucosal area was defined as the area limited by the epithelial apical membrane and the “muscularis mucosa.” Data presented are the mean of the percentage of goblet cells in their corresponding mucosal area quantified in five fields per pig. Values for total mucosal length and goblet cell quantifications were analyzed using GraphPad Prism 7.03 (GraphPad Software, Inc.). Data were tested for normality using the D’Agostino and Pearson tests, and differences among groups were determined using the Kruskal–Wallis test followed by Dunn’s multiple comparisons. For gut morphometry, pig was considered the experimental unit.
Metabolomic Analysis
Aqueous fractions of liver were prepared using the Bligh and Dyer method (Bligh and Dyer, 1959). Briefly, 100 mg of frozen liver sample were homogenized in a mixture of 0.5 ml methanol, 0.5 ml chloroform, and 0.4 ml distilled water. After 10 min of centrifugation at 18,000 × g, the top aqueous fraction was harvested and stored at −80 °C. The aqueous fraction was derived by dansyl chloride (DC) for detecting amine-containing metabolites, including amino acids. Briefly, 5 µL of sample was mixed with 100 µL of DC (3 mg/mL in acetone), five µL of 50 µM internal standard d5-tryptophan, and 50 µL of 10 mM sodium bicarbonate. This mixture was incubated at 60 °C for 15 min and subsequently centrifuged at 18,000 × g for 10 min. The supernatant was transferred into an HPLC vial for liquid chromatography-mass spectrometry analysis.
Five µL of DC-derivatized sample was injected into an Acquity Ultra-Performance Liquid Chromatography system (Waters Corporation, Milford, MA) and separated in a BEH C18 column (Waters). The mobile phase for DC-derivatized samples used a gradient ranging from water to 95% aqueous acetonitrile containing 0.1% formic acid over a 10-min run. The LC eluant was introduced into a Xevo-G2-S quadrupole time-of-flight mass spectrometer (Waters) for accurate mass measurement and ion counting. Capillary voltage and cone voltage for electrospray ionization was maintained at 3 kV and 30 V for positive-mode detection. Source and desolvation temperatures were set at 120 °C and 350 °C, respectively. Nitrogen was used as both cone gas (50 L/h) and desolvation gas (600 L/h), and argon was used as collision gas. For accurate mass measurement, the mass spectrometer was calibrated with sodium formate solution with mass-to-charge ratio (m/z) of 50–1,000 and monitored by the intermittent injection of the lock mass leucine enkephalin ([M + H]+ = m/z 556.2771) in real time. Mass chromatograms and mass spectral data were acquired and processed by the MassLynx software (Waters) in centroided format. Additional structural information was obtained by tandem MS (MS/MS) fragmentation with collision energies ranging from 15 to 40 eV.
For the metabolomic analysis, the chromatographic and spectral data were deconvoluted using the MarkerLynx software (Waters). A multivariate data matrix containing information on sample identity, ion identity (retention time and m/z), and ion abundance was generated through centroiding, deisotoping, filtering, peak recognition, and integration. The intensity of each ion was calculated by normalizing the single-ion counts vs. the total ion counts in the whole chromatogram. The processed data matrix was exported into SIMCA-P+ software (Umetrics, Kinnelon, NJ), transformed by “Pareto” scaling, and then analyzed by principal component analysis. Major latent variables in the data matrix were determined as the principal components of a multivariate model, and the relationships among examined samples were described in the scores scatter plot. Metabolite markers of MAE were identified by analyzing ions contributing to sample separation in the model. The metabolite structures were elucidated by accurate mass measurement, elemental composition analysis, database search (Human Metabolome Database, http://www.hmdb.ca), MS/MS fragmentation, and the comparisons with authentic standards. Amino acids were quantified by calculating the ratio between the peak area of amino acids and the peak area of internal standard and fitting with a standard curve using QuanLynx software (Waters).
For all analyses, significant differences were considered at P ≤ 0.05, with trends at 0.05 < P ≤ 0.20.
RESULTS
Animal Health and Mortality
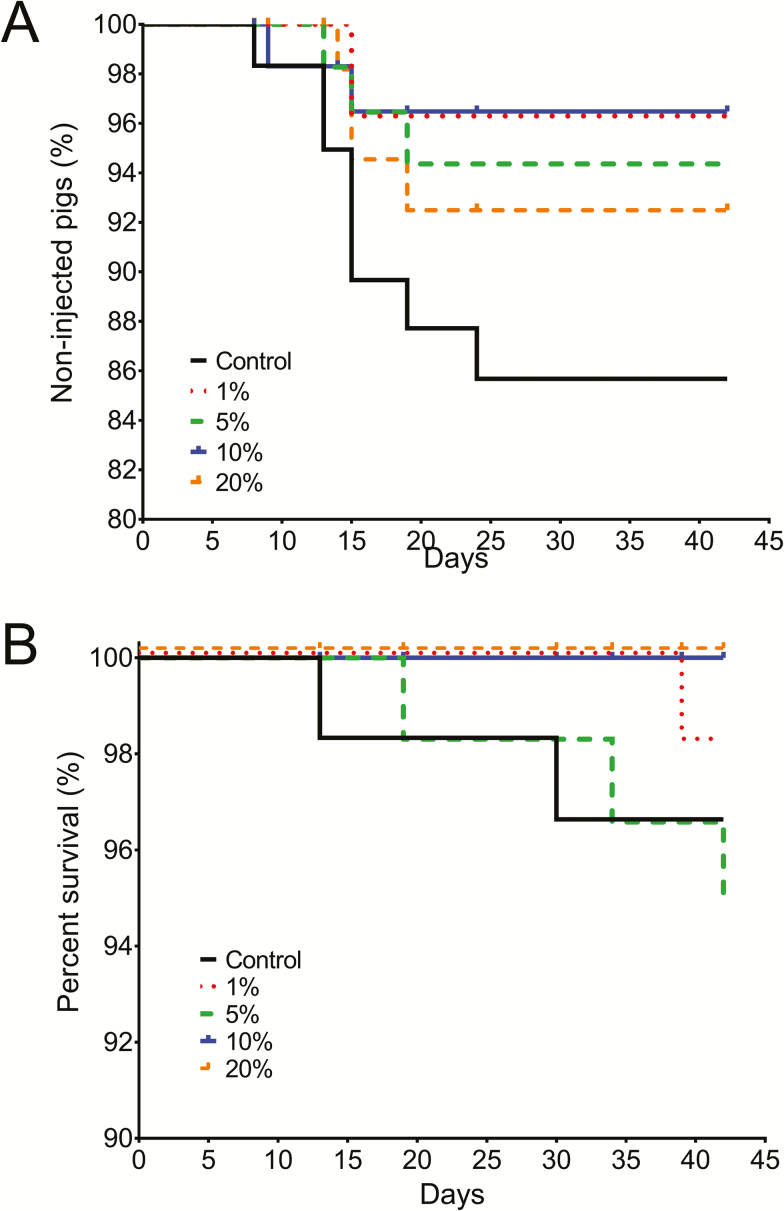
During the second week of the experiment, a high incidence of coughing and scouring was observed in pigs across all dietary treatments. Consequently, all pigs were treated with neomycin (22 mg/kg BW) by water medication from days 15 to 21 of the experiment. In addition to the neomycin treatment, 18 pigs were treated individually with enrofloxacin because of prevailing coughing and gaunt appearance. Of the 18 treated pigs, 8, 2, 3, 2, and 4 pigs were assigned to the CON, 1%, 5%, 10%, and 20% MAE diets, respectively (Figure 1). Overall mortality was 2.67% in this study, with 3, 2, 3, 0, and 0 pigs that died in the CON, 1%, 5%, 10%, and 20% MAE treatments, respectively. Trends for a greater incidence of medication treatment (P = 0.16) and mortality (P = 0.14) were observed in pigs assigned to the CON diet than for pigs fed any of the diets containing MAE. The calculated livability index (proportion of the total number of pigs that survived and did not receive additional injection treatment, expressed as percentage) were 81, 7, 93.3, 90, 96.7, and 93.3% for CON, 1%, 5%, 10%, and 20% MAE treatments, respectively.
Figure 1.
All pigs received antibiotic treatment in the drinking water from days 15 to 21, with some animals requiring additional enrofloxacin injection. (A) The magnitude of the drop at each day represents the percentage of pigs within a group that received enrofloxacin (P = 0.16). (B) Mortality of pigs fed diets with different amounts of partially de-oiled microalgae product (P = 0.14).
Growth Performance
There were no differences in initial BW among dietary treatments (Table 5). As early as day 7, there were differences in BW among pigs because feeding MAE elicited a quadratic (P < 0.05) increase in BW. The increase in BW of pigs consuming 1% MAE observed at day 7 was sustained in subsequent weigh periods. The net result was that the final BW of pigs among pens consuming MAE was greatest when consuming 1, 5, or 10% MAE compared with those fed the CON diet, but feeding 20% was not different from the CON diet (quadratic effect P < 0.05). The greater final BW appeared to be the result of greater ADG from days 1 and 7, where pigs had the greatest ADG when consuming the 1% and 10% MAE diets compared with feeding the other dietary treatments. The ADG of pigs consuming 20% MAE was less than those fed the 1, 5, or 10% MAE diets and was not different from those fed the CON diet (quadratic effect P < 0.05). This greater ADG was likely a result of greater ADFI, where on day 7, the ADFI of pigs consuming MAE diets increased with increasing levels of MAE up to 10%, but feeding the 20% MAE diet resulted in similar ADFI compared with feeding CON (quadratic effect P < 0.05). As a result of greater ADFI, there were no effects of feeding MAE on G:F during most weigh periods. However, there was a linear increase (P < 0.05) in G:F in pigs fed the MAE diets during days 15 to 21. Total pen BW of pigs consuming 10% MAE (138 kg) were heavier (P < 0.05) than pen BW of pigs consuming the control diet (126 kg), while there were no differences in pen BW among pigs consuming 1, 5, or 20% MAE diets.
Table 5.
Effects of feeding partially de-oiled microalgae extract on growth performance of nursery pigs
| Item | Control | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Number of pens | 12 | 12 | 12 | 12 | 12 |
| BW, kg | |||||
| Day 0 | 6.26 | 6.27 | 6.28 | 6.27 | 6.27 |
| Day 7† | 7.00b | 7.25a | 7.18a,b | 7.22a | 7.02b |
| Day 14*,† | 9.03b | 9.50a | 9.64a | 9.66a | 8.81b |
| Day 21† | 12.73b | 13.47a | 13.58a | 13.48a | 12.73b |
| Day 28† | 16.44b | 17.49a | 17.74a | 17.73a | 17.06a,b |
| Day 35† | 21.62y | 22.82x | 22.15x,y | 22.77x | 21.74y |
| Day 42† | 26.26y | 27.58x | 27.46x,y | 27.78x | 26.54y |
| SEM | 0.54 | ||||
| P value | |||||
| Period | <0.01 | ||||
| Diet | 0.05 | ||||
| Period × diet | <0.01 | ||||
| Final pen BW, kg2 | 126b | 136a,b | 133a,b | 138a | 133a,b |
| ADG, g | |||||
| Day 1–7 | 92b | 123a | 112a,b | 119a | 96a,b |
| Day 8–14† | 287b,c | 322a,b | 340a,b | 349a,b | 256c |
| Day 15–21 | 528 | 565 | 552 | 546 | 561 |
| Day 22–28 | 548 | 585 | 596 | 607 | 618 |
| Day 29–35 | 734a,b | 778a | 621c | 720a,b | 669b,c |
| Day 36–42 | 784 | 815 | 883 | 836 | 799 |
| Overall | 496z | 531x | 517x,y,z | 529x,y | 500y,z |
| SEM | 28.6 | ||||
| P value | |||||
| Period | <0.01 | ||||
| Diet | 0.02 | ||||
| Period × diet | 0.07 | ||||
| ADFI, g | |||||
| Day 1–7† | 120b | 147a | 138a | 139a | 122ab |
| Day 8–14† | 372c | 380b,c | 409a,b | 421a | 358c |
| Day 15–21 | 742 | 749 | 736 | 755 | 701 |
| Day 22–28 | 718b | 838a | 860a | 835a | 799a |
| Day 29–35 | 1,067 | 1,106 | 1,025 | 1,046 | 1,017 |
| Day 36–42 | 1,371 | 1,377 | 1,353 | 1,367 | 1,313 |
| Overall*,† | 732c | 766a | 754a,b | 760b,a | 718c |
| SEM | 30.8 | ||||
| P value | |||||
| Period | <0.01 | ||||
| Diet | 0.11 | ||||
| Period × diet | 0.04 | ||||
| G:F, g/kg | |||||
| Day 1–7 | 763 | 853 | 823 | 848 | 788 |
| Day 8–14 | 765 | 846 | 817 | 823 | 713 |
| Day 15–21* | 719b | 761a,b | 753a,b | 721b | 803a |
| Day 22–28 | 778 | 704 | 695 | 735 | 780 |
| Day 29–35 | 689a | 710b | 600a | 688a | 658a,b |
| Day 36–42 | 573 | 590 | 658 | 613 | 611 |
| Overall | 715 | 744 | 724 | 738 | 725 |
| SEM | 39.3 | ||||
| P value | |||||
| Period | <0.01 | ||||
| Diet | 0.36 | ||||
| Period × diet | 0.06 | ||||
*Linear effect of microalgae extract inclusion (P < 0.05).
†Quadratic effect of microalgae extract inclusion (P < 0.05).
a,b,cValues in the same row with different superscript differ (P < 0.05).
x,y,zValues in the same row with different superscripts tend to differ (0.05 < P 0.10).
Diets
Diets containing 10% and 20% MAE were difficult to mix using a vertical screw mixer. As a result, these diets were mixed using a paddle mixer. The angle of repose was used as a measure of diet flowability and increased (linear P < 0.05) with greater dietary inclusion rates of the MAE from 37.7 ± 2.3, 44.3 ± 0.6, 48.0 ± 5.2, 50.0 ± 5.0, and 51.3 ± 5.5° for 0, 1, 5, 10, and 20% MAE, respectively. Because of the poor flowability of experimental diets, all feeders were checked twice daily to ensure pigs had uninterrupted access to experimental diets.
Gut Morphology
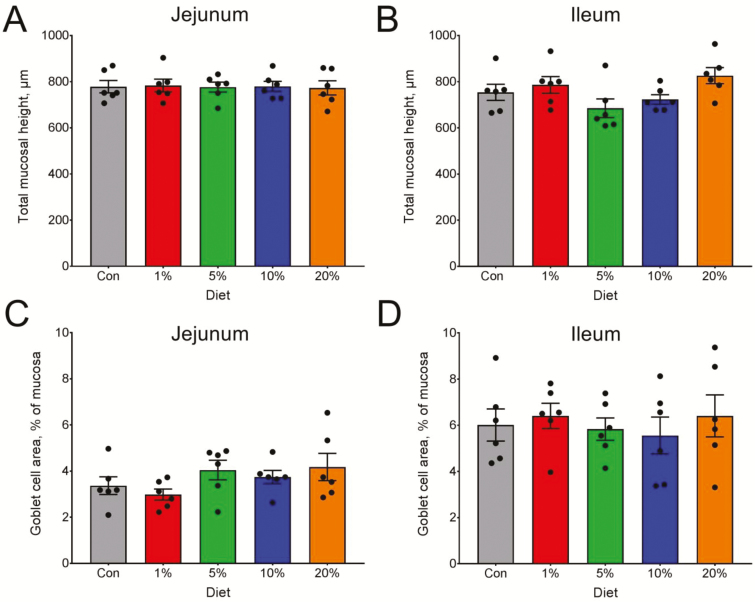
Feeding diets with MAE did not result in changes in intestinal architecture measured by the height of the intestinal mucosal (P = 0.99) or presence of mucus-producing cells (goblet cell area, P = 0.22) in the jejunum. In contrast, the ileum of pigs fed the 5% MAE diet tended (P = 0.06) to have reduced mucosal height compared with that of pigs fed 20% MAE diet. Goblet cell area of the ileum was not affected by dietary treatments (P ≥ 0.05, Figure 2).
Figure 2.
Mucosal height of the jejunum (A) and ileum (B) of pigs fed diets supplemented with partially de-oiled microalgae product. Quantification of mucin producing (Goblet) cells in jejunum (C) and ileum (D) of pigs fed the same diets. Bars heights are mean ± SEM (n = 6 pigs/group).
Liver Metabolite Analysis
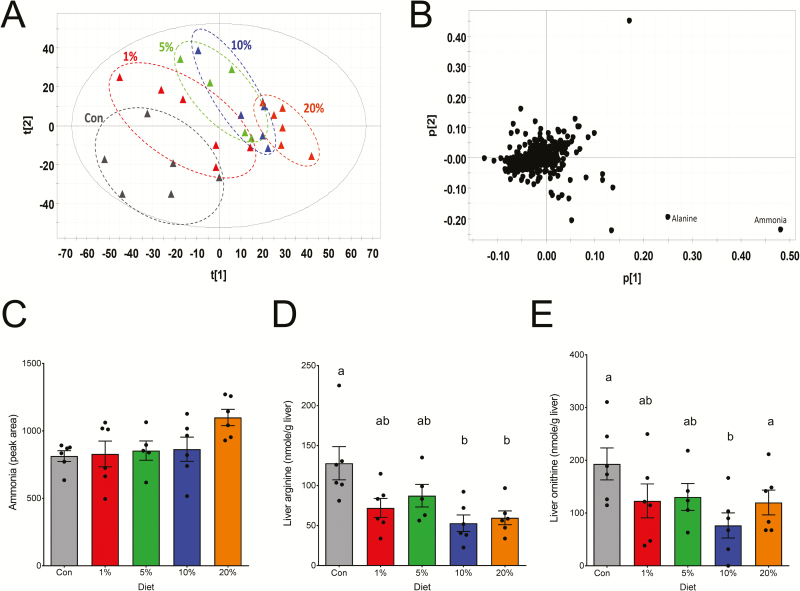
A principal component analysis model on the hepatic metabolites revealed a dose-dependent separation of CON and MAE dietary treatments (Figure 3A). The analysis of the relative abundance of metabolites derivatized by dansylation suggests that ammonia concentrations had a relatively large impact on the separation among treatments. However, these were not quantitative changes that describe the actual concentration of metabolites (data not shown). Consequently, we measured the concentration of free amino acids in the liver to determine their relative contributions to differences among dietary treatments. Our results showed differences in the quantitative concentration of hepatic alanine, arginine, histidine, ornithine, aspartic acid, citrulline, and proline (Table 6). However, these differences in amino acid concentrations did not follow a pattern or appear to be associated with dietary inclusion level of MAE.
Figure 3.
Effects of feeding partially de-oiled microalgae extract on the hepatic metabolome. (A) Score plot of a principal component analysis (PCA) model on the hepatic metabolome. The aqueous extracts of liver were derivatized by dansylation reaction to facilitate the detection of amino acids by liquid chromatography-mass spectrometry analysis. The t[1] and t[2] are the projection values of each sample in the 1st and 2nd principal components of the model, respectively. (B) Loading plot of a PCA model on the hepatic metabolome. The correlations of individual ions with the first and second components of the PCA model were indicated by their respective p[1] and p[2] values.
Table 6.
Concentrations of free amino acids in the liver of pigs fed control and diets supplemented with partially de-oiled microalgae extract*
| Amino acids | Control | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Alanine | 2,378 ± 323ab | 2,158 ± 371ab | 2,187 ± 347ab | 1936 ± 753a | 2966 ± 484b |
| Arginine | 128 ± 51a | 72 ± 29ab | 87 ± 32ab | 53 ± 25b | 60 ± 21b |
| Asparagine | 614 ± 70 | 630 ± 220 | 541 ± 147 | 448 ± 162 | 617 ± 171 |
| Aspartic acid | 5,202 ± 1,486 | 3,511 ± 1,360 | 3,364 ± 1,047 | 3,896 ± 2,757 | 5,959 ± 1,916 |
| Citrulline | 39 ± 20 | 20 ± 25 | 36 ± 33 | 29 ± 29 | 46 ± 33 |
| Glutamic acid | 2,5068 ± 5,559 | 17,504 ± 7,277 | 17,454 ± 8,369 | 18,171 ± 11,312 | 28,990 ± 6,612 |
| Glutamine | 2,529 ± 753 | 1,934 ± 575 | 2,261 ± 477 | 2,282 ± 1,018 | 3,034 ± 506 |
| Glycine | 3991 ± 860 | 3,968 ± 212 | 4,892 ± 1,008 | 4281 ± 810 | 5,077 ± 985 |
| Histidine | 451 ± 142a | 291 ± 49ab | 297 ± 50ab | 259 ± 93b | 304 ± 111a |
| Iso/Leucine | 389 ± 85 | 394 ± 56 | 399 ± 44 | 312 ± 52 | 367 ± 90 |
| Lysine | 230 ± 56 | 164 ± 65 | 169 ± 58 | 135 ± 66 | 170 ± 71 |
| Methionine | 788 ± 265 | 922 ± 180 | 966 ± 286 | 640 ± 388 | 885 ± 296 |
| Ornithine | 193 ± 74a | 123 ± 79ab | 131 ± 57ab | 77 ± 58b | 120 ± 58a |
| Phenylalanine | 93 ± 12 | 100 ± 21 | 92 ± 15 | 74 ± 12 | 89 ± 20 |
| Proline | 146 ± 33 | 132 ± 23 | 145 ± 11 | 126 ± 20 | 127 ± 30 |
| Serine | 1,598 ± 666 | 1,463 ± 266 | 1,837 ± 518 | 1,527 ± 657 | 1,953 ± 427 |
| Taurine | 1,429 ± 503 | 880 ± 326 | 1189 ± 361 | 861 ± 430 | 941 ± 308 |
| Threonine | 407 ± 58 | 340 ± 85 | 334 ± 102 | 310 ± 134 | 509 ± 177 |
| Tryptophan | 6.75 ± 3.77 | 4.88 ± 4.37 | 4.50 ± 2.76 | 4.88 ± 3.31 | 6.68 ± 3.31 |
| Tyrosine | 152 ± 93 | 90 ± 63 | 86 ± 70 | 105 ± 46 | 73 ± 38 |
| Valine | 500 ± 85 | 504 ± 75 | 508 ± 52 | 393 ± 89 | 489 ± 87 |
| Total AA | 46,332 ± 8,397 | 35,204 ± 9,326 | 36,978 ± 10,586 | 35,919 ± 1,7295 | 52,773 ± 10,021 |
*Data presented are mean ± SD as µmol/g of tissue on an as is basis.
a,bValues in the same row with different superscript differ (P < 0.05).
DISCUSSION
The production and use of microalgae have long been recognized as a means to reduce the carbon footprint in biofuels production (Lum et al., 2013), bioremediation of waste-water (Chung et al., 1978; Lu et al., 2015, 2016), and other industrial applications (Mercer and Armenta, 2011). In addition, there is increasing interest in producing and using microalgae and derived coproducts in animal feeds because large amounts of biomass can be rapidly produced from low-value substrates, and result in an environmentally sustainable, highly nutritious feed ingredient for animals. However, the nutritional benefits and applications of microalgae biomass and coproducts depend on the specific species of microalgae and industrial processes used to produce the coproducts. Few studies have been conducted to evaluate the use of microalgae coproducts in swine diets (Lum et al., 2013). Furthermore, the MAE used in this study has not been previously evaluated for use in swine diets. Consequently, we cannot discuss and compare the results obtained in this study with reference to other studies but will focus the discussion on the product composition and our observations on health and growth of nursery pigs.
The microalgae coproduct evaluated in this study was produced using proprietary mechanical lipid extraction procedures, which resulted in concentration of the carbohydrate content to about 76.62% on an as-fed basis. The concentration of total dietary fiber and glucose in the hydrolyzed extract suggested that carbohydrates in this microalgae coproduct source were mostly of soluble glucose polysaccharides, such as starch, with the potential to be digestible in the small intestine and provide energy from glucose (Chen et al., 2013). The MAE also had glucose polysaccharides that were insoluble and potentially related to cellulose, which could be fermentable in the large intestine of pigs. Because microalgae has also been studied as a potential source of prebiotics (De Jesus Raposo et al., 2016), we added it to common nursery diets at low inclusion rates (1% and 5%) to determine if potential prebiotic effects may improve growth performance and health. We also added MAE at high (10% and 20%) inclusion levels to determine its feeding value as an energy source in nursery pig diets.
One of the major findings of this study was the improvement in ADFI when pigs were fed diets containing MAE at low inclusion (1% and 5%) rates. In this preliminary experiment, we did not measure specific mechanisms that might explain this feed intake response. However, we speculate that the quadratic ADG and ADFI may be related to the carbohydrate composition of the MAE, which may have improved palatability of the diet. The quadratic response of ADFI and ADG observed may be related to dietary starch content. While increasing the inclusion of MAE, the type of starch from corn is replaced by starch from MAE. The type of starch and degradation kinetics during small intestine digestion may be different and impact feed intake and subsequent growth performance of nursery pigs (Zijlstra et al., 2012; Fouhse et al., 2017). More investigation is needed to determine the specific role of carbohydrates in MAE or other types of microalgae coproducts on diet palatability and intestinal nutrient-sensing mechanisms, both of which can affect feed intake of young pigs (Roura et al., 2016).
The fact that we observed similar feed intake and growth rate of pigs fed the 20% MAE diet and those fed the corn–soybean meal diet is encouraging, and suggests that MAE may be used to replace up to 20% of corn in diets for pigs without reducing growth performance if MAE is cost competitive with corn. In spite using a calculated value for ME, our results suggest that the estimates calculated using the NRC equation were reasonably accurate for feeding pigs at 10% and 20% inclusion (Furbeyre et al., 2017). However, experimentally derived ME values are necessary for including MAE at greater than 20% inclusion or for routine and wide use of microalgae in pig feeding programs because variation among sources is likely to affect ME as observed for other biofuel coproducts (Urriola et al. 2014). The NRC equation that calculated ME using NDF as measure of nonstarch polysaccharides will underestimate ME from MAE because a large proportion of polysaccharides in MAE are soluble. Likewise, soluble polysaccharides from MAE may have impacts on transit time, mucin production, and other physiology parameters that are not considered in the current estimates of ME. Therefore, experimental derived ME values for MAE are necessary.
Feeding diets containing up to 20% of the MAE may have beneficial environmental impacts by decreasing the carbon footprint of animal production as observed with other alternative feed ingredients fed to swine (Brune et al., 2009). This will become more important as more commercial pork production systems develop improved supply chain management of acquiring and using feed ingredients with a reduced carbon footprint (Mackenzie et al., 2016).
During this experiment, pigs developed signs of poor health and were treated with antibiotics in drinking water as well as injectable antibiotics for individual pigs. All groups of pigs fed MAE diets tended to have lower incidence of mortality and the number of pigs that required additional treatment was lower than those fed the control diet. These observations suggest that the MAE may have a health promoting effect in nursery pigs that deserves further exploration. Dietary supplementation of microalgae has been studied for their health promoting potential, especially in relation to gut morphology and integrity. Furbeyre et al. (2017) studied the effects of microalgae species of the genera Spirulina and Chlorella on intestinal development and management of digestive disorders post-weaning in pigs. They observed increases in villi height in the jejunum of piglets fed diets supplemented with either 1% Spirulina or 1% Chlorella, suggesting a positive effect on mucosal restoration and development after weaning. Similarly, Dvir et al. (2000) fed rats a polysaccharide derived from Porphyridium and observed an increase in the number of goblet cells in the small intestine. We found no differences in mucosal height or goblet cells among dietary treatments, suggesting that the MAE coproduct evaluated in the current study does not affect intestinal morphology. Likewise, we observed differences in both growth performance and health status of pigs fed the 1% and 5% MAE-supplemented diets. A possible explanation of this effect is that microalgae carbohydrates can have prebiotic effects, promoting beneficial microbiota, and production of short-chain fatty acids that favor improved growth performance and health status in pigs (De Jesus Raposo et al., 2016). Further studies are necessary to test the potential health benefits of MAE in diets for nursery pigs.
We also conducted a liver metabolomic analysis to determine if feeding MAE to nursery pigs would have beneficial metabolic effects. The analyses of hepatic metabolome and free amino acid concentrations revealed that MAE inclusion affects the concentrations of selected amino acid metabolites, including alanine, ammonia, arginine, histidine, and ornithine. Because ammonia, arginine, and ornithine are involved in the urea cycle, the decreased concentration of arginine and ornithine suggests that the urea cycle may be downregulated by feeding MAE, which may have led to increasing ammonia concentration in the liver. It is well known that feeding fermentable carbohydrates such as inulin, soybean hulls, and sugar beet pulp to pigs can shift metabolism of amino acids from urinary N to microbial N excreted in feces (Aarnink and Verstegen, 2007). The carbohydrates in the MAE may serve as a source of fermentable cellulose or resistant starch capable of shifting ammonia excretion to the large intestine of pigs, which was observed by the changes in amino acid pattern in liver that is related to the urea cycle (Jha and Berrocoso, 2016).
In a recent metabolomic study in mice, Ma et al. (2015) observed that inclusion of 5% green algae in feed increased the ratio of glutathione (GSH) to oxidized glutathione (GSSG), while inclusion level of 20% algae decreased this ratio. In the present study, levels of GSH and GSSG were not affected by dietary inclusion of MAE (data not shown). However, the MAE coproduct used in this study had a considerably lower concentration of CP than many algae preparations previously reported (Becker, 2004). Furthermore, it is probable that the lipid extraction process used to produce the MAE evaluated in this study, may have removed other nutrients which decreased its capability in altering redox balance or oxidative stress compared with other microalgae sources.
In conclusion, microalgae have the potential to serve as an environmentally sustainable nutrient source in animal production systems and may provide beneficial health effects. However, little is known about the benefits of specific fractions from microalgae on animal nutrition and health. The MAE coproduct evaluated in this study had adequate energy and nutritional value to support optimal growth of pigs when included in diets at a 20% inclusion rate. Furthermore, it was interesting to note that this source of MAE may have potential health benefits to nursery pigs when added to diets at low inclusion rates. However, repeatability of these benefits under different health conditions needs to be evaluated in future studies.
Footnotes
Funding: Solazyme Inc. (TerraVia; San Francisco, CA), NIFA MIN-16–101.
LITERATURE CITED
- Aarnink A. J. A., and Verstegen M. W. A.. 2007. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci. 109:194–203. doi:10.1016/j.livsci.2007.01.112 [Google Scholar]
- AOAC 2012. Official methods of analysis, association of official analytical chemists 19th Edition, Washington (DC). [Google Scholar]
- Becker W. 2004. Microalgae in human and animal nutrition. In: A., Richmond, editor, Handbook of microalgal culture. Oxford (UK): Blackwell Publishing Ltd; p. 312–351. doi:10.1002/9780470995280 [Google Scholar]
- Bligh E. G. and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. doi:10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Brune D. E., Lundquist T. J., and Benemann J. R.. 2009. Microalgal biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J. Environ. Eng. 135:1136–1145. doi:10.1061/(ASCE)EE.1943–7870.0000100 CE [Google Scholar]
- Chen C. Y., Zhao X. Q., Yen H. W., Ho S. H., Cheng C. L., Lee D. J., Bai F. W., and Chang J. S.. 2013. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 78:1–10. doi:10.1016/j.bej.2013.03.006 [Google Scholar]
- Chung P., Pond W. G., Kingsbury J. M., Walker E. F., and Krook L.. 1978. Production and nutritive-value of Arthrospira Platensis, Platensis, a spiral blue-Green-algae grown on swine wastes. J. Anim. Sci. 47:319–330. [Google Scholar]
- De Jesus Raposo M. F., De Morais A. M. M. B., and De Morais R. M. S. C.. 2016. Emergent sources of prebiotics: seaweeds and microalgae. Mar. Drugs. 14:1–27. doi:10.3390/md14020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir I., Chayoth R., Sod-Moriah U., Shany S., Nyska A., Stark A. H., Madar Z., and Arad S. M.. 2000. Soluble polysaccharide and biomass of red microalga porphyridium sp. Alter intestinal morphology and reduce serum cholesterol in rats. Br. J. Nutr. 84:469–476. doi:10.1017/S000711450000177X [PubMed] [Google Scholar]
- Fevrier C. and Seve B.. 1975. [Incorporation of a spiruline (spirulina maxima) in swine food]. Ann. Nutr. Aliment. 29:625–650. [PubMed] [Google Scholar]
- Fouhse J. M., Gao J., Vasanthan T., Izydorczyk M., Beattie A. D., and Zijlstra R. T.. 2017. Whole-grain fiber composition influences site of nutrient digestion, standardized ileal digestibility of amino acids, and whole-body energy utilization in grower pigs. J. Nutr. 147:29–36. doi:10.3945/jn.116.238667 [DOI] [PubMed] [Google Scholar]
- Furbeyre H., van Milgen J., Mener T., Gloaguen M., and Labussière E.. 2017. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal. 11:183–192. doi:10.1017/S1751731116001543 [DOI] [PubMed] [Google Scholar]
- Gatrell S., Lum K., Kim J., and Lei X. G.. 2014. Nonruminant nutrition symposium: potential of defatted microalgae from the biofuel industry as an ingredient to replace corn and soybean meal in swine and poultry diets. J. Anim. Sci. 92:1306–1314. doi:10.2527/jas.2013-7250 [DOI] [PubMed] [Google Scholar]
- Hintz H. F., and Heitman H.. 1967. Sewage-grown algae as a protein supplement for swine. Anim. Prod. 9:135–140. doi:10.1017/S0003356100038393 [Google Scholar]
- Isaacs R., Roneker K. R., Huntley M., and Lei X. G.. 2011. A partial replacement of soybean meal by whole or defatted algal meal in diet for weanling pigs does not affect their plasma biochemical indicators. J Anim Sci. 89(Suppl. 1):723 (abstr.). [Google Scholar]
- Jha R., and Berrocoso J. F. D.. 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212:18–26. doi:10.1016/j.anifeedsci.2015.12.002 [Google Scholar]
- Jiang X., and Rosentrater K. A.. 2015. Factors influencing feed ingredient flowability. In: Am. Soc. Ag. Biol. Eng. Annual Inter. Meeting 2015 Vol. 2 ASABE; p. 1502–1525. doi:10.13031/aim.20152184759 [Google Scholar]
- Johnston L. J., Goihl J., and Shurson G. C.. 2009. Selected additives did not improve flowability of DDGS in commercial systems. Appl. Eng. Agric. 25:75–82. doi:10.13031/2013.25422 [Google Scholar]
- Lu Q., Zhou W., Min M., Ma X., Chandra C., Doan Y. T., Ma Y., Zheng H., Cheng S., Griffith R., et al. 2015. Growing chlorella sp. On meat processing wastewater for nutrient removal and biomass production. Bioresour. Technol. 198:189–197. doi:10.1016/j.biortech.2015.08.133 [DOI] [PubMed] [Google Scholar]
- Lu Q., Zhou W., Min M., Ma X., Ma Y., Chen P., Zheng H., Doan Y. T., Liu H., Chen C., et al. 2016. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour. Technol. 201:33–40. doi:10.1016/j.biortech.2015.11.029 [DOI] [PubMed] [Google Scholar]
- Lum K. K., Kim J., and Lei X. G.. 2013. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 4:53. doi:10.1186/2049-1891-4-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhou W., Chen P., Urriola P., Gislerod H., Shurson G., Ruan R., and Chen C.. 2015. Effects of algae feeding on mouse metabolome. Faseb. J. 29:745.3.29420907 [Google Scholar]
- Mackenzie S. G., Leinonen I., Ferguson N., and Kyriazakis I.. 2016. Can the environmental impact of pig systems be reduced by utilizing co-products as feed?J. Clean Prod. 115:172–181. doi:10.1016/j.jclepro.2015.12.074 [Google Scholar]
- McGlinchey D. 2009. Bulk property characterization. In: Characterization of bulk solids. Oxford (UK): Blackwell Publishing Ltd; p. 48–84. doi:10.1002/9781444305456.ch2 [Google Scholar]
- Mercer P., and Armenta R. E.. 2011. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 113:539–547. doi:10.1002/ejlt.201000455 [Google Scholar]
- Noblet J. and Perez J. M.. 1993. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 71:3389–3398. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- Roura E., Koopmans S. J., Lallès J. P., Le Huerou-Luron I., de Jager N., Schuurman T., and Val-Laillet D.. 2016. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 29:60–90. doi:10.1017/S0954422416000020 [DOI] [PubMed] [Google Scholar]
- Urriola P. E., Li M., Kerr B. J., and Shurson G. C.. 2014. Evaluation of prediction equations to estimate gross, digestible, and metabolizable energy content of maize dried distillers’ grains with solubles (DDGS) for swine based on chemical composition. Anim. Feed Sci. Technol. 198:196–202. doi:10.1016/j.anifeedsci.2014.09.006 [Google Scholar]
- Yang Y., Kim B., Park Y-K., and Lee J-Y.. 2014. Effects of long-term supplementation of blue-green algae on lipid metabolism in C57BL/6J mice. J. Nutr. Heal. food Sci. 1:1–14. doi:10.15226/jnhfs.2014.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T. N., Wu J. F., Pond W. G., and Krook L.. 1982. Feasibility of feeding Spirulina maxima, Arthrospira platensis or Chlorella sp. to pigs weaned to a dry diet at 4 to 8 days of age. Nutr. Rep. Int. 25:543–552. [Google Scholar]
- Zijlstra R. T., Jha R., Woodward A. D., Fouhse J., and van Kempen T. A.. 2012. Starch and fiber properties affect their kinetics of digestion and thereby digestive physiology in pigs. J. Anim. Sci. 90(Suppl 4):49–58. doi:10.2527/jas.53718 [DOI] [PubMed] [Google Scholar]