Abstract
This experiment compared milk production, milk composition, and physiological responses in lactating dairy cows supplemented with or without a mixture of condensed tannins, encapsulated cinnamaldehyde, curcumin, capsaicin, and piperine. Thirty-six lactating, multiparous, pregnant ¾ Holstein × ¼ Gir cows were maintained in a single drylot pen with ad libitum access to water and a total-mixed ration and were milked twice daily (d –7 to 84). On d 0, cows were ranked by days in milk (86 ± 3 d), milk yield (27.8 ± 1.0 kg), body weight (BW; 584 ± 10 kg), and body condition score (BCS; 3.04 ± 0.06) and assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of Actifor Pro (Delacon Biotechnik GmbH; Steyregg, Austria). From d 0 to 84, SUPP cows individually received (as-fed basis) 15 g of Actifor Pro mixed with 85 g of finely ground corn through self-locking headgates before each milking of the day. Each CON cow concurrently received 85 g (as-fed basis) of finely ground corn through self-locking headgates. Throughout the experimental period (d –7 to 84), cows from both treatments were administered 500 mg of sometribove zinc at 14-d intervals and were monitored daily for morbidity, including clinical mastitis. Individual milk production was recorded daily, whereas milk samples were collected weekly for analysis of milk composition. Cow BW, BCS, and blood samples were also collected weekly. Cows receiving SUPP gained more BCS (P = 0.05) and had greater (P = 0.04) milk yield during the experiment compared with CON cows (0.22 vs. 0.07 of BCS, SEM = 0.05; 29.5 vs. 27.9 kg/d, SEM = 0.5). Milk composition did not differ (P ≥ 0.15) between SUPP and CON cows; hence, SUPP cows also had greater (P ≤ 0.02) production of fat-corrected and energy-corrected milk. Incidence of clinical mastitis did not differ (P ≥ 0.49) between SUPP and CON cows. No treatment differences were also detected (P ≥ 0.21) for serum concentrations of glucose and serum urea N. Mean serum haptoglobin concentration during the experiment was greater (P = 0.05) in CON vs. SUPP cows. Cows receiving SUPP had less (P ≤ 0.04) serum cortisol concentrations on d 21 and 42, and greater (P ≤ 0.05) serum concentrations of insulin-like growth factor-I on d 7, 35, and 63 compared with CON cows (treatment × day interactions; P ≤ 0.02). Collectively, supplementing phytogenic feed ingredients improved nutritional status and milk production of lactating ¾ Holstein × ¼ Gir cows.
Keywords: dairy cattle, milk production, physiology, phytogenic ingredients, supplementation
INTRODUCTION
Worldwide dairy production must increase 63% by 2050 to feed an additional 2.3 billion people and support a projected 20% increase in milk consumption per capita (FAO, 2009). Resources for dairy and other agricultural systems, however, will become even more limited as the planet population increases and urban areas expand. Therefore, efficiency in dairy cattle production needs to advance significantly during the next decades to meet the increasing demand for dairy products, while fostering ecological stewardship and judicious use of natural resources.
Nutritional management of dairy cows regulates not only milk production but also product quality, animal health, and environmental impacts of dairy systems (NRC, 2001). Nutritional strategies that optimize milk production efficiency, while promoting animal and ecological welfare, are thus warranted to meet the global requirements for dairy products. Phytogenic feed additives are plant-derived products with nutraceutical properties, which have been investigated across livestock production systems (Yang et al., 2015) due to increased regulations regarding feed-grade antimicrobials (US Food and Drug Administration, 2015). These include condensed tannins, essential oils, and pungent compounds known to enhance rumen function, nutrient utilization, and immunity in cattle (Tedeschi et al., 2014; Oh et al., 2017; Sharma et al., 2017).
Condensed tannins react with dietary protein and prevent microbial degradation in the rumen, increasing the passage of dietary protein for duodenal absorption (Min et al., 2003) and decreasing ammonia emissions to the environment (Koenig et al., 2018). Essential oils such as cinnamaldehyde have been shown to enhance rumen fermentation and alleviate systemic inflammation in cattle receiving a high-concentrate diet (Yang et al., 2010). Finally, compounds isolated from pungent plants such as curcumin, capsaicin, and piperine appear to have immune benefits to cattle (Oh et al., 2013) and increase synthesis of digestive enzymes in monogastrics (Platel and Srinivasan, 2000). Research is still warranted, however, to establish the benefits of supplementing phytogenic ingredients to lactating dairy cows. On the basis of the aforementioned information, we hypothesized that supplementing a combination of condensed tannins, cinnamaldehyde, and pungent compounds will enhance productivity and welfare of dairy cattle. Therefore, this experiment compared milk production, composition, and physiological responses in lactating dairy cows supplemented with or without a blend of these phytogenic ingredients.
MATERIALS AND METHODS
This experiment was conducted at the São Paulo State University—Lageado Experimental Station, located in Botucatu, São Paulo, Brazil. The animals used were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the São Paulo State University—Animal Ethics Committee (#109/2018).
Animals and Diets
Thirty-six lactating, multiparous, pregnant ¾ Holstein × ¼ Gir cows [parity = 2.5 ± 0.13 parities, body weight (BW) = 584 ± 10 kg, body condition score (BCS, according to Wildman et al., 1982) = 3.04 ± 0.06, milk yield = 27.8 ± 1.0 kg, and days in milk (DIM) = 86 ± 3 d] were assigned to this experiment (d –7 to 84). On d 0, cows were ranked in a decreasing order by DIM, milk yield, BW, and BCS and assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of Actifor Pro (Delacon Biotechnik GmbH; Steyregg, Austria). This allocation procedure was adopted to ensure that both treatment groups had similar DIM, milk yield, BW, and BCS on d 0. Actifor Pro is a patented proprietary branded product, including condensed tannins, encapsulated cinnamaldehyde, curcumin, capsaicin, and piperine, and was offered in the amount recommended by the manufacturer (Delacon Biotechnik GmbH).
From d –7 to 84, cows were maintained in a single drylot pen with ad libitum access to water and a total-mixed ration (TMR; 1.5 m of linear bunk space per cow). The TMR was formulated (Table 1) with the Spartan Dairy Ration Evaluator/Balancer (v. 3.0; Michigan State University, East Lansing, MI) to yield 30 kg of milk/d. Cows were milked twice daily in a side-by-side milking system (0600 and 1700 h). From d 0 to 84, SUPP cows individually received (as-fed basis) 15 g of Actifor Pro mixed with 85 g of finely ground corn through self-locking headgates before each milking of the day. Each CON cow concurrently received 85 g (as-fed basis) of finely ground corn through self-locking headgates. During the experiment, cows from both treatments were administered 500 mg of sometribove zinc (rBST; Lactotropin; Elanco Saúde Animal, São Paulo, Brazil) subcutaneously at 14-d intervals (d 0, 14, 28, 42, 56, and 70).
Table 1.
Composition and nutritional profile of the total-mixed ration offered for ad libitum consumption to lactating dairy cows during the experimental period
| Item | Component |
|---|---|
| Composition (dry matter basis) | |
| Corn silage, % | 50.7 |
| Ground corn, % | 16.9 |
| Soybean meal, % | 11.8 |
| Ryegrass silage, % | 9.88 |
| Wheat middlings, % | 6.90 |
| Mineral mix,a % | 3.38 |
| Urea, % | 0.44 |
| Nutritional profile (dry matter basis) | |
| Non-detergent fiber, % | 34.4 |
| Starch, % | 26.4 |
| Net energy for lactation, Mcal/kg | 1.55 |
| Net energy for maintenance, Mcal/kg | 1.70 |
| Crude protein, % | 15.1 |
aContaining 23% Ca, 2.0% P, 5.5% Na, 3.5 % K, 4.0 % Mg, 7.5 % Cl, 3.1 % S, 145 mg/kg Cu, 15 mg/kg I, 4.5 mg/kg Se, 680 mg/kg Zn, 2,400 IU/g of vitamin A, 1,100 IU/g of vitamin D3, and 37 IU/g of vitamin E.
Sampling
Cow BW and BCS were recorded weekly (d –7, 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, and 84). Cows were weighed on a platform scale (Precision Balanças; Tupã, SP, Brazil), and BCS was assessed according to Wildman et al. (1982) by the same two evaluators that were blinded to distribution of cows between treatments. Samples of the TMR were collected every 14 d during the experimental period (d 0, 14, 28, 42, 56, 70, and 84).
Individual milk production was recorded daily from d –7 to 84. Milk samples were collected weekly (d –7, 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, and 84) from each cow following each milking of the day. More specifically, 50 mL was retrieved from a composite milk sampler (#AMS/200; Ambic Equipment Limited; Oxfordshire, UK) attached to each individual milk collector (GEA Farm Technologies; Bönen, Germany), immediately mixed with a bronopol preservative, and stored at 4 °C. Samples from both milkings of the day were combined into 1 daily sample (100 mL) and shipped to a commercial laboratory (Clínica do Leite; Universidade de São Paulo, Piracicaba, Brazil) within 48 h of sampling.
Cows were monitored daily during the entire experimental period (d –7 to 84) for incidence of morbidity or mortality by trained personnel (Lima et al., 2012). Cows were screened for mastitis before every milking using the strip cup method, whereas clinical mastitis was defined as a change in milk secretion (e.g., flakes) and external evidence of udder inflammation (e.g., hardening and redness) as in Ebert et al. (2017). Cows diagnosed with mastitis were treated with ceftiofur hydrochloride (Spectramast LC; Zoetis, São Paulo, Brazil) for 5 consecutive days on diagnosis as recommended by the manufacturer, whereas milk yield was recorded but milk was discarded for 8 consecutive days after diagnosis. No other incidences of morbidity, besides mastitis, or mortality were observed during the experiment.
Blood samples were also collected weekly (d –7, 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, and 84) from each cow before the morning milking and treatment feeding of the day (0530 h). Blood was obtained from either the coccygeal vein or artery into commercial collection tubes with no anticoagulant additives (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ), placed immediately on ice, centrifuged at 3,000 × g at 4 °C for 30 min for serum collection, and stored at –20 °C on the same day of collection. All samples were centrifuged within 5 min after collection to prevent degradation of metabolites and hormones.
Laboratorial Analysis
Samples of the TMR were pooled into one sample and analyzed for nutrient content (Table 1) via wet chemistry procedures by a bromatology laboratory (3rlab, Belo Horizonte, Brazil). Milk samples were analyzed for somatic cell count (SCC) via flow cytometry (AOAC, 1990) with a Somacount 300 (Bentley Instruments Inc.; Chaska, MN), and concentrations of fat, lactose, protein, casein, urea N (MUN) and total solids via infrared spectrometry (method 972.16; AOAC, 1999). Fat-corrected milk (FCM) and energy-corrected milk (ECM) were calculated according to the NRC (2001), based on milk concentrations of fat, protein, and total solids of the concurrent week. Milk nutrient output was estimated based on milk composition, daily milk yield, and energy output (NRC, 2001).
Blood samples were analyzed for serum concentrations of urea nitrogen (SUN) and glucose (colorimetric kits #B7551 and G7521, respectively; Pointe Scientific, Inc., Canton, MI), haptoglobin (colorimetric assay; Cooke and Arthington, 2013), insulin-like growth factor-I (IGF-I; human-specific ELISA kit SG100; R&D Systems, Inc., Minneapolis, MN; validated by Cooke et al., 2013), and cortisol (radioimmunoassay #07221102; MP Biomedicals, Irvine, CA). The intra- and interassay coefficient of variation were, respectively, 2.7% and 3.2% for glucose, 7.5% and 9.4% for SUN, 4.0% and 5.8% for haptoglobin, 4.3% and 1.3% for IGF-I, and 3.5% and 5.9% for cortisol.
Statistical Analysis
All data were analyzed using SAS, version 9.3 (SAS Inst., Inc., Cary, NC) with cow as the experimental unit, cow (treatment) as the random variable, and Satterthwaite approximation to determine the denominator df for the tests of fixed effects. Quantitative data (BW, BCS, milk production, milk composition, and serum variables) were analyzed using the MIXED procedure, whereas binary data (incidence of mastitis) were analyzed using the GLIMMIX procedure with a binomial distribution and logit link function. All quantitative data were initially tested for normality with the Shapiro–Wilk test from the UNIVARIATE procedure, and milk SCC data were not normally distributed (W = 0.55). Therefore, SCC data were log transformed (base-10 log) to achieve normality (W = 0.99). The model statement used for the analysis of BW and BCS change, as well as initial and final BCS and BW during the experiment, contained the effects of treatment. The model statement used for the analysis of milk yield, milk constituents, mastitis incidence, and serum variables contained the effects of treatment, day, and the resultant interaction. Milk and serum results obtained before the beginning of treatment administration (d –7 to 0) were averaged and included as independent covariate within each respective analysis. The specified term for the repeated statements was day with cow (treatment) as subject. The covariance structure used for all repeated statements was autoregressive, which provided the best fit for these analyses according to the Akaike information criterion. Significance was set at P ≤ 0.05, and tendencies were determined if P > 0.05 and ≤ 0.10. Results are reported as least square means and were separated using least square difference. Results are reported according to treatment effects if no interactions were significant, or according to the highest-order interaction detected that contained the effects of treatment.
RESULTS
As designed, SUPP and CON cows had similar (P ≥ 0.18) DIM, BW, and BCS at the beginning of the experiment (Table 2). Cows from both treatments had similar (P = 0.85) BW change from d 0 to 84, whereas SUPP cows gained more BCS (P = 0.05) compared with CON cows. Cow BW and BCS on d 84, however, did not differ (P ≥ 0.87) between treatments (Table 2).
Table 2.
Days in milk, body weight (BW), body condition score (BCS), and incidence of mastitis in lactating ¾ Holstein × ¼ Gir dairy cows assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of a supplement containing condensed tannins, essential oils, and pungent compounds (Actifor Pro; Delacon Biotechnik GmbH; Steyregg, Austria) from d 0 to 84 of the experiment
| Item | SUPP | CON | SEM | P-value |
|---|---|---|---|---|
| Days in milk (d 0), d | 87.6 | 84.9 | 5.3 | 0.71 |
| BW, kg | ||||
| Initial (average d –7 and 0) | 583 | 582 | 15 | 0.94 |
| Final (d 84) | 604 | 604 | 15 | 0.99 |
| BW change | 21.2 | 23.0 | 6.9 | 0.85 |
| BCSa | ||||
| Initial (average d –7 and 0) | 2.96 | 3.13 | 0.08 | 0.18 |
| Final (d 84) | 3.18 | 3.21 | 0.11 | 0.87 |
| BCS change | 0.22 | 0.07 | 0.05 | 0.05 |
| Incidence of mastitisb | ||||
| Cows diagnosed with mastitis, % | 17.6 | 27.8 | 10.3 | 0.49 |
| Total mastitis per cow in trialc | 0.35 | 0.33 | 0.17 | 0.94 |
aAccording to Wildman et al. (1982).
bCows were screened for mastitis prior to every milking (d –7 to 84) using the strip cup method, whereas clinical mastitis was defined as a change in milk secretion (e.g., flakes) and external evidence of udder inflammation (e.g., hardening and redness) as in Ebert et al. (2017).
cTotal cases of mastitis divided by number of cows within each treatment group.
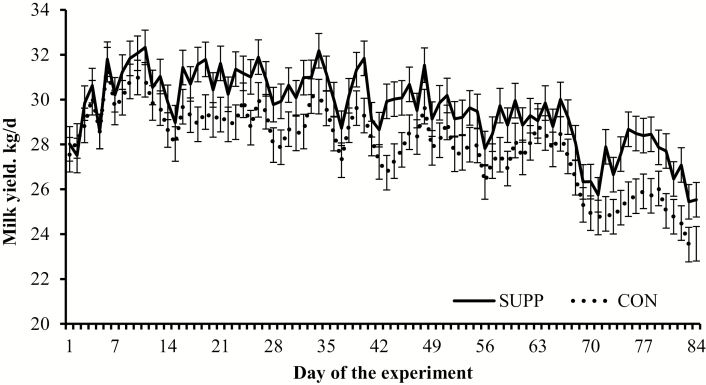
Cows receiving SUPP had greater (P = 0.04) milk yield during the experiment compared with CON cows (Table 3). Although the treatment × day interaction was not detected for milk yield (P = 0.73), treatment differences within days were mostly noted after d 14 of the experimental period (Figure 1). Milk composition did not differ (P ≥ 0.15) between SUPP and CON cows, including SCC and milk urea N (Table 3). In turn, SUPP cows had greater (P ≤ 0.04) FCM, ECM, and milk output of fat, lactose, and energy and tended (P ≤ 0.10) to have greater milk output of protein and casein compared with CON cows (Table 3).
Table 3.
Milk production from lactating ¾ Holstein × ¼ Gir dairy cows assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of a supplement containing condensed tannins, essential oils, and pungent compounds (Actifor Pro; Delacon Biotechnik GmbH; Steyregg, Austria) from d 0 to 84 of the experimenta
| Item | SUPP | CON | SEM | P-value |
|---|---|---|---|---|
| Milk production, kg/d | ||||
| Milk yield | 29.5 | 27.9 | 0.5 | 0.04 |
| Fat-corrected milk | 32.6 | 30.0 | 0.7 | 0.02 |
| Energy-corrected milk | 33.0 | 30.5 | 0.7 | 0.01 |
| Milk composition | ||||
| Fat, % | 4.10 | 4.01 | 0.10 | 0.54 |
| Protein, % | 3.34 | 3.35 | 0.03 | 0.69 |
| Casein, % | 2.58 | 2.59 | 0.02 | 0.74 |
| Milk urea N, % | 13.3 | 13.5 | 0.3 | 0.67 |
| Lactose, % | 4.66 | 4.59 | 0.04 | 0.15 |
| Total solids, % | 13.0 | 12.9 | 0.17 | 0.80 |
| Somatic cell count, cells/μLb | 5.02 | 5.11 | 0.08 | 0.45 |
| Milk nutrient output | ||||
| Fat, kg/d | 1.22 | 1.11 | 0.03 | 0.04 |
| Protein, kg/d | 0.979 | 0.927 | 0.021 | 0.10 |
| Casein, kg/d | 0.757 | 0.716 | 0.016 | 0.09 |
| Lactose, kg/d | 1.38 | 1.28 | 0.03 | 0.04 |
| Total solids, kg/d | 3.84 | 3.63 | 0.10 | 0.15 |
| Energy, Mcal/d | 22.3 | 20.5 | 0.5 | 0.01 |
aIndividual milk production was recorded daily from d –7 to 84. Milk samples were collected once weekly from each cow following each milking of the day as in Rodrigues et al. (2018). Fat-corrected milk (FCM) and energy-corrected milk (ECM) were calculated according to the NRC (2001), based on milk concentrations of fat, protein, and total solids of the concurrent week. Milk nutrient output was estimated based on milk composition, daily milk yield, and energy output (NRC, 2001). Values obtained from d –7 and 0 were averaged and included as covariate within each respective analysis; therefore, values reported are covariately adjusted least square means.
bOriginal somatic cell count results were not normally distributed (W = 0.55); therefore, were log transformed (base-10 log) to achieve normality (W = 0.99).
Figure 1.
Milk yield of lactating ¾ Holstein × ¼ Gir dairy cows assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of a supplement containing condensed tannins, essential oils, and pungent compounds (Actifor Pro; Delacon Biotechnik GmbH; Steyregg, Austria) from d 0 to 84 of the experiment. Individual milk yield from d –7 to 0 were averaged and included as covariate; therefore, values reported are covariately adjusted least square means. Treatment and day effects were detected (P = 0.04 and < 0.01, respectively), whereas the treatment × day interaction was not significant (P = 0.73). Mean milk yield was greater (P = 0.04) in SUPP vs. CON cows (29.5 vs. 27.9 kg/d, respectively, SEM = 0.5).
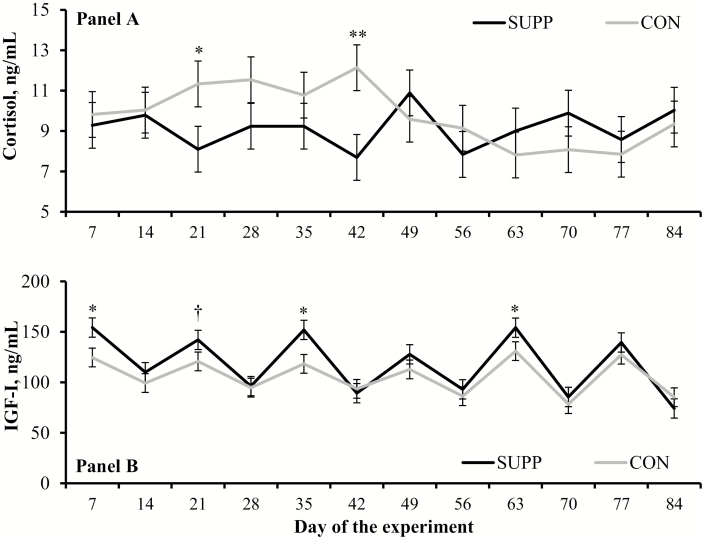
Incidence of clinical mastitis, and total number of diagnosis per cow within each experimental group, did not differ (P ≥ 0.49) between SUPP and CON cows (Table 2). No treatment differences were also detected (P ≥ 0.21) for serum concentrations of glucose and SUN (Table 4). Mean serum haptoglobin concentration during the experiment was greater (P = 0.05) in CON vs. SUPP cows (Table 4). Treatment × day interactions were detected (P ≤ 0.02) for serum cortisol and IGF-I. Cows receiving CON had greater (P ≤ 0.04) cortisol concentrations on d 21 and 42 of the experiment compared with SUPP cows (Figure 2a). Serum IGF-I concentrations were greater (P ≤ 0.05) in SUPP vs. CON cows on d 7, 35, and 63 and tended (P = 0.10) to be greater on d 21 of the experiment (Figure 2b).
Table 4.
Serum concentrations of glucose, urea nitrogen (SUN), and haptoglobin from lactating ¾ Holstein × ¼ Gir dairy cows assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of a supplement containing condensed tannins, essential oils, and pungent compounds (Actifor Pro; Delacon Biotechnik GmbH; Steyregg, Austria) from d 0 to 84 of the experimenta
| Item | SUPP | CON | SEM | P-value |
|---|---|---|---|---|
| Glucose, mg/dL | 57.6 | 55.0 | 1.4 | 0.21 |
| SUN, mg/dL | 17.6 | 17.2 | 0.5 | 0.57 |
| Haptoglobin, mg/mL | 0.097 | 0.137 | 0.015 | 0.05 |
aBlood samples were collected weekly (d –7 to 84), before the morning feeding and milking during the experiment (0530 h). Values obtained on d –7 and 0 were averaged and included as covariate within each respective analysis; therefore, values reported are covariately adjusted least square means.
Figure 2.
Serum concentrations of cortisol (A) and insulin-like growth factor-I (IGF-I) (B) in lactating ¾ Holstein × ¼ Gir dairy cows assigned to receive (SUPP; n = 18) or not (CON; n = 18) 30 g/cow daily (as-fed basis) of a supplement containing condensed tannins, essential oils, and pungent compounds (Actifor Pro; Delacon Biotechnik GmbH; Steyregg, Austria) from d 0 to 84 of the experiment. Values obtained on d –7 and 0 were averaged and included as covariate within each respective analysis; therefore, values reported are covariately adjusted least square means. Treatment × day interaction were detected (P ≤ 0.02). Treatment comparison within days; † P ≤ 0.10. * P ≤ 0.05, ** P < 0.01.
DISCUSSION
Condensed tannins are secondary plant compounds that react with dietary protein in a pH-dependent manner (Jones and Mangan, 1977). More specifically, condensed tannins form complexes with dietary protein in the rumen, preventing degradation by rumen microbes, while releasing the protein when exposed to acidic pH in the abomasum and proximal duodenum (Min et al., 2003). Accordingly, supplementing condensed tannins can increase the amount of dietary protein bypassing rumen degradation, whereas reducing ammonia release from the rumen to blood, milk, urine, and feces (Tedeschi et al., 2014; Koenig et al., 2018). Cinnamaldehyde is the main active component of cinnamon oil with antimicrobial activity, which may also inhibit peptidolysis by rumen microorganisms (Cardozo et al., 2004). Supplementing cinnamaldehyde enhanced ruminal fermentation, including greater organic matter digestibility in cattle consuming high-concentrate diets (Yang et al., 2010) and propionate production in continuous culture fermentors (Busquet et al., 2005). Cinnamaldehyde also has antioxidant and immunomodulatory effects (Sharma et al., 2017) and reduced concentrations of the acute-phase protein serum amyloid A when supplemented to feedlot steers (Yang et al., 2010). Compounds isolated from pungent plants such as curcumin, capsaicin, and piperine have also been evaluated as feed additives. Supplementing these compounds increased synthesis of digestive enzymes in rats (Platel and Srinivasan, 2000) and improved immune responses in monogastrics and ruminants (Oh et al., 2017). Overall, the commercial source of phytogenic ingredients offered to SUPP cows contained condensed tannins, cinnamaldehyde, and pungent substances with overlapping productive, digestive, and immune implications. Therefore, the physiological and productive benefits of SUPP noted herein cannot be attributed to individual components but should be associated with supplementing a blend of these phytogenic ingredients to lactating dairy cattle.
Supporting our main hypothesis, SUPP cows had greater milk yield compared with CON cows during the experiment. Differences in milk yield became evident 14 d after treatment administration began, suggesting that SUPP required a 2-wk adaption period before affecting milk production. Cows receiving SUPP also gained more BCS during the experiment, although such response was not sufficient to cause final BCS to differ. Similar outcomes were not detected for BW change, which include synthesis of body tissues as well as fluctuations in feed and water consumption. Cow BCS reflects body tissue status without being influenced by gastrointestinal tract content (Leiva et al., 2014); hence, it is considered a better indicator of nutritional status than BW for dairy cattle (West et al., 1990; Moallem et al., 2000). Together, these outcomes indicate that SUPP improved cow nutritional status, resulting in greater milk production and accumulation of body reserves (NRC, 2001). Intake of the TMR, however, was not evaluated herein to determine if SUPP increased feed intake, feed efficiency, or both. Nonetheless, the digestive benefits expected from SUPP, such as improved rumen fermentation, increased passage of dietary protein to duodenum, and enhanced N utilization are known to improve feed intake and efficiency in cattle (NRC, 2001). Reduced nutrient loss as ammonia and methane may also have contributed to increased nutrient availability to SUPP cows (Tedeschi et al., 2014; Oh et al., 2017). Additional research is warranted to determine the specific impacts of SUPP on feed intake, feed efficiency, and nutrient utilization by lactating dairy cattle.
Nutritional status is positively associated with the synthesis of glucose and IGF-I (Busato et al., 2002; Butler, 2003), which are key regulators of milk production in dairy cows (Huntington, 1997; Akers et al., 2006). Serum glucose concentrations were similar between SUPP and CON cows, which can be associated with rigorous homeostatic regulation of circulating glucose in ruminants (Huntington, 1982) and glucose uptake by the mammary gland (Bickerstaffe et al., 1974). Serum concentrations of IGF-I in SUPP and CON cows were directly influenced by rBST, peaking 7 d relative to rBST administration (Bilby et al., 1999; Lucy, 2000). Moreover, the serum IGF-I increase to rBST administration was often greater in SUPP vs. CON cows during the experimental period. Others have also reported that nutritional balance is positively associated with circulating IGF-I responses to rBST administration and subsequent milk yield in dairy cows (McGuire et al., 1991; Vicini et al., 1991). Hence, treatment differences in serum IGF-I concentrations corroborate that nutritional status was improved in SUPP cows, enhancing their metabolic response to rBST and overall milk production compared with CON cows.
Milk composition was not affected by treatments, particularly milk protein, MUN, and casein. Given the role of phytogenic ingredients in increasing ruminal bypass of dietary protein (Cardozo et al., 2004; Tedeschi et al., 2014), we theorized that SUPP would increase milk protein and casein concentrations whereas decreasing MUN (Xu et al., 1998; Giallongo et al., 2016). Alternatively, Jenkins and McGuire (2006) noted that increasing rumen bypass protein does not markedly alter milk protein content, given that transfer efficiency of dietary protein to milk is relatively low. The SUPP treatment also failed to modulate SUN concentrations, which is highly correlated with ruminal ammonia production and with MUN (Broderick and Clayton, 1997; Hammond, 1992). Both treatments had SUN and MUN values within the expected range in dairy cattle receiving diets balanced for protein and energy (Roseler et al., 1993; Baker et al., 1995). Collectively, these results suggest that SUPP did not improve cows’ nutritional status by increasing ruminal escape of dietary protein and reducing N loss as ammonia (Powell et al., 2014; Hristov et al., 2018). Moreover, SUPP increased milk production without changing milk constitution, resulting in greater FCM, ECM, and overall output of milk nutrients in SUPP vs. CON cows..
Despite the immunological benefits associated with feeding phytogenic ingredients to livestock species including dairy cattle (Oh et al., 2013; Ayrle et al., 2016), SUPP did not reduce milk SCC or alleviated the incidence of mastitis. Clinical and subclinical mastitis, the latter evidenced by elevated milk SCC, are known to impair milk production in dairy cows (Ruegg, 2003; Hadrich et al., 2018). No other health-related challenges were noted during the experiment. Cows used herein were in mid-lactation, whereas the vast majority of disease in dairy cattle occur during early lactation (LeBlanc et al., 2006). Therefore, the productive benefits yielded by SUPP were not associated with improved mammary gland health or disease mitigation in lactating dairy cattle. In turn, serum haptoglobin concentrations were less in SUPP vs. CON cows during the experiment, despite this acute-phase protein being known as biomarker for inflammatory processes such as subclinical mastitis (Murata et al., 2004; Ceciliani et al., 2012). Phytogenic ingredients have been shown to regulate inflammatory and acute-phase responses in cattle (Yang et al., 2010; Oh et al., 2017). Alternatively, circulating haptoglobin is also increased on disruption of the ruminal ecosystem and subsequent release of microbial endotoxins to the bloodstream (Marques et al., 2012), which is common in cattle consuming high-concentrate diets (Gozho et al., 2005). Hence, SUPP may have reduced serum haptoglobin concentrations due to its immunomodulatory effects, but most likely by optimizing rumen function and fermentation (Yang et al., 2010; Tedeschi et al., 2014; Oh et al., 2016).
Acute stressors can also elicit a haptoglobin response in healthy cattle, with circulating cortisol mediating this response (Cooke, 2017). One could speculate that SUPP also reduced serum haptoglobin concentrations by alleviating adrenocortical responses, although serum cortisol concentrations were less in SUPP vs. CON cows on d 21 and 42 of the experiment. It seems more plausible that serum cortisol concentrations were greater in CON vs. SUPP cows, at least transiently, due to the beneficial impacts of SUPP on rumen function and subsequent systemic inflammatory responses (Dong et al., 2013; Jia et al., 2014). Nevertheless, both neuroendocrine stress and acute-phase reactions demand a significant amount of body resources, increase maintenance requirements, and decrease nutrient intake (Elsasser et al., 1997; Johnson, 1997; Cooke, 2017). Therefore, reduced serum haptoglobin and cortisol concentrations also contributed to increased nutritional status and milk yield in SUPP vs. CON cows.
Collectively, supplementing a blend of phytogenic ingredients including condensed tannins, encapsulated cinnamaldehyde, curcumin, capsaicin, and piperine improved nutritional status, BCS accumulation, and milk production in dairy cows. These outcomes should be associated with ruminal and digestive benefits of phytogenic ingredients, and their subsequent impacts on systemic metabolic and acute-phase responses. It must be noted that this experiment was conducted with mid-lactating ¾ Holstein × ¼ Gir cows, which might not fully represent the metabolic and physiological aspects of high-producing Holstein cattle during early lactation. Nevertheless, supplementing lactating dairy cows with phytogenic ingredients appear to be a valid strategy to improve milk production and cattle welfare in dairy production systems.
Conflict of interest statement. None declared.
Footnotes
Appreciation is extended to Delacon Biotechnik GmbH (Steyregg, Austria) for providing financial support for this research project. Correspondence about this article can also be directed to Dr. Reinaldo F. Cooke at reinaldocooke@tamu.edu.
LITERATURE CITED
- Akers R. M. 2006. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci. 89:1222–1234. doi: 10.3168/jds.S0022-0302(06)72191-9. [DOI] [PubMed] [Google Scholar]
- AOAC 1990. Official method of analysis, 15th ed. Arlington (VA): Assoc. Off. Anal. Chem. [Google Scholar]
- AOAC 1999. Official method of analysis, 16th ed. Arlington (VA): Assoc. Off. Anal. Chem. [Google Scholar]
- Ayrle H., Mevissen M., Kaske M., Nathues H., Gruetzner N., Melzig M., and Walkenhorst M.. . 2016. Medicinal plants–prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet. Res. 12:89. doi: 10.1186/s12917-016-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L. D., Ferguson J. D., and Chalupa W.. . 1995. Responses in urea and true protein of milk to different protein feeding schemes for dairy cows. J. Dairy Sci. 78:2424–2434. doi: 10.3168/jds.S0022-0302(95)76871-0 [DOI] [PubMed] [Google Scholar]
- Bickerstaffe R., Annison E. F., and Linzell J. L.. . 1974. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agric. Sci. 82:71–85. doi: 10.1017/S0021859600050243. [DOI] [Google Scholar]
- Bilby C. R., Bader J. F., Salfen B. E., Youngquist R. S., Murphy C. N., Garverick H. A., Crooker B. A., and Lucy M. C.. . 1999. Plasma GH, IGF-I, and conception rate in cattle treated with low doses of recombinant bovine GH. Theriogenology 51:1285–1296. doi: 10.1016/S0093-691X(99)00072-2. [DOI] [PubMed] [Google Scholar]
- Broderick G. A., and Clayton M. K.. . 1997. A statistical evaluation of animal and nutritional factors influencing concentrations of milk urea nitrogen. J. Dairy Sci. 80:2964–2971. doi: 10.3168/jds.S0022-0302(97)76262-3. [DOI] [PubMed] [Google Scholar]
- Busato A., Faissle D., Küpfer U., and Blum J. W.. . 2002. Body condition scores in dairy cows: associations with metabolic and endocrine changes in healthy dairy cows. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 49:455–460. doi:10.1046/j.1439-0442.2002.00476.x. [DOI] [PubMed] [Google Scholar]
- Busquet M., Calsamiglia S., Ferret A., Cardozo P. W., and Kamel C.. . 2005. Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. J. Dairy Sci. 88:2508–2516. doi: 10.3168/jds.S0022-0302(05)72928-3. [DOI] [PubMed] [Google Scholar]
- Butler W. R. 2003. Energy balance relationships with follicular development ovulation and fertility in postpartum dairy cows. Livest. Prod. Sci. 83:211–218. doi: 10.1016/S0301-6226(03)00112-X. [DOI] [Google Scholar]
- Cardozo P. W., Calsamiglia S., Ferret A., and Kamel C.. . 2004. Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J. Anim. Sci. 82:3230–3236. doi: 10.2527/2004.82113230x. [DOI] [PubMed] [Google Scholar]
- Ceciliani F., Ceron J. J., Eckersall P. D., and Sauerwein H.. . 2012. Acute phase proteins in ruminants. J. Proteomics 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Cooke R. F. 2017. Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573. [DOI] [Google Scholar]
- Cooke R. F., and Arthington J. D.. . 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Bohnert D. W., Francisco C. L., Marques R. S., Mueller C. J., and Keisler D. H.. . 2013. Effects of bovine somatotropin administration on growth, physiological, and reproductive responses of replacement beef heifers. J. Anim. Sci. 91:2894–2901. doi:10.2527/jas.2012–6082. [DOI] [PubMed] [Google Scholar]
- Dong H., Wang S., Jia Y., Ni Y., Zhang Y., Zhaung S., Shen X., and Thao R.. . 2013. Long-term effects of subacute ruminal acidosis (SARA) on milk quality and hepatic gene expression in lactating goats fed a high-concentrate diet. PLoS One 8:e82850. doi: 10.1371/journal.pone.0082850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert F., Staufenbiel R., Simons J., and Pieper L.. . 2017. Randomized, blinded, controlled clinical trial shows no benefit of homeopathic mastitis treatment in dairy cows. J. Dairy Sci. 100:4857–4867. doi: 10.3168/jds.2016-11805. [DOI] [PubMed] [Google Scholar]
- Elsasser T. H., Kahl S., Steele N. C., and Rumsey T. S.. . 1997. Nutritional modulation of somatotropic axis-cytokine relationships in cattle: a brief review. Comp. Biochem. Physiol. 116A:209–221. doi: 10.1016/S0300-9629(96)00279-4. [DOI] [PubMed] [Google Scholar]
- Food and Agricultural Organization (FAO) 2009. How to feed the world in 2050. Proc. Expert Meeting on How to Feed the World in 2050 Rome: FAO Headquarters. [Google Scholar]
- Giallongo F., Harper M. T., Oh J., Lopes J. C., Lapierre H., Patton R. A., Parys C., Shinzato I., and Hristov A. N.. . 2016. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 99:4437–4452. doi: 10.3168/jds.2015-10822. [DOI] [PubMed] [Google Scholar]
- Gozho G. N., Plaizier J. C., Krause D. O., Kennedy A. D., and Wittenberg K. M.. . 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88:1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1. [DOI] [PubMed] [Google Scholar]
- Hadrich J. C., Wolf C. A., Lombard J., and Dolak T. M.. . 2018. Estimating milk yield and value losses from increased somatic cell count on US dairy farms. J. Dairy Sci. 101:3588–3596. doi: 10.3168/jds.2017-13840. [DOI] [PubMed] [Google Scholar]
- Hammond A. C. 1992. Update on BUN and MUN as a guide for protein supplementation in cattle. In: Proceedings of the 3rd Annual Florida Ruminant Nutrition Symposium; January 1992; Gainesville (FL); University of Florida; p. 9–18. [Google Scholar]
- Hristov A. N., Harper M., Oh J., Giallong F., Lopes J. C., Cudoc G., Clay J., Ward R., and Chase L. E.. . 2018. Variability in milk urea nitrogen and dairy total mixed ration composition in the northeastern United States. J. Dairy Sci. 101:1579–1584. doi:10.3168/jds.2017–12925. [DOI] [PubMed] [Google Scholar]
- Huntington G. B. 1982. Portal blood flow and net absorption of ammonia-nitrogen, urea-nitrogen, and glucose in nonlactating holstein cows. J. Dairy Sci. 65:1155–1162. doi: 10.3168/jds.S0022-0302(82)82326-6. [DOI] [PubMed] [Google Scholar]
- Huntington G. B. 1997. Starch utilization by ruminants: from basics to the bunk. J. Anim. Sci. 75:852–867. doi: 10.2527/1997.753852x. [DOI] [PubMed] [Google Scholar]
- Jenkins T. C., and McGuire M. A.. . 2006. Major advances in nutrition: impact on milk composition. J. Dairy Sci. 89:1302–1310. doi: 10.3168/jds.S0022-0302(06)72198-1. [DOI] [PubMed] [Google Scholar]
- Jia Y. Y., Wang S. Q., Ni Y. D., Zhang Y. S., Zhuang S., and Shen X. Z.. . 2014. High concentrate-induced subacute ruminal acidosis (SARA) increases plasma acute phase proteins (apps) and cortisol in goats. Animal 8:1433–1438. doi: 10.1017/S1751731114001128. [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Jones W. T., and Mangan J. L.. . 1977. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 28:126–136. doi: 10.1002/jsfa.2740280204. [DOI] [Google Scholar]
- Koenig K., Beauchemin K. A., and McGinn S. M.. . 2018. Feeding condensed tannins to mitigate ammonia emissions from beef feedlot cattle fed high-protein finishing diets containing distillers grains. J. Anim. Sci. 96:4414–4430. doi: 10.1093/jas/sky274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc S. J., Lissemore K. D., Kelton D. F., Duffield T. F., and Leslie K. E.. . 2006. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 89:1267–1279. doi: 10.3168/jds.S0022-0302(06)72195-6. [DOI] [PubMed] [Google Scholar]
- Leiva T., Cooke R. F., Aboin A. C., Drago F. L., Gennari R., and Vasconcelos J. L.. . 2014. Effects of excessive energy intake and supplementation with chromium propionate on insulin resistance parameters in nonlactating dairy cows. J. Anim. Sci. 92:775–782. doi: 10.2527/jas.2013-6852. [DOI] [PubMed] [Google Scholar]
- Lima F. S., Sá Filho M. F., Greco L. F., and Santos J. E.. . 2012. Effects of feeding rumen-protected choline on incidence of diseases and reproduction of dairy cows. Vet. J. 193:140–145. doi: 10.1016/j.tvjl.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Lucy M. C. 2000. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J. Dairy Sci. 83:1635–1647. doi: 10.3168/jds.S0022-0302(00)75032-6. [DOI] [PubMed] [Google Scholar]
- Marques R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. . 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90:5040–5046. doi: 10.2527/jas.2012-5425. [DOI] [PubMed] [Google Scholar]
- McGuire M. A., Beede D. K., Collier R. J., Buonomo F. C., DeLorenzo M. A., Wilcox C. J., Huntington G. B., and Reynolds C. K.. . 1991. Effects of acute thermal stress and amount of feed intake on concentrations of somatotropin, insulin-like growth factor (IGF)-I and IGF-II, and thyroid hormones in plasma of lactating holstein cows. J. Anim. Sci. 69:2050–2056. doi: 10.2527/1991.6952050x. [DOI] [PubMed] [Google Scholar]
- Min B. R., Barry T. N., Attwood G. T., and McNabb W. C.. . 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Technol. 106:3–19. doi:10.1016/ S0377-8401(03)00041-5. [Google Scholar]
- Moallem U., Folman Y., and Sklan D.. . 2000. Effects of somatotropin and dietary calcium soaps of fatty acids in early lactation on milk production, dry matter intake, and energy balance of high-yielding dairy cows. J. Dairy Sci. 83:2085–2094. doi: 10.3168/jds.S0022-0302(00)75090-9. [DOI] [PubMed] [Google Scholar]
- Murata H., Shimada N., and Yoshioka M.. . 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 2001. Nutrient requirements of dairy cattle. Washington, DC: National Academy of Sciences. [Google Scholar]
- Oh J., and Hristov A. N.. . 2016. Effects of plant-derived bioactive compounds on rumen fermentation, nutrient utilization, immune response, and productivity of ruminant animals. In: Jeliazkov (Zheljazkov) V.D. and Cantrell C.L., editors, Medicinal and aromatic crops: production, phytochemistry, and utilization. Washington, DC: American Chemical Society Publications; p. 167–186. doi: 10.1021/bk-2016-1218.ch011. [DOI] [Google Scholar]
- Oh J., Hristov A. N., Lee C., Cassidy T., Heyler K., Varga G. A., Pate J., Walusimbi S., Brzezicka E., Toyokawa K., . et al. 2013. Immune and production responses of dairy cows to postruminal supplementation with phytonutrients. J. Dairy Sci. 96:7830–7843. doi: 10.3168/jds.2013-7089. [DOI] [PubMed] [Google Scholar]
- Oh J., Wall E. H., Bravo D. M., and Hristov A. N.. . 2017. Host-mediated effects of phytonutrients in ruminants: a review. J. Dairy Sci. 100:5974–5983. doi: 10.3168/jds.2016-12341. [DOI] [PubMed] [Google Scholar]
- Platel K., and Srinivasan K.. . 2000. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung 44:42–46. doi:10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Powell J. M., Rotz C. A., and Wattiaux M. A.. . 2014. Potential use of milk urea nitrogen to abate atmospheric nitrogen emissions from Wisconsin dairy farms. J. Environ. Qual. 43:1169–1175. doi: 10.2134/jeq2013.09.0375. [DOI] [PubMed] [Google Scholar]
- Rodrigues R. O., Cooke R. F., Rodrigues S. M. B., Bastos L. N., de Camargo V. F. S., Gomes K. S., and Vasconcelos J. L. M.. . 2018. Reducing prepartum urine pH, by supplementing anionic feed ingredients, impacts physiological and productive responses of Holstein × Gir cows. J. Dairy. Sci. 101:9296–9308. doi: 10.3168/jds.2018-14660. [DOI] [PubMed] [Google Scholar]
- Roseler D. K., Ferguson J. D., Sniffen C. J., and Herrema J.. . 1993. Dietary protein degradability effects on plasma and milk urea nitrogen and milk nonprotein nitrogen in Holstein cows. J. Dairy Sci. 76:525–534. doi:10.3168/jds.S0022-0302(93)77372–5. [Google Scholar]
- Ruegg P. L. 2003. Investigation of mastitis problems on farms. Vet. Clin. North Am. Food Anim. Pract. 19:47–73. doi:10.1016/S0749-0720(02)00078-6. [DOI] [PubMed] [Google Scholar]
- Sharma U. K., Sharma A. K., Gupta A., Kumar R., Pandey A., and Pandey A. K.. . 2017. Pharmacological activities of cinnamaldehyde and eugenol: antioxidant, cytotoxic and anti-leishmanial studies. Cell. Mol. Biol. (Noisy-Le-Grand). 63:73–78. doi: 10.14715/cmb/2017.63.6.15. [DOI] [PubMed] [Google Scholar]
- Tedeschi L. O., Ramírez-Restrepo C. A., and Muir J. P.. . 2014. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 8:1095–1105. doi: 10.1017/S1751731114000974. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration 2015. FACT SHEET: Veterinary Feed Directive Final Rule and Next Steps [accessed November 10, 2018]. http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/ucm449019.htm
- Vicini J. L., Buonomo F. C., Veenhuizen J. J., Miller M. A., Clemmons D. R., and Collier R. J.. . 1991. Nutrient balance and stage of lactation affect responses of insulin, insulin-like growth factors I and II, and insulin-like growth factor-binding protein 2 to somatotropin administration in dairy cows. J. Nutr. 121:1656–1664. doi: 10.1093/jn/121.10.1656. [DOI] [PubMed] [Google Scholar]
- West J. W., Bondari K., and Johnson J. C. Jr. 1990. Effects of bovine somatotropin on milk yield and composition, body weight and condition score of Holstein and jersey cows. J. Dairy Sci. 73:1062–1068. doi:10.3168/jds.S0022-0302(90)78765–6. [DOI] [PubMed] [Google Scholar]
- Wildman E. E., Jones G. M., Wagner P. E., Boman R. L., Troutt H. F., and Lesch T. N.. . 1982. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 65:495–501. doi:10.3168/jds.S0022-0302(82)82223–6. [Google Scholar]
- Xu S., Harrison J. H., Chalupa W., Sniffen C., Julien W., Sato H., Fujieda T., Watanabe K., Ueda T., and Suzuki H.. . 1998. The effect of ruminal bypass lysine and methionine on milk yield and composition of lactating cows. J. Dairy Sci. 81:1062–1077. doi: 10.3168/jds.S0022-0302(98)75668-1. [DOI] [PubMed] [Google Scholar]
- Yang W. Z., Ametaj B. N., Benchaar C., He M. L., and Beauchemin K. A.. . 2010. Cinnamaldehyde in feedlot cattle diets: intake, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 88:1082–1092. doi: 10.2527/jas.2008-1608. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M. A., Huo Y., and Gong J.. . 2015. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens 4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]