Abstract
Heat stress (HS) is a major environmental stressor primarily affecting swine performance through negative effects on intestinal health. Zinc and butyric acid supplementation help maintain intestinal integrity and barrier function, and has been shown to be beneficial to swine during stress conditions. We tested a novel formulation of zinc butyrate (ZnB) to study whether it has protective effects toward swine using pig intestinal epithelial cells (IPEC-J2) and in a grower swine HS trial. IPEC-J2 cells were grown either under an inflammatory challenge (Escherichia coli lipopolysaccharide) or HS (41.5 °C for 48 h) using Transwell plates. The tight junction integrity of the cells under various treatments, including ZnB, zinc sulfate, and calcium butyrate, was followed over a period of 36 to 48 h by measuring transepithelial electrical resistance (TER). During inflammatory challenge, ZnB-treated cells had the greatest TER (P < 0.05) at 36 h. When the cells were exposed to HS at 41.5 °C, ZnB-treated cells had similar TER to the cells incubated at 37.0 °C, indicating significant protection against HS. In the swine trial (two dietary treatments, control and an encapsulated form of 40% zinc butyrate [E-ZnB] in hydrogenated palm oil pearls, 12 pigs per treatment), grower gilts (35 ± 1 kg) were supplemented with E-ZnB for 24 d before being subjected to biphasic HS for 7 d, 30 to 32 °C for 8 h and 28 °C for 16 h, for a total duration of 56 h of HS. At the end of the HS phase, half the pigs were euthanized from each treatment (n = 6 per treatment), and growth performance was calculated. During the HS phase, average daily gain (ADG; 0.53 vs. 0.79 kg) and gain-to-feed ratio (G:F; 0.33 vs. 0.43) were greater in the E-ZnB group (P < 0.05). Although in vivo intestinal permeability increased during the HS phase (P < 0.05), no differences were observed in the present study for the intestinal health parameters measured including TER, villus height:crypt depth ratio, and in vivo and ex vivo intestinal permeability between the two treatment groups. In conclusion, results presented here demonstrate that E-ZnB supplementation during HS improves ADG and G:F in grower pigs. Although we could not measure any differences, the mode of action of butyric acid and zinc suggests that the performance improvements are related to improved intestinal health.
Keywords: butyric acid, heat stress, intestinal integrity, pigs, swine, zinc
INTRODUCTION
Heat stress (HS) is a major environmental stressor costing the animal agriculture industry approximately $2.4 billion per year in the United States alone (St-Pierre et al., 2003). During HS, blood flow is diverted from the intestine to the periphery, which results in hypoxia of the intestinal epithelial cells (Yan et al., 2006). HS exposure and the resulting hypoxia lead to compromised intestinal integrity and barrier dysfunction, allowing for an influx of noxious compounds and pathogenic microbes from the intestine to the systemic circulation (Pearce et al., 2012). This leads to reduced feed intake and nutrient absorption, ultimately resulting in decreased growth (Kluger et al., 1997; Pearce et al., 2014).
Zinc is a cofactor essential for the activity of more than 300 enzymes involved in many vital biological functions necessary for the normal growth and performance of animals (Salgueiro et al., 2002). Zinc has the potential to influence immune function and has beneficial effects on intestinal health (Zhang and Guo, 2009; Haase and Rink, 2014). Zinc is usually supplemented as zinc sulfate or zinc oxide in animal diets, but other forms of zinc are also available including zinc propionate, zinc hydroxides, and zinc–amino acid combinations (Wedekind et al., 1994). Butyric acid is a short-chain fatty acid synthesized in the hindgut by bacterial fermentation of nondigestible fibers (Kien et al., 2006). Apart from serving as an energy source, butyric acid has been shown to have beneficial effects on intestinal health, including maintaining villus height and crypt depth and increasing tight junction integrity through the upregulation of the tight junction proteins (Valenzano et al., 2015). Butyric acid has also been shown to be involved in balancing the normal microbial flora, maintaining osmotic balance, and keeping the immune cells at a hyporesponsive state, which can prevent hyperinflammatory conditions (Ohata et al., 2005; Levine et al., 2013). Butyric acid is usually supplemented in animal feed as sodium or calcium butyrate (CaB).
The benefits of both zinc and butyric acid are well known. However, there are no direct studies that have focused on the impact of the combination working synergistically to improve gut health and growth performance in swine. Combining zinc and butyric acid as ZnB and delivering the molecule in an encapsulated form would release the ingredients in a controlled manner throughout the digestive system. The objective of the study was to determine whether ZnB can mitigate the negative effects of HS in vitro and in vivo. We hypothesized that the zinc and butyric acid in ZnB would improve the integrity of the intestinal barrier, which would lead to a decrease in the negative effects of HS on pig intestinal epithelial cells and a growth performance improvement in pigs. This hypothesis was tested in an in vitro cell culture model using the pig intestinal epithelial cell line, IPEC-J2, under inflammatory and HS challenge conditions, and in a HS swine trial using the encapsulated form, E-ZnB.
MATERIALS AND METHODS
Cell Culture Experiments
Chemicals.
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise stated. All cell culture experiments were conducted using pig intestinal epithelial cells (DSMZ, Braunschweig, Germany) in the cell culture facility located in Kemin Industries (Des Moines, IA). Cells were cultured in Dulbecco’s modified Eagle medium-low glucose supplemented with 10% fetal bovine serum, 1% antibiotics (Penicillin, Streptomycin, and Amphotericin B), and 5 ng/mL epidermal growth factor (Peprotech, Rocky Hill, NJ) in a humidified atmosphere with 5% CO2 at 37.0 °C. Passages 10 to 15 of the cells were used for the experiment. Cells were maintained in 175 cm2 flasks (Corning Inc., Corning, NY) with the media being changed every third day until they were plated in Transwell plates.
Transepithelial resistance measurement.
Intestinal epithelial cells were grown on a membrane in the Transwell plates, on which the IPEC-J2 cells form a single confluent monolayer (Ranaldi et al., 1992; Kalischuk et al., 2009). The cells were seeded onto the polycarbonate filter cell culture chamber inserts (diameter, 24 mm; area, 4.7 cm2; pore diameter, 0.4 µm; Transwell; Corning Inc., Corning, NY) at an approximate density of 2 × 106 cells per filter. The transepithelial electrical resistance (TER) was measured using an epithelial voltohmmeter (EVOM2, World Precision Instruments, Inc., Sarasota, FL). When the cells attained peak TER, approximately 9 d post-confluence (1,800 to 2,100 Ω cm2), they were exposed to concentrations of either 100 or 300 µM zinc sulfate, zinc chloride, ZnB, CaB, or calcium chloride for either 36 or 48 h. Initial experiments with IPEC-J2 cells were conducted to compare the effect of ZnB combination with different zinc and butyric acid sources commonly used in animal feed. Zinc sulfate and zinc chloride were chosen as zinc controls, CaB was chosen as the butyric acid control, and calcium chloride was chosen as a control for calcium. Cell culture media without added zinc or butyric acid was used as the control treatment. The CaB and ZnB used in the study were prepared by Kemin Industries. To prevent any pH changes during butyric acid addition, 1 M HEPES buffer, pH 7.4, was used to dilute the treatment solutions. Each treatment was added at 500 µL to the apical side and 1,500 µL of the media without any treatments was added to the basolateral side. The TER was measured at 0, 12, 24, 36, and 48 h, and there were eight replicates for each experiment.
Inflammatory challenge and heat stress.
Lipopolysaccharide (LPS; Sigma, St. Louis, MO; powder, isolated from Escherichia coli O55:B5 strain through phenol extraction) was used for the inflammatory challenge at a final concentration of 10 µg/mL. The cells were incubated at 37.0 °C with each treatment along with the LPS, and the TER was measured at 0, 12, 24, 36, and 48 h. For the HS experiments, a thermoneutral (TN) control plate was maintained at 37.0 °C, and cells treated with different compounds were exposed to HS in a different incubator set to 41.5 °C. The stress temperature of 41.5 °C was chosen by testing cell permeability at different temperature conditions to find a temperature that affects cell permeability without causing cell death. This temperature was also similar to the temperatures (41 to 43 °C) used for HS experiments with Caco-2 cells, human intestinal epithelial cell line (Xiao et al., 2013). To measure the TER under HS conditions, the cell plates were briefly removed from the incubators and TER was promptly measured using the voltohmmeter, then the plates were returned to the incubator. To account for the variability in the resistance values measured in different experiments, the TER measurements are expressed as a ratio to the TER control: TER treatment group in each experiment.
Claudin-4 immunofluorescence assay.
The assay was performed as previously described (Kolf-Clauw et al., 2009). Briefly, the IPEC-J2 cells were plated in Transwell plates and exposed to the respective treatments for 48 h. The cells were fixed by adding 3.7% formaldehyde for 30 min at room temperature, permeabilized with PBS-0.5% Triton-X-100 for 5 min, and then blocked with 1% bovine serum albumin at room temperature for 30 min. The cells were then incubated with Alexa Fluor conjugated claudin-4 antibody (3E2C1, ThermoFisher Scientific, Waltham, MA) for 1 h. The inserts were mounted on glass slides and counterstained with 4-6-diamidino-2-phenylindole nuclear stain for 5 min. Images were captured using a Leica fluorescent microscope, and protein expression was analyzed using ImageJ software (NCBI). To compare the expression of tight junction proteins in different treatments, images were acquired using constant acquisition parameters.
Swine Trial
Animals and management.
The experiment was conducted at the Iowa State University Swine Nutrition Farm (Ames, IA). All procedures used in this experiment were approved by the Iowa State University Institutional Animal Care and Use Committee (#5-14-7801-S). The diets were manufactured at the Iowa State University Swine Nutrition Farm. Pigs were given iso-caloric corn-soybean meal-based diets (Table 1). Each zinc molecule was bound to two butyric acid molecules in ZnB (1:2 ratio). In the E-ZnB, the same ratio was maintained but in an encapsulated form. Encapsulated ZnB, which consists of 60% hydrogenated palm oil and 40% ZnB, was added at 7.5 kg/metric ton of feed. Corn was replaced in the treatment diet to account for the E-ZnB inclusion. The dose of E-ZnB contained 819 ppm of zinc and 2,181 ppm of butyric acid per ton of feed. All animals were monitored daily, fed ad libitum, and had free access to water nipples.
Table 1.
Composition of the diets used in the grower pig study during the supplementation and HS phases
| Ingredient | Control | E-ZnB |
|---|---|---|
| Diet composition, % as fed | ||
| Corn, yellow dent | 72.37 | 71.62 |
| Soybean meal, 46.5% CP | 23.88 | 23.88 |
| Soybean oil | 0.40 | 0.40 |
| Vitamin premix1 | 0.15 | 0.15 |
| Mineral premix2 | 0.15 | 0.15 |
| Salt | 0.50 | 0.50 |
| Limestone | 1.06 | 1.06 |
| Monocalcium phosphate 21% | 1.12 | 1.12 |
| l-Lysine HCl | 0.25 | 0.25 |
| dl-Methionine | 0.05 | 0.05 |
| l-Threonine | 0.07 | 0.07 |
| E-ZnB3 | — | 0.75 |
| Total | 100 | 100 |
| Calculated composition, % as fed4 | ||
| Dry matter, % | 89.6 | 89.6 |
| DE, kcal/kg | 3,477 | 3,477 |
| ME, kcal/kg | 3,330 | 3,330 |
| NE, kcal/lb | 2,432 | 2,432 |
| Crude protein, % | 17.4 | 17.4 |
| ADF, % | 3.6 | 3.6 |
| NDF, % | 9.4 | 9.4 |
| SID Lys, % | 0.97 | 0.97 |
| Calcium, % | 0.7 | 0.7 |
| Phosphorous, % | 0.6 | 0.6 |
| Digestible phosphorous, % | 0.3 | 0.3 |
| Zinc, ppm | 166 | 985 |
| Butyrate, ppm | — | 2,181 |
| Zinc concentration, ppm, assayed | 154.6 | 1,020.7 |
| Butyrate concentration, ppm, calculated5 | — | 2,306.3 |
1Vitamins provided per kilogram of diet: 6,125 IU vitamin A, 700 IU vitamin D3, 50 IU vitamin E, 30 mg vitamin K, 0.05 mg vitamin B12, 11 mg riboflavin, 56 mg niacin, and 27 mg pantothenic acid.
2Minerals provided per kilogram of diet: 22 mg Cu (as CuSO4), 220 mg Fe (as FeSO4), 0.4 mg I (as Ca(IO3)2), 52 mg Mn (as MnSO4), 220 mg Zn (as ZnSO4), and 0.4 mg Se (as Na2SeO3).
3E-ZnB = encapsulated zinc butyrate.
4DE = digestible energy; ME = metabolizable energy; NE = net energy; ADF = acid detergent fiber; NDF = neutral detergent fiber.
5Butyrate’s value was calculated from the assayed zinc concentration of the E-ZnB diet. Background zinc values from the control diet was subtracted from the E-ZnB diet before calculating the butyrate content; w/w ratio of butyrate:zinc is 2.66.
Gilts weighing approximately 35 ± 1 kg were used for the study (n = 12 per treatment, 24 gilts, two treatments) in a randomized complete block design, with the pigs blocked by weight. The gilts began the experiment at 9 wk of age and completed the experiment 6 wk later. Pigs were penned individually (57 × 221 cm, stainless steel pens) and were on the respective feed for 24 d for supplementation of the test compounds. Individual body weights and feed intake were recorded, and blood samples were collected from all the gilts on days 0, 7, 14, 21, and 24. At the end of the 24-d supplementation phase, all pigs were moved to metabolism crates (71 × 152 cm, stainless steel crates) for adaptation to urine and feces collection during the subsequent HS phase. The pigs were given 1.25 kg of feed twice each day, and after 7 d of adaptation to metabolic crates at room temperature, they were subjected to a biphasic HS. During the acclimation period, the temperature was maintained at 19 to 20 °C and the humidity varied between 55% and 85%. During the HS phase, the temperature was maintained between 30 and 32 °C, and the humidity varied between 30% and 75%. The increase in temperature led to decreased humidity and vice versa. During the days of the HS period, the temperature was set between 30 and 32 °C, and during the night, it was set at 28 °C. HS temperatures were increased to 32 °C on day 4 of HS due to the pigs acclimating to the elevated temperatures. Blood was collected every 12 h starting on the first day of HS at 6:00 a.m. Body temperature and respiration rates were recorded daily at 8 a.m., 10 a.m., 11 a.m., and 2 p.m. during the HS phase. Body temperature was measured by rectal thermometer, and respiration rates were measured by visual observation and reported as breaths per minute. At the end of this 7-d HS phase, six pigs from each group were euthanized, and Ussing chamber studies were performed on fresh ileum and colon tissue from each individual pig. Transepithelial resistance and macromolecule transport of 4 kDa fluorescein isothiocyanate (FITC)-labeled dextran were measured to determine the effect of E-ZnB supplementation on intestinal integrity and health during HS.
In vivo intestinal integrity measurements.
The night before the initiation of HS and at the end of the HS phase, gilts were given a lactulose and mannitol (ThermoFisher Scientific, Pittsburgh, PA) bolus in a cookie dough mixture (33:3 g/g per pig, respectively) to assess intestinal permeability. Urine collections (nonacidified) started at 8 p.m. and ended at 7 a.m. for the acclimation phase and 6:15 p.m. to 7 a.m. for the HS phase. Total urine output during the specified time period was collected, weight was recorded, and the urine was frozen until the analysis. Urine lactulose and mannitol were measured by Kemin Industries, as previously described, with some modifications (Catassi et al., 1991). Briefly, mannitol and lactulose were quantified on a Perkin-Elmer Series 200 HPLC (Waltham, MA) and a Sedex 85 ELSD detector (Alfortville, France). Urine samples were thawed, mixed well, and filtered using a 1 µm filter, then 5 µL of the filtrate was injected. A Phenomenex NH2 column (4.6 × 250 mm, 5 µm; Torrance, CA) was used for separation. The mobile phase was 75% acetonitrile and 25% water. The flow rate was 1 mL/min, and total run time was 13 min. The ELSD was set at 3.5 bar N2, 80 °C and gain 9. Total recovery was calculated using equations 1 and 2.
Equation 1. Formula for calculating the recovery of lactulose or mannitol from the urine of the pigs supplemented with encapsulated zinc butyrate.
Equation 2. Formula for calculating the recovery ratio of lactulose and mannitol from the urine of the pigs supplemented with encapsulated zinc butyrate.
Ex vivo intestinal integrity measurements.
Electrophysiological measurements were taken using modified Ussing chambers as previously described (Albin et al., 2007; Gabler et al., 2009; Moeser et al., 2012). Briefly, fresh segments of the ileum and colon were removed and placed on ice in Krebs–Henseleit buffer for transport to the laboratory, while under constant aeration until clamped in the modified Ussing chambers. To assess tight junction integrity, tissues stripped of outer serosal layers were immediately mounted in a modified Ussing chamber (Physiologic Instruments, Inc., San Diego, CA, and World Precision Instruments, Inc., New Haven, CT). Each chamber was connected to a pair of dual channel current and voltage electrodes, submerged in 3% noble agar bridges, and filled with 3 M potassium chloride for electrical conductance. Each segment (0.71 cm2) was bathed on its mucosal and serosal sides with Krebs buffer and constantly gassed with a 95% O2, 5% CO2 mixture. The temperature of the tissues and apparatus was constantly maintained at 37 °C, using circulating warm water. A short circuit current was established and stabilized for about 10 min, and transepithelial resistance (TER) was measured using the included software (Acquire and Analyze, Physiologic Instruments, San Diego, CA).
After recording the basal electrophysiological measurements, the mucosal to serosal macromolecule transport of fluorescein isothiocyanate-labeled dextran (4.4 kDa; FITC-dextran, FD4-1g, Sigma–Aldrich) was assessed for 90 min to measure the integrity of both ileum and colon, as previously described (Wang et al., 2001). Briefly, the mucosal chambers were treated with 2.2 mg/mL FITC-dextran, and 200-µL chamber samples from both sides was collected for every 10 to 15 min. The relative fluorescence was then determined using a fluorescent plate reader (Bio-Tek, Winooski, VT), with excitation and emission wavelengths of 485 and 520 nm, respectively. An apparent permeability coefficient (Papp) was then calculated using the area of the membrane and rate of FITC-dextran transport, where dQ/dt = transport rate (µg/min); C0 = initial concentration in the donor chamber (µg/mL); A = area of the membrane (cm2): Papp value = dQ/(dt × A × C0).
Zinc analysis of liver.
Liver zinc concentration was analyzed for six pigs from each treatment group by the Iowa State University Veterinary Diagnostic Laboratory (Ames, IA), using inductively coupled plasma mass spectrometry. Briefly, 0.5 g of sample was weighed into Teflon vessels (MARSXpress TFM digestion vessels; CEM Corporation, Mathews, NC) with 1 mL of 18 MΩ water followed by 5 mL of trace metal grade concentrated nitric acid. Vessels were sealed, vortexed, and subsequently microwaved to digest the sample. After cooling, samples were filtered (Whatman Inc, Piscataway, NJ) and diluted to 25 mL with 18 MΩ water. Filtered samples were diluted 1:10 in 1% nitric acid for the zinc assay along with internal standards.
Statistical analysis.
Data were analyzed using the PROC MIXED procedure of SAS (SAS Institute, Cary, NC) for all models. Significance was set at a P < 0.05. Body temperature and respiratory rate, which were measured only during the HS period, were analyzed as repeated measures. For growth parameters such as average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F), P values were calculated for each phase (supplementation and HS). For the laboratory measurements such as ileum and colon TERs and FD4 Papp, P values were calculated using the F-statistic. Means and standard error of the means were calculated using the LS means function of SAS. Where appropriate, differences between the interaction of treatment and diet were calculated using the PDIFF function of SAS and corrected by using the Tukey’s adjustment.
RESULTS
Cell Culture
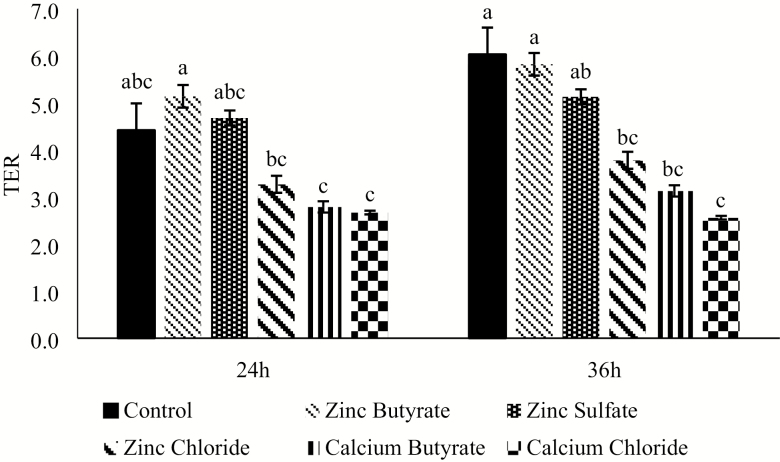
In the first experiment comparing the effect of ZnB with different zinc and butyric acid sources under normal growth conditions (Figure 1), no differences were observed at 12 h post-treatment (P > 0.05). At 24 h, the ZnB treatment had a greater TER than calcium butyrate and calcium chloride treatments, respectively (P < 0.05). The rest of the treatments were intermediate. At 36 h, the TER values of the control and ZnB treatments were greater than calcium chloride (P < 0.05), and all the other treatments had an intermediate response (Figure 1).
Figure 1.
Effect of 100 µM concentration of various treatments on transepithelial electrical resistance (TER) of IPEC-J2 cells under normal growth conditions, without any challenge after being exposed for either 24 or 36 h. TER values are expressed as a ratio to the control at 0 h. n = 8. Values are expressed as mean ± standard error of the mean. Values with different superscripts are significantly different at P < 0.05.
Inflammatory challenge.
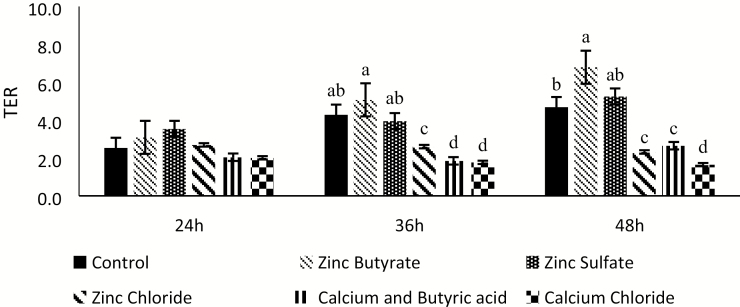
To further explore the benefits of ZnB, IPEC-J2 cells were subjected to an inflammatory challenge. The same experimental conditions as the previous experiment were maintained, except for the addition of the inflammatory challenge with bacterial LPS at a dose of 10 µg/mL of treatment media. No differences were observed until 24 h (Figure 2). At 36 h, the ZnB TER was greater than the other treatments (P < 0.05). The control and zinc sulfate treatments were intermediate, whereas calcium butyrate, zinc chloride, and calcium chloride treatments had the lowest TER values. The same trend continued at 48 h as well (Figure 2).
Figure 2.
Effect of 100 µM concentration of various treatments on transepithelial electrical resistance (TER) of the IPEC-J2 cells under inflammatory challenge (10 µg/mL) with bacterial lipopolysaccharide (LPS) after being exposed for either 24, 36, or 48 h. Values are expressed as a ratio to the control at 0 h. n = 8. Values are expressed as mean ± standard error of the mean. Values with different superscripts are significantly different at P < 0.05.
HS challenge.
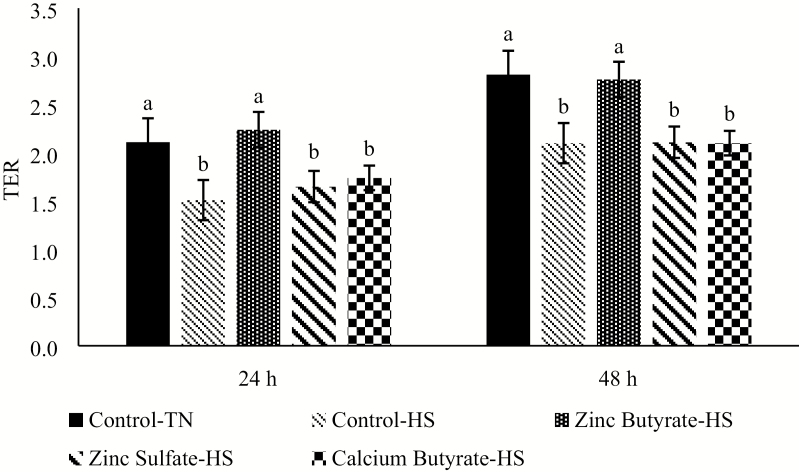
For the HS experiments, the 300 µM dose of the different treatments was chosen from preliminary experiments (data not shown). Twenty-four hours after the HS, the ZnB treatment had similar TER value to the Control-TN group, the Control-HS, zinc sulfate and calcium butyrate treatments had the lowest TER values. Similar trend was observed at 48 h as well.(Figure 3).
Figure 3.
Effect of HS challenge on transepithelial electrical resistance (TER) of the IPEC-J2 cells under various treatments at 300 µM concentration after being exposed for either 24 or 48h. Values are expressed as a ratio to the control at 0 h. n = 8. Values are expressed as mean ± standard error of the mean. Values with different superscripts are significantly different at P < 0.05; TN = thermoneutral; HS = heat stress.
Claudin-4 immunofluorescence assay.
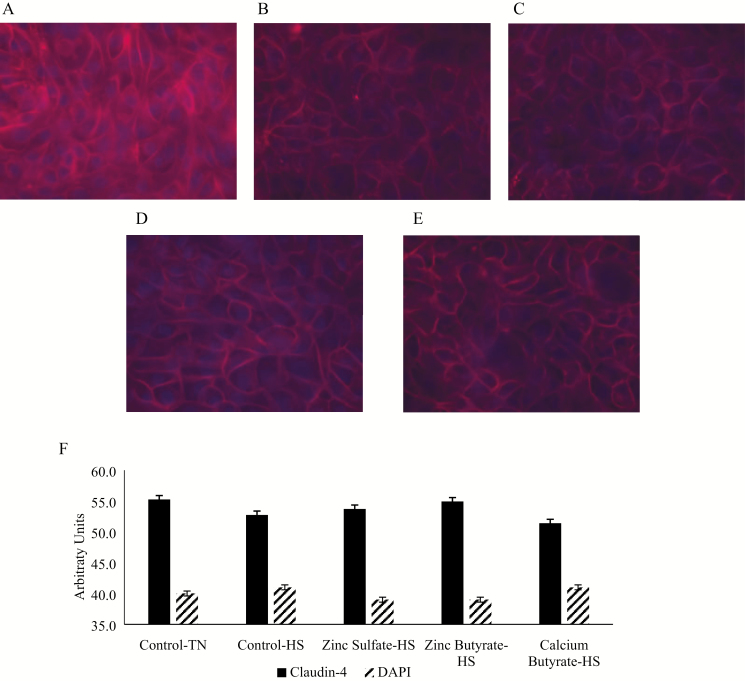
To measure the molecular impact of HS and the effect on tight junctions, claudin-4 protein was measured using immunofluorescence. The cells were exposed to HS for 48 h, then stained with Claudin-4 antibody (Figure 4). No differences were observed in the claudin-4 expression (P > 0.05), but the values followed the same trend as the TER values with Control-TN group having the greatest numerical value (55.25), followed by ZnB (54.93), zinc sulfate (53.72), and calcium butyrate heat stressed groups (51.40). Among the HS-exposed cells, the ZnB treatment had the greatest numerical value.
Figure 4.
The effects of heat stress (HS) and zinc exposure on claudin-4 expression in IPEC-J2 cells treated with different treatments and exposed to HS at 41.5 °C for 48 h. (A) Thermoneutral (TN) condition. (B) HS condition. (C) HS and treated with zinc sulfate. (D) HS and treated with ZnB. (E) HS and treated with CaB. (F) Semiquantification of claudin-4 protein abundance and nuclear stain DAPI in the IPEC-J2 cells exposed to different treatments and HS. Red color indicates claudin-4; blue color indicates nuclei. Values are expressed as mean ± standard error of the mean.
Swine Trial
Since ZnB protected the IPEC-J2 cells against HS, its efficacy was tested in a HS pig trial.
Body temperature and respiratory rate.
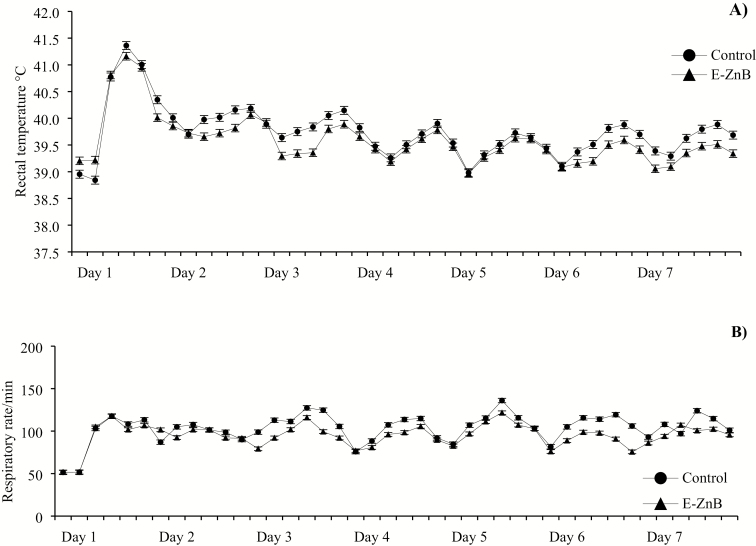
No differences were observed in body temperature and respiratory rate during the biphasic HS phase (P > 0.05, Figure 5). The body temperature increased 2.0 °C above the baseline body temperature when the HS was initiated but decreased and stabilized 1.3 °C above the baseline temperature in both treatment groups. The respiration rate increased more than 120% upon HS initiation, irrespective of the treatments. The same greater respiratory rate was observed throughout the HS phase in both treatment groups.
Figure 5.
Rectal temperature (A) and respiratory rate (B) of the pigs exposed to biphasic HS and supplemented with E-ZnB for a period of 7 d. Pigs were exposed to 30 to 32 °C during the day and 28 °C during the night to simulate a natural HS exposure. n = 12. No statistical differences were observed (P > 0.05).
Performance.
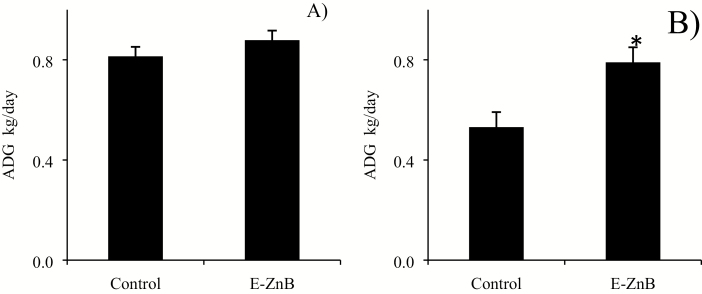
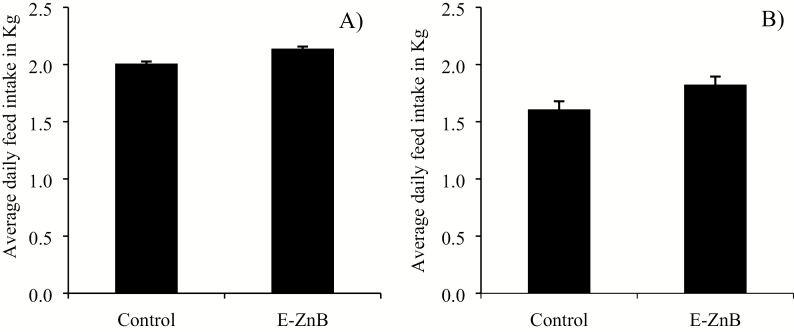
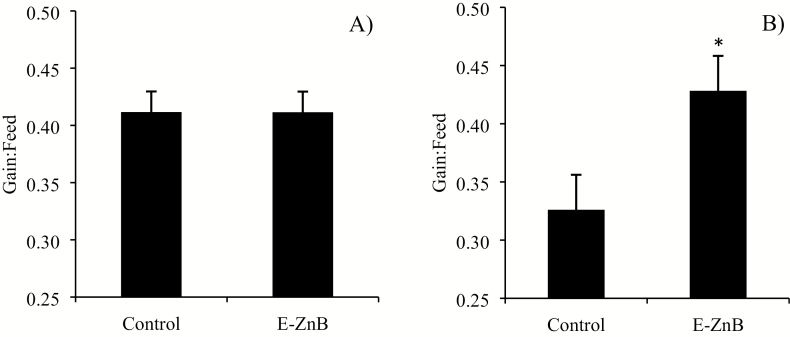
No differences were observed for ADG between the two treatments during the supplementation phase, but there was a significant improvement in ADG during the HS phase (Figure 6). No differences were observed for ADFI in both the supplementation and HS phase (Figure 7). The control group of pigs weighed 58.23 kg, and the E-ZnB group weighed 59.52 kg at the end of the trial. Encapsulated ZnB pigs weighed 1.3 kg more than control pigs at the end of the supplementation phase. Weight gain during the HS phase for the E-ZnB group was greater than the control group (P < 0.05). No difference was observed for G:F for both treatment groups during the supplementation phase (Figure 8); however, during the HS phase, G:F was greater in the E-ZnB-treated pigs (P < 0.05).
Figure 6.
Average daily gain of grower pigs supplemented with 7.5 kg of encapsulated zinc butyrate (E-ZnB)/MT of feed and heat stressed. Pigs were supplemented with E-ZnB for 24 d (A), and after the 24-d period, pigs continued supplementation with E-ZnB and heat stressed for 7 d at 30 to 32 °C during the day and 28 °C during the night (B). n = 12. *The value is significantly different at (P < 0.05). Values are expressed as mean ± standard error of the mean.
Figure 7.
Average daily feed intake (ADFI) of grower pigs supplemented with 7.5 kg of encapsulated zinc butyrate (E-ZnB)/MT of feed and heat stressed. Pigs were supplemented with E-ZnB for 24 d (A), and after the 24-d period, pigs continued supplementation with E-ZnB and heat stressed for 7 d at 30 to 32 °C during the day and 28 °C during the night (B). n = 12. Values are expressed as mean ± standard error of the mean.
Figure 8.
Gain:feed of grower pigs supplemented with 7.5 kg of encapsulated zinc butyrate (E-ZnB)/MT of feed and heat stressed. Pigs were supplemented with E-ZnB for 24 d (A), after the 24-d period pigs continued supplementation with E-ZnB and heat stressed for 7 d at 30 to 32 °C during the day and 28 °C during the night (B). *The value is significantly different at P < 0.05. Values are expressed as mean ± standard error of the mean.
In vivo and ex vivo intestinal integrity measurement.
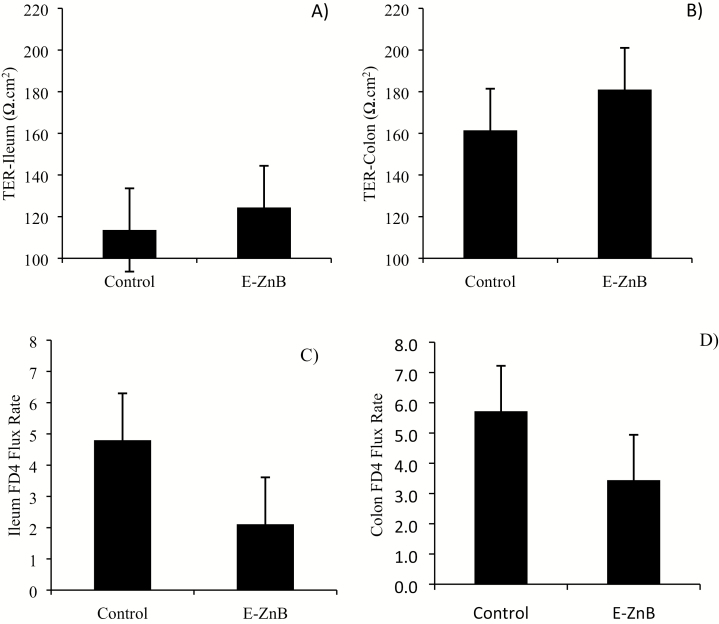
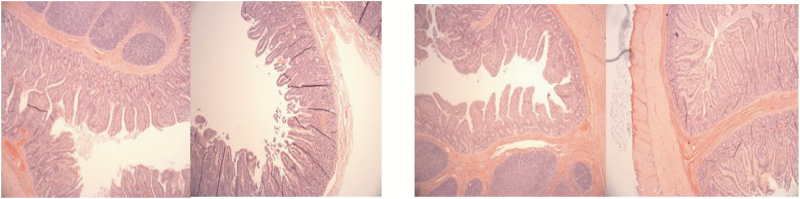
No differences were observed in terms of TER and intestinal permeability to 4 kDa FITC-dextran in both the ileum and colon samples from E-ZnB-treated pigs (Figure 9A–D). No differences were observed in terms of ileal morphology, including villus height, crypt depth, and villus height:crypt depth ratio (Figure 10; Table 2). In vivo intestinal integrity was measured by giving lactulose and mannitol to the pigs (Table 3). HS increased the overall lactulose:mannitol ratio, indicating a damaged tight junction barrier, but the E-ZnB treatment did not show any statistical improvement over the control in terms of reducing the lactulose and mannitol permeability.
Figure 9.
Intestinal health parameters of grower pigs supplemented with 7.5 kg of encapsulated zinc butyrate (E-ZnB)/MT of feed and heat stressed. Pigs were supplemented with E-ZnB for 24 d, and after the 24-d period, pigs were continued supplementation with E-ZnB and heat stressed for 7 d at 30 to 32 °C during the day and 28 °C during the night. Transepithelial electrical resistance (TER) of ileum (A) and colon (B) and macromolecule (FITC-dextran 4,000 kDa [FD4]) permeability coefficient of ileum (C) and colon (D) were measured from intestine samples collected at the end of the 7-d HS. n = 6. Values are expressed as mean ± standard error of the mean.
Figure 10.
Ileum morphology of control (left) and E-ZnB (right) supplemented pigs after 7 d of biphasic HS. Pigs were supplemented with 7.5 kg of encapsulated zinc butyrate (E-ZnB)/MT of feed for 24 d, and after the 24-d period, pigs were continued supplementation with E-ZnB and heat stressed for 7 d at 30 to 32 °C during the day and 28 °C during the night. Ileum morphology was measured from samples collected at the end of the 7-d HS period. n = 6.
Table 2.
The effects of encapsulated zinc butyrate (E-ZnB) supplementation on ileal morphology after exposure to HS
| Control diet1 | E-ZnB diet1,2 | SEM | P value | |
|---|---|---|---|---|
| Ileum villus height, µm | 362 | 377 | 26 | 0.707 |
| Ileum crypt depth, µm | 245 | 204 | 17 | 0.141 |
| Ileum villus:crypt depth ratio | 1.59 | 1.87 | 0.14 | 0.192 |
1Ileum morphology was measured from samples collected at the end of the 7-d HS period (n = 6).
2Pigs were supplemented with 7.5 kg E-ZnB/MT of feed for 24 d and exposed to biphasic heat stress for 7 d.
Table 3.
The effects of encapsulated zinc butyrate (E-ZnB) supplementation on in vivo intestinal integrity before and after HS
| Thermal neutral | Control diet1 | E-ZnB diet1,2 | SEM | P value |
|---|---|---|---|---|
| Urine mannitol, ppm | 904 | 1,001 | 166 | 0.684 |
| Urine lactulose, ppm | 141 | 206 | 39 | 0.250 |
| Mannitol recovery, % | 0.242 | 0.257 | 0.03 | 0.723 |
| Lactulose recovery, % | 0.004 | 0.004 | 0.001 | 0.864 |
| Lactulose:mannitol ratio | 0.017 | 0.019 | 0.004 | 0.678 |
| HS | ||||
| Urine mannitol, ppm | 646 | 648 | 119 | 0.990 |
| Urine lactulose, ppm | 183 | 137 | 20 | 0.121 |
| Mannitol recovery, % | 0.251 | 0.203 | 0.03 | 0.291 |
| Lactulose recovery, % | 0.007 | 0.005 | 0.001 | 0.165 |
| Lactulose:mannitol ratio | 0.030 | 0.024 | 0.004 | 0.278 |
1In vivo intestinal integrity was measured by gavaging the pigs with lactulose and mannitol through cookie dough and analyzing the urine before the hear stress was initiated and at the end of the HS (n = 6).
2Pigs were supplemented with 7.5 kg E-ZnB/MT of feed for 24 d and exposed to biphasic HS for 7 d.
Zinc level in the liver.
Zinc levels in the liver were measured from liver samples collected at the end of the HS exposure. Liver zinc concentration was not different between the two treatment groups (control 60.4 ppm vs. E-ZnB 69.6 ppm).
DISCUSSION
HS is one of the major stressors encountered by farm animals. A physiologically relevant increase in temperature has the potential to increase intestinal epithelial permeability (Dokladny et al., 2006). Although management strategies could help with mitigating HS to a certain extent, nutritional strategies need to be developed to prevent severe damage to the intestine and alleviate the growth depression. This study was conducted to test whether a novel combination product of zinc and butyric acid, ZnB, in an encapsulated form, has the potential to alleviate HS associated negative effects in grower pigs.
The effect of ZnB in maintaining the barrier integrity was tested in a cell culture model using IPEC-J2 cells under different stress conditions. The TER values and quantification of the tight junction protein claudin-4 using immunofluorescence were measured in cells grown under LPS challenge and HS. The cell culture data provided evidence that ZnB has positive effects on the intestinal epithelium during an inflammatory challenge and protects the cells from HS as well. Under both stress conditions, the ZnB-treated cells performed better than all other zinc and/or butyric acid sources, indicating that the beneficial effects of both zinc and butyric acid are additive in the combination product. Although there is no literature evidence available for IPEC-J2 cells, increasing doses of zinc have been shown to increase the barrier integrity and tight junction proteins in Caco-2 cells, human intestinal epithelial cells, even under normal conditions (Valenzano et al., 2015). Zinc has been shown to improve barrier function by activating the PI3K/AKT/mTOR pathway, which leads to increased expression of tight junctions (Shao et al., 2017). Zinc supplemented as ZnO modulated the inflammatory response of IPEC-J2 cells during an enterotoxigenic E. coli infection (Sargeant et al., 2011). These results indicate that zinc has the potential to maintain and improve the integrity of the tight junction barrier during normal and stress conditions.
Butyrate has been shown to improve the barrier integrity through upregulation of tight junction proteins claudin-3 and -4 during inflammatory challenge in IPEC-J2 cells (Yan and Ajuwon, 2017). Butyrate has also been shown to enhance the tight junction assembly by activating the AMP-activated protein kinase pathway in Caco-2 cell monolayers (Peng et al., 2009). The increase in TER during the inflammatory challenge study using the IPEC-J2 cells with different treatments conforms to these results. Although the mechanisms involved in HS-induced barrier damage in the animal may not be the same as an inflammatory challenge, from the cellular HS TER results we can speculate that some stress pathways might overlap and ZnB might have the potential to attenuate HS through acting on these pathways.
The pigs were exposed to biphasic HS for a period of 7 d after the 24-d supplementation phase. ADG and G:F were significantly greater in E-ZnB pigs during the HS phase, indicating that the E-ZnB-supplemented pigs were less stressed by the heat exposure. Zinc has been shown to improve the growth in weanling pigs when supplemented at a dose of 3,000 ppm as zinc oxide (Hill et al., 2000). Interestingly, in the referenced study, the growth response was more pronounced when supplemented in the form of zinc oxide rather than other forms of zinc sources such as zinc sulfate (Hahn and Baker, 1993). In the current trial, the zinc dose present in the E-ZnB treatment diet was 985 ppm, which is significantly lower than the pharmacological level supplemented for growth performance in the abovementioned studies. It could be argued that the growth difference seen during the HS phase can be attributed to the increased dose of supplemented zinc, but, intriguingly, the growth promoting effects were not prominently observed during the supplementation phase of 24 d.
HS has been shown to induce inflammatory signaling in the skeletal muscles of pigs (Ganesan et al., 2016). There is evidence that in addition to the benefits provided by butyrate to the intestinal tract, it can modulate the inflammatory response of piglets as well (Weber and Kerr, 2008; Bosi et al., 2009). We can speculate that butyrate might have attenuated some of the inflammatory responses associated with HS challenge, which might have contributed to a favorable growth response. The results from this study indicate that the E-ZnB treatment could conserve energy and increase efficiency during HS exposure by the combined beneficial effects of zinc and butyrate.
Interestingly, no significant differences were observed in terms of TER and in vivo and ex vivo intestinal permeability between the two treatments at the end of the HS phase. Although the beneficial effect of ZnB supplementation in the cell culture model was evident, in the swine trial despite the significant differences in growth performance and all the parameters positively trending toward intestinal health, no evidence of statistical significance was observed with regard to intestinal health. Although 12 pigs per treatment were used for the growth performance analysis during the HS phase, only 6 randomly selected pigs from the pool of 12 were used for the intestinal health measurements because of the limitations of the Ussing chamber experiments. Other researchers using zinc supplements during HS have also seen variable results with the performance results not necessarily translating to improved intestinal health parameters in the large intestine (Sanz Fernandez et al., 2014). We are uncertain about the reasons for the discrepancy between the cell culture observations and the intestinal results obtained from the swine trial. More studies need to be conducted with larger numbers of animals to ascertain whether E-ZnB would offer any benefits to the intestine during HS in pigs.
It is documented that both HS and/or decreased feed intake cause damage to the intestine (Pearce et al., 2014). Zinc and butyric acid have consistently shown benefits toward animal health (Guilloteau et al., 2010; Haase and Rink, 2014). The data from this study indicate that ZnB can mitigate the negative effects of inflammatory challenge and HS in cell culture, and E-ZnB can help to maintain the animal’s growth and may be beneficial toward the health of the intestine in HS-exposed animals. In conclusion, results from this study indicate that the E-ZnB-supplemented pigs maintained a greater growth rate and improved G:F during the HS phase when compared with the control pigs. The E-ZnB should be a suitable additive for maintaining pig health, particularly during HS and other intestinal stress conditions.
Footnotes
The authors declare following potential conflict of interests. V.M., J.R., D.S., T.P., and M.P. are employees of Kemin Industries. The dietary products used in this experiment are the proprietary products of Kemin Industries. This work was performed as a funded collaboration (ISU MSA IPA3) between Kemin Industries and the Department of Animal Sciences, Iowa State University, Ames, IA.
LITERATURE CITED
- Albin D.M., Wubben J.E., Rowlett J.M., Tappenden K.A., and Nowak R.A.. 2007. Changes in small intestinal nutrient transport and barrier function after lipopolysaccharide exposure in two pig breeds. J. Anim. Sci. 85:2517–2523. doi: 10.2527/jas.2006-237 [DOI] [PubMed] [Google Scholar]
- Bosi P., S. Messori, I. Nisi, D. Russo, L. Casini, F. Fabio, K. Schwarzer, and P. Trevisi. 2009. Effect of different butyrate supplementations on growth and health of weaning pigs challenged or not with E. coli K88. Ital. J. Anim. Sci. 8:268–270. doi:10.4081/ijas.2009.s2.268 [Google Scholar]
- Catassi C., Pierani P., Natalini G., Gabrielli O., Coppa G.V., and Giorgi P.L.. 1991. Clinical application of a simple HPLC method for the sugar intestinal permeability test. J. Pediatr. Gastroenterol. Nutr. 12:209–212. [DOI] [PubMed] [Google Scholar]
- Dokladny K., Moseley P.L., and Ma T.Y.. 2006. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G204–G212. doi: 10.1152/ajpgi.00401.2005 [DOI] [PubMed] [Google Scholar]
- Gabler N.K., Radcliffe J.S., Spencer J.D., Webel D.M., and Spurlock M.E.. 2009. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J. Nutr. Biochem. 20:17–25. doi: 10.1016/j.jnutbio.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Ganesan S., C. Reynolds, K. Hollinger, S. C. Pearce, N. K. Gabler, L. H. Baumgard, R. P. Rhoads, and J. T. Selsby. 2016. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R1288.– . doi:10.1152/ajpregu.00494.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., and Van Immerseel F.. 2010. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23:366–384. doi: 10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- Haase H., and Rink L.. 2014. Zinc signals and immune function. Biofactors 40:27–40. doi: 10.1002/biof.1114 [DOI] [PubMed] [Google Scholar]
- Hahn J.D., and Baker D.H.. 1993. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 71:3020–3024. [DOI] [PubMed] [Google Scholar]
- Hill G.M., Cromwell G.L., Crenshaw T.D., Dove C.R., Ewan R.C., Knabe D.A., Lewis A.J., Libal G.W., Mahan D.C., Shurson G.C., et al. 2000. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J. Anim. Sci. 78:1010–1016. doi:10.2527/2000.7841010x [DOI] [PubMed] [Google Scholar]
- Kalischuk L.D., Inglis G.D., and Buret A.G.. 2009. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 1:2. doi: 10.1186/1757-4749-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien C.L., Schmitz-Brown M., Solley T., Sun D., and Frankel W.L.. 2006. Increased colonic luminal synthesis of butyric acid is associated with lowered colonic cell proliferation in piglets. J. Nutr. 136:64–69. doi: 10.1093/jn/136.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger M.J., Rudolph K., Soszynski D., Conn C.A., Leon L.R., Kozak W., Wallen E.S., and Moseley P.L.. 1997. Effect of heat stress on LPS-induced fever and tumor necrosis factor. Am. J. Physiol. 273(3 Pt 2):R858–R863. doi: 10.1152/ajpregu.1997.273.3.R858 [DOI] [PubMed] [Google Scholar]
- Kolf-Clauw M., Castellote J., Joly B., Bourges-Abella N., Raymond-Letron I., Pinton P., and Oswald I.P.. 2009. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: histopathological analysis. Toxicol. In Vitro 23:1580–1584. doi: 10.1016/j.tiv.2009.07.015 [DOI] [PubMed] [Google Scholar]
- Levine U.Y., Looft T., Allen H.K., and Stanton T.B.. 2013. Butyrate-producing bacteria, including mucin degraders, from the swine intestinal tract. Appl. Environ. Microbiol. 79:3879–3881. doi: 10.1128/AEM.00589-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A.J., Borst L.B., Overman B.L., and Pittman J.S.. 2012. Defects in small intestinal epithelial barrier function and morphology associated with peri-weaning failure to thrive syndrome (PFTS) in swine. Res. Vet. Sci. 93:975–982. doi: 10.1016/j.rvsc.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Ohata A., Usami M., and Miyoshi M.. 2005. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 21:838–847. doi: 10.1016/j.nut.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Boddicker R.L., Johnson J.S., Weber T.E., Ross J.W., Baumgard L.H., and Gabler N.K.. 2012. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 90 (Suppl 4):257–259. doi: 10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Sanz-Fernandez M.V., Hollis J.H., Baumgard L.H., and Gabler N.K.. 2014. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 92:5444–5454. doi: 10.2527/jas.2014-8407 [DOI] [PubMed] [Google Scholar]
- Peng L., Li Z.R., Green R.S., Holzman I.R., and Lin J.. 2009. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J. Nutr. 139:1619–1625. doi: 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi G., Islam K., and Sambuy Y.. 1992. Epithelial cells in culture as a model for the intestinal transport of antimicrobial agents. Antimicrob. Agents Chemother. 36:1374–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgueiro M.J., Zubillaga M.B., Lysionek A.E., Caro R.A., Weill R., and Boccio J.R.. 2002. The role of zinc in the growth and development of children. Nutrition 18:510–519. doi:10.1016/S0899-9007(01)00812-7 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M.V., Pearce S.C., Gabler N.K., Patience J.F., Wilson M.E., Socha M.T., Torrison J.L., Rhoads R.P., and Baumgard L.H.. 2014. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 8:43–50. doi: 10.1017/S1751731113001961 [DOI] [PubMed] [Google Scholar]
- Sargeant H.R., Miller H.M., and Shaw M.A.. 2011. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol. Immunol. 48:2113–2121. doi: 10.1016/j.molimm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Shao Y., Wolf P.G., Guo S., Guo Y., Gaskins H.R., and Zhang B.. 2017. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 43:18–26. doi: 10.1016/j.jnutbio.2017.01.013 [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries1. J. Dairy Sci. 86 (Suppl):E52–E77. doi:10.3168/jds.S0022-0302(03)74040-5 [Google Scholar]
- Valenzano M.C., DiGuilio K., Mercado J., Teter M., To J., Ferraro B., Mixson B., Manley I., Baker V., Moore B.A., et al. 2015. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients. PLoS One 10:e0133926. doi: 10.1371/journal.pone.0133926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Fang C.H., and Hasselgren P.O.. 2001. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013 [DOI] [PubMed] [Google Scholar]
- Weber T.E., and Kerr B.J.. 2008. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim. Sci. 86:442–450. doi: 10.2527/jas.2007-0499 [DOI] [PubMed] [Google Scholar]
- Wedekind K.J., Lewis A.J., Giesemann M.A., and Miller P.S.. 1994. Bioavailability of zinc from inorganic and organic sources for pigs fed corn-soybean meal diets. J. Anim. Sci. 72:2681–2689. [DOI] [PubMed] [Google Scholar]
- Xiao G., Tang L., Yuan F., Zhu W., Zhang S., Liu Z., Geng Y., Qiu X., Zhang Y., and Su L.. 2013. Eicosapentaenoic acid enhances heat stress-impaired intestinal epithelial barrier function in caco-2 cells. PLoS One 8:e73571. doi: 10.1371/journal.pone.0073571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., and Ajuwon K.M.. 2017. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 12:e0179586. doi: 10.1371/journal.pone.0179586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.E., Zhao Y.Q., Wang H., and Fan M.. 2006. Pathophysiological factors underlying heatstroke. Med. Hypotheses 67:609–617. doi: 10.1016/j.mehy.2005.12.048 [DOI] [PubMed] [Google Scholar]
- Zhang B., and Guo Y.. 2009. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 102:687–693. doi: 10.1017/S0007114509289033 [DOI] [PubMed] [Google Scholar]