Abstract
The effects of pen location on swine thermoregulation and growth performance were determined over 6 weeks during late summer. A total of 128 mixed sex pigs [Duroc × (Landrace × Yorkshire)] were randomly assigned to 16 pens in two grow-finish barns (n = 8 pens/barn; 57.43 ± 1.33 kg initial body weight (BW)). Pen locations were determined based on orientation to ventilation fans and air inlets. Internal pens (IP; n = 4/barn) were in direct line of sight between the fans and air inlets while peripheral pens (PP; n = 4/barn) were located 0.70 ± 0.29 m to either side of a fan. Two sentinel gilts per pen were selected and vaginal temperature (TV) was measured in 10-min intervals using TV data loggers. Additionally, trunk skin temperature (TS) was measured with an infrared camera and respiration rate (RR) was measured by counting flank movements of the sentinel gilts twice daily (0800 and 1500 hours). Pen airspeed was measured twice daily (0800 and 1500 hours) at pig level with an anemometer. Individual pen ambient temperature (TA) and relative humidity (RH) were recorded daily in 10-min intervals. Feed consumption and BW were determined every 2 weeks. Data were analyzed using PROC MIXED in SAS 9.4. Although airspeed was reduced overall (P = 0.01; 11%) in PP compared with IP, no differences (P > 0.10) in TA (27.53 ± 1.73 °C) or RH (68.47 ± 5.92%) were detected. An overall increase (P ≤ 0.02) in TV (0.23 °C), minimum TV (0.18 °C), and maximum TV (0.29 °C) was detected in PP versus IP housed pigs. Similarly, from 0800 to 1900 hours and 2000 to 0700 hours, TV was greater overall (P ≤ 0.01; 0.22 and 0.25 °C, respectively) in PP compared with IP housed pigs. An overall decrease in TS (P = 0.04) was observed in PP (37.39 ± 0.14 °C) compared with IP (37.61 ± 0.14 °C) housed pigs. No RR differences (P > 0.10; 76 ± 4 breaths per minute) were detected with any comparison. While no average daily gain (ADG) and average daily feed intake (ADFI) differences were detected (P > 0.10; 0.74 ± 0.03 kg/d and 2.26 ± 0.08 kg/d, respectively), gain-to-feed ratio (G:F) was decreased (P = 0.02; 6%) in PP compared with IP housed pigs. In summary, pigs located in PP had greater body temperature and reduced G:F despite similarities in TA and RH between all pens.
Keywords: feed efficiency, pen location, pigs, productivity, thermoregulation
INTRODUCTION
In intensive production systems, pigs are reared in confinement to provide optimal environmental conditions and maximize welfare and productivity. However, higher summer temperatures can overwhelm cooling systems in swine facilities (i.e., fans, evaporative coolers), thus subjecting pigs to temperature conditions above their thermal comfort zone. Exposure to ambient temperature (TA) above the thermal comfort zone (i.e., heat stress) can negatively impact reproductive efficiency, growth rate, and health in pigs resulting in economic losses despite advances in-barn cooling technologies (Axaopoulos et al., 1992; St-Pierre et al., 2003). In addition, variation in either TA, relative humidity (RH), or airspeed creates microenvironments in swine barns (Costa et al., 2014; Massari et al., 2016). Although numerous reports have evaluated the direct effects of heat stress on production losses (as reviewed by Johnson et al., 2015a) and thermoregulation (Huynh et al., 2007), few have investigated the impact of microenvironments on swine thermoregulation and productivity.
Previous reports demonstrated that in-barn environmental variability affected pig behavior (Geers et al., 1986; Costa et al., 2014). Furthermore, the existence of microclimates within farrowing barns negatively impacts sow productivity (Morello et al., 2018). However, few studies have investigated the effects of microclimates on thermoregulation and productivity in grow-to-finish facilities. Therefore, the study objective was to ascertain the existence of microclimates in grow-finish barns and characterize their impacts on swine productivity and thermoregulation during late summer.
MATERIALS AND METHODS
Animals and Experimental Design
All procedures involving pigs were approved by the Purdue University Animal Care and Use Committee (protocol #1603001380). Animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010). The study was conducted from mid-July to mid-August 2016 over a 6-week period at the Purdue Animal Sciences Research and Education Center Swine Farm in West Lafayette, IN. Data loggers (HOBO; accuracy ±0.2 °C; data logger temp/RH; Onset; Bourne, MA) were used to monitor average daily TA (21.79 to 29.96 °C range) and RH (65.16 to 92.60% range) outside the barns. A total of 128 mixed sex (50% barrows and 50% gilts) crossbred pigs [Duroc × (Landrace × Yorkshire); 57.43 ± 1.33 kg initial body weight (BW)] were randomly assigned to 16 pens in two grow-finish barns (n = 8 pens/barn) and pens were balanced across treatments by sex and BW. Both barns (72.2 × 10.1 m) were identical and had concrete side walls, concrete slatted floors and were side ventilated with 13 hood fans (TURBO 0.10 static pressure; 61 cm diameter; 117.5 m3/min per fan) on 1 wall and 13 air inlets on the opposite wall. Throughout the study, ventilation fans were set based on in-barn TA and pig-level airspeed ranged from 0.10 to 0.60 m/s, which was within the recommended cold to hot weather ranges for finishing pigs, respectively (Midwest Plan Service, 1972). All pens (4.27 × 1.68 m) were separated by 0.91-m high panels with vertical bars. Each pen was equipped with adjustable nipple type drinker and two-hole dry feeder. Pigs were housed in pens located in two distinct locations within each barn, based on the orientation of the pens to ventilation fans and air inlets. Internal pens (IP; n = 4/barn) were directly in between the fans and the air inlets, while the peripheral pens (PP; n = 4/barn) were located 0.70 ± 0.29 m to either side of the closest ventilation fan. All pigs had ad libitum access to feed and water and were fed standard commercial corn-soybean meal based diets for two phases of 21 d each to meet nutrient requirements (NRC, 2012) based on age per standard swine industry practice (phase 3: 88% dry matter (DM), 3351.2 kcal/kg metabolizable energy (ME), 16% crude protein (CP), and 0.85% standardized ileal digestible (SID) lysine; phase 4: 88% DM, 3356.6 kcal/kg ME, 14.2% CP, and 0.73% SID lysine).
Measurements
Each individual pen was equipped with one data logger mounted at pig height to record pig-level TA and RH in 10-min intervals throughout the entire experiment. Individual pen airspeed (m/s) was measured with an anemometer (Testo Model 425; Sparta, NJ) at the pig level (approximately 0.50 m above the slatted floor) twice daily (0800 and 1500 hours) during the thermal measurement periods.
Two sentinel gilts were randomly selected per pen for vaginal temperature (TV), trunk region skin temperature (TS), and respiration rate (RR) measurements within three periods (P) lasting two weeks each (P1 = weeks 1 and 2; P2 = weeks 3 and 4; P3 = weeks 5 and 6) for 9 d per period (27 days in total). The same two sentinel gilts per pen were monitored throughout the entirety of the experiment and their data were averaged for the entire pen. Calibrated thermochron temperature recorders (iButton, accuracy ±0.1 °C; Dallas Semi-conductor, Maxim, Irving, TX) were attached to blank controlled internal drug releasing devices (Eazi-Breed CIDR; Zoetis, New York, NY) and inserted intravaginally into the two sentinel gilts selected per pen to record TV in 10-min intervals 24 h per day throughout the entire 27-d monitoring period. TV monitors were constructed in accordance with a previous report by Johnson and Shade (2017). RR and TS were assessed in the sentinel gilts twice daily (0800 and 1500 hours) throughout the entire 27-d monitoring period. RR (breaths per min; bpm) was determined by counting flank movements for 15 s and then multiplying by 4. Trunk TS was measured by taking a broadside photo of individual pigs from a distance of approximately 1.5 m using an infrared camera (FLIR Model T440, accuracy ± 0.1 °C; emissivity = 0.95; FLIR Systems Inc., USA). Care was taken to ensure that the side of the pig was dry during thermal imaging so that TS was not influenced by previous contact with the ground that could leave excess moisture on the skin. Because of this, the side of the pig in which the thermal image was taken was not always consistent. Infrared photos were analyzed with the FLIR Tools software (version 2.1). For image analysis, the minimum, maximum, and mean temperature of the trunk region of the pig (i.e., all skin caudal to the neck and dorsal to the elbow and stifle) was measured. For RR and TS, an average daily value and an average value for the morning (0800–1000 hours) and for the afternoon (1500–1700 hours) were calculated and used in the final analysis. For TV, an average daily value, a value for the daytime (0800–1900 hours) and nighttime (2000–0700 hours), a daily maximum TV, and daily minimum TV were calculated and used in the final analysis. Feed consumption and BW on a per pen basis were measured at the end of P1, P2, and P3 and were used to determine ADFI, ADG, and G:F for each period.
A thermal circulation index (TCI) was calculated using TS, TA, and TV in the following equation as described by Curtis (1983): TCI = (TS − TA)/(TV − TS). The TCI was used to determine the pig capacity to dissipate heat from the core to the skin and subsequently to its surroundings under steady-state thermal conditions. TV and TA were averaged from 0800–1000 hours and 1500–1700 hours to correspond to the timeline of TS measurements and used in the TCI calculation.
Statistics
Data were analyzed using the PROC MIXED procedure in SAS 9.4 (SAS Institute Inc., Cary, NC). Because pigs were group housed during all measures, pen was considered the experimental unit for all analyses. Barn was included in the model as a random effect, while pen location (IP, PP), day of measurement (1–27) or period (P1, P2, P3), and their interaction were considered fixed effects. A two-sample t-test was performed to compare the initial BW and final BW between PP and IP housed pigs. Day of measurement effects are only presented and discussed when there is an interaction with pen location because it was expected that overall day differences would be observed due to natural daily variation in environmental conditions and only the effects of pen location were of interest in the present study. All thermal indices data were analyzed using repeated measures and the covariance structure was determined based on goodness of fit criteria (Littell et al., 1998) with day as the repeated effect. Performance data were analyzed using repeated measures and covariance structure was selected based on goodness of fit criteria (Littell et al., 1998) with period as the repeated effect. Pen initial BW was used as covariate, but it was not significant for any of the performance parameters and was dropped from the final analysis. Values are reported as least square means ± SE, statistical differences were considered at P ≤ 0.05, and tendencies were considered at 0.05 < P ≤ 0.10.
RESULTS
Environmental Conditions
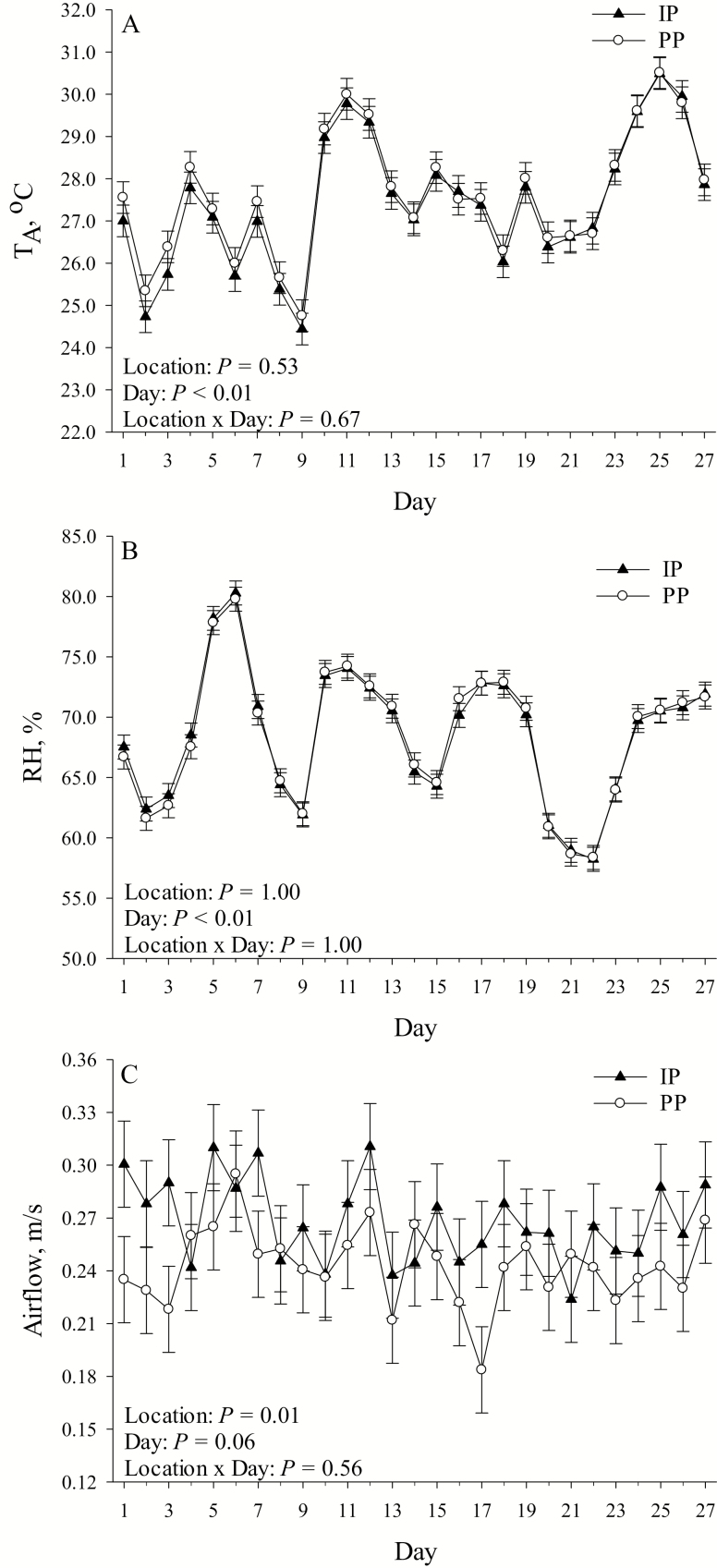
No pen location by day interaction was detected (P > 0.10) for TA, maximum daily TA, minimum daily TA, the maximum TA vs. minimum TA difference, RH, maximum daily RH, minimum daily RH, the maximum RH vs. minimum RH difference, and airspeed (Table 1; Figure 1). Overall, no differences were detected (P > 0.10) in TA, maximum daily TA, minimum daily TA, the maximum TA vs. minimum TA difference, RH, maximum daily RH, minimum daily RH, and the maximum RH vs. minimum RH difference between PP and IP (Table 1). However, airspeed was reduced overall (P = 0.01; 11%) in PP compared with IP (Table 1). Overall, day of measurement had an effect on TA (P < 0.01), where TA ranged from 24.60 ± 0.30 °C on day 9 to 30.50 ± 0.30 °C on day 25 (Figure 1A). Similarly, day of measurement had an effect on RH (P < 0.01), which ranged from 58.30 ± 0.80% on day 22 to 80.03 ± 0.80% on day 6 (Figure 1B). Airspeed tended to be greater (P = 0.06) on days 5, 6, and 12 compared with days 10, 13, 16, 17, 21, and 23 (Figure 1C). Day of measurement differed overall (P < 0.01) for maximum, minimum, and their difference for RH and TA (data not presented).
Table 1.
Effects of pen location (L) and day of measurement (D) on environmental conditions and thermal indices in grow-to-finish barn and pigs during late summer
| Pen Location | P-value | |||||
|---|---|---|---|---|---|---|
| Parameters | PPa | IPb | SEM | L | D | L × D |
| Environmental conditions | ||||||
| Airspeedc, m/s | 0.24 | 0.27 | 0.01 | 0.01 | 0.06 | 0.56 |
| RH, % | 68.46 | 68.47 | 0.70 | 1.00 | <0.01 | 1.00 |
| Max RH, % | 83.75 | 83.52 | 0.66 | 0.69 | <0.01 | 0.86 |
| Min RH, % | 54.69 | 54.28 | 0.91 | 0.33 | <0.01 | 0.99 |
| Max–Min RH, % | 27.92 | 28.24 | 0.48 | 0.58 | <0.01 | 0.50 |
| TA, °C | 27.63 | 27.43 | 0.31 | 0.53 | <0.01 | 0.67 |
| Max TA, °C | 31.65 | 31.53 | 0.35 | 0.52 | <0.01 | 0.84 |
| Min TA, °C | 23.96 | 23.66 | 0.22 | 0.34 | <0.01 | 0.99 |
| Max–Min TA, °C | 7.68 | 7.87 | 0.22 | 0.23 | <0.01 | 0.90 |
| Pig thermal indices | ||||||
| RR, bpm | 76 | 76 | 4 | 0.87 | <0.01 | 0.41 |
| RR (0800–1000 hours) | 64 | 63 | 3 | 0.75 | <0.01 | 0.75 |
| RR (1500–1700 hours) | 87 | 88 | 5 | 0.66 | <0.01 | 0.67 |
| TV, °C | 39.90 | 39.67 | 0.06 | <0.01 | <0.01 | 0.60 |
| Max TV, °C | 40.34 | 40.05 | 0.07 | <0.01 | <0.01 | 0.73 |
| Min TV, °C | 39.54 | 39.36 | 0.06 | 0.02 | 0.06 | 0.44 |
| TV (0800–1900 hours) | 40.02 | 39.80 | 0.07 | <0.01 | <0.01 | 0.83 |
| TV (2000–0700 hours) | 39.79 | 39.54 | 0.07 | 0.01 | 0.07 | 0.30 |
| TS, °C | 37.39 | 37.61 | 0.14 | 0.04 | <0.01 | 0.48 |
| TS (0800–1000 hours) | 36.23 | 36.52 | 0.13 | 0.03 | <0.01 | 0.77 |
| TS (1500–1700 hours) | 38.55 | 38.72 | 0.25 | 0.09 | <0.01 | 0.30 |
| TCI | 4.06 | 5.16 | 0.23 | <0.01 | 0.01 | 0.08 |
Max, Maximum; Min, Minimum.
aPeripheral pens, located 0.70 ± 0.29 m to either side of the closest fan
bInternal pens, located in direct line of sight between the fans and the air inlets
cAirspeed measured at pig level
Figure 1.
Effects of pen location (IP = internal pens, located in direct line of sight between the fans and the air inlets; PP = peripheral pens, located 0.70 ± 0.29 m to either side of the closest fan) on (A) average daily TA, (B) average daily RH, and (C) average daily airspeed by day of temperature measurement. Error bars indicate ±1 SEM.
Vaginal Temperature
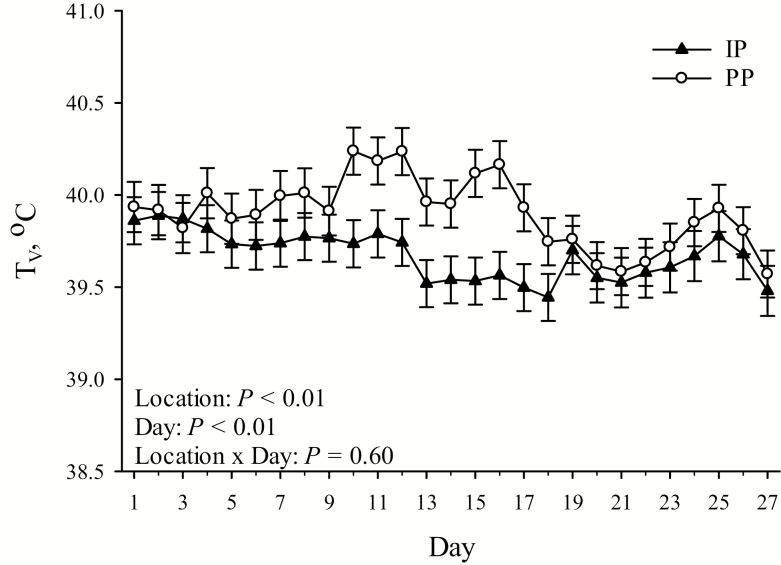
No pen location by day interaction was detected (P > 0.10) for any TV comparison (Table 1; Figure 2). TV was greater overall (P < 0.01; 0.23 °C) in PP compared with IP housed pigs (Table 1). Overall, minimum and maximum TV were greater (P ≤ 0.02; 0.18 and 0.29 °C, respectively) in PP compared with IP housed pigs (Table 1). From 0800 to 1900 hours and 2000 to 0700 hours, TV was greater (P ≤ 0.01; 0.22 and 0.25 °C, respectively) in PP compared with IP housed pigs (Table 1). Day of measurement had an overall effect on TV (P < 0.01), which ranged from 39.53 ± 0.09 °C on day 27 to 39.99 ± 0.09 °C on day 12 (Figure 2).
Figure 2.
The effects of pen location (IP = internal pens, located in direct line of sight between the fans and the air inlets; PP = peripheral pens, located 0.70 ± 0.29 m to either side of the closest fan) on average daily TV by day of temperature measurement. Error bars indicate ±1 SEM.
Skin Temperature
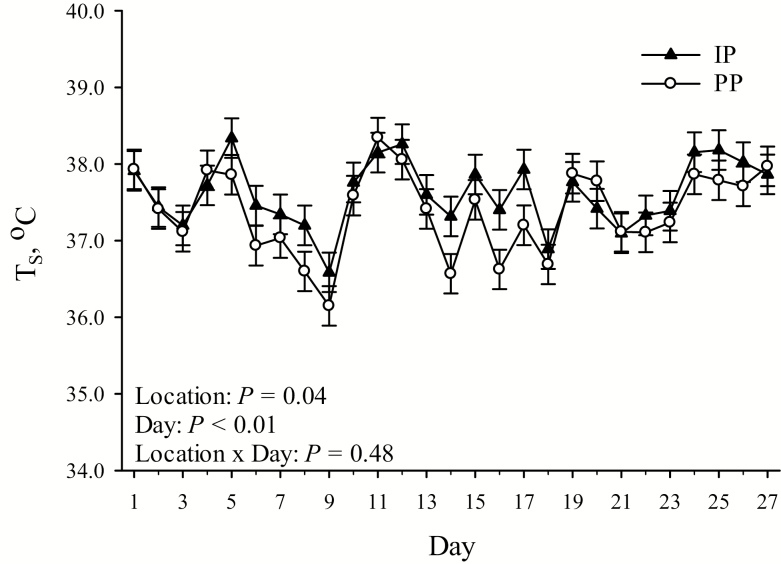
No pen location by day interaction was detected (P > 0.10) for any TS comparison (Table 1; Figure 3). TS was decreased overall (P = 0.04; 0.22 °C) in PP compared with IP housed pigs (Table 1). Day of measurement had an overall effect on TS (P < 0.01), which ranged from 36.37 ± 0.20 °C on day 9 to 38.25 ± 0.20 °C on day 11 (Figure 3). In PP compared with IP housed pigs, TS was reduced (P = 0.03; 0.30 °C) from 0800 to 1000 hours and tended to be reduced (P = 0.09; 0.17 °C) from 1500 to 1700 hours (Table 1).
Figure 3.
Effects of pen location (IP = internal pens, located in direct line of sight between the fans and the air inlets; PP = peripheral pens, located 0.70 ± 0.29 m to either side of the closest fan) on average daily TS by day of temperature measurement. Error bars indicate ±1 SEM.
Thermal Circulation Index
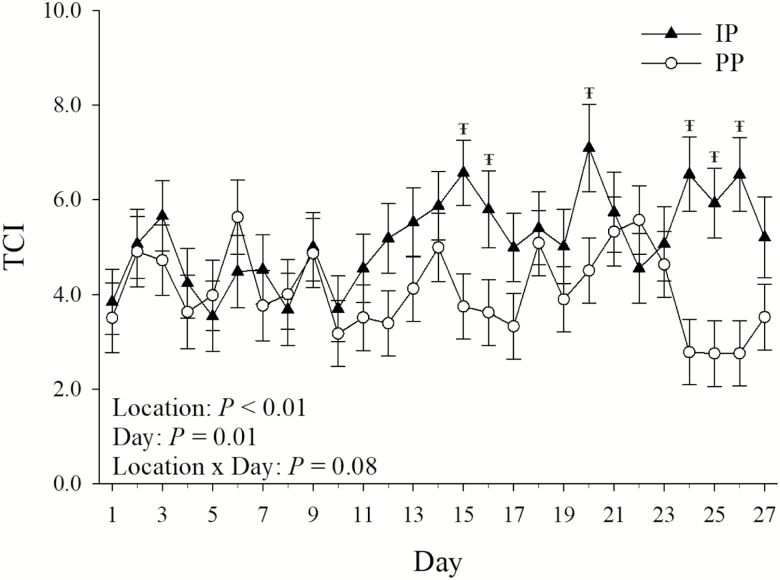
The TCI tended to be reduced (P = 0.08) in PP compared with IP housed pigs on days 15, 16, 20, 24, 25, and 26 (Figure 4). The TCI was decreased overall (P < 0.01; 21%) in PP compared with IP housed pigs (Table 1). Day of measurement had an overall effect on TCI (P = 0.01), which ranged from 3.44 ± 0.49 on day 10 to 5.80 ± 0.58 on day 20 (Figure 4).
Figure 4.
Effects of pen location (IP = internal pens, located in direct line of sight between the fans and the air inlets; PP = peripheral pens, located 0.70 ± 0.29 m to either side of the closest fan) on average daily TCI by day of temperature measurement. Error bars indicate ±1 SEM. ŦSymbol indicates location by day of measurement interaction effect.
Respiration Rate
There was no pen location by day of measurement interaction effect (P > 0.10) for RR, RR from 0800 to 1000 hours and RR from 1500 to 1700 hours (Table 1). RR was similar (P = 0.87; 76 ± 4 bpm) for PP and IP housed pigs (Table 1). Similarly, no pen location differences were observed (P > 0.10) for RR from 0800 to 1000 hours (64 ± 3 bpm) and 1500 to 1700 hours (88 ± 5 bpm; Table 1). However, day of measurement had an effect (P < 0.01) on RR, which ranged from 65 ± bpm on day 9 to 90 ± 5 bpm on day 25 (data not presented).
Growth Performance
No pen location by period interaction effect was detected (P > 0.10) for ADG, ADFI, and G:F (Table 2). No overall ADG or ADFI differences were detected (P > 0.10; 0.74 ± 0.03 kg/d and 2.26 ± 0.08 kg/d, respectively) between PP and IP housed pigs (Table 2). However, G:F was reduced overall (P = 0.02; 6%) in PP compared with IP pens (Table 2). Average daily feed intake was greater overall (P < 0.01; 15.0%) during P3 compared with P1 and P2 (Table 2). Similarly, ADG was greater (P < 0.01; 15.4%) during P3 compared with P1 and P2 (Table 2). There was no period effect detected for G:F (Table 2). When comparing PP to IP housed pigs, no differences (P > 0.10) in initial BW (57.75 ± 1.93 kg and 57.12 ± 1.97 kg, respectively) and final BW (89.72 ± 1.83 kg and 87.82 ± 1.23 kg, respectively) were detected (data not presented).
Table 2.
Effects of pen location (L) and recording period (P)a on growth parameters in grow-to-finish pigs during late summer
| P1 | P2 | P3 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | PPb | IPc | PP | IP | PP | IP | SEM | L | P | L × P |
| ADFI, kg | 2.12 | 2.11 | 2.21 | 2.17 | 2.50 | 2.45 | 0.08 | 0.71 | <0.01 | 0.96 |
| ADG, kg | 0.68 | 0.69 | 0.68 | 0.74 | 0.80 | 0.81 | 0.03 | 0.28 | <0.01 | 0.78 |
| G:F, kg/kg | 0.32 | 0.33 | 0.32 | 0.35 | 0.32 | 0.33 | 0.01 | 0.02 | 0.40 | 0.18 |
aMeasurement once every 2 weeks
bPeripheral pens, located 0.70 ± 0.29 m to either side of the closest fan
cInternal pens, located in direct line of sight between the fans and the air inlets
DISCUSSION
To mitigate the negative impacts of heat stress on swine, the in-barn environmental conditions may be improved through building design, ventilation systems, and the use of evaporative cooling techniques (Renaudeau et al., 2012). Despite these improvements, variation in environmental conditions (i.e., TA, RH, and airspeed) within facilities may occur and this can negatively affect swine productivity (Morello et al., 2018). In the present study, pigs located in PP had a reduction in G:F compared with those housed in IP, but no differences in ADG or ADFI were detected. While the lack of ADG differences are surprising considering previous research describing reduced ADG in pigs reared in pens located away from fans and air inlets (Kluzáková et al., 2013), the reduced G:F indicates that swine performance may be influenced by in-barn location. Although the specific mechanism for the reduction in G:F is currently unknown, it may be due to thermoregulatory differences between pigs located in PP versus IP since increased body temperature can reduce G:F in growing pigs (as reviewed by Johnson, 2018).
A reduced ability to dissipate body heat can result in elevated core body temperature and subsequently reduced productivity in swine (Renaudeau et al., 2008; Johnson et al., 2015a). In the present study, PP housed pigs had overall increased TV, minimum TV, and maximum TV compared with those in IP, and this may have resulted in the aforementioned reduction in G:F of PP housed pigs. Several studies have reported that heat stress negatively impacts G:F in swine (Kerr et al., 2003; Renaudeau et al., 2008; Johnson et al., 2015a), and it has been suggested that this may be due to the physiological strain caused by increased body temperature. Increasing body temperature causes morphological changes to the intestine indicative of damage (Pearce et al., 2012), and because PP housed pigs has a greater body temperature they may have had more intestinal damage compared with IP housed pigs. As a result, the absorptive capacity of the intestine may have been reduced, resulting in a decrease in digestible energy gained from the feed for PP compared with IP housed pigs. Furthermore, an increase in body temperature can increase intestinal permeability to pathogens, which activate the immune system in pigs (Baumgard et al., 2015), and this is an energetically costly process (Kvidera et al., 2017) that may re-partition energy away from growth and reduce G:F. Regardless of the mechanism, it appears that pen location may influence body temperature in pigs and negatively affect performance.
Microclimate variation within swine facilities (which can be caused by spatial differences in RH, TA, and airspeed) exists and can affect pigs’ thermoregulation (Sällvik and Walberg, 1984; Costa et al., 2014). In the present study, no pen location differences were detected in RH and TA, but TA varied by day from thermoneutral conditions to a maximum of approximately 2.5 °C below the upper temperature extreme for grow-finish pigs (Federation of Animal Science Societies, 2010). Despite the lack of pen location TA and RH differences, pig-level airspeed was significantly reduced overall in PP compared with IP. Airspeed plays an important role in convective heat loss (Curtis, 1983) and its reduction can decrease heat dissipation capacities (Bond et al., 1965; Close et al., 1981), resulting in elevated body temperature (Mitchell, 1985). In the present study, despite similarities in TA and RH between PP and IP, an 11% decrease in pig-level airspeed was detected and it is possible that this difference influenced the increase in TV for PP housed pigs. This is because heat loss is partially dependent on the movement of air across the skin (i.e., convection), and with a reduction in pig-level airspeed, it is likely that heat loss by the body would be reduced (Johnson et al., 2018). As such, TS in the present study was reduced in PP compared with IP housed pigs. Because an increase in TS is a general indicator of greater heat loss (Johnson et al., 2015b), this could help explain the increased TV in PP compared with IP housed pigs. In addition, a decrease in heat dissipation may be explained by a 21% reduction in the TCI of PP compared with IP housed pigs, which further suggests a reduced ability to dissipate body heat (Close et al., 1981).
Although statistically significant pen location pig-level airspeed differences were detected that could help explain the thermoregulatory differences, it should be mentioned that the absolute difference was relatively small (i.e., only a 0.03 m/s difference). Therefore, although specific reasons are currently unknown, it is possible that other factors that were not measured in the present study may have influenced the body temperature and production differences observed between pen locations. For example, IP housed pigs may have utilized behavioral thermoregulation (i.e., wetting of the skin with waterers, use of concrete flooring for convective cooling) more extensively than PP housed pigs, which could explain the reduced TV. Alternatively, PP housed pigs may have had a greater rate of illness compared with IP housed pigs during the study, which could explain the elevated body temperatures (i.e., pyretic response) and reduced productivity because mounting an immune response diverts nutrients away from growth and toward the immune system (Kvidera et al., 2017). Therefore, these factors should be taken into consideration in future studies on the effects of pen location on pig thermoregulation and productivity.
While PP housed pigs had an increase in body temperature compared with IP housed pigs, no RR differences were detected. This was surprising considering that RR is a sensitive indicator of heat stress in pigs (Lucy and Safranski, 2017). However, this may be because RR was not monitored during the hottest period of the day (1400 hours), and it is possible that differences would have been detected if RR measures were taken more often. Nevertheless, because the overall RR for all pigs in the present study was approximately 49% greater than levels previously reported in thermoneutral housed pigs (Johnson et al., 2015b), it is likely that both PP and IP housed pigs were suffering from heat stress due to warm summer environmental conditions (Becker et al., 1992).
Though these data may provide valuable information on the effects of microclimate variation on pig thermoregulation and productivity, some limitations are worth mentioning. Temperature measurements were only performed on gilts and may not reflect the thermal status of the barrows. However, because G:F was reduced overall for PP housed pigs and growth performance measures were taken on a per pen basis, this may suggest that the gilts and barrows have a similar growth performance response to pen location. Additionally, the experiment was conducted during a short time period (6 weeks from mid-July to mid-August) and the decrease in G:F might not reflect the entire grow-finish period. Furthermore, the study was conducted in side-ventilated barns and the findings may not be applicable to tunnel-ventilated barns. Nonetheless, these data illustrate the importance of the microclimate variability in grow-finish barns and its potential impact on thermoregulation and performance of pigs during summer months.
CONCLUSIONS
Variable thermal conditions in swine facilities can negatively affect the welfare and overall productive capacity of pigs. It was determined that pen location differences in pig thermoregulation and performance existed and that these differences may have been associated with pen-to-pen microclimate variation. While this study has furthered our understanding of the impact of microclimates within grow-finish facilities, future work should be conducted to evaluate these effects over a longer period, in different barn types, and consider other variables such as illness rate and pig thermoregulatory behavior.
Footnotes
The authors would like to acknowledge the assistance of employees at the USDA-ARS Livestock Behavior Research Unit, swine farm staff, and graduate students at the Purdue University for daily animal care and data collection. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. No conflicts of interest, financial or otherwise are declared by the authors.
LITERATURE CITED
- Axaopoulos P., Panagakis P., and Kyritsis S.. 1992. Computer simulation assessment of the thermal microenvironment of growing pigs under summer conditions. Trans. ASAE. 35:1005. doi: 10.13031/2013.28694 [DOI] [Google Scholar]
- Baumgard L. H., Keating A., Ross J. W., and Rhoads R. P.. 2015. Effects of heat stress on the immune system, metabolism and nutrient partitioning: implications on reproductive success. Rev. Bras. Reprod. Anim. 39:173–183. [Google Scholar]
- Becker B. A., Knight C. D., Buonomo F. C., Jesse G. W., Hedrick H. B., and Baile C. A.. 1992. Effect of a hot environment on performance, carcass characteristics, and blood hormones and metabolites of pigs treated with porcine somatotropin. J. Anim. Sci. 70:2732–2740. doi: 10.2527/1992.7092732x [DOI] [PubMed] [Google Scholar]
- Bond T. E., Heitman H. Jr., and Kelly A. C. F.. 1965. Effects of increased air velocities on heat and moisture loss and growth of swine. Trans. ASAE. 8:167–169. doi: 10.13031/2013.40458 [DOI] [Google Scholar]
- Close W. H., Heavens R. P., and Brown D.. 1981. The effects of ambient temperature and air movement on heat loss from the pig. Anim. Sci. 32:75–84. doi: 10.1017/S0003356100024806 [DOI] [Google Scholar]
- Costa A., Ismayilova G., Borgonovo F., Viazzi S., Berckmans D., and Guarino M.. 2014. Image-processing technique to measure pig activity in response to climatic variation in a pig barn. Anim. Prod. Sci. 54:1075–1083. doi: 10.1071/AN13031 [DOI] [Google Scholar]
- Curtis S. E. 1983. Environmental management in animal agriculture. Ames, Iowa: Iowa State University Press; p. 6–96. [Google Scholar]
- Federation of Animal Science Societies 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed.Champaign (IL): Federation of Animal Science Societies. chap. 11. [Google Scholar]
- Geers R., Goedseels V., Parduyns G., and Vercruysse G.. 1986. The group postural behaviour of growing pigs in relation to air velocity, air and floor temperature. Appl. Anim. Behav. Sci. 16:353–362. doi:10.1016/0168-1591(86)90007–9 [Google Scholar]
- Huynh T. T. T., Aarnink A. J. A., Heetkamp M. J. W., Verstegen M. W. A., and Kemp B.. 2007. Evaporative heat loss from group-housed growing pigs at high ambient temperatures. J. Therm. Biol. 32:293–299. doi: 10.1016/j.jtherbio.2007.03.001 [DOI] [Google Scholar]
- Johnson J. S. 2018. Heat stress: impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 58:1401–1413. doi: 10.1071/AN17725 [DOI] [Google Scholar]
- Johnson J. S., Abuajamieh M., Sanz Fernandez M. V., Seibert J. T., Stoakes S. K., Nteeba J., Keating A. F., Ross J. W., Rhoads R. P., and Baumgard L.. 2015a. Thermal stress alters postabsorptive metabolism during pre- and postnatal development. In: Sejian V., Gaughan J., Baumgard L., and Prasad C., editors, Climate change impact on livestock: adaptation and mitigation. New Delhi (India): Springer India; p. 61–79. [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Seibert J. T., Ross J. W., Lucy M. C., Safranski T. J., Elsasser T. H., Kahl S., Rhoads R. P., and Baumgard L. H.. 2015b. In utero heat stress increases postnatal core body temperature in pigs. J. Anim. Sci. 93:4312–4322. doi: 10.2527/jas.2015-9112 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., and Shade K. A.. 2017. Characterizing body temperature and activity changes at the onset of estrus in replacement gilts. Livest. Sci. 199:22–24. doi: 10.1016/j.livsci.2017.03.004 [DOI] [Google Scholar]
- Kerr B. J., Yen J. T., Nienaber J. A., and Easter R. A.. 2003. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 81:1998–2007. doi: 10.2527/2003.8181998x [DOI] [PubMed] [Google Scholar]
- Kluzáková E., Stupka R., Citek J., Sprysl M., Okrouhla M., Brzobohaty L., and Vehovsky K.. 2013. The influence of the stable microclimate on the pig production performance. Res. Pig Breed. 7:15–19. [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., and Baumgard L. H.. 2017. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi:10.3168/jds.2016–12001 [DOI] [PubMed] [Google Scholar]
- Littell R. C., Henry P. R., and Ammerman C. B.. 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi:10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Lucy M. C., and Safranski T. J.. 2017. Heat stress in pregnant sows: thermal responses and subsequent performance of sows and their offspring. Mol. Reprod. Dev. 84:946–956. doi: 10.1002/mrd.22844 [DOI] [PubMed] [Google Scholar]
- Massari J. M., de Moura D. J., de C. Curi T. M. R., Vercellino R. do A., and Medeiros B. B. L.. 2016. Zoning of environmental conditions inside a wean-to-finish pig facility. Eng. Agr. 36:739–748. doi: 10.1590/1809-4430-Eng.Agric.v36n5p739-748/2016 [DOI] [Google Scholar]
- Midwest Plan Service 1972. Swine handbook, housing and equipment. mwps-8, midwest plan service. Ames (IA): Iowa State University. [Google Scholar]
- Mitchell M. A. 1985. Effects of air velocity on convective and radiant heat transfer from domestic fowls at environmental temperatures of 20 degrees and 30 degrees C. Br. Poult. Sci. 26:413–423. doi: 10.1080/00071668508416830 [DOI] [PubMed] [Google Scholar]
- Morello G. M., Lay D. C. Jr, Rodrigues L. H. A., Richert B. T., and Marchant-Forde J. N.. 2018. Microenvironments in swine farrowing rooms: the thermal, lighting, and acoustic environments of sows and piglets. Sci. Agric. 75:1–11. doi: 10.1590/1678-992X-2016-0303 [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed.Washington (DC): National Academies Press. [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Baumgard L. H., and Gabler N. K.. 2012. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 90(Suppl 4):257–259. doi: 10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Kerdoncuff M., Anaïs C., and Gourdine J. L.. 2008. Effect of temperature level on thermal acclimation in large white growing pigs. Animal. 2:1619–1626. doi: 10.1017/S1751731108002814 [DOI] [PubMed] [Google Scholar]
- Sällvik K., and Walberg K.. 1984. The effects of air velocity and temperature on the behaviour and growth of pigs. J. Agric. Eng. Res. 30:305–312. doi: 10.1016/S0021-8634(84)80031-1 [DOI] [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi:10.3168/jds.S0022-0302(03)74040–5 [Google Scholar]