Abstract
The objective of this experiment was to evaluate the inclusion of a rumen-protected carbohydrate (RPC) on growth performance and blood metabolites of finishing steers during the summer. A 62-d feedlot study was conducted using 135 Angus crossbred steers (body weight = 287 ± 13 kg). All animals were fed a basal diet (BD), then treatments were top-dressed. The treatments were the same composition and only varied in ruminal degradability. Treatments were 1) a BD with 1 kg/d of a control supplement (0RPC), 2) the BD plus 0.5 kg/d of the control supplement and 0.5 kg/d of RPC (0.5RCP), and 3) the BD with 1 kg/d of RPC supplement (1RPC). Temperature humidity index and cattle panting scores (CPS) were measured daily during the experiment. Growth performance, back-fat over the 12th rib (BF), LM area, blood glucose and plasma insulin, urea, and nonesterified fatty acid concentrations were measured. Data were statistically analyzed (PROC Mixed, SAS) using treatment, time, and their interaction as a fixed variable and pen as a random variable. There were no differences (P > 0.10) between the three treatments on CPS, BF, and LM area on day 62. There was a trend (P = 0.06) for treatment effect for a greater body weight on the 0.5RPC, and a treatment effect for dry matter intake (P = 0.05). Treatment × day interactions were observed for average daily gain (ADG, P =0.04), suggesting a different response to treatments during the different sampling periods. There was a treatment effect for blood glucose concentration (P = 0.03), having the 0RPC the greatest concentration. Treatment × day interactions were found for plasma insulin concentration (P = 0.01). The results suggest that the response to RPC supplementation depends in part on environment. The use of 0.5 kg/d of RPC tends to improve overall body weight; however, the response to RPC on ADG and plasma insulin concentration depend on the time of sampling.
Keywords: blood metabolites, glucose, heat stress, protected carbohydrate
INTRODUCTION
Maintaining an increased growth rate in feedlot steers during periods of heat stress can be challenging. Weather conditions can drastically affect dry matter intake (DMI) and average daily gain (ADG) (Mader et al., 2006). Several management strategies can be adopted to mitigate the effect of heat stress, such as the use of shades and sprinklers (Gaughan et al., 2010). Altering feed delivery patterns (Mader, 2003), or the amount of feed delivered, or diet energy concentration can also be beneficial (Mader and Davis, 2004).
Decreased DMI is not the only concern during heat stress, but loss of production may also occur because the animal is metabolically challenged. For instance, in dairy cows only 30% to 50% of the reduction in milk production during heat stress can be explained by the diminished DMI (Rhoads et al., 2009). In growing bull calves exposed to heat stress, the decrease in growth performance can entirely be attributed to a decrease in DMI (O’Brien et al., 2010). Despite the different animal models, blood and plasma metabolite concentrations follow similar patterns. Even with the decrease in DMI during heat stress, plasma glucose and nonesterified fatty acids (NEFA) concentration are decreased and insulin concentrations are increased (Baumgard and Rhoads, 2012).
Glucose concentration in blood had increased by infusing starch or glucose into the abomasum (Kreikemeier et al., 1991). Efficiency of conversion of starch energy into tissue energy is improved when starch is digested in the small intestine rather than fermented in the rumen (Harmon, 1992). But due to the rumen physiology, the use of a rumen-unprotected glucose source is fermented in the rumen. Also there might be limitations in the amount of starch that the small intestine can digest (Branco et al., 1999). For example, in growing and finishing steers, Huntington et al. (2006) showed that approximately 700 g/d of starch seems to be the limit for starch digestion in the small intestine. Any excess of that amount would pass undigested contents to the lower gastrointestinal tract. This may be because of a decrease in α-amylase secretion by the pancreas (Walker and Harmon, 1995). Therefore, a rumen-protected glucose might be beneficial. However, there are no studies that show the effect of protected glucose on feedlot performance on summer time condition.
Based on the cited literature, we hypothesize that feeding rumen-protected carbohydrates (RPCs) will improve growth performance in heat-stressed animals. The objective of this study was to evaluate the inclusion of a RPC on growth performance, blood glucose, and plasma insulin and NEFA concentration in finishing steers during summer.
MATERIALS AND METHODS
Facilities
The experiment was conducted in a commercial feedlot located in Buenos Aires, Argentina (lat.: 34°43′14″ S, long.: 63°05′08″ W), during the summer of 2013–2014 (December, January, and February). Fifteen soil-surfaced pens (12 × 50 m) were used with nine steers in each pen. Water troughs were shared between two pens. All animal procedures were approved by the Animal Care and Use Committee from Veterinary College (Energy metabolism in beef cattle, 4 April 2011) and La Plata National University.
Animals, Treatments, and Sampling
One hundred thirty-five (average body weight [BW] = 287 ± 13 kg) Angus crossbred steers were used in a 62-d experiment. Steers were blocked by BW and assigned to one of three treatments in a randomized complete block design with five pens per treatment. Steers were given ad libitum access to the basal diet (BD). Treatment was top-dressed and the treatments were as follows: 1) 1 kg/d of control supplement (0RPC), 2) 0.5 kg/d of control supplement and 0.5 of kg/d RPC (0.5RPC), and 3) 1 kg/d of RPC (1RPC). The diets were formulated to meet or exceed requirements for beef (NRC, 2000). Both the control supplement and RPC supplement contained (% DM) the same ingredients (Table 1), differing only in the processing of the carbohydrate. Rumen protection of RPC and its small intestine absorption had been tested and described on the patent of the product (U.S. patent number 8,507,025). The target amount of protected dextrose for each treatment was 0, 180, or 360 g/d for 0RPC, 0.5RPC, and 1RPC, respectively (Table 1).
Table 1.
Composition and chemical analysis (on DM basis) of basal diet and RPC or supplement
| Ingredients | Basal diet | Suplement or RPCa |
|---|---|---|
| Corn silage, % | 16 | – |
| Dry-rolled corn, % | 81 | – |
| Soybean meal, % | – | 58.1 |
| Dextrose, % | – | 38.9 |
| Urea, % | 0.55 | 2.8 |
| Vitamins and mineralsb, % | 2.25 | – |
| Mineral saltsc, % | – | 1.2 |
| Diet DM,% | 57.5 | 85.7 |
| CP, % DM | 10.1 | 27.8 |
| ADF, % DM | 14.9 | 14.4 |
| NDF, % DM | 31.7 | 28.8 |
| EE, % DM | 2.9 | 4.5 |
| Ash, % DM | 4.4 | 2.8 |
aThe supplement and RPC differed only in the processing of the carbohydrate (i.e., protected or not from ruminal degradation).
bMinerals: Ca 27.74%, Mg 0.62%, Na 9.26%, Co 6.17 ppm, Cu 555 ppm, I 30.86 ppm, Mn 2037 ppm, Se 18.52 ppm, Zinc 2592 ppm, Monensin 1.03%.
cMineral salts: Na bicarbonate 35 g, K2HPO4 6 g, KH2PO4 4.5 g, ClNa 10 g.
Fifteen days before starting the experiment, BW was recorded. Steers were stratified by BW and used to establish the blocking weight criteria. Five blocks were assigned by weight, nine animals per pen.
Individual BW were scheduled to be obtained on days 1, 15, 36, and 57. Day 15 was the end of the adaptation period and then every 21 d; however, due to weather conditions (rainfall during the scheduled day), the actual BW was measured on days 1, 15, 39, and 62 relative to the starting day (day 1). Animals were individually weighed before the morning feeding.
Feed was offered daily and refusals were collected once a week (days 8, 15, 23, 29, 33, 36, 44, 49, 54, 58, and 62) to determine DMI. The DMI observations on days 1 and 15 correspond to the adaptation period to the diet, and from day 16 onwards the steers were fully adapted to the final diet.
Steers were adapted to the final diet during the first 15 d of the experiment in three stages. The first stage lasted for 5 d and the diet contained 60% corn silage, 30% dry-rolled corn, 7.5% sunflower seed meal, 0.5 urea, and 2% of mineral and vitamin mix with monensin (on DM bases). From days 6 to 11, the diet contained 53.5% corn silage, 43% dry-rolled corn, 0.5% urea, and 2% of a mineral vitamin mix with monensin (on DM bases) and at this stage, 0.5 kg of supplement or RPC was added as top-dress. From days 11 to 15, the diet contained 37% corn silage, 60% dry-rolled corn, 0.6% urea, and 2.4% of a mineral vitamin mix with monensin (on DM bases), 1 kg of supplement, RPC or half and half was added as top-dress. From days 16 to 62, end of the experiment, the animals were fed the final diets as described in Table 1.
Once in a week, individual feed ingredient samples were taken and composite to be analyzed for nutrient composition at the end of the experiment. Feed samples were analyzed for DM (60°C for 48 h), ADF and NDF (Ankom Technology methods 5 and 6, respectively; Ankom Technology, Fairport, NY), CP (method 930.15; AOAC, 1996), ether extract (method 2; Ankom Technology, Fairport, NY), and total ash (600°C for 12 h).
Back-fat at the 12th rib (BF) and LM area were measured by ultrasound on days 0 and 62 (Pie Medical mod. Aquila. Transductor 3.5 mhtz).
Blood samples were taken from the same four animals, randomly selected at the start of the trial, from each pen via jugular vein puncture before the morning feeding. A drop of blood was used for glucose analysis and the rest of the blood was placed in tubes containing disodium EDTA (1.6 mg/mL of blood) on days 0, 15, 39, and 62. Samples were maintained in a cooler until collection was finished. Blood samples were then centrifuged for 20 min at 1,000 × g to obtain plasma which was immediately frozen and stored at −20°C until analyzed for insulin, NEFA, and urea. Whole blood glucose concentrations were determined in situ with a glucometer (Optimum Xceedt, ABBOTT Lab Argentina). The plasma insulin concentration was analyzed via radioinmuno assay as described previously (Díaz-Torga et al., 2001). The minimum detectable concentration was 0.05 ng/mL. Intersample and intrasample coefficients of variation were 8% and 7%, respectively. Plasma NEFA concentration was analyzed on days 0, 15, 39, and 62, using a colorimetric assay, following the protocol described by Randox labs (FA 115 Randox Laboratories Ltd.). The minimum detectable concentration was 72 mM, and the intrasample and intersample coefficients of variation were 7.5% and 23%, respectively. Plasma urea-N concentrations were analyzed on days 0, 39, and 62, using the colorimetric protocol described by Wiener Lab city (Rosario, Santa Fe, 2R UREA Color). The minimum detectable concentration of urea was 2 mg/dL. Coefficients of variation of intrasamples and intersamples were 9.7% and 11%, respectively.
Temperature and humidity were recorded every 30 min during the entire experiment by a meteorological station (Davies instruments, San Francisco, California) placed in the city of Piedritas 14 km from the feed yard (Longitude 62°58′49″ W; latitude: 34°33′58″). Temperature humidity index (THI) was calculated according to Hubbard et al. (1999), using the following equation: THI = (0.8 × temperature) + [(% relative humidity/100) × (temperature − 14.4)] + 46.4.
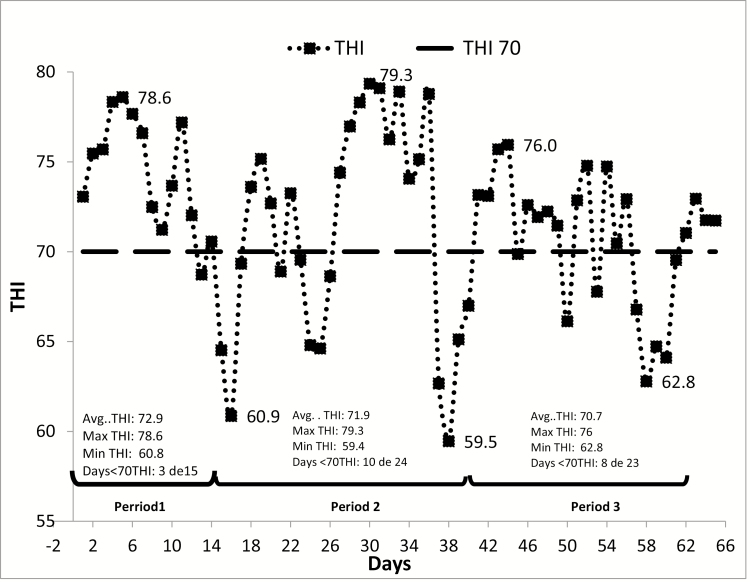
Daily average, maximum, and minimum THI values were calculated for all the experimental periods. Also, partial THI values were calculated for the periods when the animals were weighed: period 1 (days 1 to15), period 2 (days 16 to 39), and period 3 (days 40 to 62).
Cattle panting scores (CPS) were recorded from days 1 to 62, end of the experiment, between 1200 and 1600 h, as well as the time at which the observation was taken. CPS were classified as described by Mader et al. (2006) on a 0 to 4 scale with 0 being normal and 4 being severe open-mouthed panting accompanied by protruding tongue and excessive salivation, usually with neck extended forward. For the correlation between CPS and THI, only days with a THI > 70 were taken into account, and this included 29 of 62 d of the experiment.
Daily average THI measured was 72 ± 4.9 with a maximal THI of 79 and a minimal THI of 59. Animals experienced THI over 70 for 46 d of the 62-d experiment (Figure 1). When THI was sliced into periods to match BW measurements (days 0 to 15, 16 to 39, and 40 to 62), no statistical differences were observed on average THI amongst the three periods (P = 0.40).
Figure 1.
THI across days on the experiment. Marked on the figure is the THI 70 and periods 1 to 3 organized to match sampling of performance and blood metabolites.
Statistical Analysis
Data were analyzed as a randomized complete block design with repeated measurements, using the MIXED procedure of SAS, version 9.4 (SAS Institute, Inc., Cary, NC). The model included the fixed effect of treatments, days (time), and interactions between treatments and days, and the random effect of pen and block (BW). Days was considered as the repeated statement. Pen was considered as the experimental unit. Block was removed from the model when it was not significant (P > 0.1). The covariance structures compared included compound symmetry, unstructured, spatial power, autoregressive, and heterogeneous autoregressive. Dependent variables were analyzed using the covariance-structured spatial power, because it gave the best fit based on the Akaike information criterion (Littell et al., 2006). Back fat thickness at the 12th rib and LM area on day 62 did not have repeated measurements, and the initial value on day 1 was used as a co-variate. The slice option was used to separate means when the time × treatment interaction was different (P < 0.10), and mean comparisons were conducted using the PDIFF statement of SAS whenever there was only a main effect or a treatment effect for a particular day after the use of the SLICE option.
The PROC Corr of SAS, version 9.4 (SAS Institute, Inc., Cary, NC), was used to evaluate correlation between THI and CPS and CPS and time of the day when the observation was reported.
RESULTS
There was no correlation between THI and CPS (r = 0.18, P = 0.35) during the experiment. Despite the lack of correlation between THI and CPS, CPS was above 1 (on every steer) on the 29 d where THI was greater than 70.
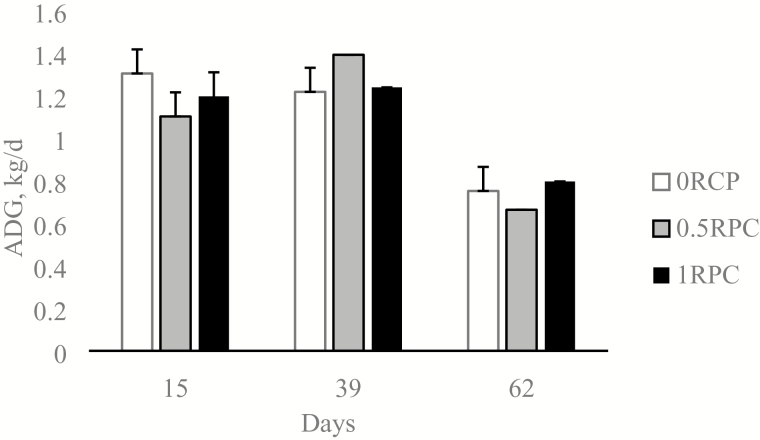
No treatment × day interaction were observed for BW, but animals fed 0.5RPC and 1RPC had a tendency for treatment effect (P = 0.06) to have a greater BW than 0RPC (Table 2). There was a treatment × day interaction (P = 0.04, Figure 2) for ADG. In period 2, steers fed 0.5RPC reported the largest ADG of 1.42 kg/d followed by the steers fed 1RPC with 1.23 kg/d and the steers fed 0RPC 1.13 kg/d, but there were no differences at the end of the experiment on the total ADG comparing the animals fed the three treatments (Figure 2). There was a treatment effect (P = 0.05, Table 2) for DMI. There were no differences amongst animals fed the different treatments for back fat 12th rib on day 62 (P > 0.10) or LM area on day 62 (P = 0.15; Table 2).
Table 2.
Effect of increasing dose of RPC on DMI, BW, back fat (BF) at 12th rib on 63 d and Longissimus muscle (LM) area on day 62 in finishing steers during heat stress.
| Item | Treatment (Trt) | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0RPC | 0.5RPC | 1RPC | Trt | Day (d) | Trt × d | ||
| Initial BW, kg | 281 | 285 | 284 | 1.6 | 0.06 | <0.01 | 0.76 |
| Final BW, kg | 350 | 355 | 352 | ||||
| DMI, kg/d | 9.8a | 9.9ab | 10.1b | 0.07 | 0.05 | <0.01 | 0.14 |
| BF, cm | 0.59 | 0.6 | 0.6 | 0.015 | 0.82 | – | – |
| LM on day 62, cm2 | 57.39 | 54.56 | 56.56 | 0.967 | 0.15 | – | – |
Figure 2.
Effects of increasing dose of RPC on ADG in finishing steers during heat stress. The dotted line represents the daily THI. 0RPC steers received the basal diet plus 1 kg/d of supplement top dressed (58.1% soybean meal, 38.9% dextrose, 2% urea, and 1% minerals salts DM basis, without the ruminal protection). 0.5RPC steers received the basal diet plus top dressed 0.5 kg/d of supplement and 0.5 kg/d of RPC (58.1% soybean meal, 38.9% dextrose, 2% urea, and 1% minerals salts DM basis, with ruminal protection). 1RPC steers received the basal diet plus 1 kg/d of RPC. Data are presented as least square means and SEM. P-value for the treatment by time interaction = 0.04.
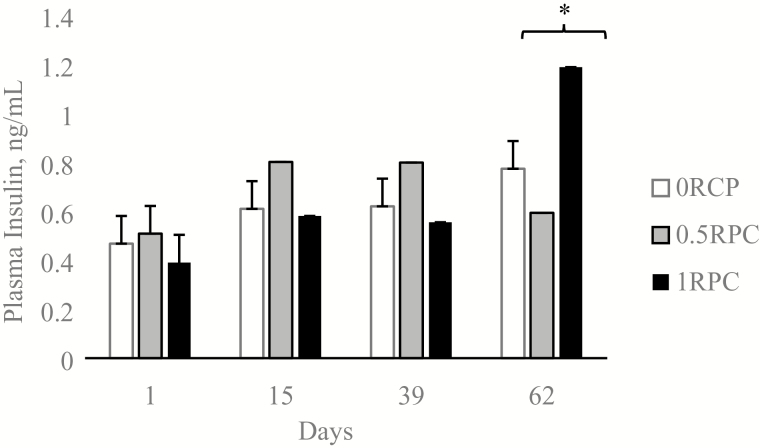
There was a treatment effect (P = 0.03) for blood glucose concentration. Steers fed 0RPC had the greater overall blood glucose concentration than the other two treatments (P < 0.03; Table 3). There was a treatment × day interaction (P < 0.01; Table 3, Figure 3) for plasma insulin concentration. Plasma insulin concentration was similar on the steers fed the different treatments on days 0, 15, and 39, but it was different on day 62, where the steers fed 1RPC had the greatest plasma insulin concentration (Figure 3). Plasma urea concentration also had a treatment × day interaction (P = 0.02, Table 3). The main difference in urea concentration was found on day 1. Plasma urea concentration was different before the treatment supplementation started (P < 0.01, Table 3), but no differences were found during the rest of the experiment. On day 1 plasma urea concentration was greater (P < 0.05) for 0.5RPC (45.0 mg/dL) compared with 0RPC and 1RPC (34.7 and 29.1 mg/dL, respectively). Plasma NEFA concentration was not different amongst the steers fed the different treatments (P = 0.15, Table 3).
Table 3.
Effect of increasing dose of RPC on blood glucose concentration, plasma insulin, NEFA, and urea concentrations on finishing steers during heat stress
| Item | Treatments (Trt) | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0RPC | 0.5RPC | 1RPC | Trt | Day (d) | Trt × d | ||
| Glucose, mg/dL | 88a | 83b | 83b | 2.4 | 0.03 | <0.01 | 0.35 |
| Insulin, ng/mL | 0.62 | 0.68 | 0.68 | 0.056 | 0.69 | <0.01 | 0.01 |
| NEFA, mM | 184 | 191 | 161 | 17.2 | 0.42 | <0.01 | 0.50 |
| Urea, mg/dL | 24 | 27 | 22 | 0.1 | 0.15 | <0.01 | 0.02 |
Figure 3.
Effects of rumen-protected carbohydrate on plasma insulin concentration in finishing steers during heat stress. Steers on treatment 0RPC received the basal diet plus 1 kg/d of supplement top dressed (58.1% soybean meal, 38.9% dextrose, 2% urea, and 1% minerals salts DM basis, without the ruminal protection). Steers on treatment 0.5RPC received the basal diet plus top dressed 0.5 kg/d of supplement and 0.5 kg/d of RPC (58.1% soybean meal, 38.9% dextrose, 2% urea, and 1% minerals salts DM basis, with ruminal protection). Steers on treatment 1RPC received the basal diet plus 1 kg/d of RPC. Data are presented as least square means and SEM. P-value for the treatment by time interaction <0.01; *P < 0.05 using the SLICE option of SAS.
DISCUSSION
Diets were designed to be isoenergetic and isonitrogenous. All treatments received the same amount of dextrose (360 g) and the difference was in the ruminal protection of the carbohydrate. In 0RPC, none of the 360 g of dextrose were protected from ruminal degradation and all of the soluble carbohydrates were assumed to fuel microbial growth in the rumen. In 0.5RPC, only 180 g of dextrose were protected and 180 g were unprotected, and cattle fed 1RPC received 360 g of rumen-protected dextrose (Russi et al., 2011).
Period 1 lasted for 15 d and had an average THI of 72.9 ± 5 with a maximum and minimum THI of 78.6 and 60.8, respectively, and 12 d of the 15-d period recorded above 70 THI. In period 2 lasting 24 d, average THI was 71.9 ± 5.8 with a maximum THI of 79.3 and a minimum of 59.4, 14 d of the 24 d above 70 THI. Period 3 lasted for 23 d and had a maximum THI of 75.95 and a minimum of 62.7 with an average THI of 70.7 ± 3.7. Eight of 23 d were above 70 THI (Figure 1). When blood samples were taken from the animals, THI was different each time: for 1 d, THI was 73; for 15 d, THI 69; for 39 d, THI 66; and for 62 d, THI 73. Despite no differences for THI, period 1 had the greatest average THI, period 2 had the maximal THI and was the most variable (greater standard deviation), and period 3 had the least THI and was the least variable.
Temperature and humidity index averaged 72 ± 4.9, and according to Mader and Davis (2004), animals experienced mild heat stress (THI between 70 and 73.9) for 20 d and were heat-stressed (THI over 74) for 22 of 62 d of the experiment. These values confirm that at least for ¾ of the experiment, animals experienced some degree of heat stress. Despite this, THI was not correlated with CPS. Mader et al. (2010) suggested that including wind speed and solar radiation increased the correlation between CPS and modified THI. This could have explained the lack of correlation between CPS and THI in our study. It is also possible that the THI was not great enough for the need of the animals to dissipate heat by increasing the CPS.
It is well documented that animals subjected to heat stress alter their eating behavior, which results in a decrease of DMI and growth performance (Mader, 2003). On periods of heat stress, cattle might benefit from the more energy dense diets, due to the depressed DMI. However, the increase in diet lipid concentration did not show that benefit (Gaughan and Mader, 2009). The inclusion of RPC in our study had a time by treatment interaction for growth performance, with an erratic pattern among treatments. Our study also shows a dose increase in DMI. However, the increase on DMI was not associated with changes on ADG or overall growth performance. O’Brien et al. (2010) showed that changes in growth performance were associated with the decrease of DMI. However, our findings do not corroborate such association. Independently of the DMI, steers fed 0.5RPC had a tendency to have a greater ADG at the end of the experiment.
Growth performance traits varied widely between treatments from days 15 to 39. During this time, the steers had elevated average THI values and reported the greatest maximal THI from the whole study (79.3). Also, 0.5RPC was the treatment that showed the greatest ADG. This difference in growth performance traits on this period could be partially explained by the site of carbohydrate digestion and absorption which may have been modified by treatments. Even though treatments and diets were planned to be isoenergetic and isonitrogenous, differences in site of digestion and absorption of the protected carbohydrate were expected in the metabolic utilization of the energy.
The experimental approach was to increase the amount of glucose reaching the small intestine using protected glucose instead of unprocessed grain. Energetically, it is clear that glucose is more efficiently used by the animal when it is directly absorbed in the small intestine, rather than fermented in the rumen to VFA (Rodriguez et al., 2004). When glucose reaches the small intestine, it is fully absorbed, unlike what happens with starch (Kreikemeier et al., 1991). Depending on the animal model (dairy cow or finishing steer), there are limitations in the amount of starch that can be digested in the small intestine (Branco et al., 1999). In growing and finishing steers, approximately 700 g/d of starch seems to be the limit for starch digestion in the small intestine (Huntington et al., 2006). Any excess of that amount would pass undigested contents to the lower gastrointestinal tract. This may be because of a decrease in α-amylase secretion by the pancreas (Walker and Harmon, 1995; Swanson et al, 2004). Although we did not measure glucose absorption per se, unlike starch, glucose is readily absorbed in the small intestine with no limitations (Krehbiel et al., 1996; Rodriguez et al., 2004). Using glucose as a source of energy appears to be a sound theory to enhance growth performance, but no benefit has been found when abomasal glucose infusion was compared with other sources of energy in growing steers (Schroeder et al., 2006). Similarly, no benefits were observed on more milk production when starch or glucose was infused to lactating dairy cows (Reynolds et al., 2001; Larsen and Kristensen, 2009). However, when the animal is metabolically challenged, glucose as a nutrient may have a chance to enhance performance, such as observed in this current study during heat stress.
From days 15 to 39, the animals experienced heat stress for the longest time. During this time, feeding 0.5RPC had a greater ADG. It appears that the use of glucose as a nutrient from RPC may have benefited the animal. Despite the positive response, it is unclear why dosing 180 g of protected glucose (0.5RPC) vs. 360 g (1RPC) elicited an improved growth. It is possible that the combination of a portion of ruminally protected and unprotected glucose may have achieved a balance between enhanced ruminal fermentation and small intestine digestion considering the responses observed with 0.5RPC. Another explanation could be that 1RPC with a greater dose of protected glucose (360 g) triggered endocrine gut responses, such as an increase in glucagon like peptide-1 or glucose-dependent insulinotropic polypeptide plasma concentration (Relling and Reynolds, 2008). These peptides had been associated with DMI regulation (Relling and Reynolds, 2007) and energy efficiency (Relling et al., 2014), respectively; however, the current experiment was not designed to measure them.
Differences in growth performance (BW and ADG) during the third period (from days 39 to 62) were not observed in the experiment. The decreased severity of THI recorded in the third period may have allowed a compensatory growth, or may have created an effect of acclimatization of the animals to milder heat stress conditions (O’Brien et al., 2008).
Besides the ability of RPC to enhance growth performance in certain moments of the experiment, we would have expected 0.5RPC to alter the pattern of tissue deposition, such as more intramuscular fat, larger LM area, and less BF on day 62 of the experiment (Smith and Crouse, 1984). This was not the case with these treatments, and a possible cause may be that heat stress altered physiological glucose partitioning behavior in steer’s metabolism (O’Brien et al., 2010; Rhoads et al., 2013). In McLeod et al. (2007) and Baldwin et al. (2007) companion experiments, infusion of up to 800 g/d of glucose into the abomasum decreased DMI and resulted in greater adipose accretion, particularly the omental depot–stimulating lipogenesis from glucose and acetate is more pronounced in abdominal depots relative to subcutaneous depots (Baldwin et al., 2007; McLeod et al., 2007). In our experiment, the maximal dose of protected dextrose was 360 g and there were no differences on the subcutaneous adipose tissue accretion. Therefore, perhaps there is a need of a greater dose or time of supplementation of protected carbohydrate to obtain the same response observed previously (Baldwin et al., 2007; McLeod et al., 2007). It is also possible that heat stress affected energy partitioning on a different manner or the duration of the experiment was not enough to see differences in BF or LM.
Homoheretic mechanisms are irremediably challenged when animals are exposed to heat stress and nutrients may not be all used for production. Blood and plasma metabolites are good descriptors of this diversion (O’Brien et al., 2010). In this experiment, blood glucose, insulin, and NEFA concentration had different responses depending the sampling conditions such as THI, which described a similar pattern observed in heated-stress dairy cows (Wheelock et al., 2010) and other species exposed to heat stress (Baumgard and Rhoads, 2012). Despite the decrease in DMI observed on 0RPC, and the fact that such treatment did not have protected glucose, blood glucose concentration was the greatest compared with the other two treatments. This response was not expected and is not associated with changes in plasma insulin concentration. Our current data do not allow us to find a physiological explanation, but it is possible that the glucose–insulin metabolism could be changed due to heat stress (Baumgard and Rhoads, 2012). It is also possible that the absorption of the glucose on the small intestine increased the secretion of GLP-1 and GIP (Relling and Reynolds, 2008), which play a role in glucose metabolism. Therefore, the increase of these two hormones facilitates the tissues to uptake the glucose, decreasing blood glucose concentration. But more research needs to be conducted to understand glucose metabolism when glucose is absorbed in the small intestine during heat stress periods in finishing cattle. Dosing RPC at the greatest dose (1RPC, 360 g of dextrose) registered a peak in insulin decreasing glucose on day 62. These data indicate that at least on the day of sampling, the dose of RPC was affecting blood metabolite concentration. It is interesting to note that from the 3 d that blood was sampled, when the animals were already adapted to the diet (sampling on day 1 was taken before the adaptation period), day 62 was the day that reported the greatest THI (73.3). Therefore, on day 62, the animals were exposed to heat stress at the moment of sampling, and this may be the reason we found greater insulin concentration for the treatment that had the full dose of rumen-protected carbohydrate (1RPC). It is also possible that longer time on RPC supplementation changes the metabolic status of the animals for more insulin resistance (Joy et al., 2017). For plasma urea concentration, we were expecting a decrease due to RPC supplementation, associated with an increase in plasma insulin concentration (Reynolds and Maltby, 1994); however, we did not find an association between plasma urean and insulin concentration.
In conclusion, the addition of RPC to the diet, animals change growth performance and plasma metabolites in finishing feedlot steers during times with moderate THI. Despite a tendency for a better growth in an intermediate dose that delivers 180 g/d of glucose to the small intestine, the results might depend on the THI; however, more studies need to be conducted to understand the role of RPC on growth and metabolism.
LITERATURE CITED
- Baldwin R. L. 6th, McLeod K. R., McNamara J. P., Elsasser T. H., and Baumann R. G.. 2007. Influence of abomasal carbohydrates on subcutaneous, omental, and mesenteric adipose lipogenic and lipolytic rates in growing beef steers. J. Anim. Sci. 85:2271–2282. doi:10.2527/jas.2006-588 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H. and Rhoads R. P.. 2012. Ruminant nutrition symposium: ruminant production and metabolic responses to heat stress. J. Anim. Sci. 90:1855–1865. doi:10.2527/jas.2011-4675 [DOI] [PubMed] [Google Scholar]
- Branco A. F., D. L. Harmon D. W. Bohnert B. T. Larson, and Bauer M. L.. 1999. Estimating true digestibility of nonstructural carbohydrates in the small intestine of steers. J. Anim. Sci. 77:1889–1895. doi:10.2527/1999.7771889x [DOI] [PubMed] [Google Scholar]
- Díaz-Torga G. S., M. E. Mejia A. González-Iglesias N. Formia D. Becú-Villalobos, and Lacau-Mengido I. M.. 2001. Metabolic cues for puberty onset in free grazing holstein heifers naturally infected with nematodes. Theriogenology 56:111–122. doi:10.1016/S0093-691X(01)00547-7 [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., S. Bonner I. Loxton T. L. Mader A. Lisle, and Lawrence R.. 2010. Effect of shade on body temperature and performance of feedlot steers. J. Anim. Sci. 88:4056–4067. doi:10.2527/jas.2010-2987 [DOI] [PubMed] [Google Scholar]
- Gaughan J. B. and Mader T. L.. 2009. Effects of sodium chloride and fat supplementation on finishing steers exposed to hot and cold conditions. J. Anim. Sci. 87:612–621. doi:10.2527/jas.2008-1125 [DOI] [PubMed] [Google Scholar]
- Harmon D. L. 1992. Dietary influences on carbohydrases and small intestinal starch hydrolysis capacity in ruminants. J. Nutr. 122:203–210. doi:10.1093/jn/122.1.203 [DOI] [PubMed] [Google Scholar]
- Hubbard K. G., Stooksbury D. E., Hahn G. L., and Mader T. L.. 1999. A climatological perspective on feedlot cattle performance and mortality related to the temperature-Humidity index. Journal of Production Agriculture. 12:650–653. doi: https://doi.org/10.2134/jpa1999.0650 [Google Scholar]
- Huntington G. B., D. L. Harmon, and Richards C. J.. 2006. Sites, rates, and limits of starch digestion and glucose metabolism in growing cattle. J. Anim. Sci. 84 (Suppl):E14–E24. doi: https://doi.org/10.2527/2006.8413_supplE14x [DOI] [PubMed] [Google Scholar]
- Joy F., J. J. McKinnon S. Hendrick P. Górka, and Penner G. B.. 2017. Effect of dietary energy substrate and days on feed on apparent total tract digestibility, ruminal short-chain fatty acid absorption, acetate and glucose clearance, and insulin responsiveness in finishing feedlot cattle. J. Anim. Sci. 95:5606–5616. doi:10.2527/jas2017.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel C. R., R. A. Britton D. L. Harmon J. P. Peters R. A. Stock, and Grotjan H. E.. 1996. Effects of varying levels of duodenal or midjejunal glucose and 2-deoxyglucose infusion on small intestinal disappearance and net portal glucose flux in steers. J. Anim. Sci. 74:693–700. doi: https://doi.org/10.2527/1996.743693x [DOI] [PubMed] [Google Scholar]
- Kreikemeier K. K., D. L. Harmon R. T. Brandt T. B. Jr Avery, and Johnson D. E.. 1991. Small intestinal starch digestion in steers: effect of various levels of abomasal glucose, corn starch and corn dextrin infusion on small intestinal disappearance and net glucose absorption. J. Anim. Sci. 69:328–338. doi: https://doi.org/10.2527/1991.691328x [DOI] [PubMed] [Google Scholar]
- Larsen M. and Kristensen N. B.. 2009. Effect of abomasal glucose infusion on splanchnic and whole-body glucose metabolism in periparturient dairy cows. J. Dairy Sci. 92:1071–1083. doi:10.3168/jds.2008-1453 [DOI] [PubMed] [Google Scholar]
- Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., and Schabenberger O.. 2006. SAS for Mixed Models. 2nd ed SAS Institute, Cary, NC. [Google Scholar]
- Mader T. L. 2003. Environmental stress in confined beef cattle. J. Anim. Sci. 81: (Suppl):E110–E119. doi: https://doi.org/10.2527/2003.8114_suppl_2E110x [Google Scholar]
- Mader T. L. and Davis M. S.. 2004. Effect of management strategies on reducing heat stress of feedlot cattle: feed and water intake. J. Anim. Sci. 82:3077–3087. doi:10.2527/2004.82103077x [DOI] [PubMed] [Google Scholar]
- Mader T. L., M. S. Davis, and Brown-Brandl T.. 2006. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 84:712–719. doi:10.2527/2006.843712x [DOI] [PubMed] [Google Scholar]
- Mader T. L., L. J. Johnson, and Gaughan J. B.. 2010. A comprehensive index for assessing environmental stress in animals. J. Anim. Sci. 88:2153–2165. doi:10.2527/jas.2009-2586 [DOI] [PubMed] [Google Scholar]
- McLeod K. R., R. L. Baldwin 6th, Solomon M. B., and Baumann R. G.. 2007. Influence of ruminal and postruminal carbohydrate infusion on visceral organ mass and adipose tissue accretion in growing beef steers. J. Anim. Sci. 85:2256–2270. doi:10.2527/jas.2006-359 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th revised ed Washington, D.C: National Academy Press. [Google Scholar]
- O’Brien M. D., Baumgard L. H., Rhoads L. H., Duff G. C., Bilby T. R., Collier R. J., and Rhoads R. P.. 2008. The effects of heat stress on production, metabolism, and energetics of lactating and growing cattle. Gainesville, FL:Florida Ruminant Nutrition Symposium, Best Western Gateway Grand [Google Scholar]
- O’Brien M. D., R. P. Rhoads S. R. Sanders G. C. Duff, and Baumgard L. H.. 2010. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 38:86–94. doi:10.1016/j.domaniend.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Relling A. E., Crompton L. A., Loerch S. C., and Reynolds C. K.. 2014. Short communication: plasma concentration of glucose-dependent insulinotropic polypeptide may regulate milk energy production in lactating dairy cows. J Dairy Sci. 97:2440–2443. doi:http://dx.doi.org/10.3168/jds.2013–7574 [DOI] [PubMed] [Google Scholar]
- Relling A. E., and Reynolds C. K.. 2007. Feeding rumen-inert fats differing in their degree of saturation decreases intake and increases plasma concentrations of gut peptides in lactating dairy cows. J Dairy Sci. 90:1506–1515. doi: http: doi.org/10.3168/jds.s0022-0302(07)71636-3 [DOI] [PubMed] [Google Scholar]
- Relling A. E. and Reynolds C. K.. 2008. Abomasal infusion of casein, starch and soybean oil differentially affect plasma concentrations of gut peptides and feed intake in lactating dairy cows. Domest. Anim. Endocrinol. 35:35–45. doi:10.1016/j.domaniend.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Reynolds C. K., S. B. Cammell D. J. Humphries D. E. Beever J. D. Sutton, and Newbold J. R.. 2001. Effects of postrumen starch infusion on milk production and energy metabolism in dairy cows. J. Dairy Sci. 84:2250–2259. doi:10.3168/jds.S0022-0302(01)74672-3 [DOI] [PubMed] [Google Scholar]
- Reynolds C. K. and Maltby S. A.. 1994. Regulation of nutrient partitioning by visceral tissues in ruminants. J. Nutr. 124(8 Suppl):1399S–1403S. doi:10.1093/jn/124.suppl_8.1399S [DOI] [PubMed] [Google Scholar]
- Rhoads R. P., L. H. Baumgard, and Suagee J. K.. 2013. 2011 and 2012 early careers achievement awards: metabolic priorities during heat stress with an emphasis on skeletal muscle. J. Anim. Sci. 91:2492–2503. doi:10.2527/jas.2012-6120 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., R. P. Rhoads M. J. VanBaale R. J. Collier S. R. Sanders W. J. Weber B. A. Crooker, and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating holstein cows. I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi:10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Rodriguez S. M., K. C. Guimaraes J. C. Matthews K. R. McLeod R. L. Baldwin 4th, and Harmon D. L.. 2004. Influence of abomasal carbohydrates on small intestinal sodium-dependent glucose cotransporter activity and abundance in steers. J. Anim. Sci. 82:3015–3023. doi:10.2527/2004.82103015x [DOI] [PubMed] [Google Scholar]
- Russi J. P., Russi P. F., Simondi J. M., Bonetto G. M., Nasser Marzo C., Di Rienzo J. A., and Castillo A. R.. 2011. Evaluation of a rumen protected carbohydrate supplement prototype feed with fresh lactation dairy cows. J. Dairy Sci. 94(E-Suppl. 1)(Abstr.):623. [Google Scholar]
- Schroeder G. F., E. C. Titgemeyer M. S. Awawdeh J. S. Smith, and Gnad D. P.. 2006. Effects of energy source on methionine utilization by growing steers. J. Anim. Sci. 84:1505–1511. doi:https://doi.org/10.2527/2006.8461505x [DOI] [PubMed] [Google Scholar]
- Smith S. B. and Crouse J. D.. 1984. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 114:792–800. doi:10.1093/jn/114.4.792 [DOI] [PubMed] [Google Scholar]
- Swanson K. C., J. A. Benson J. C. Matthews, and Harmon D. L.. 2004. Pancreatic exocrine secretion and plasma concentration of some gastrointestinal hormones in response to abomasal infusion of starch hydrolyzate and/or casein. J. Anim. Sci. 82:1781–1787. doi:10.2527/2004.8261781x [DOI] [PubMed] [Google Scholar]
- Walker J. A. and Harmon D. L.. 1995. Influence of ruminal or abomasal starch hydrolysate infusion on pancreatic exocrine secretion and blood glucose and insulin concentrations in steers. J. Anim. Sci. 73:3766–3774. https://doi.org/10.2527/1995.73123766x [DOI] [PubMed] [Google Scholar]
- Wheelock J. B., R. P. Rhoads M. J. Vanbaale S. R. Sanders, and Baumgard L. H.. 2010. Effects of heat stress on energetic metabolism in lactating holstein cows. J. Dairy Sci. 93:644–655. doi:10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]