Abstract
Each year 500.000 sows, equal to 50% of Danish sows, are culled and transported to slaughter. However, the clinical condition, behavior, and welfare of cull sows have received almost no scientific attention. The aim of the current observational study was to describe the clinical condition of cull sows on the day of transportation to slaughter, including examination of possible differences between lactating and nonlactating sows. On the day of transportation, the participating farms were visited by trained technicians who conducted a thorough clinical examination of all sows selected by the farmer for slaughter. Four sows could not be transported because they were unfit according to the European Council Regulation regarding fitness for transportation, and they were not included in the present data. A total of 522 sows, with a median parity of 5 (range: 1–11), from 12 Danish farms were included in the study. Approximately, 10% showed signs of changed gait, and 0.8% were obvious lame. Wounds were observed in 54.6% of the sows, and 11% had decubital shoulder ulcers. Almost 40% of the cull sows were lactating. At culling, the lactating sows were of higher parity than the nonlactating sows, and lactating sows were at higher risk of having deviations from normal on clinical variables related to examination of the udder, such as udder swellings and inflammations. Nonlactating sows had 3.5 times more superficial skin lesions than lactating sows. Our findings warrant for further studies exploring different aspects of the life of cull sows in what is here defined as the The Cull Period, which is the interval from the culling decision is made until the sows are slaughtered
Keywords: animal transportation, animal welfare, cull sows, preslaughter logistic chain, slaughter
INTRODUCTION
International pig production is characterized by increasing herd sizes and changes in the slaughter industry toward fewer and larger units and, as a consequence hereof, transportation distances from farm to slaughter are increasing (as discussed by Lambooij, 2014). Especially cull swine may be vulnerable to transportation stress (Grandin, 2016; McGee et al., 2016). However, despite the large proportion of cull sows each year (500.000 sows, equal to 50% of the population, Statistics Denmark, https://www.statistikbanken.dk/ANI9) and the focus on the economic aspects of culling strategies (Lucia et al. 2000a, 2000b; Engblom et al., 2007; Zhao et al., 2015), behavior, and welfare of cull sows have received almost no scientific attention.
Reproductive problems, reduced health, and age are among the major reasons reported for culling (de Jong et al., 2014; Zhao et al., 2015), and a large proportion of sows are culled right after weaning their last litter (Engblom et al., 2007; de Hollander et al., 2015). The post-transportation clinical condition of cull sows has been reported (Cleveland-Nielsen et al., 2004; Knauer et al., 2007), and McGee et al. (2016) found that cull sows—as compared with market weight animals—made up the majority of swine arriving as fatigued, lame, or in a very low body condition at U.S. buying stations. However, only very little is known about the clinical condition of cull sows before being loaded onto trucks destined for slaughter. This may seem paradoxical, as such information is highly relevant for the assessment of fitness for transportation. According to EU legislation (Council Regulation, EC 1/2005) as well as industry guidelines and OIE guidelines (as reviewed by Grandin, 2016), sows must be fit for transportation, and obviously, ill or injured sows are not considered fit. Sows that are slightly ill or injured may, though, be considered fit for transportation.
The aim of the current study was to describe the clinical condition of cull sows on the day of transportation to slaughter and to examine possible differences between lactating and nonlactating sows. The study was part of an observational study of transportation of cull sows (Herskin et al., 2017; Thodberg et al., 2017).
MATERIALS AND METHODS
Herds and Animals
The study was designed as an observational study involving sows from private Danish herds and with a target sample size of 500 sows and a maximum of five loads per herd.
The primary inclusion criterion for involved herds was their geographical location stratified according to the distance to a large slaughter plant (four distance categories of expected duration of transportation: 0–2, 2–4, 4–6, or 6–8 h). These distance categories were selected to cover the range of transportation time (up to 8 h) allowed for cull sows in Denmark (Law nr 520, 26/05/2010; Anonymous, 2014). Within each distance category, six municipalities were randomly selected, and within each of these, a postal code area was randomly chosen. Potential experimental herds were identified within the resulting 24 areas based on database information about sow herds and livestock transportation publically available from the Danish Ministry of Environment and Food. Further inclusion criteria for the herds were use of the preselected large slaughter plant and delivery of minimum eight cull sows per load. All herds used either electronic sow feeders, sow feeding systems with feed stalls, wet feeding in long troughs, or floor feeding for their gestating sows. Within the group of potential candidate herds, one herd at a time was randomly chosen within each postal code area and distance category and contacted by email or phone. Study enrollment depended upon acceptance from the herd owner. For each of the involved herds, selection of animals to be slaughtered was made by the farmer. Based on an approval from the Danish Animal Experiments Inspectorate (license number: 2014-15-0201-00172), we were allowed not to comply with specific Danish requirements regarding sow fitness for transportation (Law nr 520, 26/05/2010; Anonymous, 2014) and only had to take into account the European Council Regulation (Council Regulation, EC 1/2005). Hence, sows with large uncrusted wounds (diameter >5 cm), prolapses, rectal temperature >40.5 °C, sows not being able to take support on all four legs, sows that had given birth in the previous week, and sows for whom at least 90% of the pregnancy period had passed could not be included in the data set.
Clinical Protocol
On the day of transportation, each herd was visited, and sow-related information such as parity was obtained from the farmer. Trained technicians conducted a thorough clinical examination of all animals selected for slaughter. The clinical examination was divided into three parts: 1) scored from a distance of 1–2 m before touching the sow, 2) scored while the sow was standing, and 3) scored while the sow was walking in the aisle.
“Part 1” included measures of general body condition. First, any deviation from normal condition was described in words. Afterward, respiration frequency (number per 30 s), respiration quality (normal [8–18 breaths/min], strained/forced [including abdominal movements], or superficial [involving only thorax]), and abnormalities in the head region, such as asymmetry, swellings, or flux from the snout, were registered. Body condition was scored on a scale from 1–4, including 0.5 measures (modified from SEGES I/S, Denmark). If the sow was lying, we noted her ease of getting up as either normal or not, and the technicians assessed the sow’s eyesight and hearing by waving and clapping the hands in front of her.
“Part 2” of the clinical examination included rectal temperature (°C), heart rate (beats per 20 s), possible abnormality in the shape and volume of the belly (yes/no), and if an umbilical outpouching was present, we noted its height and perimeter. For all sows, the udder was examined, including distance between floor and the lowest part of the udder, number of teats with milk, and number of udder lesions. Acute inflammation of the udder was noted as well as signs of swelling, asymmetry, soreness, flux, color, and signs of chronic udder inflammation.
Hair cover (normal/long) and the color of the body skin were registered (normal, pale, red, or cyanotic). Elasticity of the skin, as a sign of dehydration status, was recorded by pinching the body skin carefully and quantifying the interval until the skin recoiled (s). To gain information about the blood circulation, skin temperature of the distant third part of the ear and a hind limb below the knee was noted as either warmer or colder than the palpating hand. Additionally, a mucosa pressure test was done by pressing the mucosa skin and subsequently quantifying the interval to color normalization. If present, vulva lesions and color of flux from vulva were recorded together with the color of the vaginal mucosa. We noted the presence of abnormal muscle volume on the legs, the number of torn hoofs, damaged hoofs, and coronary band lesions.
To quantify the number of skin lesions, we divided the body of each sow into three parts: front (from snout to shoulder), middle (from shoulder until hind legs), and hind (from hind legs to tail). For each body part, the number of wounds (skin lesions involving at least dermis and larger than 1 cm) and elongated superficial skin lesions (restricted to epidermis and longer than 5 cm) was counted. The latter was right censored if 15 or more superficial skin lesions were counted per body part. For each wound, we noted whether crust, bleeding, redness, swelling, or flux was present. Wounds located in the shoulder area were described by use of a specific protocol (described by Jensen et al., 2011). The shoulder area was restricted to the area of the size of a hand surrounding the Spina scapula, and the number of wounds within this area was counted (criteria for two wounds: crusts visually separable). The type of wound was categorized as either a decubital shoulder ulcer (relatively rounded wound) or other types of wounds. Redness and swelling (including categorization as either soft or hard based on palpation) of the skin area surrounding a shoulder wound were registered. Differences between left and right shoulder, resulting in asymmetrical shoulders, were recorded when the sow was standing normally on all four legs. Redness of the skin close to the wound as well as presence of crust were noted, and we measured the wound diameter (boarder to boarder).
“Part 3” of the clinical examination was conducted when the sows were moved from their home pen to the pick-up facility just before being loaded for transportation, and lameness was scored on a 4-point scale (Karlen et al., 2007; Table 1). According to the European Council Regulation (Council Regulation, EC 1/2005), sows with lameness score 3 cannot be transported.
Table 1.
Description of the lameness scoring scale applied to access lameness in sows
| SCORE | Description |
|---|---|
| 0 | Normal ability to stand and move; symmetrical limb movements |
| 1 | Normal ability to stand and move; legs bearing weight similarly but compromised movement |
| 2 | Moderately lame: obviously reduced ability to stand; movement diminished or difficult; unwillingness to bear weight on affected leg(s); frequent weight shifting |
| 3 (Not fit for transport) | Severely lame: compromised ability to stand and move; one or more nonweight-bearing limbs, often with swollen joints; stiffness; frequent vocalizations if made to move. |
Scale modified from Karlen et al., 2007.
Statistical Analysis
Descriptive statistics were generated by the procedures PROC Freq and PROC Means in SAS Enterprise Guide software version 5.1. (SAS Institute Inc., Cary, NC). The results are presented as means and SE for normally distributed variables and as medians (MED) and interquartile ranges (IQR) for the remaining variables.
For each of the following continuous variables: rectal temperature, respiration rate, heart rate, parity, latency to normalization in the mucosa pressure test, latency to recoil in the skin elasticity test, as well as distance between floor and lowest part of udder, a mixed model was used to test the effect of lactation status (lactating/nonlactating) (PROC MIXED). Herd was included as a random factor in all models. Variance homogeneity and distribution of the residuals were used to evaluate the validity of the statistical model for each variable. Degrees of freedom were calculated using Satterthwaite’s approximation. Results from the mixed models are reported as least squares means (LSMEANS) and SE as well as F (dftreatment, dferror) and the corresponding P values.
For categorical variables, the category describing the healthy or normal condition was set to zero. To examine differences between lactating and nonlactating cull sows, a new binomial (0/1) variable was created for each measure. We analyzed this binomial variable in a generalized linear mixed model (PROC GLIMMIX) with lactation status as dependent and herd treated as a random factor. This was done for the following variables: occurrence of wounds, superficial skin lesions or reddened skin areas summed across the whole body, lesions on udder or vulva, signs of acute udder inflammation, abnormal leg muscle volume, and lameness score. In addition, a new udder variable was created (due to low representation in the data) summing udder abnormality, redness, and signs of chronic udder inflammation. The results are presented as odds ratios and SE.
All statistical analyses were performed with SAS Enterprise Guide software (version 5.1, SAS Institute Inc.). Across all analyses, differences were considered statistically significant if P < 0.05 and considered a tendency if 0.05 ≤ P < 0.1.
RESULTS
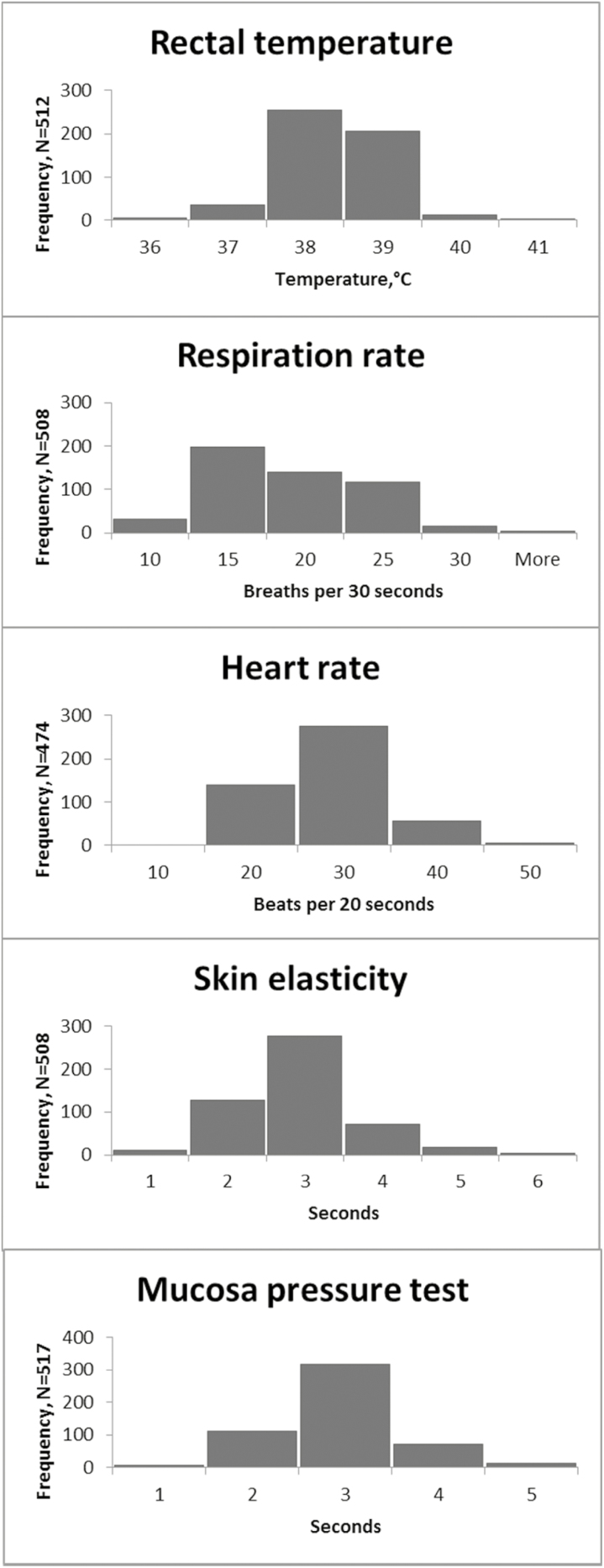
Four sows could not be transported because they were unfit according to the European Council Regulation regarding fitness for transportation (Council Regulation, EC 1/2005) (one sow with a large shoulder ulcer, two severely lame sows [lameness score 3], and one sow with a rectal temperature above 40.5 °C). These sows stayed on-farm, and their clinical examinations were terminated as soon as the criteria for unfit for transportation were met and therefore excluded from the data. We included 522 sows from 12 private sow herds located in Jutland and Funen, Denmark and visited in the period from January 2015 to February 2016. A total of 47 visits were performed with an average of 3.9 visits per herd of which three herds were visited only once. On average, 11.1 sows were examined per visit. Mean herd size consisted of 729 (410–1400) animals, including sows, boars, and gilts. Information on parity was available from 70% of the sows. Within these, parity ranged from 1 to 11 with a median of 5 (IQR = 3). An overview of the main results from the clinical examination is presented in Table 2 and Figure 1.
Table 2.
Results of the on-farm clinical examination of 522 cull sows on the day of transportation to slaughter
| Clinical measure | Percentage of sows, % | |
|---|---|---|
| General condition | Normal | 97.3 |
| Other | 1.0 | |
| NA | 1.7 | |
| Body condition score | ≤2 | 3.4 |
| >2–<4 | 88.8 | |
| 4 | 13.0 | |
| NA | 1.7 | |
| Respiration quality | Normal | 97.9 |
| Forced | 0.6 | |
| Superficial | 0.8 | |
| NA | 0.8 | |
| Head shape | Normal | 99.2 |
| Asymmetry/swellings | 0.4 | |
| NA | 0.4 | |
| Belly shape | Normal | 99.0 |
| Abnormal | 0.2 | |
| NA | 0.8 | |
| Body hair cover | Normal | 92.5 |
| Long | 3.8 | |
| NA | 3.6 | |
| Hoof length | Normal | 92.3 |
| Long | 7.3 | |
| NA | 0.4 | |
| Length of accessory digits | Normal | 92.7 |
| Long | 6.9 | |
| NA | 0.4 | |
| Lameness scorea | 0:Normal | 87.0 |
| 1:Abnormal gait | 9.6 | |
| 2:Lame | 0.8 | |
| NA | 2.7 | |
| Leg muscle volume | Normal | 84.5 |
| Abnormal on one leg | 8.8 | |
| Abnormal on more than one leg | 5.9 | |
| NA | 0.8 | |
| Vulva lesions | None | 92.3 |
| >1 | 7.3 | |
| NA | 0.4 | |
| Vulva smell | No smell | 96.2 |
| Smell | 1.0 | |
| NA | 2.7 | |
| Color of mucosa | Normal | 95.4 |
| Pinkish | 1.7 | |
| Pale | 1.9 | |
| Very red | 0.4 | |
| Bluish | – | |
| NA | 0.6 | |
NA = data not available for this measure.
aScale 0–3; Two sows could not be transported due to lameness score 3 and stayed on-farm. This data set contains only animals sent for slaughter.
Figure 1.
Results of the on-farm clinical examination of cull sows on the day of transportation to slaughter. The number of sows examined for each variable is noted on the y-axis. One sow could not be transported due to rectal temperature above 40.5 °C. This data set contains only animals sent for slaughter.
Body and Limbs
Almost 99% of the sows had normal skin color, and none were categorized as cyanotic based on skin color (Table 2). Only one sow had an umbilical outpouching. Overall, 54.6% of all sows had at least one wound on the body, excluding wounds in the shoulder and udder region, and the number of wounds on the body was not affected by lactation status (N = 522, MED = 1, IQR = 1, P > 0.1). Characteristics of the wounds are shown in Table 3. For 97.5% of the sows, no redness or swelling of the skin was identified (N = 519, range: 0–4 red/swollen areas). Due to the low prevalence, redness was not analyzed further. A total of 73 wounds were found in the shoulder region of 60 sows, and descriptive characteristics of these are presented in Table 3. Seventy-one of the wounds were categorized as decubital shoulder ulcers. A little less than one-third of the sows (30.8%) had at least one superficial skin lesion.
Table 3.
Results of the on-farm clinical examination of 522 cull sows on the day of transportation to slaughter
| Wounds (shoulder excluded) | Percentage of wounds,a % | |
|---|---|---|
| Wound characteristic | Crust | 45.8 |
| Bleeding | 21.4 | |
| Redness | 2.8 | |
| Swollen | 38.7 | |
| NA | 0.9 | |
| Wounds in shoulder region | Percentage of wounds,b % | |
| Type of wound | Decubital ulcer | 97.2 |
| Other | 1.4 | |
| Not determined | 1.4 | |
| Decubital shoulder ulcers | Percentage of wounds,c % | |
| Skin area reddened | No | 76.1 |
| Yes | 19.7 | |
| NA | 4.2 | |
| Swelling in skin area | No | 80.3 |
| Yes, soft | 16.9 | |
| Yes, hard | 0.0 | |
| NA | 2.8 | |
| Asymmetrical shoulder | No | 94.4 |
| Yes | 2.8 | |
| NA | 2.8 | |
| Crust on wound | No | 21.1 |
| Yes | 76.1 | |
| NA | 2.8 | |
| Redness on edge of wound | No | 83.9 |
| Yes | 18.3 | |
| NA | 2.8 | |
| Diameter of woundd | 0–2 cm | 53.5 |
| 2–5 cm | 46.5 | |
| >5 | – | |
| NA | – | |
| Median | 2.0 | |
| IQR | 1.0 | |
| Range | 1–5 cm | |
This table covers the descriptive characteristics for wounds and decubital shoulder ulcers. NA = Data not available for this measure.
aTotal of 533 wounds (excluding wounds in the shoulder area) from 285 sows.
bTotal of 73 wounds located in the shoulder region of 60 sows.
cTotal of 71 decubital shoulder ulcers from 58 sows.
dDiameter of decubital shoulder lesions. One sow could not be transported due to a decubital shoulder ulcer larger than 5 cm in diameter. This data set contains only animals sent for slaughter.
Discharge from vulva is typically seen in the days after farrowing and was found in 12% (N = 62) of all sows with around half of these having transparent fluid (N = 30) and the remaining white or brownish fluid (N = 32). Although expected, there was no effect of lactation status (F1507 = 0.9, P = 0.35) on the occurrence or type of vulva discharge. Findings related to vulva are listed in Table 2.
The examination of the udders revealed that 25.5% of the sows had at least one udder lesion. We recorded acute udder inflammation in 4% of the sows, udder soreness in 1.3%, and 17.4% of the sows had other udder problems (e.g., chronic udder inflammation [2.5%], asymmetrical udder [11.5%], or swollen mammary glands [3.4%]).
Most sows had normal hoof length, but 7.3% had long hoofs (Table 2). Furthermore, three sows had had at least one hoof torn off or damaged, and one sow had a wound on the coronary band. Of the 293 sows that were lying at the beginning of the clinical examination, 3% were not able to get up in a normal way, but all sows were able to stand on their own. Lameness scores ranged from 0 to 2 on the 4-point scale (Table 2).
Effect of Lactation Status
Almost 4 out of 10 (39%) sows were lactating at the time of the clinical examination. At culling, the lactating sows had a higher parity than the nonlactating sows (LSM: 5.5 ± 0.3 vs. 4.6 ± 0.3 for lactating and nonlactating, respectively; F1,349 = 12.0, P = 0.0006). To evaluate the possible differences between lactating and nonlactating cull sows regarding a range of categorical variables, we calculated the odds of deviating from the clinical healthy category. The results show that nonlactating cull sows had 3.5 times more superficial skin lesions (MEDlactating = 0, IQRlactating = 0; MEDnonlactating = 0, IQRnonlactating = 3; F1,507 = 24.8, P < 0.0001) but 0.13 times fewer udder lesions (MEDlactating = 0.5, IQRlactating = 2; MEDnonlactating = 0, IQRnonlactating = 0; F1,499 = 63.6, P < 0.0001) compared with lactating sows. Furthermore, nonlactating sows were 2.3 times more likely to have a lameness score different from 0 compared with lactating sows (N = 508, F1,495 = 5.2, P = 0.02). The rectal temperature (37.7 °C [range: 33.2–40.4] vs. 38.0 °C [range: 36.6–40.1], F1,484 = 6.7, P = 0.01) and respiration frequency (16 vs. 17 breaths per 30 s, F1,505 = 4.6, P = 0.03) were significantly lower for lactating compared and nonlactating sows.
Likewise, the odds for udder swellings, asymmetrical udder, and chronical udder inflammation were 0.19 for nonlactating compared with lactating sows (F1,506 = 27.9, P < 0.0001). The distance from the floor to the lowest part of the udder was found to be 19.7 cm in lactating sows and was higher (24.3 cm) for nonlactating (F1,475 = 99.9, P < 0.0001).
DISCUSSION
The present study is among the first to examine the clinical condition of cull sows from commercial herds before mixing and transportation to slaughter and included data from 522 sows collected over a year from 12 private Danish herds. The parity of the sows ranged from 1 to 11, and almost 40% of the cull sows came directly from the farrowing unit and were lactating on the day of transportation. Four sows were unfit for transportation, according to the European Council Regulation regarding fitness for transportation (Council Regulation, EC 1/2005), and were not included in this data set. Among the remaining sows, 10% showed signs of changed gait, and 0.9% were lame. We observed wounds in 54.6%, decubital shoulder ulcers in 11%, superficial skin lesions in 30.8%, and vulva lesions in 7% of the sows. The observed occurrence of lesions calls for further investigation of the welfare of sows in the last part of their life, which until now has received almost no scientific attention.
To the best of our knowledge, no studies have described the clinical condition of cull sows while still on-farm. Due to the study design of the present data set, this paper does not provide a representative picture of the general condition of cull sows in Denmark. However, some inferences can be drawn based on the random selection of the farms and the sample size of more than 500 animals from different herds destined for 47 transportations to slaughter. Until now, the clinical condition of sows has been described during production, like in studies of gestating sows (Chapinal et al., 2010; Nalon et al., 2013), or upon arrival at a slaughter plant (Cleveland-Nielsen et al., 2004; Knauer et al., 2007; de Jong et al., 2014; McGee et al., 2016). When comparing our findings with such studies, it seems that the parity range of the present data set (1–11, with a median of 5) corresponds to other studies of cull sows (de Jong et al., 2014; Zhao et al., 2015). Despite this similarity, and the knowledge from the present study, we need future epidemiological studies in order to fully determine the prevalence of the different clinical conditions among cull sows while they are still on-farm.
In the present data set, decubital shoulder ulcers were observed in 11% of the sows. To date, no studies have provided information about the presence of decubital shoulder ulcers in cull sows before transportation to slaughter but only described the condition in studies focusing on lactating sows (Bonde, 2008; Ivarsson et al., 2009; Kilbride et al., 2009). Decubital shoulder ulcers have been associated with behavioral changes (Larsen et al., 2015) and are suggested to be painful (Herskin et al., 2011; Dahl-Pedersen et al., 2013). The consequences of pretransportation conditions/activities and actual transportation for sows with decubital shoulder ulcers are not studied. Mixing, fighting, and standing in the moving truck are likely to result in worsening of the ulcers, as the ulcers might be torn open or to be perceived more painful due to mechanical pressure/“bumping into inventory.” This means that even though the ulcers observed in the present study would not, according to the present legal practice, render the animals unfit for transportation due to the size of the ulcers (Council Regulation, EC 1/2005), the presence of ulcers and perhaps even scars may have adverse consequences for the sows in the period from the decision to cull until slaughter. The relatively high percentage of sows with decubital shoulder ulcers in the present data set shows that not only the period from the clinical examination until slaughter but also the weeks before the clinical examination, where the ulcers are developing (as reviewed by Herskin et al., 2011), need increased focus in terms of prevention of ulcer development.
Cull sows have been suggested to be more vulnerable to transportation stress than other groups of swine (Nielsen et al., 2011; Grandin, 2016) based on increased mortality upon arrival at a slaughter plant (Lykke et al., 2007; Malena et al., 2007; Peterson et al., 2017). This is supported by more specific characteristics, such as increased occurrence of fatigue and lameness or very low BCS among cull sows compared with swine of market weight (Lykke et al., 2007; McGee et al., 2016), and the fact that a considerable proportion of the cull animals are lactating at the time of transportation (OIE, 2016). As cull sows and market weight pigs differ in a range parameters (e.g., weight, maturity, health), it may not be unexpected that no direct comparisons are available, but the clinical characteristics of the two groups may differ considerably (as exemplified by, i.e., the occurrence of decubital shoulder ulcers and udder lesions in the sows and a reported relatively high prevalence of tail injury in market weight pigs; Harley et al., 2012).
Further studies, involving the collection of data from the same animals before and after transportation, are needed to clarify the vulnerability of cull sows toward transportation, potentially including comparison with other groups of swine that are transported (such as roaster pigs (Peterson et al., 2017), heavy slaughter pigs (Martelli et al., 2005), or weaners (Sutherland et al., 2009)). Such knowledge is highly relevant, as the European legislation regarding fitness for transportation is not specified for certain types of animals. In addition, such studies would provide knowledge about the involved risk factors of a potential worsening of the clinical condition of slightly injured sows (as would probably be considered the case for the sows with lesions from the present data set) and hence knowledge about the risk of violating EU legislation on animal fitness for transportation (Council Regulation, EC 1/2005).
Even though almost half of the sow population is culled yearly as part of modern sow production (Lucia et al., 2000a and 2000b; Rodriguez-Zas et al., 2003), the management and welfare of this group of animals have received very limited scientific attention. At present, very little is known about the duration of the interval from the decision to cull a sow is taken until she is either slaughtered or euthanized, and no studies have examined typical housing of sows during this period, lasting hours to weeks, or examined how sows should be managed in order to maximize animal welfare and carcass quality. The present results show that even though only very few sows were in a condition leaving them unfit for transportation according to European legislation (Council Regulation, EC 1/2005), a considerable proportion of the sows had shoulder and vulva injuries. This suggests that increased attention directed toward the health and welfare of these animals in their last days or weeks of life is needed in order to optimize the quality of the carcasses and avoid foregone revenue (as discussed by Peterson et al., 2017). We suggest a more clearly delimitation of the period in the sow’s life—The Cull Period—from when the decision is made by the farmer that she should be culled, regardless of the reason, until the sow is dead.
The clinical condition of sows in The Cull Period is highly important and may be subjective to rapid changes due to the different links in the preslaughter chain in the days and hours before being picked up by a commercial truck transporting sows to slaughter. Recently, Herskin et al. (2017) studied behavior of cull sows while kept—for biosecurity reasons—in transfer vehicles before being picked up by a commercial truck. By use of data from 106 of the sows from the present data set, Herskin et al. (2017) suggested that the use of such vehicles can be associated with limited rest for the animals and a high level of aggression. Engaging in aggressive behavior will entail a greater risk of skin lesions for sows compared with market weight pigs, as the risk of lesions increases with increasing BW (Turner et al., 2006).
Future studies should focus on different aspects of the life of cull sows in the interval from the culling decision is made until the animals have died. Only this way, we can establish knowledge and a basis for evidence-based decisions aiming to optimize the welfare of the animals in their last days or weeks as well as to optimize the meat quality and value of the end product.
In the present data, almost 4 out of 10 sows were lactating on the day of transportation and came directly from the farrowing barn. Based on results from de Hollander et al. (2015) and Engblom et al. (2007), this was not unexpected, but the consequences in terms of clinical differences and potential differences in vulnerability toward transportation stress have not been examined before. The present results show a number of differences between the lactating and nonlactating cull animals, such as fewer superficial skin lesions in the lactating sows, signs of increased rectal temperature, and respiration as well as larger udder size and increased occurrence of udder swellings and udder inflammation. In sows, discomfort related to the abrupt cessation of lactation taking place at weaning has received very limited scientific attention, as compared with, for example, dairy cows where milk leakage and reduced lying time have been reported after abrupt cessation of milking (e.g., Leitner et al., 2007; Zobel et al., 2013). For cull sows, which are typically mixed with other sows and transported to slaughter either on the day of weaning or shortly after, the challenge from the abrupt cessation of lactation might be even larger than for sows that are moved to another part of the barn to continue production. In their recommendations regarding transportation of livestock, OIE specifies the need of lactating females for special protection before as well as during transportation (OIE, 2016). Future studies should focus on management of newly weaned sows in order to examine whether these animals can sustain the challenges associated with mixing and transportation this early after cessation of lactation.
The lactating sows in our study had a higher body temperature compared with the nonlactating sows, which could suggest that they were at a greater risk of hyperthermia during a subsequent transportation. Modern lactating sows are, due to the large litter sizes and increased genetic potential for milk production, more sensitive toward heat stress than sows just a few decades ago (Brown-Brandl et al., 2014) and especially just before commercial weaning (approximately 4 weeks after farrowing) when milk production is peaking (Williams et al., 2013). Examination of heat production of sows has shown a steady increase from farrowing until weaning (Brown-Brandl et al., 2014). Recently, this knowledge has led to increased focus on the susceptibility of lactating sows toward heat stress (e.g., Rosero et al., 2012; Jeon and Kim, 2014; Cabezon et al., 2017). However, despite the large proportion of lactating animals, no studies have focused on the susceptibility of cull sows toward heat stress. Hyperthermia has been suggested to be among the main reasons for cull sow mortality before arrival to a slaughter plant (or immediately upon arrival) (Peterson et al., 2017), emphasizing that possible links between sow fitness for transportation, lactation, and environmental temperature need further attention.
We suggest that future research should focus on whether cull sows, taken almost directly from the farrowing barn into the trucks transporting them to slaughter, are able to cope with the different links in the preslaughter logistic chain and whether they may benefit in terms of animal welfare or carcass quality from being rested for some days on-farm before transportation to slaughter.
CONCLUSION
The present study examined the clinical condition of cull sows from commercial herds before mixing and transportation to slaughter and included data from 522 sows. The present findings warrant for further studies exploring different aspects of the life of cull sows in the interval from the culling decision is made until the animals have died, which we here denote The Cull Period. This in order to establish a knowledge base for evidence-based decisions to optimize the welfare of sows in their last days or weeks as well as to optimize the meat quality and value of the end product.
LITERATURE CITED
- Anonymous 2014. Law no. 520, 26/05/2010; Ministerial order number 470 of 15/5/2014 https://www.retsinformation.dk/pdfPrint.aspx?id=162875.
- Bonde M. Prevalence of decubital shoulder lesions in Danish sow herds (In Danish). Aarhus, Denmark: University of Aarhus, Faculty of Agricultural Sciences; 2008. Internal Report 12; p. 8. [Google Scholar]
- Brown-Brandl Tami M., Hayes Morgan D., Xin Hongwei, Nienaber John A., Li Hong, Eigenberg Roger A., Stinn John P., and Shepherd Timothy. 2014. Heat and moisture production of modern swine. ASHRAE Transactions 120: 469. [Google Scholar]
- Cabezon F. A., Schinckel A. P., Smith A. J., Marchant-Forde J. N., Johnson J. S., and Stwalley R. M.. 2017. Effect of floor cooling on lactating sows under acute heat stress. Livest. Sci. 206:113–120. doi:10.1016/j.livsci.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapinal N., Ruiz de la Torre J. L., Cerisuelo A., Gasa J., Baucells M. D., Coma J., Vidal A., and Manteca X.. 2010. Evaluation of welfare and productivity in pregnant sows kept in stalls or in 2 different group housing systems. J. Vet. Behav. 5:82–93. doi:10.1016/j.jveb.2009.09.046 [DOI] [PubMed] [Google Scholar]
- Cleveland-Nielsen A., Christensen G., and Ersbøll A. K.. 2004. Prevalences of welfare-related lesions at post-mortem meat-inspection in Danish sows. Prev. Vet. Med. 64:123–131. doi:10.1016/j.prevetmed.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Council Regulation.. (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97 (OJ L 3, 5.1.2005, pp. 1–44). [Google Scholar]
- Dahl-Pedersen K., Bonde M. K., Herskin M. S., Jensen K. H., Kaiser M., and Jensen H. E.. 2013. Pathogenesis and pathology of shoulder ulcerations in sows with special reference to peripheral nerves and behavioural responses to palpation. Vet. J. 198:666–671. doi:10.1016/j.tvjl.2013.09.059 [DOI] [PubMed] [Google Scholar]
- De Jong E., Appeltant R., Cools A., Beek J., Boyen F., Chiers K., and Maes D.. 2014. Slaughterhouse examination of culled sows in commercial pig herds. Livest. Sci. 67:362–369. doi:10.1016/j.livsci.2014.07.001 [Google Scholar]
- Engblom L., Lundeheim N., Dalin A. M., and Andersson K.. 2007. Sow removal in Swedish commercial herds. Livest. Sci. 106:76–86. doi:10.1016/j.livsci.2006.07.002 [Google Scholar]
- Grandin T. 2016. Transport fitness of cull sows and boars: a comparison of different guidelines on fitness for transport. Animals 6:77. doi:10.3390/ani6120077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley S., More S. J., O’Connell N. E., Hanlon A., Teixeira D., and Boyle L.. 2012. Evaluating the prevalence of tail biting and carcase condemnations in slaughter pigs in the Republic and Northern Ireland, and the potential of abattoir meat inspection as a welfare surveillance tool. Vet. Rec. 171:621. doi:10.1136/vr.100986 [DOI] [PubMed] [Google Scholar]
- Herskin M. S., Bonde M. K., Jørgensen E., and Jensen K. H.. 2011. Decubital shoulder ulcers in sows: a review of classification, pain and welfare consequences. Animal 5:757–766. doi:10.1017/S175173111000203X [DOI] [PubMed] [Google Scholar]
- Herskin S. M., Fogsgaard K. K., Erichsen D., Bonnichsen M., Gaillard C., and Thodberg K.. 2017. Housing of cull sows in the hours before transport to the abattoir-an initial description of sow behaviour while waiting in a transfer vehicle. Animals 7:1. doi:10.3390/ani7010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hollander C. A., Knol E. F., Heuven H. C. M., and van Grevenhof E. M.. 2015. Interval from last insemination to culling: II. Culling reasons from practise and the correlation with longevity. Livest. Sci. 181:25–30. doi:10.1016/j.livsci.2015.09.018 [Google Scholar]
- Ivarsson E., Mattson B., Lundeheim N., and Holmgren N.. Decubital shoulder ulcers—prevalence and risk factors (In Swedish). 2009. Svenska Pig, Report 42, p. 8. [Google Scholar]
- Jensen H. E., Bonde M. K., Bådsgaard N. P., Dahl-Pedersen K., Andersen P. H., Herskin M. S., Jørgensen E., Kaiser M., Lindahl J., Nielsen J. P.,. et al. 2011. En enkel og valideret skala for klinisk vurdering af skuldersår. Dansk Veterinærtidsskrift. 94:6–12. [Google Scholar]
- Jeon J. H., and Kim D. H.. 2014. Methods to supply chilled drinking water for lactating sows during high ambient temperatures. Ital. J. Anim. Sci. 13:3431. doi:10.4081/ijas.2014.3431 [Google Scholar]
- Karlen G. A. M., Hemsworth P. H., Gonyou H. W., Fabrega E., Strom A. D., and Smits R. J.. 2007. The welfare of gestating sows in conventional stalls and large groups on deep litter. Appl. Anim. Behav. Sci. 105: 87–101. doi:10.1016/j.applanim.2006.05.014 [Google Scholar]
- KilBride A. L., Gillman C. E., Ossent P., and Green L. E.. 2009. A cross sectional study of prevalence, risk factors, population attributable fractions and pathology for foot and limb lesions in preweaning piglets on commercial farms in England. BMC Vet. Res. 5:31. doi:10.1186/1746-6148-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer M., Stalder K. J., Karriker L., Baas T. J., Johnson C., Serenius T., Layman L., and McKean J. D.. 2007. A descriptive survey of lesions from cull sows harvested at two Midwestern U. S. facilities. Pre. Vet. Med. 82: 198–212. doi: 10.1016/j.prevetmed.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Lambooij E. B. 2014. Transport of pigs. In: Grandin T, editor. Livestock handling and transport. Oxfordshire (UK): CAB International; p. 280–298. [Google Scholar]
- Larsen T., Kaiser M., and Herskin M. S.. 2015. Does the presence of shoulder ulcers affect the behaviour of sows?Res. Vet. Sci. 98:19–24. doi:10.1016/j.rvsc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Leitner G., Jacoby S., Maltz E., and Silanikove N.. 2007. Casein hydrolyzate intramammary treatment improves the comfort behavior of cows induced into dry-off. Livest. Sci. 110:292–297. doi:10.1016/j.livsci.2007.02.016 [Google Scholar]
- Lucia J., Dial G. D., and Marsh W. E.. 2000a. Lifetime reproductive performance in female pigs having distinct reasons for removal. Livest. Prod. Sci. 63:213–222. doi:10.1016/S0301-6226(99)00142-6 [Google Scholar]
- Lucia T. Jr, Dial G. D., and Marsh W. E.. 2000b. Lifetime reproductive and financial performance of female swine. J. Am. Vet. Med. Assoc. 216:1802–1809. doi: 10.2460/javma.2000.216.1802 [DOI] [PubMed] [Google Scholar]
- Lykke L., Blaabjerg L., and Hartung J.. Investigation of pig transports for more than 8 hours in cold and warm weather conditions and of the requirements for ventilation during the transport. 2007. Taastrup, Denmark: Report from Danish Meat Research Institute, p. 81 [accessed April 2017]. www.teknologisk.dk. [Google Scholar]
- Malene M., Voslárovaa E., Kozák A., Belobrádek P., Bedánová I., Steinhauser L., and Vecerek V.. 2007. Comparison of mortality rates in different categories of pigs and cattle during transport for slaughter. Acta Vet. Brno. 76: S109–S116. doi: 10.2754/avb200776S8S109 [DOI] [Google Scholar]
- Martelli G., Sardi L., Parisini P., Badiani A., Parazza P., and Mordenti A.. 2005. The effects of a dietary supplement of biotin on Italian heavy pigs’ (160 kg) growth, slaughtering parameters, meat quality and the sensory properties of cured hams. Livest. Prod. Sci. 93:117–124. doi:10.1016/j.livprodsci.2004.09.006 [Google Scholar]
- McGee M., Johnson A. K., O’ Connor A. M., Tapper K. R., and Millman S. T.. 2016. An assessment of swine marketed through buying stations and development of fitness for transport guidelines. J. Anim. Sci. 94:9. doi:10.2527/msasas2016-019 [Google Scholar]
- Nalon E., Conte S., Maes D., Tuyttens F. A. M., and Devillers N.. 2013. Assessment of lameness and claw lesions in sows. Livest. Sci. 156:10–23. doi:10.1016/j.livsci.2013.06.003 [Google Scholar]
- OIE World Organization for Animal Health 2016. Terrestrial animal health code [accessed December 12, 2017]. http://www.oie.int/international-standard-setting/terrestrial-code/access-online/.
- Peterson E., Zemmenga M., Hagerman A., and Akkina J.. 2017. Use of temperature, humidity and slaughter condemnation data to predict increases in transport losses in three classes of swine and resulting foregone revenue. Front. Vet. Sci. 4:67 doi:10.3389/fvets.2017.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Zas S. L., Southey B. R., Knox R. V., Connor J. F., Lowe J. F., and Roskamp B. J.. 2003. Bioeconomic evaluation of sow longevity and profitability. J. Anim. Sci. 81:2915–2922. doi:10.2527/2003.81122915x [DOI] [PubMed] [Google Scholar]
- Rosero D. S., van Heugten E., Odle J., Arellano C., and Boyd R. D.. 2012. Response of the modern lactating sow and progeny to source and level of supplemental dietary fat during high ambient temperatures. J. Anim. Sci. 90:2609–2619. doi:10.2527/jas.2012-4242 [DOI] [PubMed] [Google Scholar]
- Statistics Denmark (The code following StatBank/dk indicates the specific table from which the figures are extracted). https://www.statistikbanken.dk/ANI9.
- Sutherland M. A., Krebs N., Smith J. S., Dailey J. W., Carroll J. A., and McGlone J. J.. 2009. The effect of three space allowances on the physiology and behavior of weaned pigs during transportation. Livest. Sci. 126:183–188. doi:10.1016/j.livsci.2009.06.021 [Google Scholar]
- Thodberg K., Fogsgaard K. K., Erichsen D., Bonnichsen M., and Putzer A. H. M. S.. 2017. Proceedings of the are sows sent for slaughter fit for transport?HSA International Symposium 2015; July 16 to 17;Zagreb, Croatia. [Google Scholar]
- Turner P. T., Farnworth M. J., White I. M. S., Brotherstone S., Mendl M., Knap P., Penny P., and Lawrence A. B.. 2006. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Appl. Anim. Behav. Sci. 96:245–259. doi:10.1016/j.applanim. 2005.06.009 [Google Scholar]
- Williams A. M., Safranski T. J., Spiers D. E., Eichen P. A., Coate E. A., and Lucy M. C.. 2013. Effects of a controlled heat stress during late gestation, lactation, and after weaning on thermoregulation, metabolism, and reproduction of primiparous sows. J. Anim. Sci. 91:2700–2714. doi:10.2527/jas.2012-6055 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liu X., Mo D., Chen Q., and Chen Y.. 2015. Analysis of reasons for sow culling and seasonal effects on reproductive disorders in Southern China. Anim. Reprod. Sci. 159:191–197. doi:10.1016/j.anireprosci.2015.06.018 [DOI] [PubMed] [Google Scholar]
- Zobel G., Leslie K., Weary D. M., and von Keyserlingk M. A.. 2013. Gradual cessation of milking reduces milk leakage and motivation to be milked in dairy cows at dry-off. J. Dairy Sci. 96:5064–5071. doi:10.3168/jds.2012-6501 [DOI] [PubMed] [Google Scholar]