Abstract:
This study determined whether feeding the immunomodulating supplement, OmniGen-AF, to feedlot heifers would alter metabolic profiles to a glucose tolerance test. Heifer calves (n = 32; 217 ± 2 kg) were allocated into two treatment diets: 1) Control, fed a standard receiving ration, and 2) OmniGen, fed the Control diet supplemented with OmniGen at 4.54 g/45 kg BW/d. Heifers were fed for 42 d. On d 42, Heifers were processed through a working facility for placement of indwelling jugular catheters. After these procedures, heifers were moved into individual stanchions in an enclosed barn and all heifers were fed their treatment diets at 1400 h. All orts were removed at 2000 h to allow for a 12-h fast prior to first blood collection. The following day, heifers were administered 0.5 mL/kg BW of a 50% dextrose solution at 0900 h (0 min). Blood samples were collected for serum isolation at −60, −45, −30, −15, 0, 10, 20, 30, 45, 60, 90, 120, and 150 min relative to bolus dextrose infusion. Serum was stored at −80 oC until analyzed for cortisol, glucose, insulin, non-esterified fatty acid (NEFA) and urea N concentrations. There was a treatment × time interaction for post-challenge cortisol (P = 0.004) such that cortisol was greater in OmniGen heifers than Control heifers from 10- to 45- min post-infusion. Glucose concentrations increased post-infusion (P < 0.01) and were reduced in OmniGen compared to Control heifers at 10-, 45-, and 90-min after challenge (treatment × time P < 0.001). Similarly, there was a treatment × time interaction for post-challenge insulin concentrations (P = 0.04) such that insulin was greater in OmniGen-fed heifers than Control heifers from 10 to 30 min. In addition, there was a treatment × time interaction (P = 0.01) for NEFA concentrations such that concentrations were reduced in OmniGen-supplemented heifers from 10 to 30 min following administration of the dextrose bolus. Serum urea N concentrations were greater in Control heifers at 150 min compared to OmniGen-fed heifers (post-challenge treatment × time interaction: P < 0.001). These data suggest that OmniGen-fed heifers were more responsive to changes in glucose, perhaps affecting the storage and/or redistribution of energy deposits and provide further evidence for altered metabolism in OmniGen-supplemented cattle. The differences observed may explain differences observed in the immune response in OmniGen-supplemented calves.
Keywords: cattle, glucose, glucose tolerance, insulin, non-esterified fatty acids, OmniGen-AF
INTRODUCTION
The use and study of feed additives is growing exponentially. Producers are being encouraged to find alternatives to long-standing management resources in order to address concerns and demands of consumers. Specifically, change is being driven by consumers wanting more “natural” products as well as by increased regulations like the Veterinary Feed Directive. There are a plethora of feed additives available to producers, ranging from phytochemicals, vitamin/mineral blends, and probiotics, several of which boast the ability to improve health, feed efficiency, and growth, among other claims (Broadway et al., 2014). Research is necessary in order to provide producers information on how these products work, and which products may be the best to introduce into their management systems based on their needs.
Research in our laboratory has studied several commercially available products that have the potential to influence animal health (Burdick Sanchez et al., 2013, 2018; Broadway et al., 2015). Previous research using the OmniGen-AF product demonstrated changes in aspects of the acute phase response to a lipopolysaccharide (LPS) challenge (Buntyn et al., 2016). However, the immune system does not perform its actions without the help of energy, in the form of metabolites such as glucose, fatty acids, and protein. There has been a surge in recent literature associated with the study of interactions between immunity and metabolism, and the role of energy availability on immune responsiveness (Huntley et al., 2017; Kvidera et al., 2017). Specifically, the aforementioned studies have focused on the amount of energy and/or glucose needed by the body to mount a response to an LPS challenge. In the study by Buntyn et al. (2016), significant differences were observed in the metabolic response of cattle supplemented with OmniGen prior to an LPS challenge, such that OmniGen-fed heifers had greater glucose concentrations post-challenge yet reduced NEFA and urea nitrogen (SUN) concentrations. On the basis of these data, we hypothesized that OmniGen alters metabolic regulation, which may drive the differences observed in the acute phase response. Therefore, the current study was designed to determine whether feeding OmniGen to feedlot heifers would alter the metabolic profiles to a glucose tolerance test.
MATERIALS AND METHODS
All experimental procedures were in compliance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committee of the Livestock Issues Research Unit (Protocol #2014-01-JAC16).
Experimental Design
Angus-cross heifer calves (n = 32; 217 ± 2 kg BW; approximately 5 to 8 mo of age) were acquired from an order buying facility in west-central Texas and received by a commercial feedlot in the Texas panhandle. Two days after arrival at the feedlot, heifers were processed using the normal processing procedure of the feedlot which included vaccination (Pyramid 3 and Pyramid IBR, Boehringer Ingelheim, St. Joseph, MO; Covexin 8, Merck Animal Health, Millboro, DE; and Polybac, Texas Vet Labs, Inc., San Angelo, TX), deworming (Long Range, Merial and Safeguard drench, Merck Animal Health), metaphylaxis (Draxxin, Zoetis, Parsippany, NJ), individual identification, and individual BW collection. Heifers were then allotted randomly to two treatment diets (3 pens per treatment) for 42 d: 1) Control, fed a standard receiving ration (Table 1) OmniGen-AF (OmniGen), fed the Control diet supplemented with OmniGen-AF at 4.54 g/45 kg BW/d mixed into the total mixed ration (TMR) (OmniGen-AF, Phibro Animal Health Corporation, Teaneck, NJ). OmniGen-AF is an immunomodulating feed additive that contains a mixture of silicon dioxide, calcium aluminosilicate, sodium aluminosilicate, brewers dehydrated yeast, mineral oil, calcium carbonate, rice hulls, niacin supplement, biotin, dicalcium pantothenate, vitamin B-12 supplement, choline chloride, thiamine mononitrate, pyridoxine hydrochloride, riboflavin-5-phosphate and folic acid, but full formulation is proprietary. Heifers were fed a diet containing decreasing amounts of hay and increasing amounts of a TMR for the first 8 d, followed by increasing amounts of the TMR using slick bunk management to ensure no refusals. The ration in Table 1 indicates the final TMR that was fed from d 8 through the end of the study. Calves were weighed on d 2 following arrival at the feedlot, after 16 d on feed, and after 42 d on feed on a full basis. The heifers had no health issues during the feeding period. On d 42, heifers were transported approximately 130 km (1.5 h) to the USDA-ARS Livestock Issues Research Unit’s Bovine Immunology Research and Development Complex in New Deal, TX. Upon arrival, heifers were placed by treatment in outdoor pens (7.6 × 18.3 m) with access to treatment diets and water ad libitum. The heifers were then processed through an indoor working facility where they were weighed, and each fitted with an indwelling jugular catheter. The heifers were then moved into individual stanchions (2.28 m in length, 0.76 m in width, 1.67 m in height) in an enclosed, environmentally controlled barn for the remainder of the study. Following processing, all heifers received the remainder of their daily allotment of feed at 1400 h. At 2000 h, all remaining orts were removed to allow for a 12-h fast prior to the first blood collection. No feed was offered to the heifers until the completion of the glucose tolerance test the following morning.
Table 1.
Ration ingredients included in the diet of heifer calves fed for 42 d prior to a glucose tolerance test
| Ingredient | % Dry matter |
|---|---|
| Steam-flaked corn | 30 |
| Wet distiller’s grains with solubles | 27 |
| Sorghum-sudan silage | 19 |
| Wheat hay | 20 |
| Supplement1 | 4 |
1Supplement contained (on as-fed basis): 23.8% crude protein, 17.5% Ca, 0.32% P, 0.26% Mg, 0.48% K, 5.75% salt, 10 mg/kg Co, 500 mg/kg Cu, 25 mg/kg I, 2,000 mg/kg Mn, 7.5 mg/kg Se, 112 IU vitamin A/g, 496 IU vitamin E/kg, and 750 g/ton lasalocid.
On the following day at 0900 h (0 min), a glucose tolerance test was conducted on the heifers by administration of an intravenous bolus dose of dextrose (0.5 mL/kg BW 50% dextrose solution) (Burdick Sanchez et al., 2016). Whole blood samples were collected into Sarstedt tubes containing no additive (Sarstedt, Inc., Newton, NC) at −60 (0800 h), −45, −30, −15, 0, 10, 20, 30, 45, 60, 90, 120, and 150 min (1130 h) relative to the dextrose infusion. For blood sample collection, a 3-mL waste sample was collected from the catheter extension (approximately 1.5 m in length, approximately 2 mL in volume), followed by collection of the blood sample (9 mL). Next, 10 mL of sterile saline was administered to replace fluid loss, and 2.5 mL of heparinized saline (10 USP/mL) was administered to maintain catheter patency. The entire sample collection procedure was completed within 2–5 min. The dextrose bolus was administered immediately after collection of the 0 min sample. Whole blood samples were allowed to clot at room temperature for 30 min and were then centrifuged at 1,500 × g for 20 min at 4 oC. Isolated serum was stored at −80 oC until analyzed for serum concentrations of cortisol, glucose, insulin, NEFA, and SUN.
Serum Analysis
All serum analyses were performed in duplicate. Cortisol concentrations were determined using a commercially available enzyme immunoassay kit according to the manufacturer’s directions (Arbor Assays, Ann Arbor, MI) by comparison of unknowns to standard curves generated with known concentrations of cortisol. Intra- and inter-assay coefficients of variation were less than 8.5% and 5.5%, respectively.
Glucose concentrations were determined by modification of the enzymatic Autokit Glucose (Wako Diagnostics, Richmond, VA) to fit a 96-well format as previously described (Burdick Sanchez et al., 2014). Briefly, 300 µL of prepared working solution was added to 2 µL of serum or prepared standards in a 96-well plate. Plates were incubated at 37 oC for 5 min and absorption was recorded at 505 nm. The plate reader used for this assay (BioTek Powerwave 340; BioTek Instruments, Winooski, VT) has an incubating and timing feature and therefore ensured that the sample absorbances were read immediately following the 5-min incubation. Concentrations of glucose were determined by comparing unknown samples to a standard curve of known glucose concentrations. The intra- and inter-assay coefficients of variation were less than 13.9% and 12.5%, respectively. Glucose clearance rates (k) were calculated from glucose concentrations measured between 10 (t1) and 30 min (t2), and between 30 (t1) and 120 min (t2) following dextrose infusion as previously described (Bernhard et al., 2012). Insulin concentrations were determined by a bovine-specific insulin ELISA according to the manufacturer’s instructions (Cat # 80-INSBO-E01; Alpco Diagnostics, Salem, NH). The intra- and inter-assay coefficients of variation were less than 7.1% and 9.0%, respectively.
Concentrations of NEFA were determined by modification of the enzymatic HR Series NEFA-HR (2) assay (Wako Diagnostics) to fit a 96-well format as previously described (Burdick Sanchez et al., 2014). Briefly, 200 µL of the prepared Color Reagent A were added to 5 µL of serum or prepared standards in a 96-well plate. Plates were incubated at 37 oC for 5 min and then the absorbance was read using a plate reader at 550 nm. Next, 100 µL of prepared Color Reagent B was added to all wells on the 96-well plate. Plates were incubated for an additional 5 min and read for a second time using a plate reader at 550 nm. The plate reader used for this assay has an incubating timing feature and therefore ensured that the sample absorbances were read immediately following the 5-min incubation. A final absorbance was obtained by subtracting the first reading, which was multiplied by a factor of 0.67 to account for changes in volume, from the second reading. The final absorbance values were used for all calculations (i.e., standard curve, sample concentrations). Concentrations of NEFAs were determined by comparing unknown samples to a standard curve of known NEFA concentrations. The intra- and inter-assay coefficients of variation were less than 13.8% and 14.0%, respectively.
Concentrations of SUN were determined by a colorimetric assay according to the manufacturer’s directions (K024-H1; Arbor Assays) by comparison of unknowns to standard curves generated with known concentrations of urea nitrogen. The intra- and inter-assay coefficients of variation were less than 6.3% and 19.4%, respectively.
Statistical Analysis
Data were analyzed as repeated measure over time with the use of the MIXED procedure of SAS (v. 9.4; SAS Institute, Inc., Cary, NC). Treatment, time, and the treatment × time interaction were included as fixed effects with heifer within treatment included as the experimental unit. Autoregressive 1 covariance structure was used based on having the lowest AICC fit statistic value. When main effects were significant, means were separated using the PDIFF option in SAS, with P ≤ 0.05 considered significant and trends toward significance were considered at 0.05 ≤ P ≤ 0.10. All data are presented as the LSM ± SEM.
RESULTS
Body weights during the 42-d feeding period increased over time (P < 0.01). Although there was no treatment effect (P = 0.12), there was a tendency (P = 0.06) for a treatment × time interaction. There was no difference in body weight upon arrival (216 and 218 ± 3 kg for Control and OmniGen, respectively; P = 0.74) or on d 16 (240 and 244 ± 3 kg for Control and OmniGen, respectively; P = 0.35), final body weights on d 42 were different between the two treatments (266 and 277 ± 3 kg for Control and OmniGen, respectively; P = 0.01).
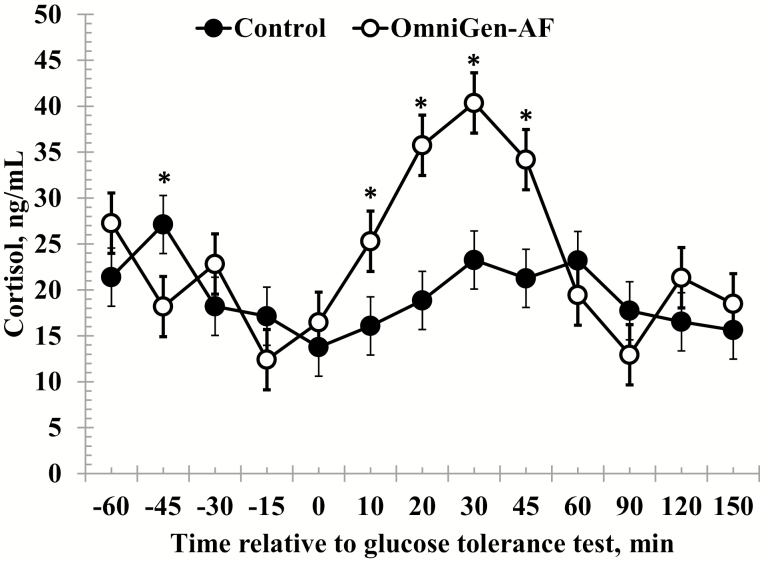
Pre- and post-challenge average values are summarized in Table 2. Prior to administration of the challenge, there was a treatment × time interaction (P < 0.01) for serum cortisol concentrations, such that cortisol was reduced in OmniGen-fed heifers compared to Control heifers at −45 min (Fig. 1). Following administration of the dextrose bolus there was a treatment × time interaction (P < 0.01), where cortisol concentrations were greater in OmniGen fed heifers from 10- to 45-min post-challenge compared to Control heifers.
Table 2.
Serum cortisol, glucose, insulin, non-esterified fatty acid (NEFA), and serum urea nitrogen (SUN) concentrations measured in beef heifers fed a control (Control) diet or supplemented with OmniGen-AF at 4.54 g/45 kg BW/d for 42 d prior to a glucose tolerance test (0.5 mL/kg BW of a 50% dextrose solution)
| Variable | Control | OmniGen | SEM | TRT1 | Time | Interaction2 |
|---|---|---|---|---|---|---|
| Pre-challenge | ||||||
| Cortisol, ng/mL | 19.53 | 19.43 | 1.84 | 0.97 | 0.01 | 0.008 |
| Glucose, mg/dL | 121.14 | 113.20 | 3.74 | 0.14 | 0.07 | 0.21 |
| Insulin, ng/mL | 0.57 | 0.58 | 0.06 | 0.95 | 0.36 | 0.12 |
| NEFA, mmol/L | 0.47 | 0.39 | 0.01 | <0.001 | <0.001 | 0.27 |
| SUN, mg/dL | 9.58 | 8.88 | 0.32 | 0.13 | <0.001 | 0.55 |
| Post-challenge | ||||||
| Cortisol, ng/mL | 18.48 | 24.92 | 1.47 | 0.004 | <0.001 | 0.004 |
| Glucose, mg/dL | 178.93 | 160.42 | 4.19 | 0.004 | <0.001 | 0.003 |
| Insulin, ng/mL | 1.27 | 1.42 | 0.11 | 0.34 | <0.001 | 0.04 |
| NEFA, mmol/L | 0.27 | 0.23 | 0.01 | <0.001 | <0.001 | 0.01 |
| SUN, mg/dL | 9.86 | 8.92 | 0.34 | 0.06 | <0.001 | <0.001 |
Significant values represented by bold text.
1TRT: treatment.
2Interaction: treatment × time.
Figure 1.
Effect of OmniGen-AF supplementation (4.54 g/45 kg BW/d for 42 d) on the serum cortisol response to a glucose tolerance test (0.5 mL/kg of a 50% dextrose solution at 0 min). There was a treatment × time interaction (P = 0.008) for serum cortisol prior to the challenge such that cortisol was greater in Control heifers at −45 min. In addition, there was a treatment × time interaction post-challenge (P = 0.004) where cortisol was greater in OmniGen-AF fed heifers from 10- to 45-min post-challenge compared to Control heifers (P < 0.001). *Treatments differ at P ≤ 0.05.
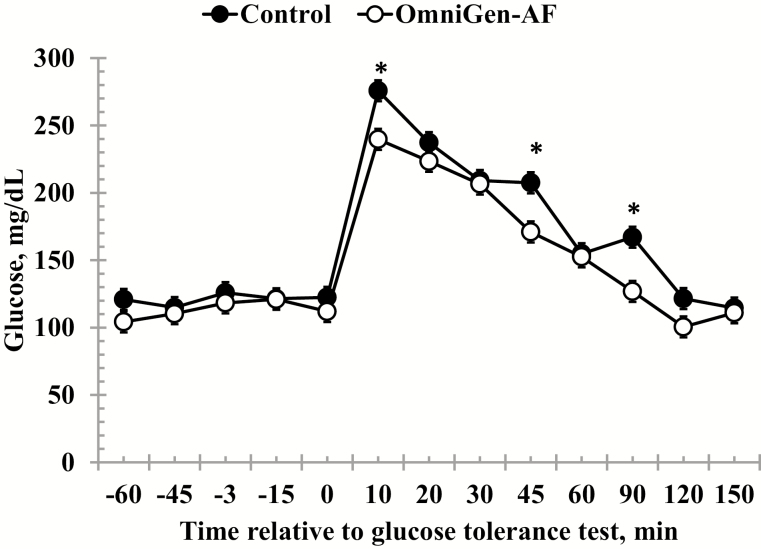
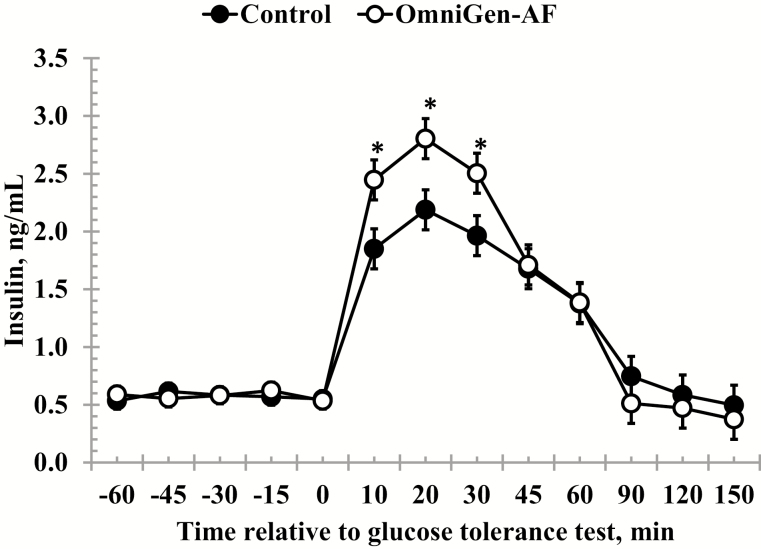
Serum glucose concentrations measured prior to administration of the dextrose bolus were unaffected by treatment, time, or a treatment × time interaction (P ≥ 0.07; Table 2). However, following the challenge, there was a treatment × time interaction (P < 0.01) for glucose concentrations, such that glucose was reduced in OmniGen-supplemented heifers at 10-, 45-, and 90-min post-challenge (Fig. 2). Glucose clearance rates (k) did not differ between Control and OmniGen-supplemented heifers from 10 to 30 min (2.79 ± 0.60 vs. 3.23 ± 0.87 %/min, respectively; P = 0.68) nor from 30 to 120 min after dextrose infusion (3.02 ± 0.65 vs. 3.45 ± 0.87 %/min, respectively; P = 0.70). In a similar manner, no differences were observed for treatment or time, nor was a treatment × time interaction observed for pre-challenge insulin concentrations (P ≥ 0.12; Table 2). However, there was a treatment × time interaction (P = 0.04) for insulin concentrations following administration of the dextrose bolus. Specifically, insulin concentrations were greater in OmniGen-supplemented heifers from 10- to 30-min post-challenge compared to Control heifers (Fig. 3).
Figure 2.
Effect of OmniGen-AF supplementation (4.54 g/45 kg BW/d for 42 d) on the serum glucose response to a glucose tolerance test (0.5 mL/kg of a 50% dextrose solution at 0 min). Serum glucose was unaffected by treatment prior to the challenge (P = 0.14). Following administration of the dextrose bolus, there was a treatment × time interaction for serum glucose such that concentrations were reduced at 10, 45, and 90 min in OmniGen-AF supplemented compared to Control heifers (P < 0.001). *Treatments differ at P ≤ 0.001.
Figure 3.
Effect of OmniGen-AF supplementation (4.54 g/45 kg BW/d for 42 d) on the serum insulin response to a glucose tolerance test (0.5 mL/kg of a 50% dextrose solution at 0 min). Insulin concentrations did not differ between treatments prior to the challenge (P = 0.95). However, following administration of the dextrose bolus, there was a treatment × time interaction such that insulin concentrations were greater in OmniGen-AF fed heifers from 10- to 30-min post-challenge (P = 0.04). *Treatments differ at P ≤ 0.03.
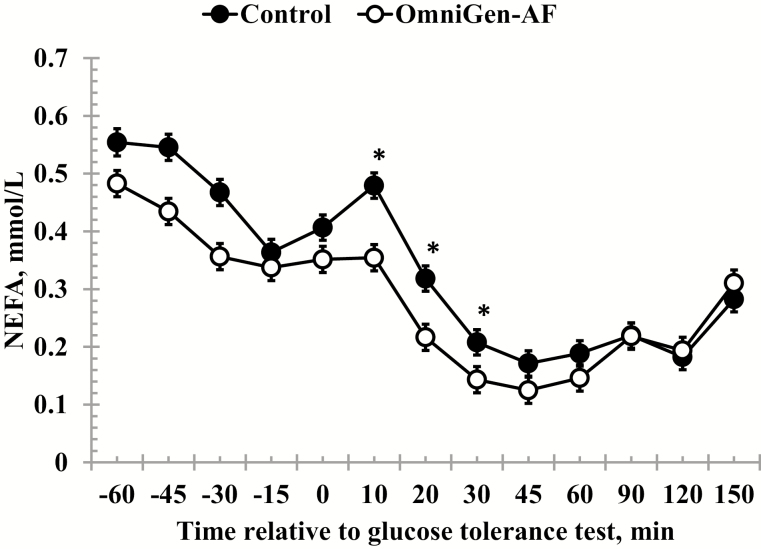
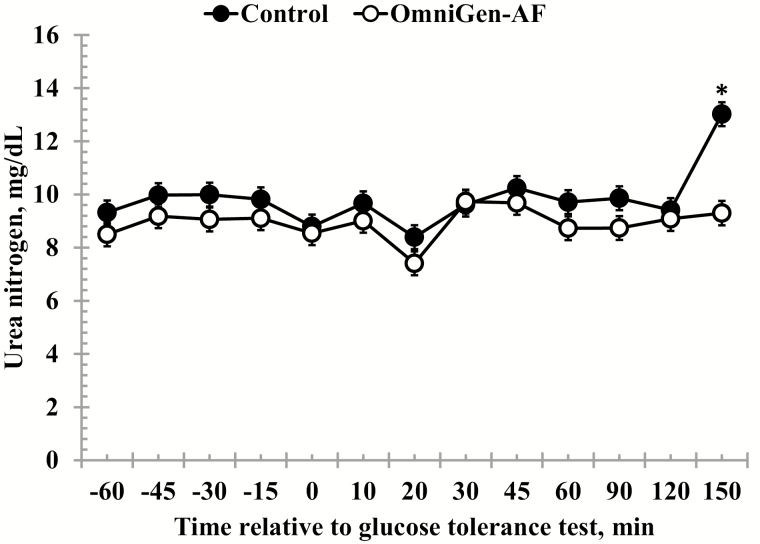
Serum NEFA concentrations were reduced in OmniGen-fed heifers compared to Control heifers prior to administration of the dextrose bolus (treatment P < 0.01; Table 2; Fig. 4). In addition, average NEFA concentrations decreased from −45 to −15 min. Following administration of the dextrose bolus there was a treatment × time interaction (P = 0.01) for NEFA concentrations, where concentrations were reduced in OmniGen-supplemented heifers from 10- to 30-min post-challenge. Average pre-challenge SUN concentrations changed over time (P < 0.01), where SUN concentrations increased from −60 to −45 min, and decreased from −15 to 0 min (Table 2; Fig. 5). There was a treatment × time interaction (P < 0.01) for post-challenge SUN concentrations, where concentrations were reduced in OmniGen-fed heifers compared to Control heifers at 150 min.
Figure 4.
Effect of OmniGen-AF supplementation (4.54 g/45 kg BW /d for 42 d) on the serum non-esterified fatty acid (NEFA) response to a glucose tolerance test (0.5 mL/kg of a 50% dextrose solution at 0 min). Prior to administration of the dextrose bolus, serum NEFA concentrations were reduced in OmniGen-AF-supplemented heifers compared to Control heifers (P < 0.001). Post-challenge, there was a treatment × time interaction (P = 0.01) for NEFA concentrations such that concentrations were reduced in OmniGen-fed heifers from 10 to 30 min after challenge. *Treatments differ at P ≤ 0.04.
Figure 5.
Effect of OmniGen-AF supplementation (4.54 g/45 kg BW /d for 42 d) on the serum urea nitrogen (SUN) response to a glucose tolerance test (0.5 mL/kg of a 50% dextrose solution at 0 min). Pre-challenge, SUN concentrations were unaffected by treatment (P = 0.13). However, there was a treatment × time interaction (P < 0.001) for SUN following administration of the dextrose bolus such that SUN concentrations were reduced in OmniGen-AF fed heifers compared to Control heifers at 150 min. *Treatments differ at P < 0.001.
DISCUSSION
Body weights increased over the 42-d feeding period prior to the glucose tolerance test, as expected. Interestingly, OmniGen calves weighed more than Control calves on the final weigh day. Another study in steers fed OmniGen found no difference in body weight compared to control steers when fed for 28 d or for 215 d (Buntyn et al., 2016). In addition Lippolis et al. (2017) observed a reduction in final BW and ADG in crossbred steers fed OmniGen compared to Control calves. It is interesting that the two aforementioned studies used steers and the current study used heifers, which may be partially responsible for the differences observed yet unlikely. Overall, there is limited information on the effect of OmniGen on performance in beef cattle.
A previous study observed reduced basal cortisol in OmniGen-fed calves, as well as a reduced cortisol response to LPS challenge in beef heifers (Buntyn et al., 2016). No differences in basal cortisol concentration were observed in the current study, with the exception of one time point (−45 min). It is possible that the short transportation period and/or the cannulation process the day prior to the challenge may have affected the basal cortisol response. However, previous studies in our laboratory have found that an overnight rest (≥12 h) is sufficient time to allow cattle to recover from the stress associated with short duration transportation and/or cannulation (Carroll et al., 2009; Burdick et al., 2010, 2011). This is supported by the low cortisol concentrations observed during the baseline period. In response to LPS or environmental challenges, products designed to support the immune system have been observed to decrease the cortisol response (Collier et al., 2011; Burdick Sanchez et al., 2013; Finck et al., 2014). Cortisol is regarded as a stress hormone or an anti-inflammatory mediator. However, due to the focus of cortisol and the stress response, it may be disregarded as a glucocorticoid, i.e., a regulator of glucose. Some of the early work in characterizing cortisol was through its direct effects on glucose (Long et al., 1940). Specifically, increased cortisol concentrations result in increased glycogenolysis and gluconeogenesis, and thus increased circulating concentrations of glucose (Long et al., 1940). However, studies using glucose tolerance tests observed a decrease or no change in cortisol concentrations in response to dextrose administration (Vierhapper et al., 2003; Gordon and McKeever, 2006). Thus, it was unexpected to see an increase in cortisol in response to the glucose tolerance test, and furthermore, to observe such a difference between the treatment groups. We have reported an increase in cortisol in response to administration of a dextrose bolus in Angus-cross calves (Burdick Sanchez et al., 2016), but not to the magnitude that was observed in the current study. However, a study by Chalmeh et al. (2015) found no difference in cortisol concentrations in lactating Holstein cows following a glucose tolerance test. One explanation for the increased cortisol response in OminGen-fed heifers is that the heifers may be more sensitive to changes in glucose. More specifically, although the rapid increase in serum glucose concentrations observed in response to administration of the dextrose bolus was less in OmniGen-fed than Control heifers, it resulted in greater stimulation of cortisol production compared to Control calves. A study in humans found an increase in cortisol in response to varying doses of insulin (Greenwood et al., 1966). This is interesting as cortisol is typically associated with reducing insulin concentrations (Plat et al., 1996). However, when following the temporal patterns of secretion in the current study, glucose peaked first, followed by insulin and lastly cortisol. Thus, it is possible that the increase in cortisol was influenced as much by increasing insulin concentrations, which were greater in OmniGen-fed heifers, as it was by the bolus dextrose infusion (Gross et al., 2015). Interestingly, greater cortisol area under the curve (AUC) has been associated with greater glucose AUC in humans (Reynolds et al., 2003), which contrasts with the results of the current study which found greater cortisol but reduced glucose concentrations in OmniGen-fed heifers. Ultimately, the cortisol response to the glucose tolerance test in the OmniGen-supplement heifers appears to be an anomaly that conflicts with published literature. However, much of the literature available is in humans, and thus this relationship must be studied further in a bovine model.
Reduced glucose concentrations in OmniGen-supplemented heifers suggests that these heifers exhibited increased uptake or clearance of glucose compared to Control heifers. Although numerically increased in OmniGen-supplemented heifers, glucose clearance rates from 10 to 30 min and 30 to 120 min were not different between treatments. In addition, insulin concentrations were greater in OmniGen-supplemented heifers compared to Control heifers. Ruminant animals absorb very little glucose from the gastrointestinal tract, and therefore rely on gluconeogenesis from volatile fatty acid substrates, mainly propionate, for production of glucose. Based on this, there is an altered relationship between glucose and insulin in ruminants when compared to nonruminants. Insulin appears to be less important for removing large amounts of glucose in cattle as compared to nonruminants (Prior and Smith, 1982). In addition, it has been reported that ruminants are less sensitive to the actions of insulin, requiring approximately twice as much insulin to reduce glucose concentrations compared to humans (Brockman and Laarveld, 1986). Therefore, the greater insulin concentrations observed in OmniGen-fed heifers may have been partially responsible for the reduction in glucose concentrations observed compared to Control steers. As glucose concentrations observed in the current study typically result in glucosuria, it would be interesting to determine whether glucose concentrations in the urine differed between treatments. Unfortunately, urine samples were not collected, but this offers an avenue for further investigation. Overall, the reduced glucose and increased insulin concentrations suggest that calves fed OmniGen may be more responsive to changes in glucose, which is supported by the increased cortisol concentrations discussed earlier.
As OmniGen is a proprietary blend of yeast, minerals, and other supplements, it is difficult to make direct comparisons between OmniGen and other commercially available supplements. Further, there have been limited studies aimed at determining whether immune modulating products can alter metabolism. Chromium is one of the few products studied where differences in immunity and metabolism have been observed (Burton, 1995; Kegley and Spears, 1995). Similar glucose and insulin responses to the glucose tolerance test were observed in cattle supplemented with chromium propionate or chromium nicotinic acid (Kegley and Spears, 1995; Bernhard et al., 2012). The aforementioned study and the data from the current study highlight the need to continue to study the involvement of metabolism in the modulation of the immune system when feeding supplements with immunomodulating properties.
Reduced NEFA and SUN concentrations have been observed previously in steers fed OmniGen (Buntyn et al., 2016). Elevated concentrations of fatty acids and urea nitrogen are typically viewed as indicators of adipose and protein catabolism, respectfully. Therefore, reduction in these variables suggests less tissue catabolism in OmniGen-fed heifers in the current study. This is supported by the increased insulin concentrations observed in response to the dextrose bolus, as insulin promotes protein and triglyceride production in muscle and adipose tissue (Brockman, 1978; Brockman and Laarveld, 1986). It appears insulin has greater effects on adipose and muscle tissue, particularly on uptake of fatty acids and amino acids and substrates that allow for their synthesis (Prior and Smith, 1982; Brockman and Laarveld, 1986). Administration of insulin to sheep resulted in reduced free fatty acids (Prior and Smith, 1982); therefore, it is possible that the greater insulin response observed in the current study was at least in part responsible for the reduced NEFA concentrations. Yet, NEFA concentrations were reduced prior to the administration of the dextrose bolus, which cannot be explained by the increased insulin response to the dextrose challenge. Regardless, OmniGen was able to reduce NEFA concentrations in supplemented heifers, suggesting a reduction in lipolysis or an increase in lipogenesis in these calves. In addition, the effects of OmniGen on SUN concentrations are likely minimal, as concentrations differed at only one time point post-challenge. Review of all the heifers within this treatment did not find any single heifer influencing this response, but all heifers demonstrated an increase in urea N to varying degrees at this time point and is likely an anomaly in the current study. However, it is possible that SUN concentrations were beginning to increase at this time point, which were not captured in the OmniGen treatment group as this was the last sample collected.
CONCLUSION
In summary, supplementation of heifer calves with OmniGen was able to improve their metabolic response to a glucose tolerance test. Specifically, OmniGen-fed heifers produced greater cortisol and insulin responses, yet had reduced glucose, NEFA, and SUN (tendency) responses. These data suggest that calves supplemented with OmniGen may be more responsive to changes in glucose, as indicated by their greater cortisol and insulin responses, and these calves may not need to catabolize as much adipose tissue during period of feed restriction or immunological challenges for energy. Ultimately, data from this study suggest that OmniGen supplementation may affect the storage and/or redistribution of energy deposits and provides further evidence for improved metabolic responses in OmniGen-fed cattle.
ACKNOWLEDGMENTS
We would like to thank J. W. Dailey and J. R. Carroll (USDA-ARS) for their outstanding technical support throughout the study. This research was funded by Phibro Animal Health.
Footnotes
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Bernhard B. C., Burdick N. C., Rathmann R. J., Carroll J. A., Finck D. N., Jennings M. A., Young T. R., and Johnson B. J.. . 2012. Chromium supplementation alters both glucose and lipid metabolism in feedlot cattle during the receiving period. J. Anim. Sci. 90:4857–4865. doi: 10.2527/jas.2011-4982 [DOI] [PubMed] [Google Scholar]
- Broadway P., Carroll J., and Callaway T.. . 2014. Alternative antimicrobial supplements that positively impact animal health and food safety. Agri. Food Anal. Bacteriol. 4:109–121. [Google Scholar]
- Broadway P. R., Carroll J. A., and Sanchez N. C.. . 2015. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: a review. Microorganisms 3:417–427. doi: 10.3390/microorganisms3030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. P. 1978. Roles of glucagon and insulin in the regulation of metabolism in ruminants. A review. Can. Vet. J. 19:55–62. [PMC free article] [PubMed] [Google Scholar]
- Brockman R. P., and Laarveld B.. . 1986. Hormonal regulation of metabolism in ruminants: a review. Livest. Prod. Sci. 14:313–334. doi: 10.1016/0301-6226(86)90012-6 [DOI] [Google Scholar]
- Buntyn J. O., Sieren S. E., Bittner C. J., Burken D., Erickson G. E., Burdick Sanchez N. C., Carroll J. A., Jones S. J., Schmidt T. B., . et al. 2016. Effects of feeding OmniGen-AF on immune function, performance, and carcass characteristics during the feeding period. Nebraska Beef Cattle Reports (864). University of Nebraska-Lincoln. https://digitalcommons.unl.edu/animalscinbcr/864/ [Google Scholar]
- Burdick N. C., Carroll J. A., Hulbert L. E., Dailey J. W., Willard S. T., Vann R. C., Welsh T. H., and Randel R. D.. . 2010. Relationships between temperament and transportation with rectal temperature and serum concentrations of cortisol and epinephrine in bulls. Livest. Sci. 129:166–172. doi: 10.1016/j.livsci.2010.01.020 [DOI] [Google Scholar]
- Burdick N. C., Carroll J. A., Randel R. D., Willard S. T., Vann R. C., Chase C. C., Lawhon S. D., Hulbert L. E., and Welsh T. H.. . 2011. Influence of temperament and transportation on physiological and endocrinological parameters in bulls. Livest. Sci. 139:213–221. doi: 10.1016/j.livsci.2011.01.013 [DOI] [Google Scholar]
- Burdick Sanchez N. C., Carroll J. A., Broadway P. R., Bass B. E., and Frank J. W.. . 2018. Modulation of the acute phase response following a lipopolysaccharide challenge in pigs supplemented with an all-natural Saccharomyces cerevisiae fermentation product. Livest. Sci. 208:1–4. doi: 10.1016/j.livsci.2017.11.022 [DOI] [Google Scholar]
- Burdick Sanchez N. C., Carroll J. A., Broadway P. R., Hughes H. D., Roberts S. L., Richeson J. T., Schmidt T. B., and Vann R. C.. . 2016. Cattle temperament influences metabolism: metabolic response to glucose tolerance and insulin sensitivity tests in beef steers. Domest. Anim. Endocrinol. 56:85–95. doi: 10.1016/j.domaniend.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez N. C., Carroll J. A., Randel R. D., Vann R. C., and Welsh T. H. Jr. 2014. Associations between endotoxin-induced metabolic changes and temperament in Brahman bulls. J. Anim. Physiol. Anim. Nutr. (Berl). 98:178–190. doi: 10.1111/jpn.12071 [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez N. C., Young T. R., Carroll J. A., Corley J. R., Rathmann R. J., and Johnson B. J.. . 2013. Yeast cell wall supplementation alters aspects of the physiological and acute phase responses of crossbred heifers to an endotoxin challenge. Innate Immun. 19:411–419. doi: 10.1177/1753425912469673 [DOI] [PubMed] [Google Scholar]
- Burton J. L. 1995. Supplemental chromium: its benefits to the bovine immune system. Anim. Feed Sci. Tech. 53:117–133. doi: 10.1016/0377-8401(95)02016-S [DOI] [Google Scholar]
- Carroll J. A., Reuter R. R., Chase C. C. Jr, Coleman S. W., Riley D. G., Spiers D. E., Arthington J. D., and Galyean M. L.. . 2009. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immun. 15:81–89. doi: 10.1177/1753425908099170 [DOI] [PubMed] [Google Scholar]
- Chalmeh A., Hajimohammadi A., and Nazifi S.. . 2015. Endocrine and metabolic responses of high producing Holstein dairy cows to glucose tolerance test based on the stage of lactation. Livest. Sci. 181:179–186. doi: 10.1016/j.livsci.2015.09.014 [DOI] [Google Scholar]
- Collier C. T., Carroll J. A., Ballou M. A., Starkey J. D., and Sparks J. C.. . 2011. Oral administration of Saccharomyces cerevisiae boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs. J. Anim. Sci. 89:52–58. doi: 10.2527/jas.2010-2944 [DOI] [PubMed] [Google Scholar]
- Finck D., Ribeiro F., Burdick N., Parr S., Carroll J., Young T., Bernhard B., Corley J., Estefan A., and Rathmann R.. . 2014. Yeast supplementation alters the performance and health status of receiving cattle. Prof. Anim. Sci. 30:333–341. doi: 10.15232/S1080-7446(15)30125-X [DOI] [Google Scholar]
- Gordon M. E., and McKeever K. H.. . 2006. Oral and intravenous carbohydrate challenges decrease active ghrelin concentrations and alter hormones related to control of energy metabolism in horses. J. Anim. Sci. 84:1682–1690. doi: 10.2527/jas.2005-484 [DOI] [PubMed] [Google Scholar]
- Greenwood F. C., Landon J., and Stamp T. C.. . 1966. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin. I. In control subjects. J. Clin. Invest. 45:429–436. doi: 10.1172/JCI105357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. J., Wellnitz O., and Bruckmaier R. M.. . 2015. Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows. J. Anim. Sci. 93:3395–3401. doi: 10.2527/jas.2015-8903 [DOI] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. . 2017. Immune system stimulation increases nursery pig maintenance energy requirements. J. Anim. Sci. 95:68–69. doi: 10.2527/asasmw.2017.12.145 [DOI] [Google Scholar]
- Kegley E. B., and Spears J. W.. . 1995. Immune response, glucose metabolism, and performance of stressed feeder calves fed inorganic or organic chromium. J. Anim. Sci. 73:2721–2726. doi: 10.2527/1995.7392721x [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Fernandez M. V. S., and Baumgard L. H.. . 2017. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi: 10.3168/jds.2016-12001 [DOI] [PubMed] [Google Scholar]
- Lippolis K. D., Cooke R. F., Schumaher T., Brandão A. P., Silva L. G. T., Schubach K. M., Marques R. S., and Bohnert D. W.. . 2017. Physiologic, health, and performance responses of beef steers supplemented with an immunomodulatory feed ingredient during feedlot receiving. J. Anim. Sci. 95:4945–4957. doi: 10.2527/jas2017.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. N. H., Katzin B., and Fry E. G.. . 1940. The adrenal cortex and carbohydrate metabolism. Endocrinology. 26(2):309–344. doi: 10.1210/endo-26-2-309 [DOI] [Google Scholar]
- Plat L., Byrne M. M., Sturis J., Polonsky K. S., Mockel J., Féry F., and Van Cauter E.. . 1996. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am. J. Physiol. 270(1 Pt 1):E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36 [DOI] [PubMed] [Google Scholar]
- Prior R. L., and Smith S. B.. . 1982. Hormonal effects on partitioning of nutrients for tissue growth: role of insulin. Fed. Proc. 41:2545–2549. [PubMed] [Google Scholar]
- Reynolds R. M., Syddall H. E., Walker B. R., Wood P. J., and Phillips D. I.. . 2003. Predicting cardiovascular risk factors from plasma cortisol measured during oral glucose tolerance tests. Metabolism. 52:524–527. doi: 10.1053/meta.2003.50090 [DOI] [PubMed] [Google Scholar]
- Vierhapper H., Heinze G., Gessl A., and Exner M.. . 2003. Adrenocortical tumors: prevalence of impaired glucose tolerance and of “Paradoxical Rise” of cortisol during an oral glucose tolerance test. Exp. Clin. Endocrinol. Diabetes 111:415–420. doi: 10.1055/s-2003-44288 [DOI] [PubMed] [Google Scholar]