Abstract
Purpose
Serous cystic neoplasm (SCN) of the pancreas is considered benign in most cases. However, some SCN patients undergo surgical resection because lesions could not be differentiated preoperatively. This study evaluated causes of resection for SCN, investigated clinical and radiological features of surgically resected SCNs, and compared characteristics of SCNs diagnosed accurately and those misdiagnosed.
Methods
One hundred patients, who underwent surgery for pancreatic cystic tumors with pathological confirmation of SCN between 2000 and 2014 were retrospectively analyzed.
Results
The mean patient age was 52.9 years, 67 (67%) were female, and most lesions (72%) were located in the pancreatic body or tail. Fifty-one (51%) pathologically confirmed SCNs were preoperatively diagnosed as non-SCNs. Patients underwent surgery due to uncertain diagnosis (58%) or symptomatology (18%). According to radiological examination, most lesions were macrocystic (85%), exhibited septation (58%), or were enhancing lesions (48%). Compared with preoperatively diagnosed non-SCNs, accurately diagnosed SCNs exhibited septation (75.5% vs. 41.2%, P = 0.001) and central scar (36.7% vs. 11.8%, P = 0.003) more frequently in radiological examinations. In terms of macrocystic tumors (n = 85), most parameters did not differentiate preoperative diagnoses, although lesions accurately diagnosed as SCN exhibited septation more frequently than those preoperatively misdiagnosed as mucinous cystic neoplasm or intraductal papillary mucinous neoplasm (70.7% vs. 38.9% vs. 33.3%, respectively, P = 0.009).
Conclusion
It is difficult to accurately distinguish macrocystic SCNs from other cystic tumors using conventional radiological methods. For more accurate diagnosis, new biomarkers and/or other diagnostic modalities are needed and warrant further investigation.
Keywords: Pancreatic cyst, Radiology, Serous cystadenoma
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) account for approximately 30% of resected pancreatic tumors [1,2,3]. Due to the increased use of sophisticated imaging modalities and advances in imaging techniques, detection rates for PCNs have gradually increased [4]. There are 4 common categories of PCN including: intraductal papillary mucinous neoplasm (IPMN); serous cystic neoplasm (SCN); mucinous cystic neoplasm (MCN); and solid pseudopapillary neoplasm [1]. Of these, IPMN, MCN, and SCN are recommended to be surgically removed due to their malignant potential [5,6,7,8].
Although symptomatic SCNs should be surgically removed, regular surveillance of asymptomatic SCNs is generally recommended due to lower malignant potential compared with other PCNs [9]. However, some SCNs do not exhibit the typical honeycomb pattern, and their atypical appearance makes it difficult to discriminate SCNs from other potentially malignant PCNs and to decide whether to perform the surgery [10]. If surgeons could accurately distinguish SCNs from other PCNs, unnecessary operations would be decreased. Therefore, the aim of this study was to evaluate the causes of resection for SCN, to investigate the clinical and radiological features of SCNs that are surgically resected, and to compare the characteristics of SCNs that were diagnosed accurately and those misdiagnosed.
METHODS
Patients and materials
This was a retrospective study that analyzed prospectively collected medical data. Between 2000 and 2014, patients who underwent surgical resection and were diagnosed with pathologically confirmed SCN were included. Patients with incomplete radiological data were excluded. All patients underwent preoperative multidetector CT using either the Brilliance 64 (Philips Medical Systems, Cleveland, OH, USA) or LightSpeed Ultra (GE Healthcare, Little Chalfont, UK) instruments. Additional MRI using the Magnetom Verio (Siemens Healthcare, Erlangen, Germany) system was performed as required.
Clinical factors included age, sex, roots of detection of the lesions, preoperative diagnosis, tumor location, reason(s) for surgery, and operation type. The department of radiology of the authors' institution provided the reporting form for PCNs, which included radiological characteristics including type of cyst (microcystic or macrocystic), tumor size, calcification, central scar, solid component(s), cystic wall thickening, septation in the cyst, enhancing lesion, pancreatic duct dilatation, bile duct dilatation and pancreas atrophy, and suggested all possible diagnoses according to these radiological characteristics. Type of cyst, and other radiological characteristics were defined based on the authors' previous report [10]. Briefly, cysts were classified as microcystic if they were < 2 cm in size, and macrocystic when ≥ 2 cm. The typical “honeycomb” appearance was included among the microcystic features. All radiological images were reviewed by one specialized pancreatobiliary radiologist.
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. 1606-086-771), and all patients consented to participate in this study.
Statistical analysis
Categorical variables were compared using the chi-square test and continuous variables were compared using Student t-test. Differences were considered to be statistically significant at P < 0.05 in 2-tailed testing. All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
Clinical variables and radiological features
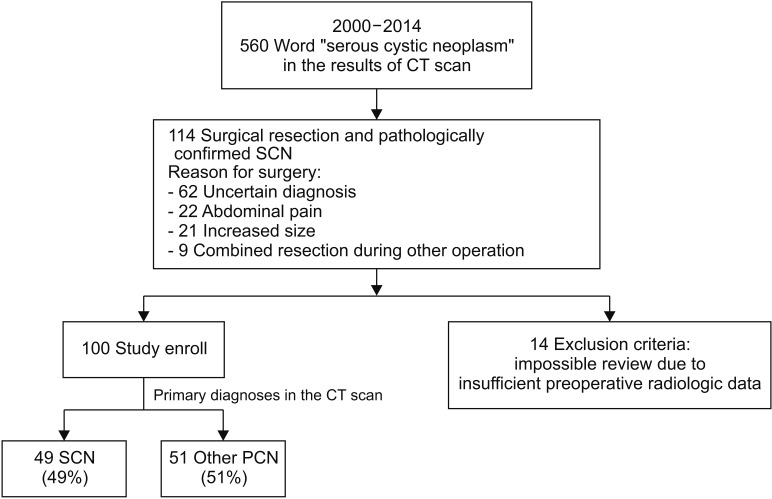
A flowchart illustrating patient enrollment is presented in Fig. 1. Overall, 560 patients were potentially diagnosed with SCN, which was described in the CT scan reports. Most patients underwent regular surveillance, and 114 underwent surgical resection and SCN was pathologically confirmed. All of the excluded patients (n = 14) underwent preoperative radiological examinations in another hospital; therefore, imaging data were not recorded in the medical database and could not be reviewed. Ultimately, 100 patients were included in the present study. There were no cases of serous cystadenocarcinoma.
Fig. 1. Study design and patient enrollment. Finally, one hundred consecutive patients underwent surgical resection and had pathologic confirmation of serous cystic neoplasm (SCN). In regards to preoperative diagnoses, 49 patients were accurately diagnosed of SCN, and 51 patients were misdiagnosed of other pancreatic cystic neoplasm. SCN serous cystic neoplasm.
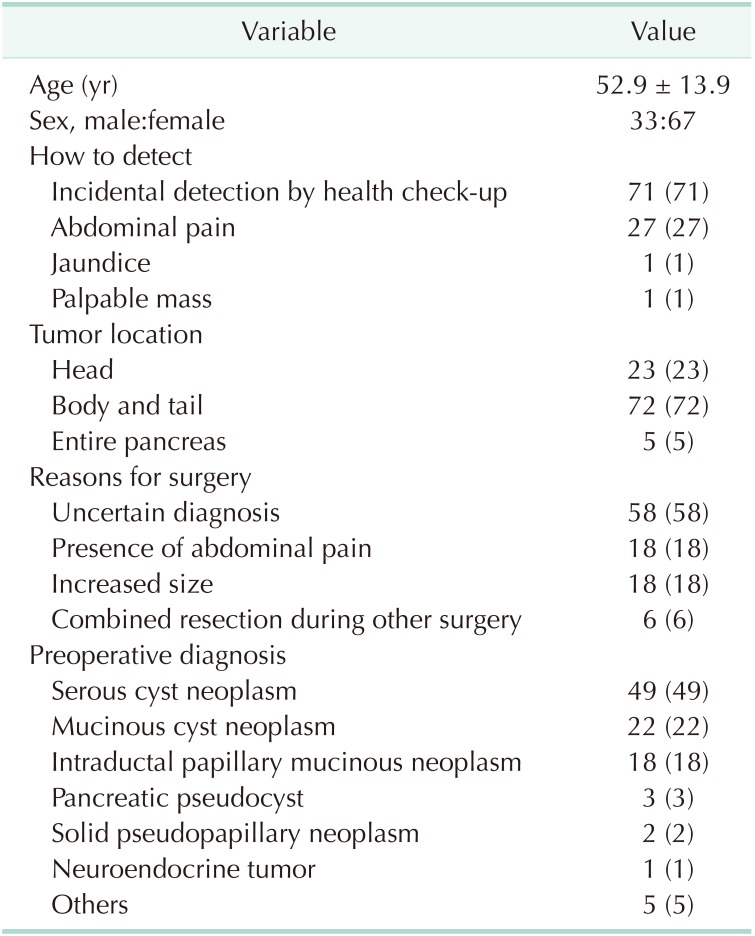
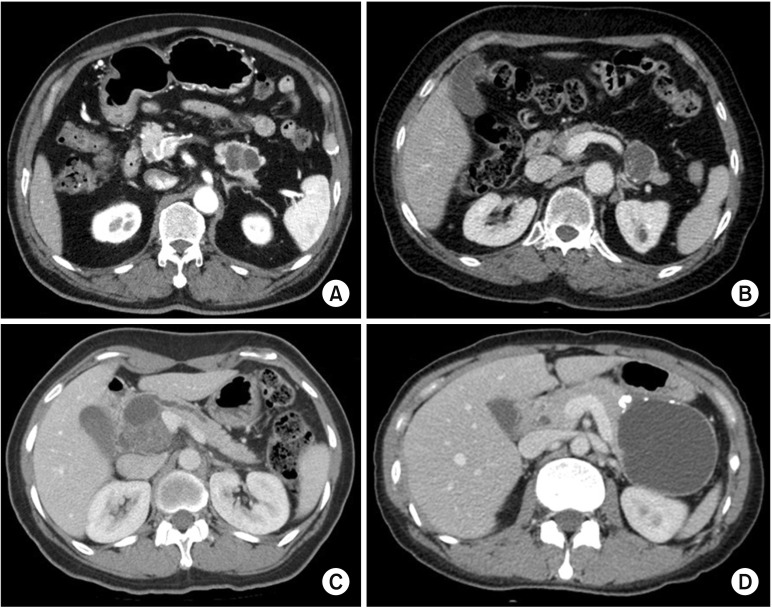
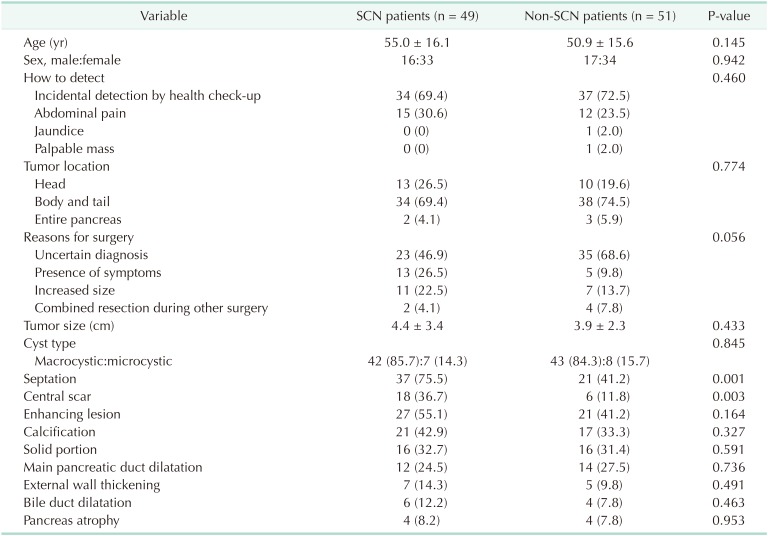
The mean age of the patients was 52.9 years and 67 (67%) were female (Table 1). Seventy-one patients (71%) had their PCNs incidentally detected at a regular health check-up, 27 (27%) were due to abdominal pain, 1 (1%) due to jaundice, and 1 (1%) due to a palpable mass. Seventy-two patients (72%) had the PCN in the pancreatic body and tail. Reasons for surgery included uncertain diagnosis (n = 58, 58%), symptomatology (n = 18, 18%), and combined resection during other surgery (n = 8, 8%). Of patients with an uncertain diagnosis, 10 were preoperatively diagnosed with possible malignant lesions, in which the most probable preoperative diagnoses were: IPMN with invasive carcinoma (n = 5); pancreatic head cancer (n = 2); pancreatic tail cancer (n = 2); and multiple metastases from renal cell carcinoma (n = 1). The preoperative diagnoses were SCN (n = 49, 49%) (Fig. 2A), misdiagnosed MCN (n = 22, 22%) (Fig. 2B), misdiagnosed IPMN (n = 18, 18%) (Fig. 2C), and misdiagnosed pancreatic pseudocyst (n = 3, 3%) (Fig. 2D).
Table 1. Clinical variables in total patients (n = 100).
Values are presented as mean ± standard deviation or number (%).
Fig. 2. Preoperative image according to the preoperative diagnoses. All these lesions were pathologically confirmed with serous cystic neoplasm (SCN) after surgical resection. (A) SCN. 3.8-cm-sized cystic lesion with internal septation in pancreas tail. (B) Preoperatively misdiagnosed mucinous cystic neoplasm. 3.8-cm-sized well-defined septated cystic lesion with wall calcification. (C) Preoperatively misdiagnosed intraductal papillary mucinous neoplasm. 6.3-cm-sized multiloculated cystic mass with solid component and the presence of upstream pancreatic duct dilatation. (D) Preoperatively misdiagnosed pseudocyst. 9-cm-sized loculated cystic lesion with evenly-thickened wall with multiple calcification.
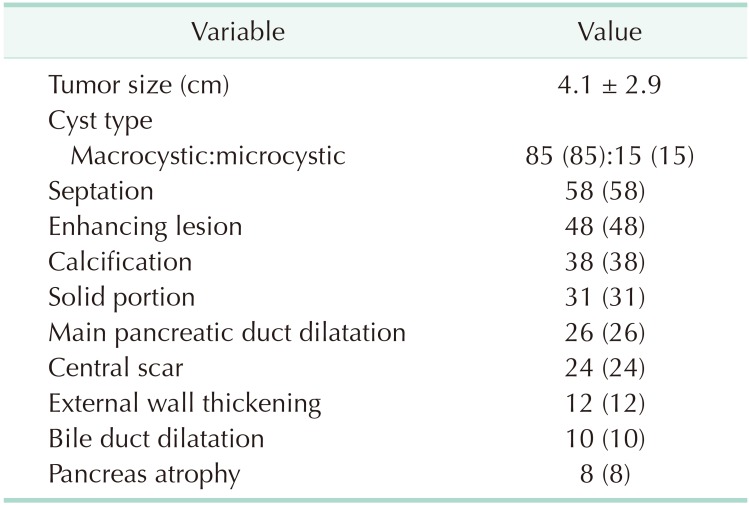
The radiological characteristics of all patients are summarized in Table 2. The mean tumor size was 4.1 cm. Because only patients with surgically resected SCNs were enrolled in this study, most lesions exhibited macrocystic features (n = 85, 85%), followed by microcystic appearance (n = 15, 15%). Septation in the cyst was evident in 58 (58%) patients, enhancing lesions in the cyst in 48 (48%), and calcification in the cyst in 38 (38%).
Table 2. Radiologic features in total patients (n = 100).
Values are presented as mean ± standard deviation or number (%).
Comparisons of clinical and radiological features according to preoperative diagnoses
Patients were divided into 2 groups: those preoperatively diagnosed with SCN (n = 49, 49%); and those who were diagnosed with lesions other than SCN (n = 51, 51%). The clinical and radiological characteristics of the 2 groups are compared in Table 3. There were no significant differences in clinical features between the 2 groups. In terms of radiological features, septation (75.5% vs. 41.2%, P = 0.001) and central scar (36.7% vs. 11.8%, P = 0.003) were more prevalent in patients preoperatively diagnosed with SCN.
Table 3. Comparisons of characteristics between preoperatively diagnosed serous cystic neoplasm (SCN) patients and non-SCN patients.
Values are presented as mean ± standard deviation or number (%).
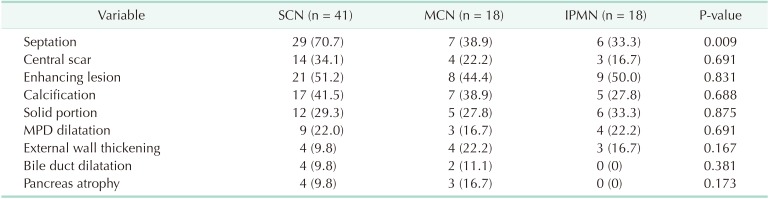
A comparison of radiological features according to preoperative diagnoses for those with macrocystic neoplasms is shown in Table 4. Macrocystic lesions did not exhibit different radiological features among the preoperative diagnoses, although septation in the cyst was more prevalent in those accurately diagnosed with SCN compared with those preoperatively misdiagnosed with MCN or IPMN (70.7 vs. 38.9 vs. 33.3%, respectively, P = 0.009).
Table 4. Radiologic features according to the preoperative diagnoses in the macrocystic tumor.
Values are presented as number (%).
SCN, serous cystic neoplasm; MCN, mucinous cystic neoplasm; IPMN, intraductal papillary mucinous neoplasm; MPD, main pancreatic duct.
DISCUSSION
PCNs are increasingly being detected due to increased use of radiological examinations [4,11]. Whereas IPMNs and MCNs consist of columnar epithelium, which produces mucin, and have a relatively high malignant potential, SCNs have a single layer of either cuboidal or flattened cells, and malignant SCNs have been reported in a very limited number of cases [2,7,12,13,14]. Therefore, surgical indications for SCNs have not been based on oncological potential, but generally on large size, symptomatology, and difficult or impossible discrimination from other potentially malignant lesions [15,16]. However, because pancreatectomies are associated with a high morbidity rate, surgical resections of benign SCNs have also caused postoperative complications that would be preventable if the benign SCNs had been initially accurately diagnosed [17,18,19].
The present study investigated 100 consecutive patients who were diagnosed with pathologically confirmed benign SCN after complete surgical resection. Most lesions were discovered incidentally at health check-up (71%) or due to abdominal pain (27%) (Table 1). Most patients underwent surgical resection due to uncertain preoperative diagnosis (58%), with 51 lesions (51%) preoperatively diagnosed as non-SCNs. In other words, if the benign SCNs had been accurately diagnosed, more than onehalf of patients could have undergone close observation instead of surgical resection.
In our previous report, we mentioned that the opposite of “microcystic” was “macrocystic,” not “oligocystic,” and proposed that the “oligocystic SCN” according to the World Health Organization classification should be revised to “macrocystic SCN” [10,20]. We also suggested that macrocystic SCN could be divided into 2 subtypes: “unilocular” and “multilocular.” The most representative radiological feature of SCN (i.e., honeycomb appearance) was included in the microcystic SCN [16]. Generally, macrocystic SCNs are detected in <10% of all SCN patients [16,21]. However, one large-scale study recently reported that macrocystic SCNs were detected in 32% of cases, and mixed macrocystic/microcystic in 18% [22]. In this study, 85% of SCNs exhibited macrocystic features (Table 2). This was because the present study enrolled surgically resected SCNs, not those that had undergone regular surveillance. Although we attempted to evaluate radiological differences in macrocystic tumors among the preoperative diagnoses, no significant difference was revealed, except for septation (Table 4). This result was in accordance with our previous study, in which CT could differentiate macrocystic unilocular SCN with low diagnostic accuracy [10].
Furthermore, the enrolled SCNs exhibited other radiological features such as enhancing lesions (48%), calcification (38%), solid component (31%), and main pancreatic duct dilatation (26%) that were representative of other PCNs including MCN, IPMN, and pseudocyst (Table 2). Microcystic honeycomb appearance is a representative feature of typical SCNs (Fig. 2A). However, some radiological features, such as a macrocystic lesion with calcification (Fig. 2B), a cystic lesion with a solid component and the presence of main pancreatic duct dilatation (Fig. 2C), and a cystic lesion with evenly-thickened wall with multiple calcifications (Fig. 2D), made it difficult to distinguish between SCN and other PCNs. Although 49 patients were preoperatively diagnosed with SCN, 23 (46.9%) underwent surgical resection due to uncertain diagnoses (Table 3). Conventional radiological examination provides fundamental information to the clinician, but cannot exactly diagnose atypical SCNs, which can mimic other PCNs.
As mentioned earlier, surgical indications for SCNs remain controversial. Tumor size > 4 cm has been generally accepted because larger lesions tend to cause symptoms [14,23]. Furthermore, laparoscopic surgery becomes more difficult if tumors grow larger than a specific size due to small available operation field [24,25], or chronic inflammation around the cystic mass. Hwang et al. [24,25] suggested the timely and active surgery, especially for pancreatic body or tail SCN of more than 3 cm at detection, because laparoscopic distal pancreatectomy already proved to be safe and feasible. Two studies reported that cyst growth rate was not related to initial cyst size [26,27]. Recently, one large-scale European study reported that cyst size ≥ 4 cm exhibited a faster cyst growth rate than those < 4 cm [22]. Despite these results, the authors argued that whether size or growth rate criteria was an appropriate surgical indication remained questionable [22,27,28]. Although symptom resolution is also an essential goal of surgery, not all SCNs are associated with abdominal pain [22]. Therefore, surgical resection could be considered if SCNs are associated with abdominal symptoms or growth into a large cyst, even if the diagnosis of SCN is highly suspected.
The distinction between SCNs and other PCNs is worldwide, long-term task. Recently, endoscopic ultrasonography (EUS) or EUS-guided fine-needle aspiration (FNA) biopsy has been performed to diagnose PCNs accurately. However, EUS or EUSguided FNA biopsy was not helpful in diagnosing benign SCNs that it had low sensitivity with high specificity that was not superior to CT or MRI [22,28,29]. In addition, EUS-guided FNA biopsy had always potential peritoneal dissemination in case of malignant tumor [30]. Therefore, EUS or EUS-guided biopsy would be helpful for selected lesions that could not be distinguished from other potentially malignant diseases.
In conclusion, it is difficult to accurately distinguish atypical SCNs from other PCNs using conventional radiological examinations, especially macrocystic SCNs. For more accurate diagnosis, new biomarkers and/or other diagnostic modalities are needed and, thus, warrant further investigation.
Footnotes
Fund/Grant Support: This research was supported by grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C2037), and by the Collaborative Genome Program for Fostering New Post-Genome Industry of the National Research Foundation funded by the Ministry of Science and ICT (NRF-2017M3C9A5031591).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: JSK, JYJ.
- Formal Analysis: JSK, HJK.
- Investigation: YJC, YB, YH.
- Methodology: JML, HK, WK.
- Project Administration: JSK, JML, JYJ.
- Writing — Original Draft: JSK, HJK.
- Writing — Review & Editing: JSK, JYJ.
References
- 1.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 2.Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 3.Tampi C, Mullerpatan P, Shah R, Jagannath P, Zimmermann A. Microcystic serous cystadenoma of the pancreas: a report of two cases with one of diffuse presentation. Pancreatology. 2006;6:248–253. doi: 10.1159/000092028. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butte JM, Brennan MF, Gonen M, Tang LH, D'Angelica MI, Fong Y, et al. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350–357. doi: 10.1007/s11605-010-1337-1. [DOI] [PubMed] [Google Scholar]
- 6.Estrella JS, Li L, Rashid A, Wang H, Katz MH, Fleming JB, et al. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol. 2014;38:147–157. doi: 10.1097/PAS.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 7.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: part II. Mucinous cystic neoplasms. Surg Oncol. 2011;20:e93–e101. doi: 10.1016/j.suronc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Strobel O, Z'graggen K, Schmitz-Winnenthal FH, Friess H, Kappeler A, Zimmermann A, et al. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24–33. doi: 10.1159/000073222. [DOI] [PubMed] [Google Scholar]
- 10.Lee SE, Kwon Y, Jang JY, Kim YH, Hwang DW, Kim MA, et al. The morphological classification of a serous cystic tumor (SCT) of the pancreas and evaluation of the preoperative diagnostic accuracy of computed tomography. Ann Surg Oncol. 2008;15:2089–2095. doi: 10.1245/s10434-008-9959-1. [DOI] [PubMed] [Google Scholar]
- 11.Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319–323. doi: 10.1007/s00268-002-6570-7. [DOI] [PubMed] [Google Scholar]
- 12.King JC, Ng TT, White SC, Cortina G, Reber HA, Hines OJ. Pancreatic serous cystadenocarcinoma: a case report and review of the literature. J Gastrointest Surg. 2009;13:1864–1868. doi: 10.1007/s11605-009-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto T, Hirano S, Yada K, Shibata K, Sasaki A, Kamimura T, et al. Malignant serous cystic neoplasm of the pancreas: report of a case and review of the literature. J Clin Gastroenterol. 2005;39:253–256. doi: 10.1097/01.mcg.0000152749.64526.38. [DOI] [PubMed] [Google Scholar]
- 14.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol. 2011;20:e84–e92. doi: 10.1016/j.suronc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Galanis C, Zamani A, Cameron JL, Campbell KA, Lillemoe KD, Caparrelli D, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–826. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 16.Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandezdel Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okano K, Hirao T, Unno M, Fujii T, Yoshitomi H, Suzuki S, et al. Postoperative infectious complications after pancreatic resection. Br J Surg. 2015;102:1551–1560. doi: 10.1002/bjs.9919. [DOI] [PubMed] [Google Scholar]
- 18.Pugalenthi A, Protic M, Gonen M, Kingham TP, Angelica MI, Dematteo RP, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2016;113:188–193. doi: 10.1002/jso.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeliger H, Christians S, Angele MK, Kleespies A, Eichhorn ME, Ischenko I, et al. Risk factors for surgical complications in distal pancreatectomy. Am J Surg. 2010;200:311–317. doi: 10.1016/j.amjsurg.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Li ZS, Li Q. The latest 2010 WHO classification of tumors of digestive system. Zhonghua Bing Li Xue Za Zhi. 2011;40:351–354. [PubMed] [Google Scholar]
- 21.Chu LC, Singhi AD, Haroun RR, Hruban RH, Fishman EK. The many faces of pancreatic serous cystadenoma: radiologic and pathologic correlation. Diagn Interv Imaging. 2017;98:191–202. doi: 10.1016/j.diii.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas) Gut. 2016;65:305–312. doi: 10.1136/gutjnl-2015-309638. [DOI] [PubMed] [Google Scholar]
- 23.Werner JB, Bartosch-Harlid A, Andersson R. Cystic pancreatic lesions: current evidence for diagnosis and treatment. Scand J Gastroenterol. 2011;46:773–788. doi: 10.3109/00365521.2011.551892. [DOI] [PubMed] [Google Scholar]
- 24.Hwang HK, Chung YE, Kim HK, Park JY, Choi HJ, Kang CM, et al. Serous cystic neoplasm: do we have to wait till it causes trouble? Korean J Hepatobiliary Pancreat Surg. 2011;15:134–138. doi: 10.14701/kjhbps.2011.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang HK, Kim H, Kang CM, Lee WJ. Serous cyst adenoma of the pancreas: appraisal of active surgical strategy before it causes problems. Surg Endosc. 2012;26:1560–1565. doi: 10.1007/s00464-011-2070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Hayek KM, Brown N, O'Rourke C, Falk G, Morris-Stiff G, Walsh RM. Rate of growth of pancreatic serous cystadenoma as an indication for resection. Surgery. 2013;154:794–800. doi: 10.1016/j.surg.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Malleo G, Bassi C, Rossini R, Manfredi R, Butturini G, Massignani M, et al. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut. 2012;61:746–751. doi: 10.1136/gutjnl-2011-300297. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XP, Yu ZX, Zhao YP, Dai MH. Current perspectives on pancreatic serous cystic neoplasms: diagnosis, management and beyond. World J Gastrointest Surg. 2016;8:202–211. doi: 10.4240/wjgs.v8.i3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins BT. Serous cystadenoma of the pancreas with endoscopic ultrasound fine needle aspiration biopsy and surgical correlation. Acta Cytol. 2013;57:241–251. doi: 10.1159/000346911. [DOI] [PubMed] [Google Scholar]
- 30.Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, et al. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323–1324. doi: 10.1046/j.1440-1746.2003.03040.x. [DOI] [PubMed] [Google Scholar]