Abstract
Glaucoma is characterized by retinal ganglion cell (RGC) death and axonal loss. Therefore, neuroprotection is important in treating glaucoma. In this study, we explored whether exoenzyme C3 transferase (C3)-based gene therapy could protect retinas in an ischemia/reperfusion (I/R) injury rat model. Self-complementary adeno-associated virus 2 (scAAV2) vectors encoding either C3 protein (scAAV2-C3) or enhanced green fluorescence protein (scAAV2-EGFP) were intravitreally delivered into both eyes of rats, and I/R models (acute ocular hypertension) were made in one eye of each rat at day 7 after the injection. The rats were divided into six groups: scAAV2-C3, scAAV2-C3 with I/R, scAAV2-EGFP, scAAV2-EGFP with I/R, blank control, and blank control with I/R. TUNEL (terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling), immunohistochemistry of cleaved caspase-3, NeuN and Brn-3a, and H&E staining were used to detect apoptotic cells and other changes in the retina. The results showed that scAAV2-C3 significantly reduced the number of apoptotic RGCs and decreased cell loss in the ganglion cell layer after I/R injury, and the I/R-injured retinas treated with scAAV2-C3 were the thickest in all I/R groups. These results suggest that scAAV2-mediated C3 gene therapy is able to protect the rat retina from I/R injury and has potential in the treatment of glaucoma in the future.
Keywords: self-complementary AAV, exoenzyme C3 transferase, gene therapy, ischemia/reperfusion injury, optic neuroprotection, rat
Graphical Abstract
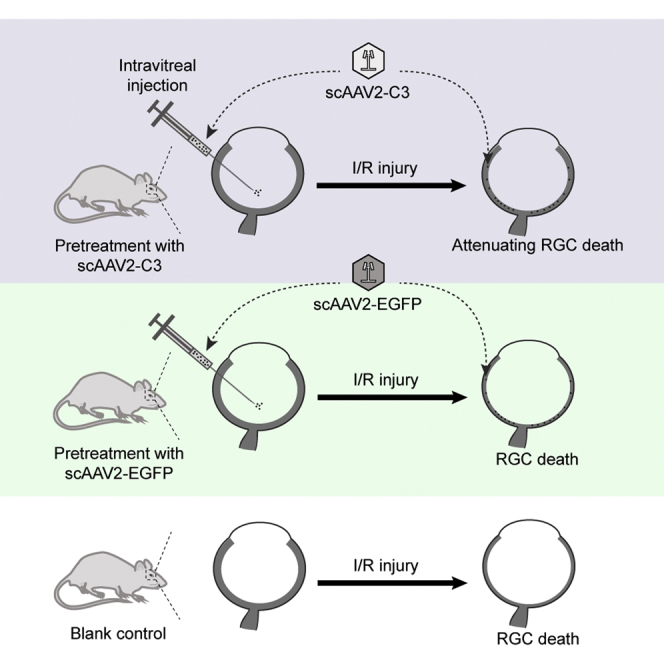
Fan and colleagues showed that scAAV2-mediated C3 gene therapy is able to protect the rat retina from I/R injury and has potential in the treatment of glaucoma in the future.
Introduction
Glaucoma is characterized by retinal ganglion cell (RGC) death and axonal loss, and therefore it is defined as a neurodegenerative disease, with elevated intraocular pressure (IOP) as the main risk factor. Even though IOP-lowering treatment is an effective therapeutic approach to treat glaucoma, it is generally known that IOP lowering only is insufficient to prevent the glaucomatous neurodegeneration in patients with glaucoma. At present, however, there are no clinically approved neuroprotective strategies available for glaucomatous optic neuropathy. Therefore, effective approaches of neuroprotection for glaucoma treatment are in urgent need. Exoenzyme C3 transferase (C3) isolated from Clostridium botulinum can inactivate all the three Rho GTPases (Rhos), i.e., RhoA, RhoB, and RhoC, by adenosine diphosphate (ADP)-ribosylation. Rhos are involved in various cellular processes, such as regulation of actin cytoskeleton, cell proliferation, and apoptosis.1 Our previous work showed that C3 protein, as a Rho inhibitor, was able to protect RGCs from excitotoxic damage induced by N-methyl-d-aspartate (NMDA) in rats.2 After central nervous system (CNS) injury, C3 reduced the number of TUNEL (terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling)-labeled cells by 50% in both injured mice and rats.3 H-1152P, a downstream inhibitor of the Rho pathway, was also neuroprotective in both organotypic cultures of mouse retinas under serum deprivation and rats treated with optic nerve crush (ONC).4
Currently, recombinant adeno-associated viral vector (AAV) has been commonly used in gene therapy trials for ocular diseases, particular for the retina, due to their stable, relatively long-term expression and their low immunogenicity.5 Self-complementary AAV (scAAV) is formed with complementary double-stranded DNA, which is capable of inducing a faster and stronger transgene expression in neurons and their axons.6
Retinal neurons, particularly RGCs, are vulnerable to ischemia, and thinning of the nerve fiber layer will occur as a result of ganglion cell loss.7,8 As mentioned above, our previous study showed that C3 protein could protect RGCs from excitotoxic damage induced by NMDA in rats. In present study, we established an ischemia-reperfusion (I/R) injury model by anterior chamber perfusion and explored whether scAAV2-mediated C3 gene therapy could protect the retina from I/R injury in a rat acute ocular hypertensive model.
Results
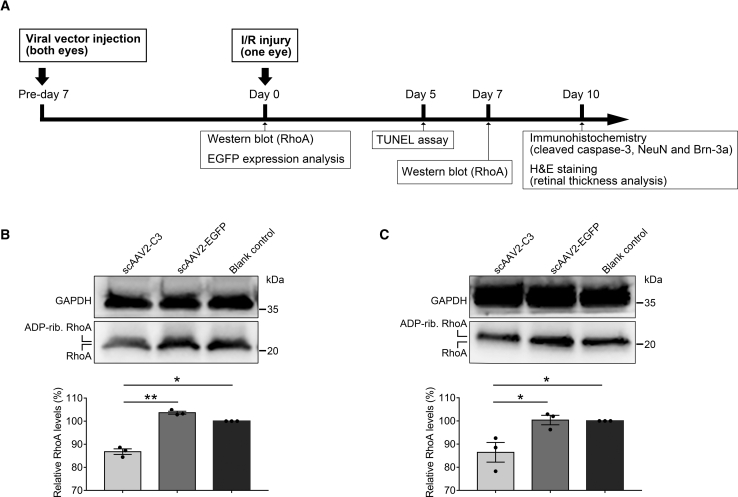
RhoA Inhibition in Rat Retina after Intravitreal Injection of scAAV2-C3
Figure 1A shows the overall experimental workflow. RhoA expression in rat retinas was examined on days 7 and 14 by western blot following scAAV2-C3 delivery. As shown in Figures 1B and 1C, a slight increase in molecular weight and significant decrease in protein level of RhoA were found in the scAAV2-C3 group at two time points (p < 0.01 at day 7 or p < 0.05 at day 14 versus the scAAV2-EGFP group, p < 0.05 at days 7 and 14 versus the blank control group; n = 3), which was consistent with our previous studies.2,9 No statistically significant difference (p > 0.05) was observed between the scAAV2-EGFP group and the blank control group (Figures 1B and 1C; n = 3).
Figure 1.
Western Blot and Densitometric Analysis for Total RhoA Expression in Rat Retina after Delivery
(A) Design of the experiment of the study. (B and C) Western blots examined RhoA expression at days 7 (B) and 14 (C) after delivery. The results were normalized to the reference protein GAPDH, and these values were further standardized to those of the blank control group. ∗p < 0.05 by an LSD post hoc test (n = 3); ∗∗p < 0.01 by an LSD post hoc test (n = 3). No statistically significant difference (p > 0.05) was detected by an LSD post hoc test between the scAAV2-EGFP group and the blank control group (n = 3). Error bars show SEM. ADP-rib. RhoA, ADP-ribosylated RhoA.
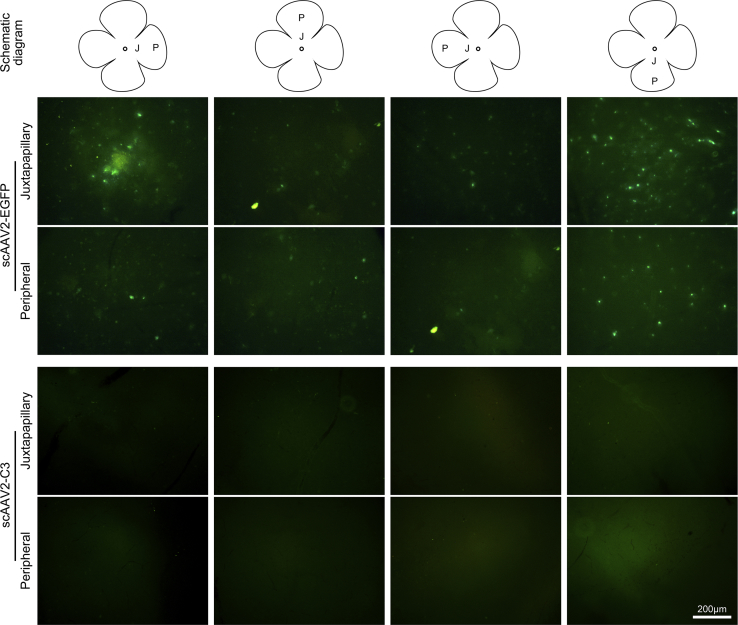
EGFP Expression in Rat Retina following Vector Delivery
In order to confirm the transduction of scAAV2 vectors used in the study, detection of EGFP in the retina was done after intravitreal delivery of the vectors. Because the EGFP fluorescence is lost easily during the tissue processing of histopathology,10 we chose direct fluorescence detection to examine the EGFP expression in the retinal flat mount. On day 7 post-delivery, there was an obvious EGFP fluorescence throughout a large area in flat-mounted retina treated by scAAV2-EGFP, not present in the scAAV2-C3-treated retinal flat mount (Figure 2).
Figure 2.
Direct Green Fluorescence Detection of EGFP in Rat Flat-Mounted Retinas on Day 7 after Delivery of scAAV2 Vectors
Schematic diagram of a flat-mounted retina showing the analyzed regions (890 × 670 μm), including juxtapapillary (J) and peripheral (P) regions. The green fluorescence was found only in the scAAV2-EGFP-treated retina and was not present in the scAAV2-C3-treated retina.
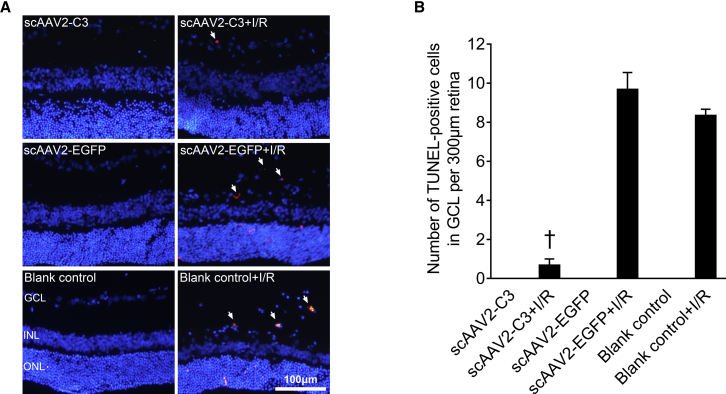
Intravitreal Delivery of scAAV2-C3 Prevented Retinal Cells from I/R Injury-Induced Apoptosis
TUNEL-positive cells were localized in the whole retinal layer in all I/R injury groups with weak fluorescence, although they did not exist in all uninjured control groups (Figure 3A). The mean numbers of TUNEL-positive cells in the ganglion cell layer (GCL) per 300 μm of retina length of the scAAV2-C3 + I/R, scAAV2-EGFP + I/R, and blank control + I/R groups were 0.7 ± 0.3, 9.7 ± 0.9, and 8.3 ± 0.3 cells/300 μm, respectively. The number of TUNEL-positive cells per 300 μm of retina length in the scAAV2-C3 + I/R group was significantly decreased compared to that in the scAAV2-EGFP + I/R group (Figure 3B, p < 0.05; n = 3) and in the blank control + I/R group (Figure 3B, p < 0.05; n = 3).
Figure 3.
Intravitreal Delivery of scAAV2-C3 Reduced the Number of TUNEL-Positive Cells in the GCL of Rat Retinas
(A) Representative images of TUNEL staining in the six groups. Blue, DAPI; red, TUNEL-positive cells. Arrows denote TUNEL-positive cells in the ganglion cell layer (GCL). (B) Quantitative analysis of the number of TUNEL-positive cells per 300 μm of retina length in each group. †p < 0.05 versus the scAAV2-EGFP + I/R group by an LSD post hoc test (n = 3), and the blank control + I/R group by an LSD post hoc test (n = 3). No statistically significant difference (p > 0.05) was detected by an LSD post hoc test between the scAAV2-EGFP + I/R and the blank control + I/R groups (n = 3). Error bars show SEM. INL, inner nuclear layer; ONL, outer nuclear layer.
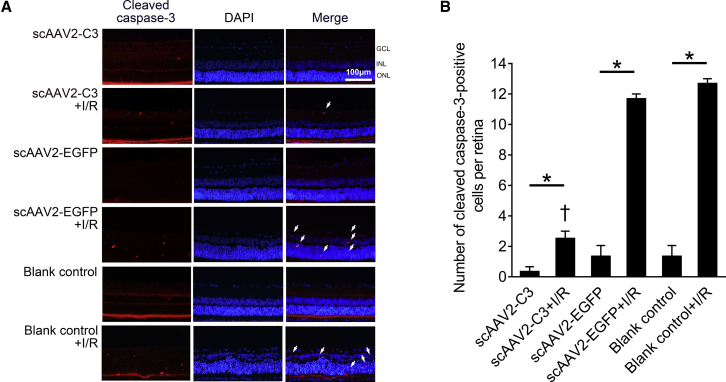
Cleaved caspase-3 is a classic apoptotic marker commonly used in RGC apoptosis. The cleaved caspase-3-positive cells were located diffusely in the neurosensory retina, including the GCL, inner nuclear layer (INL), and outer nuclear layer (ONL), with weak fluorescence in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups (Figure 4A). The mean numbers of cleaved caspase-3-positive cells in each neurosensory retina of the six groups were 0.3 ± 0.3, 2.5 ± 0.5, 1.3 ± 0.7, 11.7 ± 0.3, 1.3 ± 0.7, and 12.7 ± 0.3 cells, respectively. The positive cells in I/R injury groups were significantly more than those in corresponding uninjured control groups (Figure 4B, p < 0.05; n = 3). I/R-injured retinas treated with scAAV2-C3 showed the least positive cells than those in the other I/R injury groups (Figure 4B, p < 0.05; n = 3).
Figure 4.
Intravitreal Delivery of scAAV2-C3 Reduced the Expression of Cleaved Caspase-3
(A) Representative images of cleaved caspase-3 immunofluorescence in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups. Blue, DAPI; red, cleaved caspase-3-positive cell (arrow). (B) Quantitative analysis of cleaved caspase-3-positive cells in each neurosensory retina. ∗p < 0.05 by an LSD post hoc test (n = 3); †p < 0.05 versus the scAAV2-EGFP + I/R group by an LSD post hoc test (n = 3) and the blank control + I/R group by an LSD post hoc test (n = 3). No statistically significant difference (p > 0.05) was detected by an LSD post hoc test between the scAAV2-EGFP + I/R and the blank control + I/R groups (n = 3), or among the scAAV2-C3, scAAV2-EGFP, and blank control groups (n = 3). Error bars show SEM.
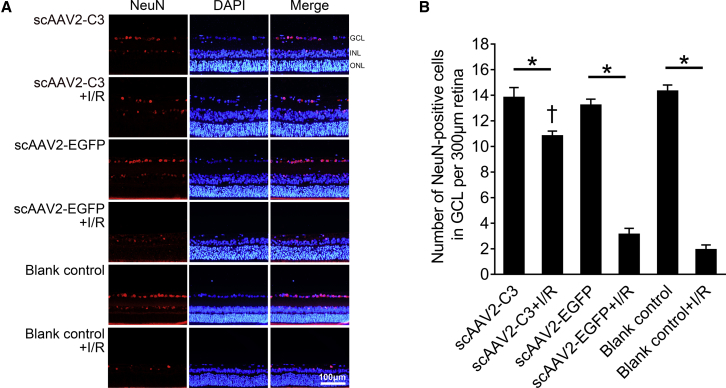
Intravitreal Delivery of scAAV2-C3 Attenuated I/R-Inducing Retinal Neurons and Reduction of RGCs
The nuclei-stained NeuN-positive cells localized predominantly in the GCL in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups (Figure 5A). The mean numbers of NeuN-positive cells in the GCL of the six groups were 13.8 ± 0.8, 10.8 ± 0.4, 13.2 ± 0.5, 3.1 ± 0.5, 14.3 ± 0.5, and 1.9 ± 0.4 cells/300 μm of retina length, respectively. I/R injury induced significant reduction of retinal neurons in the GCL in the scAAV2-C3 + I/R, scAAV2-EGFP + I/R, and blank control + I/R groups (Figure 5B, p < 0.05; n = 3). The numbers of surviving neurons in the GCL in the scAAV2-C3 + I/R group were significantly more than those in the scAAV2-EGFP + I/R group and blank control + I/R group (Figure 5B, p < 0.05; n = 3). Furthermore, there was no statistically significant difference of surviving neurons in the GCL between the scAAV2-EGFP + I/R and blank control + I/R groups (Figure 5B, p > 0.05; n = 3).
Figure 5.
Intravitreal Delivery of scAAV2-C3 Eased Neuronal Cell Loss in the GCL after I/R Injury
(A) Representative images of NeuN immunofluorescence in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups. The NeuN-positive cells localized predominantly in the GCL and showed weak fluorescence in the INL. Blue, DAPI; red, NeuN-positive cell. (B) Quantitative analysis of NeuN-positive cells per 300 μm of retina length in each group. ∗p < 0.05 by an LSD post hoc test (n = 3); †p < 0.05 versus the scAAV2-EGFP + I/R group by an LSD post hoc test (n = 3), and the blank control + I/R group by an LSD post hoc test (n = 3). No statistically significant difference (p > 0.05) was detected by an LSD post hoc test between the scAAV2-EGFP + I/R and the blank control + I/R groups (n = 3), or among the scAAV2-C3, scAAV2-EGFP, and blank control groups (n = 3). Error bars show SEM.
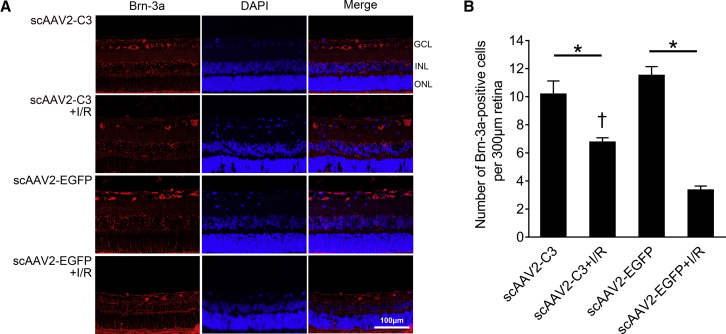
In order to observe RGCs in the GCL, immunohistochemical analysis of Brn-3a (RGC marker) was performed in rat retinas treated with the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, and scAAV2-EGFP + I/R groups. The Brn-3a immunofluorescence was expressed only in the GCL in the four groups (Figure 6A). The mean numbers of Brn-3a-positive cells were 10.2 ± 1.0, 6.8 ± 0.3, 11.5 ± 0.6, and 3.3 ± 0.3 cells/300 μm of retina length in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, and scAAV2-EGFP + I/R groups, respectively. Similar to the results of NeuN immunofluorescence, I/R injury induced significant reductions of RGCs in the scAAV2-C3 + I/R group and scAAV2-EGFP + I/R group (Figure 6B, p < 0.05; n = 4). The surviving RGCs in the scAAV2-C3 + I/R group was significantly more than that in the scAAV2-EGFP + I/R group (Figure 6B, p < 0.05; n = 4), and no statistically significant difference in the number of RGCs was found between the scAAV2-C3 group and the scAAV2-EGFP group (Figure 6B, p > 0.05; n = 4).
Figure 6.
Intravitreal Delivery of scAAV2-C3 Reduced the Loss of RGCs after I/R Injury
(A) Representative images of Brn-3a immunofluorescence in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, and scAAV2-EGFP + I/R groups. Brn-3a immunofluorescence was only detected in the GCL of the four groups. Blue, DAPI; red, Brn-3a-positive cell. (B) Quantitative analysis of Brn-3a-positive cells in the GCL per 300 μm of retina length. ∗p < 0.05 by an LSD post hoc test (n = 4); †p < 0.05 versus the scAAV2-EGFP + I/R group by an LSD post hoc test (n = 4). No statistical difference (p < 0.05) was detected by an LSD post hoc test between the scAAV2-C3 and the scAAV2-EGFP groups (n = 4). Error bars show SEM.
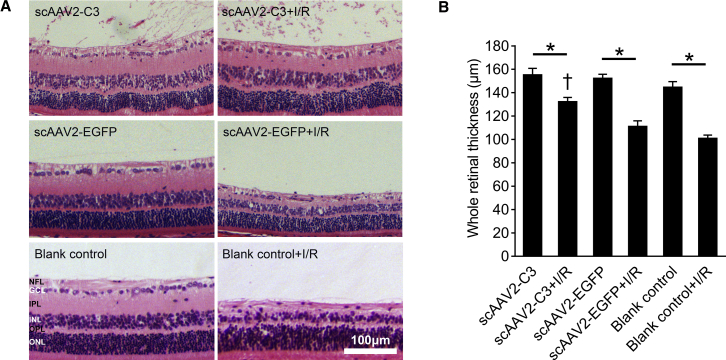
Intravitreal Delivery of scAAV2-C3 Suppressed the I/R Injury-Induced Retinal Thinning
Hematoxylin and eosin (H&E) staining was performed on paraffin section of the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups, and whole retinal thickness of each section was measured. scAAV2 vectors had no obvious effect on the retinal morphology and structure (Figure 7A). Remarkable retinal tissue loss was observed in the scAAV2-EGFP + I/R and blank control + I/R groups, whereas the morphology and structure of scAAV2-C3-treated retinas almost resembled the uninjured retinas (Figure 7A).
Figure 7.
Intravitreal Delivery of scAAV2-C3 Mitigated the Reduction in Retinal Thickness
(A) Representative images of H&E staining of retinas in the scAAV2-C3, scAAV2-C3 + I/R, scAAV2-EGFP, scAAV2-EGFP + I/R, blank control, and blank control + I/R groups. (B) Quantitative analysis of the whole retina thickness in each group. ∗p < 0.05 by an LSD post hoc test (n = 4); †p < 0.05 versus the scAAV2-EGFP + I/R group, and the blank control + I/R group by an LSD post hoc test (n = 4). No statistical difference (p > 0.05) was detected by an LSD post hoc test between the scAAV2-EGFP + I/R and the blank control + I/R groups (n = 4), or among the scAAV2-C3, scAAV2-EGFP, and blank control groups (n = 4). NFL, nerve fiber layer; IPL, internal plexiform layer; OPL, outer plexiform layer. Error bars show SEM.
The mean whole retinal thicknesses of the six groups were 155.0 ± 5.8, 132.1 ± 3.8, 152.0 ± 3.7, 110.9 ± 5.0, 144.4 ± 5.1, and 100.7 ± 3.1 μm, respectively (Figure 7B; n = 4). The mean whole retinal thicknesses were significantly reduced in all I/R groups compared to those in the corresponding uninjured control groups (Figure 7B, p < 0.05; n = 4), but the decrease of whole retinal thickness was significantly attenuated by scAAV2-mediated C3 expression in the scAAV2-C3 + I/R group (Figure 7B, p < 0.05 versus the scAAV2-EGFP + I/R group and the blank control + I/R group; n = 4).
Discussion
The I/R rat model used in this study is a retinal circulatory disorder induced by elevating the IOP for a definite period of time, a major risk factor involved in the pathogenesis of several ocular disorders, including glaucoma. The elevated IOP compresses retinal blood vessels and causes impaired blood flow. Upon IOP reduction, the following reperfusion induces increased oxidative stress, and a higher concentration of the reactive oxygen species (ROS) ensues, which triggers the formation of free radicals. These radicals attack the cellular structures and proteins and cause additional cell/tissue damage.11 The necrotic and apoptotic retinal cell death induced by I/R injury are mostly due to the elevated levels of ROS, including hydroxyl radical, superoxide, singlet oxygen, and hydrogen peroxide.12,13 The excessive levels of ROS result in hypersecretion of excitatory amino acids, including glutamate and aspartate, which will lead to an influx of excessive free calcium by stimulation of the NMDA receptor-operated channels afterward.14 The secretion of glutamate and calcium influx, which leads to mitochondrial dysfunction, will ultimately induce apoptosis.15, 16, 17, 18
Rho and Rho kinase (ROCK) play a role in I/R-induced cellular injuries. Previous studies demonstrated that ischemia and subsequent reperfusion enhanced ROCK activity in myocardium and lung,19, 20, 21 and inhibition of ROCK exerted a protective effect against I/R injury.20,22,23 Yamamoto et al.24 suggested that K-115, a ROCK inhibitor, attenuated RGC death in mice after ONC by inhibiting nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)-mediated ROS production. Other ROCK inhibitors, such as Y-27632 and fasudil, exert their neuroprotective effect against I/R injury in rat retinas by regulating leukocyte infiltration and inhibiting apoptosis.25,26 Moreover, Cen et al. reported that short hairpin RNA against RhoA promoted RGC axon regeneration in rats after ONC.27 As a Rho inhibitor, C3 inactivates RhoA, RhoB, and RhoC by transferring an ADP-ribose moiety from nicotinamide adenine dinucleotide (NAD+) onto asparagine 41 of Rho.28,29 The dysfunctional Rho causes a serials of cellular consequences, including disorganization and disruption of the actin cytoskeleton and cellular adhesions, leading to morphological changes and impaired formation of the contractile ring.30,31 Our previous studies and those of other groups have shown that C3 protein is able to promote RGC survival and axon regeneration in animal models, including NMDA-induced excitotoxic damage and autologous peripheral nerve grafts.2,32 The present study indicated that C3 exhibited neuroprotective effects in the rat I/R model via inhibition of the Rho-activating pathway. Our results by TUNEL assay and immunohistochemistry of cleaved caspase-3 found that scAAV2-mediated C3 expression could protect retinal neuronal cells from I/R injury by preventing retinal apoptosis. To detect the surviving neuronal cells in the GCL, in addition, immunohistochemistry of NeuN was performed. Brn-3a is a member of POU-domain transcription factors and plays an important role in differentiation, survival, and axonal extension of RGCs during their development.33 As an endogenous marker of RGCs, the expression level of Brn-3a could reflect the physiological status of RGCs, which will diminish as the cells enter the process of apoptosis.34 These results show that scAAV2-mediated C3 gene therapy could increase the survival of neuronal cells, including RGCs after I/R injury, and alleviate the reduction of retinal thickness after I/R injury, suggesting that C3 may hold potential as a novel therapeutic strategy for glaucomatous and probably other ischemia-related optic neuropathies.
AAV vectors play a crucial role in the delivery of therapeutic genes in gene therapy studies. Single-stranded AAV vectors (ssAAVs) have been used in gene therapy trials such as inherited retinal and optic nerve diseases.35, 36, 37 Previous studies suggested that ssAAV vectors were a safe and efficient tool for gene therapy.38,39 Transduction efficiency in trabecular meshwork (TM) tissue upon intracameral delivery of ssAAVs seems very low because it is hard for AAVs to form the double-stranded DNA by themselves.40 The scAAV2 is modified so as to bypass the required second-strand DNA synthesis and therefore exhibits efficient transduction in the cultured human anterior segments and in the TM of rats and monkeys.5,40 Furthermore, as long as the serotype of AAV is considered, our previous studies demonstrated that intracameral injection of scAAV2-C3 induced a long-term IOP-lowering effect in monkey eyes.41 Bogner et al.42 suggested that scAAV2-based vectors transduced the TM of mice and rats more effectively than did scAAV8-based vectors. In addition, the scAAV2 vector transduces RGCs around the optic nerve head more efficiently than do scAAV5 and scAAV8 after intravitreal injection.43,44 Intravitreally delivered ssAAV2 had a marginally higher efficiency of transduction of both outer and inner retinas than did scAAV2, while scAAV2 showed a more rapid expression onset than for ssAAV2.45,46 Our results are in line with these observations, as an obvious green fluorescence over a large area of flat-mounted retina was observed and a significant change in RhoA expression was detected as early as day 7 after delivery of the vectors. The AAV2 vectors, either ssAAV2 or scAAV2, can mediate the expression of the reporter gene in various types of inner retinal cells, including the ganglion cell layer neurons, amacrine cells, horizontal cells, and even Müller cells or bipolar cells after intravitreal injection.45,47, 48, 49 In our previous study,2 intravitreal injection of C3 protein induced an increase in molecular weight and a decrease in protein level (lower than PBS-treated or NMDA-treated control) of RhoA in the rat retina of the NMDA-induced model. The present study also showed that the RhoA expression was reduced by about 15% in the rat retina following the scAAV3-C3 treatment. Therefore, the inhibition of RhoA expression caused by either C3 protein or scAAV2-mediated C3 expression was clear in the rat retina. scAAV2 encoding exogenous brain-derived neurotrophic factor resulted in high transduction in the neural retina of rats and protection to RGCs from I/R injruy.50 Feuer et al.51 reported that intravitreal injection of scAAV2 expressing a normal ND4 complementary DNA could delay or improve visual acuity in the patients with Leber hereditary optic neuropathy (LHON) and did not induce serious safety issues. Our current study also showed that scAAV2-mediated C3 delivery protected the inner retinas of rats from I/R injury, likely by inhibiting the Rho/ROCK pathway. Taken together, scAAV2 appears to be a suitable gene therapy vector for retinal degenerative diseases and glaucomatous optic neuropathies.
In summary, the current study confirmed the neuroprotective effects of intravitreal delivery of C3 in rat models with I/R injuries. These results, together with our previous studies showing that C3 protein attenuated NMDA-induced excitotoxic damage,2 suggest that C3 holds great potential in the treatment of glaucomatous optic neuropathy. In addition, an scAAV2 vector seems to be promising for use in studies of ocular neuroprotective gene therapies. More studies are essentially needed with other animal models.
Materials and Methods
Vector Construction
scAAV2-EGFP (1 × 1012 viral genomes [vg]/mL) and scAAV2-C3 (5 × 1011 vg/mL) vectors were prepared by the Beijing FivePlus Molecular Medicine Institute (FMMI) (Beijing, China), as previously described.41
Animals and Animal Groups
All experimental procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The study protocol was approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Sciences, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital. Sprague-Dawley (SD) rats weighing 200–240 g were purchased from Charles River Laboratory Animal Technology Company (Beijing, China). Rats were fed with a standard diet, had unlimited water, and were kept in a 12-h light/12-h dark cycle. Viral vectors were injected intravitreally into both eyes of each rat. Meanwhile, rat eyes receiving no intravitreal injection were defined as the “blank control” group. Seven days after intravitreal injection, the I/R injury was performed in one eye of each rat, as described below, while the contralateral eye served as the uninjured control. The rats were then divided into six groups: (1) scAAV2-C3, (2) scAAV2-C3 with I/R injury (scAAV2-C3 + I/R), (3) scAAV2-EGFP, (4) scAAV2-EGFP with I/R injury (scAAV2-EGFP + I/R), (5) blank control, and (6) blank control with I/R injury (blank control + I/R).
Intravitreal Injections
10% chloral hydrate (Sigma-Aldrich, St. Louis, MO, USA) with a dose of 4 mL/kg bodyweight was intraperitoneally injected for anesthesia. 1% tropicamide and 0.5% tetracaine hydrochloride were topically applied to the operative eyes for mydriasis and topical anesthesia, respectively, before intravitreal injection. Hamilton microsyringes (33G, Hamilton Company, Reno, NV, USA) were used for intravitreal injections under an ophthalmic surgical microscope (Leica Heerbrugg, Switzerland). Both eyes were injected with the same vector for each rat. A dose of 2.5 × 109 vg scAAV2-C3 or scAAV2-EGFP vectors was injected into both eyes of each rat. Ofloxacin ophthalmic ointment (Santen, Tokyo, Japan) was topically administered to prevent ocular infection.
Western Blot Analysis
Retinal tissues transduced with scAAV2 vectors were harvested in cold RIPA (radioimmunoprecipitation assay) buffer (CWBio, Beijing, China) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (CWBio). Each retina served as an individual sample (n = 3 per group). Equal amounts of protein (50 μg/lane) were separated on a commercial polyacrylamide gel (4%–20% Mini-PROTEAN or Criterion TGX [Tris-glycine extended] precast gel; Bio-Rad, Hercules, CA, USA) and transferred onto a polyvinylidene fluoride membrane (Millipore, Burlington, MA, USA). After blocking for 1 h in 5% non-fat dry milk in Tris-buffered saline with Tween 20 (TBS-T), membranes were incubated with primary antibodies against RhoA (2117, rabbit monoclonal antibody, 1:1,000; Cell Signaling Technology, Danvers, MA, USA) or GAPDH (AF5718, goat polyclonal antibody, 1 mg/mL; R&D Systems, Minneapolis, MN, USA) at 4°C overnight. After washing with TBS-T three times, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000; ZSGB-Bio) at room temperature for 1 h. The membrane was then washed three times with TBS-T at room temperature. Bands were exposed with a Fluor ChemE (92-14860-00; ProteinSimple, San Jose, CA, USA). The signal densitometry was analyzed with ImageJ software.
Direct GFP Detection in the Flat-Mounted Retina
Rats were sacrificed and the eyes were enucleated immediately. Eyes were fixed with 4% paraformaldehyde (PFA) in 1× PBS at pH 7.4 for 0.5 h, after which the anterior segments were removed, and the entire retinas were carefully dissected from the eyecups. Four radial cuts were made on retina to flatten the tissue, and the flat-mounted retinas (inner retina facing up) were sealed with an antifade mounting medium and the coverslip. Green fluorescence was detected on the juxtapapillary and peripheral regions of retina with an Olympus CKX53 inverted fluorescence microscope (Olympus, Tokyo, Japan).
Retinal I/R Model
At 7 days post-injection, retinal I/R injury was performed in one eye of each rat and the model was made as described previously.52 10% chloral hydrate with a dose of 4 mL/kg bodyweight was intraperitoneally injected for anesthesia. Briefly, a 26G needle was cannulated into the anterior chamber of one eye. The needle was connected to a saline reservoir containing 0.9% NaCl, which was elevated a height by which the IOP raised to 110 mm Hg and was maintained for 90 min. Retinal ischemia was confirmed by whitening of the iris and loss of red reflex from the fundus. Reperfusion was confirmed by returning of iris color and fundus red reflex after withdrawal of the needle from the anterior chamber. The contralateral eye remained untreated and served as the uninjured control. 9 of the rats were sacrificed on day 5 after I/R injury and frozen sections of eyeballs were prepared, and 12 of the rats were sacrificed on day 10 after I/R injury and paraffin section of eyeballs were prepared for further analysis.
TUNEL Assay on the Retinal Frozen Section
After fixation in 4% PFA for 60 min, the eyeballs were equilibrated in 30% sucrose overnight, then embedded in optimal cutting temperature compound (OCT; Sakura Finetek USA, Torrance, CA, USA) on the 5th day after acute retinal ischemia. Cryosections (6 μm) were obtained. Then, a TUNEL assay was performed using an In Situ Cell Death Detection Kit (TMR red; Roche Diagnostics, Manheim, Germany) according to the manufacturer’s instructions. After washing with PBS, nuclei were counterstained with Fluoroshield containing DAPI (4′,6′-diamidino-2-phenylindole; Sigma-Aldrich, Poole, UK). The TUNEL-positive cells were examined on the juxtapapillary retinas with a confocal laser scanning microscope (Leica TCS SP5; Leica Microsystems, Wetzlar, Hesse, Germany), then the average of TUNEL-positive cells in the GCL of each visual field were calculated and presented as cells/300 μm. Images were analyzed with Photoshop 7.0 (Adobe, San Jose, CA, USA).
H&E Staining and Immunohistochemistry
Eye tissue samples were fixed in a formaldehyde, acetic acid, and saline (FAS) fixative (Wuhan Servicebio, Wuhan, China) and embedded in paraffin on the 10th day after acute retinal ischemia. 5-μm-thick sections were made by a microtome (RM2245; Leica Biosystems, Nussloch, Germany). Slides were stained with H&E to evaluate the retinal thickness. Images of juxtapapillary retina were taken from each section with an Olympus inverted fluorescence microscope (CKX53; Olympus, Central Valley, PA, USA), and the whole retinal thickness was measured. Immunohistochemical staining was performed on the eye sections that were deparaffinized by xylene and rehydrated by three concentrations of absolute ethyl alcohol (100%, 95% and 75%). To unmask the antigen epitopes, heat-induced antigen retrieval was performed with 0.1 M citrate buffer (pH 6.0) (Wuhan Servicebio) for 10 min at almost 100°C. The slides were then rinsed in water and blocked with 5% bovine serum albumin in PBS, which contained 1% Triton X-100, for 1 h at room temperature. Next, slides were incubated with primary antibodies (Table 1) overnight at 4°C. After washing with PBS three times, slides were incubated with secondary antibodies (Table 1) at room temperature and washed with PBS three times again. Finally, the Fluoroshield containing DAPI (Sigma-Aldrich) was used to mount the cover slides. Immunohistochemistry of cleaved caspase-3, NeuN, and Brn-3a was performed to evaluate the changes of apoptosis, retinal neurons, and RGCs, respectively. All sections were examined on the juxtapapillary retinas with a confocal laser scanning microscope (Leica TCS SP5), then the average of positive cells per field was calculated and presented as cells/300 μm. Images were analyzed with Photoshop 7.0 (Adobe).
Table 1.
Primary and Corresponding Secondary Antibodies Used for Immunohistochemistry.
| Primary Antibodies | Host/Catalog No. | Company | Dilution | Secondary Antibodies | Company | Dilution |
|---|---|---|---|---|---|---|
| Cleaved caspase-3 | rabbit/9661 | Cell Signaling Technology | 1:50 | Alexa Fluor 594 donkey anti-rabbit IgG (H+L) | Life Technologies | 1:500 |
| Brn-3a | mouse/sc-8429 | Santa Cruz Biotechnology | 1:50 | Alexa Fluor 594 goat anti-mouse IgG (H+L) | Life Technologies | 1:500 |
| NeuN | rabbit/ab177487 | Abcam | 1:200 | Alexa Fluor 594 donkey anti-rabbit IgG (H+L) | Life Technologies | 1:500 |
Statistical Analysis
Data are presented as means ± SEM (stand error of the mean). SPSS 18 software (SPSS, Chicago, IL, USA) was used for all statistics. One-way analysis of variance (ANOVA) followed by a least significant difference (LSD) post hoc test for multiple comparisons was used to compare differences among the groups when the variance was homogeneous. p < 0.05 was considered to be statistically significant.
Author Contributions
N.F., X.L., and X. Zhu conceived the study and experiments and secured funding. J.T., X. Zhang, and D.L. monitored the study, performed experiments, and wrote the manuscript. N.F. and G.L. wrote the study protocol and monitored experiments. Y.W., D.Z., X.W., W.T., X.D., and L.Z. provided help, expertise, and feedback.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81770924, 81900829 and 81770950), the Science and Technology Plan Project in Medical and Health of Xiamen (3502Z20194066 and 3502Z20194069), the Sanming Project of Medicine in Shenzhen (SZSM201512045), the Science and Technology Innovation Committee of Shenzhen (JCYJ20160428144701106), and the Guangdong Basic and Applied Basic Research Foundation (2019A1515011234).
Contributor Information
Xianjun Zhu, Email: xjzhu@uestc.edu.cn.
Xuyang Liu, Email: xliu1213@126.com.
Ning Fan, Email: szfanning@126.com.
References
- 1.von Elsner L., Hagemann S., Just I., Rohrbeck A. C3 exoenzyme impairs cell proliferation and apoptosis by altering the activity of transcription factors. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389:1021–1031. doi: 10.1007/s00210-016-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Wang Y., Yang Q., Guo L., Yin Y., Fan N., Zhou X., Cai S.P., Kaufman P.L., Liu X. Neuroprotective effects of C3 exoenzyme in excitotoxic retinopathy. Exp. Eye Res. 2014;125:128–134. doi: 10.1016/j.exer.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubreuil C.I., Winton M.J., McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J. Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tura A., Schuettauf F., Monnier P.P., Bartz-Schmidt K.U., Henke-Fahle S. Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Invest. Ophthalmol. Vis. Sci. 2009;50:452–461. doi: 10.1167/iovs.08-1973. [DOI] [PubMed] [Google Scholar]
- 5.Buie L.K., Rasmussen C.A., Porterfield E.C., Ramgolam V.S., Choi V.W., Markovic-Plese S., Samulski R.J., Kaufman P.L., Borrás T. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 2010;51:236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Keefe K., Tang X., Lin S., Smith G.M. Use of self-complementary adeno-associated virus serotype 2 as a tracer for labeling axons: implications for axon regeneration. PLoS ONE. 2014;9:e87447. doi: 10.1371/journal.pone.0087447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukaida Y., Machida S., Masuda T., Tazawa Y. Correlation of retinal function with retinal histopathology following ischemia-reperfusion in rat eyes. Curr. Eye Res. 2004;28:381–389. doi: 10.1080/02713680490503679. [DOI] [PubMed] [Google Scholar]
- 8.Hayreh S.S., Zimmerman M.B., Kimura A., Sanon A. Central retinal artery occlusion. Retinal survival time. Exp. Eye Res. 2004;78:723–736. doi: 10.1016/s0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 9.Tan J., Liu G., Zhu X., Wu Z., Wang N., Zhou L., Zhang X., Fan N., Liu X. Lentiviral vector-mediated expression of exoenzyme C3 transferase lowers intraocular pressure in monkeys. Mol. Ther. 2019;27:1327–1338. doi: 10.1016/j.ymthe.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swenson E.S., Price J.G., Brazelton T., Krause D.S. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- 11.Belforte N., Sande P.H., de Zavalía N., Fernandez D.C., Silberman D.M., Chianelli M.S., Rosenstein R.E. Ischemic tolerance protects the rat retina from glaucomatous damage. PLoS ONE. 2011;6:e23763. doi: 10.1371/journal.pone.0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agardh C.D., Gustavsson C., Hagert P., Nilsson M., Agardh E. Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion. Metabolism. 2006;55:892–898. doi: 10.1016/j.metabol.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Di Mascio P., Murphy M.E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991;53(1, Suppl):194S–200S. [PubMed] [Google Scholar]
- 14.Bresnick G.H. Excitotoxins: a possible new mechanism for the pathogenesis of ischemic retinal damage. Arch. Ophthalmol. 1989;107:339–341. doi: 10.1001/archopht.1989.01070010349021. [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Chen M., Forrester J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett S.G., Lin H., Godley B.F., Boulton M.E. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog. Retin. Eye Res. 2008;27:596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Headley P.M., Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol. Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 18.Osborne N.N., Ugarte M., Chao M., Chidlow G., Bae J.H., Wood J.P., Nash M.S. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv. Ophthalmol. 1999;43(Suppl 1):S102–S128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 19.Bian H., Zhou Y., Yu B., Shang D., Liu F., Li B., Qi J. Rho-kinase signaling pathway promotes the expression of PARP to accelerate cardiomyocyte apoptosis in ischemia/reperfusion. Mol. Med. Rep. 2017;16:2002–2008. doi: 10.3892/mmr.2017.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohata K., Chen-Yoshikawa T.F., Menju T., Miyamoto E., Tanaka S., Takahashi M., Motoyama H., Hijiya K., Aoyama A., Date H. Protective effect of inhaled Rho-kinase inhibitor on lung ischemia-reperfusion injury. Ann. Thorac. Surg. 2017;103:476–483. doi: 10.1016/j.athoracsur.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Xu F., Liu X.B., Bi S.J., Lu Q.H. Increased Rho kinase activity in patients with heart ischemia/reperfusion. Perfusion. 2019;34:15–21. doi: 10.1177/0267659118787432. [DOI] [PubMed] [Google Scholar]
- 22.Qian X., Zhu M., Qian W., Song J. Vitamin D attenuates myocardial ischemia-reperfusion injury by inhibiting inflammation via suppressing the RhoA/ROCK/NF-κB pathway. Biotechnol. Appl. Biochem. 2019;66:850–857. doi: 10.1002/bab.1797. [DOI] [PubMed] [Google Scholar]
- 23.Dong L.Y., Qiu X.X., Zhuang Y., Xue S. Y-27632, a Rho-kinase inhibitor, attenuates myocardial ischemia-reperfusion injury in rats. Int. J. Mol. Med. 2019;43:1911–1919. doi: 10.3892/ijmm.2019.4097. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K., Maruyama K., Himori N., Omodaka K., Yokoyama Y., Shiga Y., Morin R., Nakazawa T. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55:7126–7136. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]
- 25.Hirata A., Inatani M., Inomata Y., Yonemura N., Kawaji T., Honjo M., Tanihara H. Y-27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:51–59. doi: 10.1007/s00417-007-0666-6. [DOI] [PubMed] [Google Scholar]
- 26.Song H., Gao D. Fasudil, a Rho-associated protein kinase inhibitor, attenuates retinal ischemia and reperfusion injury in rats. Int. J. Mol. Med. 2011;28:193–198. doi: 10.3892/ijmm.2011.659. [DOI] [PubMed] [Google Scholar]
- 27.Cen L.P., Liang J.J., Chen J.H., Harvey A.R., Ng T.K., Zhang M., Pang C.P., Cui Q., Fan Y.M. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience. 2017;343:472–482. doi: 10.1016/j.neuroscience.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Chardin P., Boquet P., Madaule P., Popoff M.R., Rubin E.J., Gill D.M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekine A., Fujiwara M., Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J. Biol. Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- 30.Wiegers W., Just I., Müller H., Hellwig A., Traub P., Aktories K. Alteration of the cytoskeleton of mammalian cells cultured in vitro by Clostridium botulinum C2 toxin and C3 ADP-ribosyltransferase. Eur. J. Cell Biol. 1991;54:237–245. [PubMed] [Google Scholar]
- 31.Kishi K., Sasaki T., Kuroda S., Itoh T., Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J. Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Cui Q., Harvey A.R. Interactive effects of C3, cyclic AMP and ciliary neurotrophic factor on adult retinal ganglion cell survival and axonal regeneration. Mol. Cell. Neurosci. 2007;34:88–98. doi: 10.1016/j.mcn.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang S.W., Mu X., Bowers W.J., Kim D.S., Plas D.J., Crair M.C., Federoff H.J., Gan L., Klein W.H. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development. 2002;129:467–477. doi: 10.1242/dev.129.2.467. [DOI] [PubMed] [Google Scholar]
- 34.Nadal-Nicolás F.M., Jiménez-López M., Sobrado-Calvo P., Nieto-López L., Cánovas-Martínez I., Salinas-Navarro M., Vidal-Sanz M., Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest. Ophthalmol. Vis. Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- 35.Dalkara D., Byrne L.C., Klimczak R.R., Visel M., Yin L., Merigan W.H., Flannery J.G., Schaffer D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 36.den Hollander A.I., Black A., Bennett J., Cremers F.P. Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J. Clin. Invest. 2010;120:3042–3053. doi: 10.1172/JCI42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDougald D.S., Dine K.E., Zezulin A.U., Bennett J., Shindler K.S. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Invest. Ophthalmol. Vis. Sci. 2018;59:1212–1220. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., McCague S., Pierce E.A., Chen Y., Bennicelli J.L. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson S.G., Cideciyan A.V., Ratnakaram R., Heon E., Schwartz S.B., Roman A.J., Peden M.C., Aleman T.S., Boye S.L., Sumaroka A. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borrás T., Xue W., Choi V.W., Bartlett J.S., Li G., Samulski R.J., Chisolm S.S. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. 2006;8:589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 41.Tan J., Wang X., Cai S., He F., Zhang D., Li D., Zhu X., Zhou L., Fan N., Liu X. C3 transferase-expressing scAAV2 transduces ocular anterior segment tissues and lowers intraocular pressure in mouse and monkey. Mol. Ther. Methods Clin. Dev. 2019;17:143–155. doi: 10.1016/j.omtm.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogner B., Boye S.L., Min S.H., Peterson J.J., Ruan Q., Zhang Z., Reitsamer H.A., Hauswirth W.W., Boye S.E. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS ONE. 2015;10:e0128759. doi: 10.1371/journal.pone.0128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S.H., Colosi P., Lee H., Ohn Y.H., Kim S.W., Kwak H.W., Park T.K. Laser photocoagulation enhances adeno-associated viral vector transduction of mouse retina. Hum. Gene Ther. Methods. 2014;25:83–91. doi: 10.1089/hgtb.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay C.N., Ryals R.C., Aslanidi G.V., Min S.H., Ruan Q., Sun J., Dyka F.M., Kasuga D., Ayala A.E., Van Vliet K. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE. 2013;8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mowat F.M., Gornik K.R., Dinculescu A., Boye S.L., Hauswirth W.W., Petersen-Jones S.M., Bartoe J.T. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natkunarajah M., Trittibach P., McIntosh J., Duran Y., Barker S.E., Smith A.J., Nathwani A.C., Ali R.R. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. 2008;15:463–467. doi: 10.1038/sj.gt.3303074. [DOI] [PubMed] [Google Scholar]
- 47.Dalkara D., Kolstad K.D., Caporale N., Visel M., Klimczak R.R., Schaffer D.V., Flannery J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomita H., Sugano E., Yawo H., Ishizuka T., Isago H., Narikawa S., Kügler S., Tamai M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest. Ophthalmol. Vis. Sci. 2007;48:3821–3826. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- 49.Bi A., Cui J., Ma Y.P., Olshevskaya E., Pu M., Dizhoor A.M., Pan Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igarashi T., Miyake K., Kobayashi M., Kameya S., Fujimoto C., Nakamoto K., Takahashi H., Igarashi T., Miyake N., Iijima O. Tyrosine triple mutated AAV2-BDNF gene therapy in a rat model of transient IOP elevation. Mol. Vis. 2016;22:816–826. [PMC free article] [PubMed] [Google Scholar]
- 51.Feuer W.J., Schiffman J.C., Davis J.L., Porciatti V., Gonzalez P., Koilkonda R.D., Yuan H., Lalwani A., Lam B.L., Guy J. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang R., Liang S., Fang L., Wu M., Cheng H., Mi X., Ding Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018;24:367–378. [PMC free article] [PubMed] [Google Scholar]