Abstract
Adaptation to stress is a fundamental requirement to cope with changing environmental conditions that pose a threat to the homeostasis of cells and organisms. Post-translational modifications (PTMs) of proteins represent a possibility to quickly produce proteins with new features demanding relatively little cellular resources. FK506 binding protein (FKBP) 51 is a pivotal stress protein that is involved in the regulation of several executers of PTMs. This mini-review discusses the role of FKBP51 in the function of proteins responsible for setting the phosphorylation, ubiquitination and lipidation of other proteins. Examples include the kinases Akt1, CDK5 and GSK3β, the phosphatases calcineurin, PP2A and PHLPP, and the ubiquitin E3-ligase SKP2. The impact of FKBP51 on PTMs of signal transduction proteins significantly extends the functional versatility of this protein. As a stress-induced protein, FKBP51 uses re-setting of PTMs to relay the effect of stress on various signaling pathways.
Keywords: FKBP51, post-translational modification, signal transduction, stress response
Introduction
The physiological stress response is elicited whenever a change in the environment is sensed and interpreted as threat to homeostasis. Effector systems comprise the autonomic nervous system and the hypothalamic-pituitary-adrenocortical (HPA) axis [1]. The effector molecules of these systems are noradrenaline and adrenaline for the autonomic nervous system, and cortisol for the HPA axis (corticosterone in rodents). Adrenaline and noradrenaline act through G protein-coupled receptors that are hooked to various intracellular pathways involving determinants of post-translational modifications (PTMs) such as kinases and phosphatases [2]. These determinants are also referred to as ‘writers' and ‘erases' of PTMs [3,4]. Using PTMs for signal transduction comes with the obvious advantage of allowing for quickly changing the mode of action of pre-synthesized proteins in a vast functional space defined by the huge number of possible combinations of PTMs at different amino acids [4,5]. Moreover, PTMs are reversible at a small time scale, which is another important feature for the stress response, in particular when stress exposure is short [5].
The HPA axis also activates G protein-coupled receptors, namely the receptors for the hormones corticotropin-releasing factor and adenocorticotropic hormone [1]. However, its final effector cortisol operates through the steroid receptors glucocorticoid receptor (GR) and mineralocorticoid receptor [6]. These receptors are transcription factors, and thus this part of the overall stress response typically takes longer than the time required to redefine the function of proteins through re-setting their PTMs. Nevertheless, the action of these receptors also is intertwined with PTMs: they are subject to regulation by PTMs and they impact writers and erasers of PTMs [7–9]. In part, this is achieved through the synthesis of specific proteins that take part in the orchestration of proteome function through PTMs. FK506 binding protein (FKBP) 51 turned out to be one of these proteins which is subject of this review.
The stress protein FKBP51, promoted through translational research
FKBP51 is a show-case of translational research where clinical and basic science approaches stimulated each other. The background of FKBP51 is laid out here only shortly, and the reader is referred to the numerous recent reviews for more detailed information [10–15]. Originally discovered as part of steroid receptor-heat shock protein 90 hetero-complexes, FKBP51 was shown to be a potent inhibitor of GR by several laboratories [16–19]. By virtue of its binding to immune suppressive drugs such as FK506, FKBP51 also has been classified as ‘immunophilin' [15]. Biochemically, FKBP51 is able to isomerize peptidyl-prolyl bonds [20]; the physiological relevance, if any, of this function is not clear [11,12,21]. However, the peptidylprolyl isomerase domain of FKBP51 is engaged in protein interactions, and drug binding to this domain likely affects several functions of this protein [13].
The inducibility of FKBP51 gene (named FKBP5) expression by the activated GR [22–27] gives rise to an intracellular ultra-short negative feedback loop, as one of the hallmarks of adaptive molecular circuits [11]. Of particular interest for neuropsychiatric research was the observation that FKBP51 was overexpressed in squirrel monkeys featuring altered set-points of the HPA axis [28]. Thus, the molecular settings in these animals were considered a model for GR-resistance [29]. This was related to the situation in patients suffering from depression where malfunction of GR was hypothesized to be causal for the development of the disease [30]. Based on these considerations, FKBP5 was included as candidate gene in an early gene association study in depression that found this gene linked to the response to antidepressant treatment [31]. Later, FKBP5 could be linked to additional stress-related diseases such as post-traumatic stress disorder [12,14]. These findings strongly amplified the interest in FKBP51 and stimulated research on its function and regulation in several laboratories; this greatly expanded the knowledge base on FKBP51's (patho)physiological role, regulation on several levels and involvement in multiple molecular pathways, going beyond stress regulation [12,13]. The variety of its physiological functions goes along with its association with several proteins, including proteins involved in writing and erasing PTMs, as detailed in the subsequent sections.
Post-translational modifications
More than 200 PTMs are known with a major impact in the configuration of protein networks [32–34]; the vast majority of these modifications are reversible with prominent examples being the attachment of chemical groups (e.g. phosphorylation, acetylation, methylation, nitrosylation, sulfonation), the conjugation with polypeptides (e.g. ubiquitination, NEDD8 [neural-precursor-cell-expressed developmentally down-regulated 8], and ubiquitin-like peptides such as SUMO [small ubiquitin-like modifier] or ATG8 [autophagy-related gene 8]) and the addition of a complex group of molecules including, e.g. prenylation, farnesylation, glycosylation, palmitoylation, myristoylation, glutamylation, ADP-ribosylation and AMPylation [4,35]. Protein phosphorylation is one of the first known PTM [36,37] and probably the best studied one affecting almost all biological processes [38]. In eukaryotes, proteins are phosphorylated primarily through phosphor-ester bonds formed at the residues serine, threonine and tyrosine, and up to 2% of the protein-coding genes produce the enzymatic machinery governing this process [39].
While this review focusses on the effect of FKBP51 on PTMs of other proteins, it should be noted that FKBP51 also is subject to PTMs itself. This has been reviewed very recently [40], and thus is mentioned here only briefly: Not surprisingly, the first reported PTM of FKBP51 was phosphorylation. Originally, it was inferred from the pattern of this protein in 2D gel electrophoresis and the modulation of this pattern by the use of kinase inhibitors or phosphatases [40–42]. More recently, PTEN-induced putative kinase 1 (PINK1) was found to phosphorylate FKBP51 at yet to be mapped serine residues [43]. Thereby, PINK1 regulates the interaction of FKBP51 with the kinase Akt1 and the phosphatase PHLPP [43]. This interaction further is controlled by acetylation of FKBP51 at lysines 28 and 155 [44]. The sirtuin SIRT7 has been identified as deacetylase acting at these sites [44]. SUMOylation of FKBP51 was detected and mapped to lysine 422 [45]. It regulates the inhibitory action of FKBP51 on GR [45].
On or off? FKBP51 associates with both kinases and phosphatases
The first evidence for the involvement of FKBP51 in the regulation of the phosphoproteome was provided by the observation that FK506-bound FKBP51 inhibits the serine/threonine-phosphatase calcineurin (also known as protein phosphatase 2B), thereby inhibiting nuclear factor of activated T cells (NFAT) [46,47]. It also has been reported that FKBP51 interacts with calcineurin in the absence of FK506 [48], which was not observed by others [47]. Nevertheless, impacting PTMs through re-arranging protein association of kinases and phosphatases as in the case of calcineurin/NFAT is the mode of action also revealed for the effect of FKBP51 on many other signaling pathways.
The inhibition of calcineurin by FKBP51 is reported to also affect the nuclear factor (NF)κB pathway [49]. In this pathway, phosphorylation of the inhibitor of κB (IκB) by the kinase of IκB (IKK) leads to activation of NFκB [50]. Therefore, the protein associations of FKBP51 with calcineurin as well as with IKKα and other kinases of the NFκB pathway [51] support a model where FKBP51 impacts NFκB activity through re-setting phosphorylation at multiple levels with variable outcome [11]. For example, a direct association between FKBP51 and both TNF receptor-associated factor 2 (TRAF2) and IKKγ was found [52]. TRAF2 catalyzes K63-linked poly-ubiquitination of receptor-interacting protein 1 (RIP1) in response to TNFα, thereby facilitating the recruitment of the IKK complex to its upstream activating kinase TAK1 (transforming growth factor-beta activated kinase 1) [53,54]. FKBP51 enhances and shapes this polyubiquitin-mediated interaction, thereby changing the phosphorylation of IKK and IκB and thus the activity of NFκB [52].
The serine/threonine kinase Akt (also known as protein kinase B) is another well-examined example of FKBP51's impact on PTMs through organizing protein complexes. Akt is activated by step-wise phosphorylation in response to extracellular signals that involves its translocation from the cytoplasm to the cell membrane [55,56]. FKBP51 employs its ability to interact with various proteins, frequently referred to as scaffolding, to recruit the phosphatase PHLPP that de-phosphorylates and thereby inactivates Akt [57,58] (Figure 1A). Akt is a central pathway regulator that inhibits apoptosis and promotes cell growth [59]. Accordingly, FKBP51 expression is enhanced in most cancer types and is linked to resistance to chemotherapy [57,60–62]. Evidence has also been provided that FKBP51 mediates the inactivation of Akt induced by stress [58]. Thus, FKBP51 may also relay the effect of stress on the phosphoproteome [11].
Figure 1. Protein associations of FKBP51 impact PTMs.
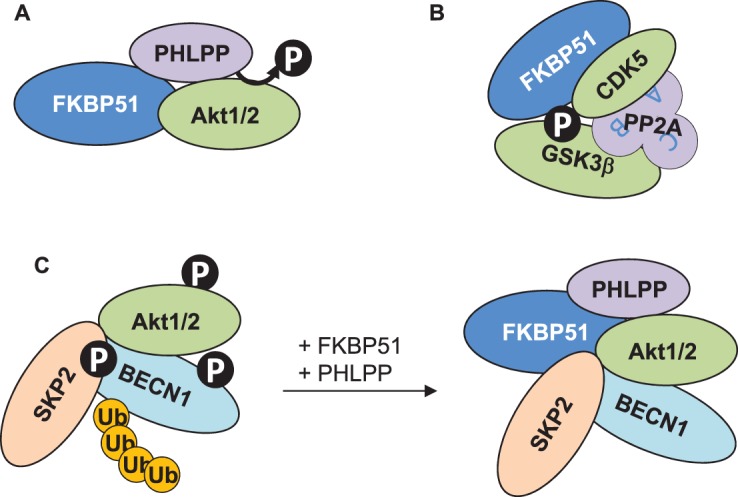
(A) FKBP51 associates with the phosphatase PHLPP and the kinase Akt, which leads to de-phopshporylation and thus inactivation of Akt [57,58]. (B) The association of FKBP51 with CDK5, GSK3β and PP2A (with the subunits A–C) presumably results in increased phosphorylation of GSK3β [67]. (C) Through association with PHLPP, Akt, SKP2 and BECN1, FKBP51 changes two types of PTMs of BECN1, phosphorylation and ubiquitination [58,64,65]. The recruitment of PHLPP leads to lower Akt phosphorylation and activity, entailing less phosphorylation of BECN1 and of the E3-ligase SKP2. Thereby, SKP2 is less active resulting in lower ubiquitination of BECN1. Whether or not FKBP51 associates with all these proteins in one complex remains to be elucidated.
FKBP51's effect on Akt also alters downstream PTM-dependent pathways. For example, through Akt1 FKBP51 impacts p38 MAPK, thereby differentially regulating the transcription factors GR and peroxisome proliferator-activated receptor-γ [63]. FKBP51 also governs the effects of Akt1 on its targets Beclin 1 (BECN1) and S-phase kinase-associated protein 2 (SKP2), constituting a link to autophagy as detailed below [58,64,65]. Another downstream target of Akt is glycogen synthase kinase 3β (GSK3β) [66]. Consistent with the inhibitory effect of FKBP51 on Akt1, it has been reported that overexpression of FKBP51 decreased the phosphorylation of GSK3β at serine 9 [57]. However, it also has been found that FKBP51 associates with GSK3β, and increases its phosphorylation [67]. This appears to be accomplished through rearrangement of the protein heterocomplex governing phosphorylation and thus the activity of GSK3β. More specifically, FKBP51 recruits cyclin-dependent kinase 5 (CDK5) and furthermore associates with the three subunits of the phosphatase PP2A [67] (Figure 1B), which acts in concert with CDK5 to regulate GSK3β affecting downstream targets [68]. Thus, FKBP51 redefines signaling pathway connections through protein associations.
FKBP51's impact on protein phosphorylation furthermore provides a link to epigenetic regulation as well as to metabolic function. The link to epigenetics is evidenced by its impact on phosphorylation, and thus the activity of DNA methyltransferase 1 (DNMT1) [69]. Mechanistically, this effect appears to be achieved through the differential association of FKBP51 and its close homolog FKBP52 with CDK5 and its regulatory protein p35 [69]. Metabolic function is impacted by FKBP51 through protein associations that dephosphorylate and thus inhibit Akt2, leading to dephosphorylation and thus inhibition of AS160 and reduced glucose uptake [70].
PTMs are also involved in the effect of FKBP51 on microtubule dynamics. It is assumed that phosphorylation of tau leads to its dissociation from microtubules and adoption of the trans configuration of specific peptidylprolyl bonds, while the association of phosphorylated tau with FKBP51 promotes its dephosphorylation and cis configuration that is required for microtubule association [71–73].
Another mass spectrometry-based screen for FKBP51-associated proteins revealed the Rho (Ras homologous) GTPase-activating proteins deleted in liver cancer (DLC) 1 and DLC2 as novel interaction partners [74]. Accordingly, FKBP51 enhances RhoA activity and signaling through the serine/threonine kinase ROCK (rho-associated coiled-coil containing protein kinase) along with the linked processes cell migration and invasion [74]. The exact mechanism remains to be elucidated. It appears that this is an example for a more indirect effect of FKBP51 on the phosphoproteome: it inhibits a protein, DLC, that serves as a GTPase activator for members of the Rho family of GTPases that regulate downstream kinases [74,75].
Ubiquitination and lipidation
Ubiquitination is an essential PTM in all eukaryotes [3,76]. It determines protein stability as well as protein function through changing protein–protein interaction. Biochemically, ubiquitination is a process where the 76 amino acid protein ubiquitin is covalently linked to other proteins, in most cases through the formation of an amide bond between its carboxy terminus and the ε amino group of lysine residues in the substrate proteins [34,76]. In addition to this isopeptide bond, ubiquitin forms other links to target diverse protein residues such as cysteines, serines or threonines [3]. Ubiquitination comes in the form of mono-ubiquitination and poly-ubiquitination, where distinct lysines of one ubiquitin serve as attachment sites for additional ubiquitin moieties. The site of linkage destines the modified protein to different functions. For example, poly-ubiquitination through the lysines at position 11 and 48 typically lead to degradation of the protein through the 26S proteasome [77,78].
The first indication that FKBP51 influences the ubiquitination of other proteins came from the observation that it stabilizes tau and protects it from becoming ubiquitinated [71]. The mode of action appears to be an indirect mechanism where FKBP51 influences the conformation and/or phosphorylation of tau to prevent the access of ubiquitinating enzymes [71,73,79]. For the differential effect of FKBP51 and FKBP52 on NFκB, it has been proposed that a mechanism is involved that is similar to Pin1's promotion of the ubiquitin-mediated proteolysis of the NFκB subunit p65/RelA [80]. Further experimental evidence is awaited; in any case, this effect also would be indirect. The reported effect of FKBP51 on NFκB signaling through the association with TRAF2 and IKK is dependent on the K63-poly-ubiquitination of RIP1, but does not appear to impact ubiquitination in this context [52]. The E3 ubiquitin-protein ligase complex members TRAF3 and TRAF6 also were discovered as FKBP51 associating proteins, with currently unknown consequences for their enzymatic activity [81].
More recently, a direct association of FKBP51 with Glomulin, a regulator of the SCF (Skp1-CUL1-F-box protein) E3 ubiquitin-protein ligase complex, has been described in detail and in comparison with other FKBPs [82]. It is likely that this interaction affects the ubiquitination activity of SCF; experimental investigation of this potential impact of FKBP51 on the ubiquitination machinery has not been reported yet.
A yeast two-hybrid screen indicated protein associations of FKBP51 with the ubiquitin-specific peptidases (USPs) 18, 36 and 49 as well as with the E3 ubiquitin ligases RING Finger Protein 219 and SKP2 [83]. While the functional consequences were not explored in this report, another study revealed that USP49 stabilizes FKBP51 by the removal of ubiquitin chains [84]. Thus, in this case, the association with a PTM executer results in FKBP51 being a target rather than a modifier. Conversely, FKBP51 regulates the ubiquitination activity of SKP2 through phosphorylation, probably by functioning as protein scaffolder for the association with PHLPP and AKT1 [65]. This novel FKBP51 function on protein ubiquitination has far-reaching consequences: The study further discovered the autophagy regulator BECN1 as novel target of SKP2 executing K48-linked poly-ubiquitination at this autophagy regulator [65]. Thus, FKBP51 drives autophagy involving at least two types of PTMs of BECN1, ubiquitination and phosphorylation [58,64,65] (Figure 1C). Given the multiple targets of SKP2 [85–88], it is assumed that the ubiquitination of more proteins will be changed by FKBP51 with diverse functional consequences.
Autophagy is another fundamental cellular process essential for protein, organelle and energy homeostasis [89]. It involves several ATG products that through several steps form autophagosomes, vesicles that engulf material destined for degradation which is accomplished upon the fusion with lysosomes [89,90]. An important step in this process is the lipidation of ATG8 (also known as microtubule associated protein 1 light chain 3 beta), a ubiquitin-like protein that is integrated into the autophagosomal membrane upon formation of an amide bond with phosphatidylethanolamine [91,92]. Similar to the process of ubiquitination, the formation of this amide bond is executed by a set of ligases (E1–E3) that use cysteine linked thioesters as intermediates. The last step, conjugation to phosphatidylethanolamine, is mediated by the E3-like Atg12-Atg5:Atg16 complex [93]. The effect of FKBP51 on this protein lipidation probably is indirect through driving autophagy by regulating BECN1. However, FKBP51 also leads to enhanced levels of ATG12, a member of the conjugation system [58], pointing to different pathways used by FKBP51 to enhance ATG8 lipidation.
Perspective
FKBP51 is receiving increased attention for its pivotal role in research on stress and stress-related diseases, but also several additional fields such as immunology, metabolism, oncology, neurology, etc.
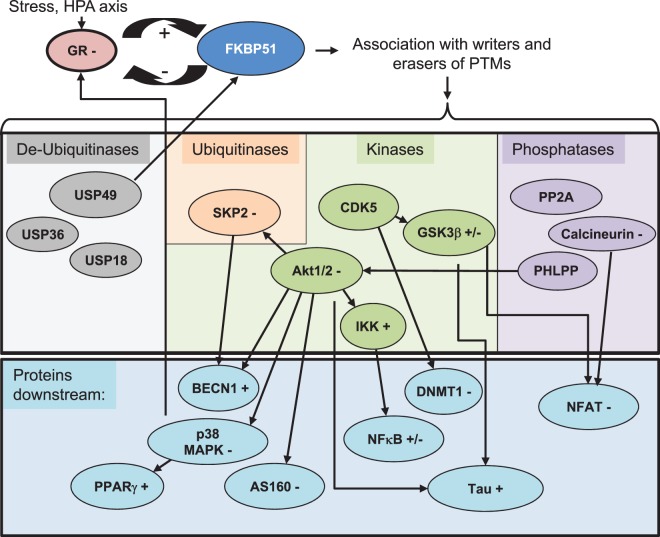
The stress protein FKBP51 is engaged in various signaling pathways through diverse protein interactions. It both affects the stress response and is affected by the stress response, and furthermore relays stress to multiple pathways. Likewise, it is the subject of PTMs and affects the activity of PTM writers and erasers (Figure 2).
Protein associations of FKBP51 with PTM writers and erasers could affect PTMs of FKBP51 itself or of other proteins, and deciphering these scenarios will significantly contribute to our mechanistic understanding of this versatile protein. Furthermore, it will be of high interest to elucidate which of the PTM-mediated downstream effects of FKBP51 contribute to the physiological stress reaction.
Figure 2. FKBP51 impacts signaling pathways through PTMs.
The stress protein FKBP51 is intertwined with GR as both inhibitor and target. As target, it has the potential to relay the stress response to downstream pathways through the association with writers and erasers of PTMs. Known associations include de-ubiquitinases, ubiquitinases, protein kinases and protein phosphatases (first box, writers and erasers of PTMs are grouped according to the type of their biochemical activity). Some of the associated proteins are changed in their activity, others are redirected to certain targets. Examples of target proteins affected by the altered activities of PTM writers and erasers are provided in the box below. ‘+' and ‘−' after the protein names indicate the overall effect of FKBP51 on the activity of associating proteins or downstream proteins. Not all potential interactions are displayed. BECN1 is a downstream protein that also forms a complex with FKBP51. However, most of the downstream target proteins are indirectly affected in the sense that they were not shown to associate with FKBP51.
Abbreviations
- ATG
autophagy-related gene
- BECN1
Beclin 1
- CDK
cyclin-dependent kinase
- DLC
deleted in liver cancer
- DNMT1
DNA methyltransferase 1
- FKBP
FK506 binding protein
- GR
glucocorticoid receptor
- GSK
glycogen synthase kinase
- HPA axis
hypothalamic-pituitary-adrenocortical axis
- IκB
inhibitor of κB
- IKK
kinase of IκB
- MAPK
mitogen-activated protein kinase
- NFκB
nuclear factor kappa B
- NFAT
nuclear factor of activated T cells
- PHLPP
pleckstrin homology domain leucine-rich repeat protein phosphatase
- PP2A
protein phosphatase 2A
- PPARγ
peroxisome proliferator-activated receptor-γ
- PTM
post-translational modification
- RIP1
receptor-interacting protein 1
- SCF
Skp1-CUL1-F-box protein
- SKP2
S-phase kinase-associated protein 2
- TNF
tumor necrosis factor
- TRAF
TNF receptor-associated factor
- USP
ubiquitin-specific peptidase
Competing Interests
The author declares that there are no competing interests associated with this manuscript.
References
- 1.Deussing J.M. and Chen A. (2018) The corticotropin-releasing factor family: physiology of the stress response. Physiol. Rev. 98, 2225–2286 10.1152/physrev.00042.2017 [DOI] [PubMed] [Google Scholar]
- 2.Cole S.W. and Sood A.K. (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res. 18, 1201–1206 10.1158/1078-0432.CCR-11-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh E., Akopian D. and Rape M. (2018) Principles of ubiquitin-dependent signaling. Annu. Rev. Cell Dev. Biol. 34, 137–162 10.1146/annurev-cellbio-100617-062802 [DOI] [PubMed] [Google Scholar]
- 4.Spoel S.H. (2018) Orchestrating the proteome with post-translational modifications. J. Exp. Bot. 69, 4499–4503 10.1093/jxb/ery295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q., Bhattacharya S., Pi J., Clewell R.A., Carmichael P.L. and Andersen M.E. (2015) Adaptive posttranslational control in cellular stress response pathways and its relationship to toxicity testing and safety assessment. Toxicol. Sci. 147, 302–316 10.1093/toxsci/kfv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N. et al. (2015) Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faresse N. (2014) Post-translational modifications of the mineralocorticoid receptor: how to dress the receptor according to the circumstances? J. Steroid. Biochem. Mol. Biol. 143, 334–342 10.1016/j.jsbmb.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 8.Kumar R. and Thompson E.B. (2019) Role of phosphorylation in the modulation of the glucocorticoid receptor's intrinsically disordered domain. Biomolecules 9, E95 10.3390/biom9030095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weikum E.R., Knuesel M.T., Ortlund E.A. and Yamamoto K.R. (2017) Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 18, 159–174 10.1038/nrm.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storer C.L., Dickey C.A., Galigniana M.D., Rein T. and Cox M.B. (2011) FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 22, 481–490 10.1016/j.tem.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rein T. (2016) FK506 binding protein 51 integrates pathways of adaptation: FKBP51 shapes the reactivity to environmental change. Bioessays 38, 894–902 10.1002/bies.201600050 [DOI] [PubMed] [Google Scholar]
- 12.Fries G.R., Gassen N.C. and Rein T. (2017) The FKBP51 glucocorticoid receptor co-chaperone: regulation, function, and implications in health and disease. Int. J. Mol. Sci. 18, E2614 10.3390/ijms18122614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hähle A., Merz S., Meyners C. and Hausch F. (2019) The many faces of FKBP51. Biomolecules 9, E35 10.3390/biom9010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criado-Marrero M., Rein T., Binder E.B., Porter J.T., Koren J. III and Blair L.J. (2018) Hsp90 and FKBP51: complex regulators of psychiatric diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160532 10.1098/rstb.2016.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zgajnar N.R., De Leo S.A., Lotufo C.M., Erlejman A.G., Piwien-Pilipuk G. and Galigniana M.D. (2019) Biological actions of the Hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules 9, E52 10.3390/biom9020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wochnik G.M., Ruegg J., Abel G.A., Schmidt U., Holsboer F. and Rein T. (2005) FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 280, 4609–4616 10.1074/jbc.M407498200 [DOI] [PubMed] [Google Scholar]
- 17.Riggs D.L., Roberts P.J., Chirillo S.C., Cheung-Flynn J., Prapapanich V., Ratajczak T. et al. (2003) The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22, 1158–1167 10.1093/emboj/cdg108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denny W.B., Valentine D.L., Reynolds P.D., Smith D.F. and Scammell J.G. (2000) Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 141, 4107–4113 10.1210/endo.141.11.7785 [DOI] [PubMed] [Google Scholar]
- 19.Scammell J.G., Denny W.B., Valentine D.L. and Smith D.F. (2001) Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 124, 152–165 10.1006/gcen.2001.7696 [DOI] [PubMed] [Google Scholar]
- 20.Pirkl F. and Buchner J. (2001) Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol 308, 795–806 10.1006/jmbi.2001.4595 [DOI] [PubMed] [Google Scholar]
- 21.Rein T. (2020) Peptidylprolylisomerases, protein folders or scaffolders? The example of FKBP51 and FKBP52. Bioessays 42, 10.1002/bies.201900250 [DOI] [PubMed] [Google Scholar]
- 22.Baughman G., Wiederrecht G.J., Chang F., Martin M.M. and Bourgeois S. (1997) Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineurin inhibition. Biochem. Biophys. Res. Commun. 232, 437–443 10.1006/bbrc.1997.6307 [DOI] [PubMed] [Google Scholar]
- 23.Harrigan M.T., Baughman G., Campbell N.F. and Bourgeois S. (1989) Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol. Cell. Biol. 9, 3438–3446 10.1128/MCB.9.8.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubler T.R. and Scammell J.G. (2004) Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones 9, 243–252 10.1379/CSC-32R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds P.D., Roveda K.P., Tucker J.A., Moore C.M., Valentine D.L. and Scammell J.G. (1998) Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Lab. Anim. Sci. 48, 364–370 PMID: [PubMed] [Google Scholar]
- 26.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M. et al. (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16, 33–41 10.1038/nn.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paakinaho V., Makkonen H., Jaaskelainen T. and Palvimo J.J. (2010) Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol. Endocrinol. 24, 511–525 10.1210/me.2009-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrousos G.P., Renquist D., Brandon D., Eil C., Pugeat M., Vigersky R. et al. (1982) Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc. Natl. Acad. Sci. U.S.A. 79, 2036–2040 10.1073/pnas.79.6.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chrousos G.P., Loriaux D.L., Tomita M., Brandon D.D., Renquist D., Albertson B. et al. (1986) The new world primates as animal models of glucocorticoid resistance. Adv. Exp. Med. Biol. 196, 129–144 10.1007/978-1-4684-5101-6_9 [DOI] [PubMed] [Google Scholar]
- 30.Holsboer F. (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501 10.1016/S0893-133X(00)00159-7 [DOI] [PubMed] [Google Scholar]
- 31.Binder E.B., Salyakina D., Lichtner P., Wochnik G.M., Ising M., Putz B. et al. (2004) Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36, 1319–1325 10.1038/ng1479 [DOI] [PubMed] [Google Scholar]
- 32.Krishna R.G. and Wold F (1998). Posttranslational modifications In Proteins: Analysis and Design (Angeletti R.H., ed.), pp. 121–207, Academic, San Diego [Google Scholar]
- 33.Duan G. and Walther D. (2015) The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol. 11, e1004049 10.1371/journal.pcbi.1004049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deribe Y.L., Pawson T. and Dikic I. (2010) Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 17, 666–672 10.1038/nsmb.1842 [DOI] [PubMed] [Google Scholar]
- 35.Consortium T.U. (2017) Uniprot: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fi E.H. and Krebs E.G. (1955) Conversion of phosphorylase b to phosphorylase a in muscle extracts. J. Biol. Chem. 216, 121–132 PMID: [PubMed] [Google Scholar]
- 37.Hunter T. and Cooper J.A. (1985) Protein-tyrosine kinases. Annu. Rev. Biochem. 54, 897–930 10.1146/annurev.bi.54.070185.004341 [DOI] [PubMed] [Google Scholar]
- 38.Gelens L. and Saurin A.T. (2018) Exploring the function of dynamic phosphorylation-dephosphorylation cycles. Dev. Cell 44, 659–663 10.1016/j.devcel.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 39.Manning G., Plowman G.D., Hunter T. and Sudarsanam S. (2002) Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 27, 514–520 10.1016/S0968-0004(02)02179-5 [DOI] [PubMed] [Google Scholar]
- 40.Daneri-Becerra C., Zgajnar N.R., Lotufo C.M., Ramos Hryb A.B., Piwien-Pilipuk G. and Galigniana M.D. (2019) Regulation of FKBP51 and FKBP52 functions by post-translational modifications. Biochem. Soc. Trans. 47, 1815–1831 10.1042/BST20190334 [DOI] [PubMed] [Google Scholar]
- 41.Nair S.C., Rimerman R.A., Toran E.J., Chen S., Prapapanich V., Butts R.N. et al. (1997) Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol. Cell. Biol. 17, 594–603 10.1128/MCB.17.2.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo L.I., Lagadari M., Piwien-Pilipuk G. and Galigniana M.D. (2011) The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 286, 30152–30160 10.1074/jbc.M111.256610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boonying W., Joselin A., Huang E., Qu D., Safarpour F., Iyirhiaro G.O. et al. (2019) Pink1 regulates FKBP5 interaction with AKT/PHLPP and protects neurons from neurotoxin stress induced by MPP+. J. Neurochem. 150, 312–329 10.1111/jnc.14683 [DOI] [PubMed] [Google Scholar]
- 44.Yu J., Qin B., Wu F., Qin S., Nowsheen S., Shan S. et al. (2017) Regulation of serine-threonine kinase Akt activation by NAD(+)-dependent deacetylase SIRT7. Cell Rep. 18, 1229–1240 10.1016/j.celrep.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antunica-Noguerol M., Budziñski M.L., Druker J., Gassen N.C., Proto-Cassina L., Senin S. et al. (2016) The activity of the glucocorticoid receptor is regulated by SUMO conjugation to FKBP51. Cell Death Differ. 23, 1579–1591 10.1038/cdd.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baughman G., Wiederrecht G.J., Campbell N.F., Martin M.M. and Bourgeois S. (1995) FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol. Cell. Biol. 15, 4395–4402 10.1128/MCB.15.8.4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiwad M., Edlich F., Kilka S., Erdmann F., Jarczowski F., Dorn M. et al. (2006) Comparative analysis of calcineurin inhibition by complexes of immunosuppressive drugs with human FK506 binding proteins. Biochemistry 45, 15776–15784 10.1021/bi061616p [DOI] [PubMed] [Google Scholar]
- 48.Li T.K., Baksh S., Cristillo A.D. and Bierer B.E. (2002) Calcium- and FK506-independent interaction between the immunophilin FKBP51 and calcineurin. J. Cell. Biochem. 84, 460–471 10.1002/jcb.10026 [DOI] [PubMed] [Google Scholar]
- 49.Giraudier S., Chagraoui H., Komura E., Barnache S., Blanchet B., LeCouedic J.P. et al. (2002) Overexpression of FKBP51 in idiopathic myelofibrosis regulates the growth factor independence of megakaryocyte progenitors. Blood 100, 2932–2940 10.1182/blood-2002-02-0485 [DOI] [PubMed] [Google Scholar]
- 50.Hacker H. and Karin M. (2006) Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- 51.Bouwmeester T., Bauch A., Ruffner H., Angrand P.O., Bergamini G., Croughton K. et al. (2004) A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 6, 97–105 10.1038/ncb1086 [DOI] [PubMed] [Google Scholar]
- 52.Romano S., Xiao Y., Nakaya M., D'Angelillo A., Chang M., Jin J. et al. (2015) FKBP51 employs both scaffold and isomerase functions to promote NF-κB activation in melanoma. Nucleic Acids Res. 43, 6983–6993 10.1093/nar/gkv615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao G., Zhang M., Harhaj E.W. and Sun S.C. (2004) Regulation of the NF-κB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279, 26243–26250 10.1074/jbc.M403286200 [DOI] [PubMed] [Google Scholar]
- 54.Uhlik M., Good L., Xiao G., Harhaj E.W., Zandi E., Karin M. et al. (1998) NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J. Biol. Chem. 273, 21132–6 10.1074/jbc.273.33.21132 [DOI] [PubMed] [Google Scholar]
- 55.Cole P.A., Chu N., Salguero A.L. and Bae H. (2019) AKTivation mechanisms. Curr. Opin. Struct. Biol. 59, 47–53 10.1016/j.sbi.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C. and Abraham R.T. (2017) The PI3K pathway in human disease. Cell 170, 605–635 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pei H., Li L., Fridley B.L., Jenkins G.D., Kalari K.R., Lingle W. et al. (2009) FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 16, 259–266 10.1016/j.ccr.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gassen N.C., Hartmann J., Zschocke J., Stepan J., Hafner K., Zellner A. et al. (2014) Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. 11, e1001755 10.1371/journal.pmed.1001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manning B.D. and Cantley L.C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romano S., D'Angelillo A., Pacelli R., Staibano S., De L.E., Bisogni R. et al. (2010) Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 17, 145–157 10.1038/cdd.2009.115 [DOI] [PubMed] [Google Scholar]
- 61.Lagadari M., Zgajnar N.R., Gallo L.I. and Galigniana M.D. (2016) Hsp90-binding immunophilin FKBP51 forms complexes with hTERT enhancing telomerase activity. Mol. Oncol. 10, 1086–1098 10.1016/j.molonc.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Russo D., Merolla F., Mascolo M., Ilardi G., Romano S., Varricchio S. et al. (2017) Fkbp51 immunohistochemical expression: a new prognostic biomarker for OSCC? Int. J. Mol. Sci. 18, E443 10.3390/ijms18020443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stechschulte L.A., Hinds T.D. Jr, Khuder S.S., Shou W., Najjar S.M. and Sanchez E.R. (2014) FKBP51 controls cellular adipogenesis through p38 kinase-mediated phosphorylation of GRα and PPARν. Mol. Endocrinol. 28, 1265–1275 10.1210/me.2014-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gassen N.C., Hartmann J., Schmidt M.V. and Rein T. (2015) FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 11, 578–580 10.1080/15548627.2015.1017224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gassen N.C., Niemeyer D., Muth D., Corman V.M., Martinelli S., Gassen A. et al. (2019) SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 10, 5770 10.1038/s41467-019-13659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M. and Hemmings B.A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- 67.Gassen N.C., Hartmann J., Zannas A.S., Kretzschmar A., Zschocke J., Maccarrone G. et al. (2016) FKBP51 inhibits GSK3β and augments the effects of distinct psychotropic medications. Mol. Psychiatry 21, 277–289 10.1038/mp.2015.38 [DOI] [PubMed] [Google Scholar]
- 68.Louis J.V., Martens E., Borghgraef P., Lambrecht C., Sents W., Longin S. et al. (2011) Mice lacking phosphatase PP2A subunit PR61/B′δ (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3β. Proc. Natl. Acad. Sci. U.S.A. 108, 6957–6962 10.1073/pnas.1018777108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gassen N.C., Fries G.R., Zannas A.S., Hartmann J., Zschocke J., Hafner K. et al. (2015) Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 8, ra119 10.1126/scisignal.aac7695 [DOI] [PubMed] [Google Scholar]
- 70.Balsevich G., Hausl A.S., Meyer C.W., Karamihalev S., Feng X., Pohlmann M.L. et al. (2017) Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat. Commun. 8, 1725 10.1038/s41467-017-01783-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jinwal U.K., Koren J. III, Borysov S.I, Schmid A.B., Abisambra J.F., Blair L.J. et al. (2010) The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J. Neurosci. 30, 591–599 10.1523/JNEUROSCI.4815-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cioffi D.L., Hubler T.R. and Scammell J.G. (2011) Organization and function of the FKBP52 and FKBP51 genes. Curr. Opin. Pharmacol. 11, 308–313 10.1016/j.coph.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koren J. III, Jinwal U.K., Davey Z., Kiray J., Arulselvam K. and Dickey C.A. (2011) Bending tau into shape: the emerging role of peptidyl-prolyl isomerases in tauopathies. Mol. Neurobiol. 44, 65–70 10.1007/s12035-011-8182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takaoka M., Ito S., Miki Y. and Nakanishi A. (2017) FKBP51 regulates cell motility and invasion via RhoA signaling. Cancer Sci. 108, 380–389 10.1111/cas.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulherkar S. and Tolias K.F. (2020) RhoA-ROCK signaling as a therapeutic target in traumatic brain injury. Cells 9, E245 10.3390/cells9010245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hershko A. and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- 77.Ikeda F. and Dikic I. (2008) Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 9, 536–542 10.1038/embor.2008.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pohl C. and Dikic I. (2019) Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 366, 818–822 10.1126/science.aax3769 [DOI] [PubMed] [Google Scholar]
- 79.Blair L.J., Nordhues B.A., Hill S.E., Scaglione K.M., O'Leary J.C. III, Fontaine S.N. et al. (2013) Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Invest. 123, 4158–4169 10.1172/JCI69003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erlejman A.G., De Leo S.A., Mazaira G.I., Molinari A.M., Camisay M.F., Fontana V. et al. (2014) NF-κB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J. Biol. Chem. 289, 26263–26276 10.1074/jbc.M114.582882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akiyama T., Shiraishi T., Qin J., Konno H., Akiyama N., Shinzawa M. et al. (2014) Mitochondria-nucleus shuttling FK506-binding protein 51 interacts with TRAF proteins and facilitates the RIG-I-like receptor-mediated expression of type I IFN. PLoS One 9, e95992 10.1371/journal.pone.0095992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hähle A., Geiger T.M., Merz S., Meyners C., Tianqi M., Kolos J. et al. (2019) FKBP51 and FKBP12.6-Novel and tight interactors of Glomulin. PLoS One 14, e0221926 10.1371/journal.pone.0221926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taipale M., Tucker G., Peng J., Krykbaeva I., Lin Z.Y., Larsen B. et al. (2014) A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 158, 434–448 10.1016/j.cell.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo K., Li Y., Yin Y., Li L., Wu C., Chen Y. et al. (2017) USP49 negatively regulates tumorigenesis and chemoresistance through FKBP51-AKT signaling. EMBO J. 36, 1434–1446 10.15252/embj.201695669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carrano A.C., Eytan E., Hershko A. and Pagano M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 10.1038/12013 [DOI] [PubMed] [Google Scholar]
- 86.Lim M.S., Adamson A., Lin Z., Perez-Ordonez B., Jordan R.C., Tripp S. et al. (2002) Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood 100, 2950–2956 10.1182/blood.V100.8.2950 [DOI] [PubMed] [Google Scholar]
- 87.Amati B. and Vlach J. (1999) Kip1 meets SKP2: new links in cell-cycle control. Nat. Cell Biol. 1, E91–E93 10.1038/12087 [DOI] [PubMed] [Google Scholar]
- 88.Kulinski M., Achkar I.W., Haris M., Dermime S., Mohammad R.M. and Uddin S. (2018) Dysregulated expression of SKP2 and its role in hematological malignancies. Leuk. Lymphoma 59, 1051–1063 10.1080/10428194.2017.1359740 [DOI] [PubMed] [Google Scholar]
- 89.Mizushima N. and Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 90.Nakatogawa H., Suzuki K., Kamada Y. and Ohsumi Y. (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 10.1038/nrm2708 [DOI] [PubMed] [Google Scholar]
- 91.Sou Y.S., Waguri S., Iwata J., Ueno T., Fujimura T., Hara T. et al. (2008) The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol. Biol. Cell 19, 4762–4775 10.1091/mbc.e08-03-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie Z., Nair U. and Klionsky D.J. (2008) Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell 19, 3290–3298 10.1091/mbc.e07-12-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noda N.N., Ohsumi Y. and Inagaki F. (2009) ATG systems from the protein structural point of view. Chem. Rev. 109, 1587–1598 10.1021/cr800459r [DOI] [PubMed] [Google Scholar]