Abstract
Remodeling of distal pulmonary arterioles (PAs) associated with marked accumulation of pulmonary artery smooth muscle cells (PASMCs) represents one of the major pathologic features of pulmonary hypertension (PH). We have reported that the transcription factor Twist1 mediates hypoxia-induced PH. However, the mechanism by which endothelial Twist1 stimulates SMC accumulation to distal PAs in PH remains unclear. Here, we have demonstrated that Twist1 overexpression increases the expression of platelet-derived growth factor (PDGFB) in human pulmonary arterial endothelial (HPAE) cells. Hypoxia upregulates the levels of Twist1 and PDGFB in HPAE cells. When we implant hydrogel supplemented with endothelial cells (ECs) on the mouse lung, these ECs form vascular lumen structures and hypoxia upregulates PDGFB expression and stimulates accumulation of αSMA–positive cells in the gel, while knockdown of endothelial Twist1 suppresses the effects. The levels of Twist1 and PDGFB are higher in PAE cells isolated from idiopathic pulmonary arterial hypertension (IPAH) patients compared to those from healthy controls. IPAH patient-derived PAE cells stimulate accumulation of αSMA–positive cells in the implanted gel, while Twist1 knockdown in PAE cells inhibits the effects. Endothelial Twist1-PDGFB signaling plays a key role in αSMA–positive cell proliferation and migration in PH.
Subject terms: Growth factor signalling, Mechanisms of disease
Introduction
Pulmonary hypertension (PH) is a multifactorial life-threatening cardiopulmonary disorder1,2. It is characterized by a sustained increase in pulmonary arterial (PA) pressure, leading to right-sided heart failure1,2. The current therapeutic options only partially improve symptoms and survival1,3. Uncontrolled pulmonary artery smooth muscle cell (PASMC) proliferation and accumulation of PASMCs to normally non-muscularized distal PAs are the major histological characteristics of PH2,4. Thus, we need to understand the mechanism of abnormal SMC proliferation, migration, and their accumulation to distal PAs. In addition to lining vascular structures, endothelial cells (ECs) regulate various physiological functions by secreting angiocrine factors5. Deregulation of this paracrine mechanism leads to the development of diseases5. In PH pathology, abnormal ECs release factors that stimulate SMC proliferation (e.g., FGF-26, serotonin7) or fail to produce factors that physiologically suppress SMC proliferation (e.g., apelin8), indicating that aberrant pulmonary arterial EC signaling plays key roles in abnormal SMC accumulation to distal PAs2.
The transcription factor Twist1 controls physiological and pathological angiogenesis in various organs including the lungs9–14. The levels of Twist1 are upregulated in the lungs of patients with pulmonary arterial hypertension (PAH; WHO group1 PH)15,16 and mice with type II bone morphogenetic protein receptor (Bmpr2) gene mutation15, a gene mutated in familial and idiopathic PAH17. Twist1 expression also increases in the patients with chronic lung diseases associated with PH such as pulmonary fibrosis18,19. We have demonstrated that endothelial Twist1 contributes to the pathogenesis of pulmonary fibrosis in a bleomycin-induced mouse pulmonary fibrosis model12. Twist1 is also involved in endothelial to mesenchymal transition (EndMT)14,15,20, which potentially contributes to the pathogenesis of PH2,15,16,20–23. We have reported that hypoxia-induced pathological accumulation of α-smooth muscle actin (αSMA)-positive cells, including SMCs and myofibroblasts, to distal PAs and increases in right ventricular systolic pressure (RVSP) are attenuated in Tie2-specific Twist1 conditional knockout (Twist1fl/fl/Tie2-cre) mouse lungs20. However, the mechanistic role of endothelial Twist1 in the vascular remodeling in PH has not been fully understood. Twist1 controls the expression of multiple angiogenic factors and receptors in ECs12,13 and exhibits crosstalk with other angiogenesis-related signaling pathways such as hypoxia-inducible factor-1α (HIF-1α)24, Wnt25,26, Notch27, phosphoinositide 3-kinase (PI3K)-AKT28, and transforming growth factor (TGF)-β20,29, which are involved in the pathogenesis of PH1,2. Twist1 also controls the expression of platelet-derived growth factor (PDGF)-related genes in tumor cells30. PDGFB controls SMC proliferation and migration, and excessive PDGFB expression contributes to PH pathology1,31–33.
Here we have demonstrated that endothelial Twist1 stimulates SMC DNA synthesis and migration by increasing PDGFB expression in vitro and mediates hypoxia-induced αSMA-positive cell accumulation in the gel implanted on the mouse lungs. Knockdown of endothelial Twist1 also inhibits accumulation of αSMA-positive cells in the gel supplemented with human IPAH patient-derived ECs and implanted on the mouse lungs. Endothelial Twist1-PDGFB signaling could therefore be one of the key pathways in the pathogenesis of PH.
Materials and Methods
Materials
Anti-Twist1, -PDGFB, -HIF-1α, and -αSMA antibodies were from Abcam (Cambridge, MA). HIF-1α antibody (Abcam; ab1) was validated in MCF7 (human breast adenocarcinoma cell line) cells treated with metformin hydrochloride, which decreases HIF1α expression, to decrease the levels of HIF1α in a dose dependent manner by immunocytochemistry (ICC). PDGFB antibody (Abcam; ab23914) was validated by detecting recombinant human PDGFBB protein. Anti-β-actin monoclonal antibody was from Sigma (St. Louis, MO). Anti-Twist1 antibody was from Santa Cruz Biotechnology (Dallas, TX). Staining with secondary antibody alone confirmed that there was no non-specific binding of the secondary antibody for immunohistochemistry (IHC) (Supplementary Fig. 3a). Recombinant PDGFB and PDGF blocking antibody were purchased from R&D (Minneapolis, MN). Human pulmonary arterial endothelial (HPAE) cells (Lonza) were cultured in EBM2 medium containing 5% FBS and growth factors (VEGF, bFGF and PDGF). Human pulmonary artery smooth muscle cells (HPASMCs) were purchased from Lonza and cultured in DMEM containing 5% FBS. De-identified human IPAH patient ECs were obtained from unused donor control lungs at time of transplantation via the Pulmonary Hypertension Breakthrough Initiative (PHBI) Network, which is funded by the Cardiovascular Medical Research and Education Fund (CMREF) and NIH-NHLBI. The study using these de-identified human cells has been determined and approved as Non-Human Subjects Research by the Medical College of Wisconsin Institutional Review Board (IRB PRO00029154). We obtained ECs isolated from PA (>5 mm in diameter) from females and males (4 control samples; 44.25 +/− 2.86 years old, 6 IPAH samples; 32.5 +/− 2.79 years old). The patient demographic information is in Table 1. These ECs were cultured in EBM2 medium containing 5% FBS and growth factors (VEGF, bFGF and PDGF).
Table 1.
Sample demographics.
| ID | Age | Sex | Race | Passage |
|---|---|---|---|---|
| Con-1 | 36 | Female | White | 3–5 |
| Con-2 | 45 | Female | White | 4–6 |
| Con-3 | 47 | Male | White | 4–6 |
| Con-4 | 49 | Female | White | 4–6 |
| PAH-1 | 27 | Female | White | 4–6 |
| PAH-2 | 23 | Female | White | 3–5 |
| PAH-3 | 32 | Male | White | 3–5 |
| PAH-4 | 33 | Female | White | 3–5 |
| PAH-5 | 40 | Male | White | 4–6 |
| PAH-6 | 40 | Female | White | 3–5 |
Plasmid construction and gene knockdown
Human Twist1 siRNA was previously described20. Lentiviral construct targeting human Twist1 (Twist1 shRNA,CCGGGCTGGACTCCAAGATGGCAAGCTCGAGCTTGCCATCTTGGAGTCCAGCTTTTT) was obtained from Sigma. The full-length Twist1 plasmid was from Addgene (Cambridge, MA) and pHAGE-Twist1-full was constructed as described20. As a control, plasmid with vector only was used. Generation of lentiviral vectors was accomplished by a five-plasmid transfection procedure as reported34. Viral supernatants were collected starting 48 h after transfection for four consecutive times every 12 h, pooled, and filtered through a 0.45 μm filter. Viral supernatants were then concentrated 100-fold by ultracentrifugation in a Beckman centrifuge for 1.5 h at 16500 rpm. HPAE cells were incubated with viral stocks in the presence of 5 μg/ml polybrene (Sigma) and 90–100% infection was achieved 3 days later34.
Molecular biological and biochemical methods
Quantitative reverse transcription (qRT)-PCR was performed with the iScript reverse transcription and iTaq SYBR Green qPCR kit (BioRad, Hercules, CA) using the BioRad real time PCR system (BioRad). β2 microglobulin controlled for overall cDNA content as a reference gene. The primers used for human β2 microglobulin and Twist1 were previously described12,13,20,34. The primers used for human PDGFB were forward; 5′-CTCGATCCGCTCCTTTGATGA-3′ and reverse; 5′-CGTTGGTGCGGTCTATGAG-3′. The protein levels of human PDGFB in ECs were measured using ELISA (R&D systems) and normalized by the protein levels of total cell lysate. When we measured the PDGFB protein levels in the conditioned media (CM), we normalized the levels by the cell numbers.
Mouse EC isolation
Mouse lung ECs were isolated from B6-GFP mouse lungs (Jackson Laboratories, stock # 004353, 2–3 week old) using anti-CD31 conjugated magnetic beads20. We cut mouse lung tissue from B6-GFP mouse into small pieces using small scissors and treated the tissue with 5 ml collagenase A (1 mg/ml) for 30 min at 37 °C. The tissue suspension was filtered through a 40 μm cell strainer (Falcon) to remove the undigested cell clumps and separate single cells. Cells were centrifuged (1000 rpm, 5 min) at room temperature (RT) and the pellet was resuspended into 0.5 ml RBC Lysis Buffer (Sigma, 1 min, RT). The lysis reaction was stopped by adding 10 ml 10% FBS/DMEM, centrifuged (1000 rpm, 5 min, RT), and the pellet was resuspended into 0.5 ml 4% FBS/PBS with APC anti-mouse CD31 (Biolegend, 1/100), incubated (20 min, on ice) and washed three times with 4% FBS/PBS. Cells were centrifuged (1000 rpm, 5 min, RT) and resuspended into 0.1 ml 4% FBS/PBS with anti-APC conjugated microbeads (Miltenyl Biotec, Somerville, MA), incubated (10 min, on ice) and washed three times with 4% FBS/PBS. The cells were then resuspended in 0.5 ml 4% FBS/PBS and CD31-positive ECs were magnetically separated using MACS column (Miltenyl Biotec) according to manufacturer’s instruction. To increase the purity of the magnetically separated fraction, the eluted fraction was enriched over a second new MACS column. Using this method, we obtained 5 × 105 cells/mouse and FACS analysis confirmed that 82.6% of the cells are CD31+ and VE-cadherin+ cells (Supplementary Fig. 2a).
In vitro hypoxia assay
At 80% confluence, HPAE cells were exposed to 1% O2 for 48 h in a hypoxia chamber (Billups-Rothenberg, Del Mar, CA). Cells were lysed for molecular and biochemical analysis. DNA synthesis of SMCs was analyzed by a BrdU incorporation assay. HPASMCs (DMEM with 2% serum) were treated with CM collected from HPAE cells with or without manipulation of Twist1 or in combination with PDGFB (10 ng/ml), pulsed with 5 μM BrdU for 2 h, immunostained and imaged using a confocal Leica SP5 microscope20. DNA synthesis of HPASMCs was also analyzed by measuring the number of BrdU+ cells using FACS (BD Biosciences BrdU flow kit)35. Since counting of BrdU+ cells and FACS analysis revealed similar trends (Supplementary Fig. 2b), we used counting of BrdU+ cells in this study. SMC migration was analyzed using a modified transwell migration assay36. The cells that migrated towards the conditioned media collected from HPAE cells with or without manipulation of Twist1 in 0.5% serum DMEM or supplemented with PDGFB (10 ng/ml) through the membrane were stained with Giemsa, counted and averaged in three independent experiments.
Fibrin gel implantation on the mouse lung
The in vivo animal study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were reviewed and approved by the Animal Care and Use Committee of Medical College of Wisconsin. Fibrin gel was fabricated as described20,37. Briefly, we added thrombin (2.5 U/ml) with angiogenic factors (VEGF and bFGF at 100 ng/ml) to the fibrinogen solution (12.5 mg/ml), mixed well, and supplemented the gel with HPAE cells labeled with GFP using lentiviral transduction or ECs isolated from B6-GFP mouse lungs (1 × 106 cells). When we supplemented the gel with human ECs and implanted on nonobese diabetic/severe combined immunodeficiency gamma (NSG, Jackson Laboratories, stock # 005557) mouse lungs, commercially available human lung fibroblasts (ATCC, Old Town Manassas, VA, 2.5 × 105 cells) were mixed in the gel to induce robust vascular network formation in the gel38. These fibroblasts were not labeled with GFP. We did not mix the gel with fibroblasts when we implanted gel supplemented with ECs isolated from B6-mGFP (background C57BL6) mouse lungs on syngenic B6-mRFP (Jackson Laboratories, stock # 005884) or C57BL6 (Jackson Laboratories, stock # 000664, 8–10 week old) mouse lungs, because similar vessel-like structures were formed without fibroblasts. Immune cells recruited from host C57BL6 mice may help forming vessel-like structures without supplementing fibroblasts by secreting angiogenic factors or stimulating other cells. Drops of the mixture were incubated at 37 °C for 30 min until they solidified. For gel implantation on the mouse lungs, NSG, B6-mRFP, or C57BL6 mice were mechanically ventilated and thoracotomy was performed in the fifth left intercostal space12,37. After thoracotomy, a small area of the left visceral pleura (0.5 mm2) was scraped using forceps and the fabricated fibrin gel was implanted on the mouse lung surface using fibrin glue. To evaluate the effects of hypoxia in vivo, NSG, B6-mRFP or C57BL6 mice, in which fibrin gel supplemented with HPAE cells or ECs isolated from B6-GFP mouse lungs was implanted, were housed in plexiglass chambers and exposed to 8.5 ± 0.5% O2 for 3 days20. For histological analysis, gels were fixed in 4% PFA overnight at 4 °C followed by OCT embedding and cryosectioning. Fluorescent images were taken on a Leica TCS SP5 confocal laser scanning microscope or a Zeiss LSM 510 confocal imaging system. Fluorescently labeled EC-derived vascular lumen structures and accumulation of αSMA-positive cells in the gel were evaluated in five different areas of the gel using ImageJ software12,20,34,37.
Statistical analysis
All phenotypic analysis was performed by masked observers unaware of the identity of experimental groups. Error bars (SEM) and p values were determined from the results of three or more independent experiments. The F test (for two samples) or the Levene test (for more than two samples) was performed to confirm that the variances are homogeneous. Student’s t-test was used for statistical significance for two groups. For more than two groups, one-way ANOVA with a post-hoc analysis using the Bonferroni test was conducted.
Results
Endothelial Twist1 stimulates DNA synthesis and migration of SMCs through PDGFB in vitro
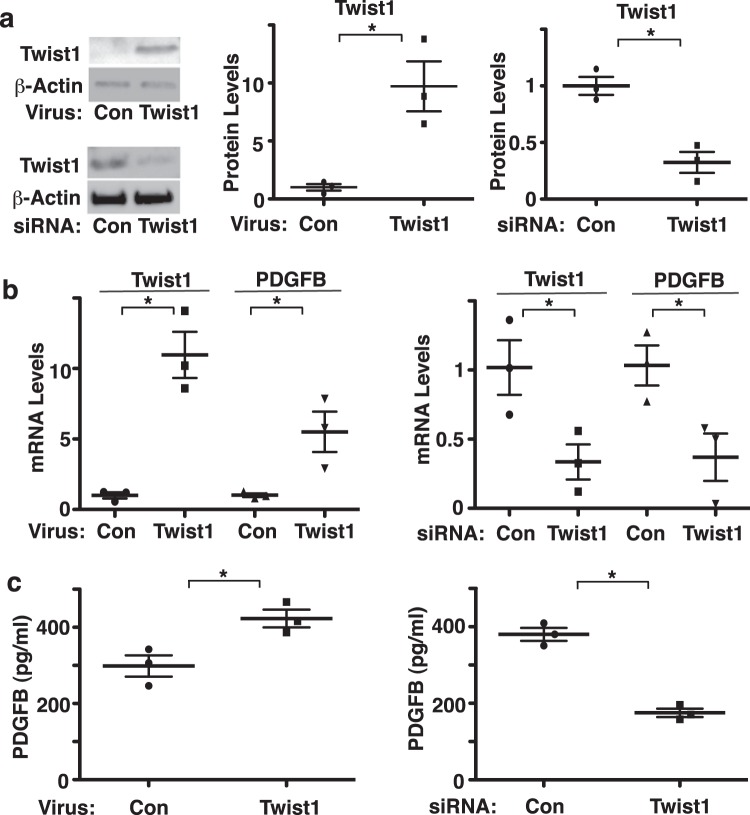
The expression of Twist1 is upregulated in the lungs of patients with PAH15,16. Knockdown of endothelial Twist1 prevents hypoxia-induced increases in RVSP and accumulation of αSMA-positive cells to distal PAs in the mouse model20. However, the mechanism by which endothelial Twist1 controls SMC accumulation has not been fully understood. PDGFB is known to stimulate SMC proliferation and migration, and contributes to PH pathology1,31–33. Overexpression of Twist1 using lentiviral transduction (Fig. 1a, Supplementary Fig. 1) increased the mRNA and protein levels of PDGFB in HPAE cells by 5.2- and 1.3-times, respectively, compared to those treated with control virus (vector alone) as analyzed by qRT-PCR and ELISA, respectively (Fig. 1b,c). In contrast, Twist1 knockdown using siRNA transfection (Fig. 1a, Supplementary Fig. 1) decreased the mRNA and protein levels of PDGFB in HPAE cells, compared to those treated with control siRNA with irrelevant sequences (Fig. 1b,c). When we treated HPASMCs with CM collected from Twist1-knocked down HPAE cells in combination with supplementation with PDGFB, HPASMC DNA synthesis and migration induced by CM and PDGFB, analyzed using a BrdU incorporation assay and a transwell migration assay, respectively, were inhibited by 29% and 16%, respectively (Fig. 2a,b). Overexpression of Twist1 in HPAE cells stimulated SMC DNA synthesis and migration by 1.5- and 1.4- times, respectively, while treatment with a PDGF neutralizing antibody suppressed the effects (Fig. 2c,d), suggesting that endothelial Twist1 is required for SMC DNA synthesis and migration through PDGFB.
Figure 1.
Twist1 induces PDGFB expression in HPAE cells in vitro. (a) Representative immunoblots showing Twist1 and β-actin protein levels in HPAE cells treated with lentivirus expressing Twist1 or control virus (vector alone; left top). Representative immunoblots showing Twist1 and β-actin protein levels in HPAE cells treated with Twist1 siRNA or control siRNA with irrelevant sequences (left bottom). Graphs showing the protein levels of Twist1 in HPAE cells treated with lentivirus expressing Twist1 or control virus (vector alone; middle) or Twist1 siRNA or control siRNA with irrelevant sequences (right; n = 3, *p < 0.05). Error bars represent s.e.m. (b) Graph showing the mRNA levels of Twist1 and PDGFB in HPAE cells treated with lentivirus expressing full-length Twist1 or control virus (left; n = 3, *p < 0.05). Graph showing the mRNA levels of Twist1 and PDGFB in HPAE cells treated with Twist1 siRNA or control siRNA with irrelevant sequences (right; n = 3, *p < 0.05). Error bars represent s.e.m. (c) Graph showing the protein levels of PDGFB in CM collected from HPAE cells treated with lentivirus expressing full-length Twist1 or control virus (left; n = 3, *p < 0.05). Graph showing the protein levels PDGFB in CM collected from HPAE cells treated with Twist1 siRNA or control siRNA with irrelevant sequences (right; n = 3, *p < 0.05). Error bars represent s.e.m.
Figure 2.
Endothelial Twist1 stimulates SMC DNA synthesis and migration through PDGFB in vitro. (a) Graph showing BrdU-positive HPASMCs treated with CM collected from HPAE cells treated with Twist1 siRNA or control siRNA with irrelevant sequences or in combination with PDGFB (n = 4, *p < 0.05). Error bars represent s.e.m. (b) Graph showing HPASMCs migrating towards CM collected from HPAE cells treated with Twist1 siRNA or control siRNA with irrelevant sequences or in combination with PDGFB (n = 5, *p < 0.05). Error bars represent s.e.m. (c) Graph showing BrdU-positive HPASMCs treated with CM collected from HPAE cells treated with lentivirus expressing Twist1 or in combination with PDGF inhibitory antibody or PDGFB (n = 4, *p < 0.05). As a control, CM was collected from HPAE cells treated with control virus (vector alone). Error bars represent s.e.m. (d) Graph showing HPASMCs migrating towards CM collected from HPAE cells treated with lentivirus expressing Twist1 or in combination with PDGF inhibitory antibody or PDGFB (n = 5, *p < 0.05). As a control, CM was collected from HPAE cells treated with control virus (vector alone). Error bars represent s.e.m.
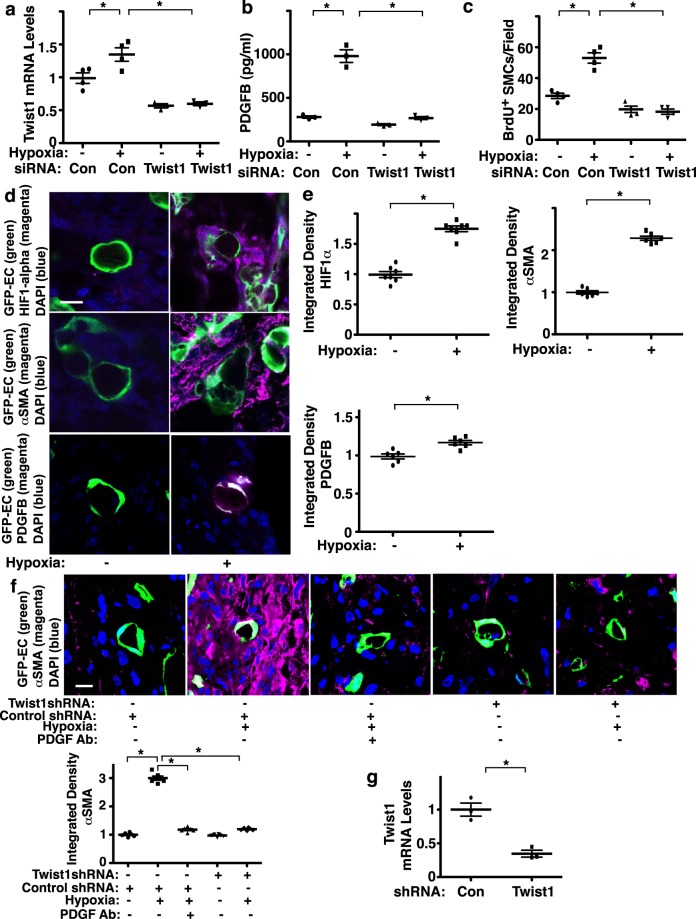
To explore more physiological relevance of endothelial Twist1 in vitro, we next examined the effects of hypoxia or in combination with manipulation of Twist1 expression on PDGFB expression in ECs. When we treated HPAE cells with hypoxia (1% O2) for 2 days, hypoxia increased the mRNA levels of Twist1 by 1.3 times and the protein levels of PDGFB measured by ELISA by 3.2 times, while Twist1 knockdown suppressed these effects (Fig. 3a,b). We also examined whether hypoxia treatment of HPAE cells stimulates SMC DNA synthesis. When we treated HPASMCs with CM collected from HPAE cells treated with hypoxia, BrdU incorporation was stimulated by 1.9-fold compared to those treated with CM of HPAE cells treated with normoxia (Fig. 3c). These effects were attenuated when HPASMCs were treated with CM collected from ECs treated with hypoxia in combination with Twist1 siRNA, suggesting that endothelial Twist1 mediates hypoxia-induced PDGFB expression and SMC DNA synthesis.
Figure 3.
Endothelial Twist1 mediates hypoxia-induced DNA synthesis and accumulation of αSMA-positive cells in the gel implanted on the mouse lung through PDGFB. (a) Graph showing the mRNA levels of Twist1 in HPAE cells treated with hypoxia or in combination with control siRNA or Twist1 siRNA. As a control HPAE cells were treated with control siRNA with irrelevant sequences under normoxia (n = 4, *p < 0.05). Error bars represent s.e.m. (b) Graph showing the protein levels of PDGFB in CM collected from HPAE cells treated with hypoxia or in combination with control siRNA or Twist1 siRNA. As a control HPAE cells were treated with control siRNA with irrelevant sequences under normoxia (n = 3, *p < 0.05). (c) Graph showing BrdU-positive HPASMCs treated with CM collected from HPAE cells treated with hypoxia or in combination with control siRNA or Twist1 siRNA. As a control HPASMCs were treated with CM collected from HPAE cells treated with control siRNA with irrelevant sequences under normoxia (n = 4, *p < 0.05). Error bars represent s.e.m. (d) IF micrographs of fibrin gel supplemented with GFP-labeled B6-GFP mouse lung ECs implanted on C57BL6 mouse lung for 7 days and treated with hypoxia for the last 3 days; GFP-labeled blood vessel lumen structure, HIF1α expression and DAPI (top), GFP-labeled blood vessel lumen structure, αSMA expression and distribution and DAPI (middle), and GFP-labeled blood vessel lumen structure, PDGFB expression and distribution and DAPI (bottom) in the fibrin gel. Scale bar, 10 μm. (e) Graphs showing integrated fluorescent density of HIF1α (n = 7, *p < 0.05), αSMA(n = 6, *p < 0.05), and PDGFB (n = 6, *p < 0.05). Error bars represent s.e.m. (f) IF micrographs of fibrin gel supplemented with GFP-labeled HPAE cells treated with control shRNA or Twist1 shRNA, implanted on mouse lung for 7 days or in combination with exposure to hypoxia for the last 3 days and/or treatment with PDGF neutralizing antibody showing GFP-labeled blood vessel lumen structure, αSMA expression and distribution and DAPI in the fibrin gel. Scale bar, 10 μm. Graph showing the integrated fluorescent density of αSMA (n = 6, *p < 0.05). Error bars represent s.e.m. (g) Graph showing the mRNA levels of Twist1 in HPAE cells treated with lentivirus targeting Twist1 (Twist1 shRNA) or control virus (n = 3, *p < 0.05).
Endothelial Twist1 mediates hypoxia-induced accumulation of αSMA-positive cells in the gel implanted on the mouse lungs
Blood vessels interact with other cellular and non-cellular components and build complex structures in an organ-specific manner39–41. Thus, to study vascular structures and cellular interactions in the lung, we developed a system to implant fibrin gel on the lung surface of living mice12,20,37,41. To study the effects of hypoxia on PDGFB expression and SMC accumulation in the lung, we implanted fibrin gel supplemented with ECs isolated from B6-GFP mouse lungs (background, C57BL6) on the C57BL6 mouse lung (8–10 week old) for 7 days and treated the mice with hypoxia (8.5%) for the last 3 days12,37. Confocal fluorescence images show that GFP-labeled ECs supplemented into the gel form a vascular lumen structure 7 days after implantation (Fig. 3d). Hypoxia treatment for the last 3 days stimulated HIF-1α expression, and induced PDGFB expression in ECs and accumulation of αSMA-positive cells in the gel (Fig. 3d,e). These αSMA-positive cells in the gel seem to be recruited from host mouse lungs; when we implanted fibrin gel supplemented with ECs isolated from B6-GFP mouse lungs to B6-mRFP mouse lungs and treated the mice with hypoxia for 3 days, αSMA-stained mRFP-positive cells accumulated in the gel (Supplementary Fig. 3b).
Consistent with previous report20 and the results using mouse lung ECs, when the gel was supplemented with HPAE cells, implanted on the mouse lung and treated with hypoxia for 3 days, hypoxia induced accumulation of αSMA-positive cells in the gel (Fig. 3f, Supplementary Fig. 3c). Consistent with previous report20, hypoxia also induced EndMT in the gel (Fig. 3f). Twist1 knockdown using lentivirus expressing Twist1 shRNA (Fig. 3g) inhibited the hypoxia-induced EndMT and accumulation of αSMA-positive cells in the gel (Fig. 3f). A PDGF neutralizing antibody also suppressed the hypoxia-induced accumulation of αSMA-positive cells in the gel (Fig. 3f). These results suggest that Twist1-PDGFB signaling mediates hypoxia-induced αSMA-positive cell migration and DNA synthesis in the lung.
Twist1 controls PDGFB expression in IPAH patient PAECs
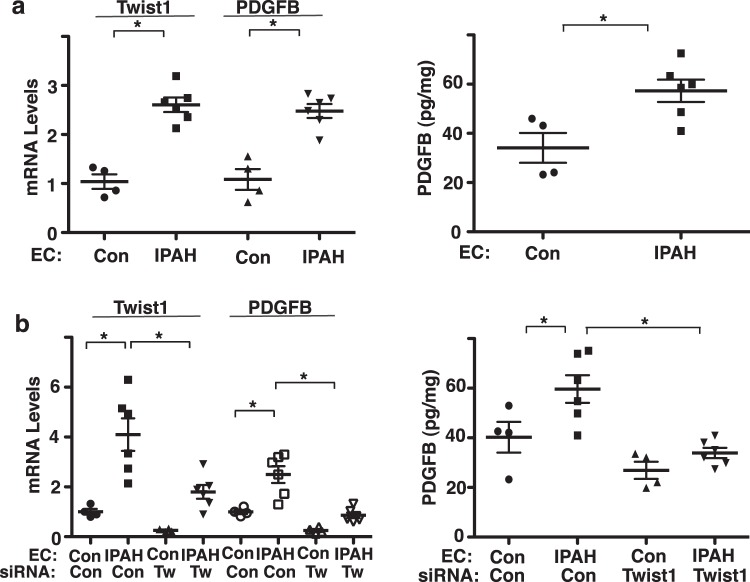
The expression of Twist1 is upregulated in the lungs of patients with PAH15,16 and knockdown of endothelial Twist1 prevents hypoxia-induced accumulation of αSMA-positive cells to distal PAs in the mouse model20. The mRNA levels of Twist1 and PDGFB were 2.6- and 2.5- times higher, respectively in IPAH patient-derived PAECs compared to those in control healthy PAECs (Fig. 4a). The protein levels of PDGFB measured by ELISA were also 1.5- times higher in IPAH patient-derived PAECs compared to those in control healthy PAECs (Fig. 4a). Consistent with hypoxia-treated HPAE cells (Fig. 3b), Twist1 knockdown using siRNA transfection inhibited the PDGFB mRNA and protein expression in IPAH patient PAECs (Fig. 4b).
Figure 4.
Twist1 mediates PDGFB expression in IPAH patient ECs in vitro. (a) Graph showing the mRNA levels of Twist1 and PDGFB in PAECs derived from IPAH patients or healthy controls (left; n = 4–6, *p < 0.05). Graph showing the protein levels of PDGFB in PAECs derived from IPAH patients or healthy controls (right; n = 4–6, *p < 0.05). Error bars represent s.e.m. (b) Graph showing the mRNA levels of Twist1 and PDGFB in PAECs derived from IPAH patients or healthy controls or in combination with treatment with control siRNA or Twist1 siRNA (left; n = 4–6, *p < 0.05). Graph showing the protein levels of PDGFB in PAECs derived from IPAH patients or healthy controls or in combination with treatment with control siRNA or Twist1 siRNA (right; n = 4–6, *p < 0.05). Error bars represent s.e.m.
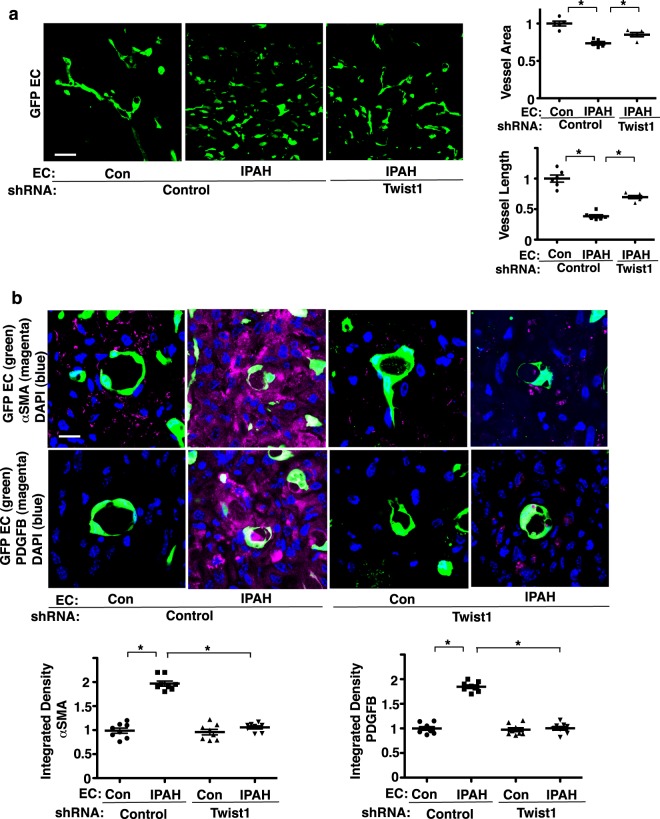
Hypoxia induces accumulation of αSMA-positive cells in the gel implanted on the mouse lungs (Fig. 3d–f). Thus, we next examined the IPAH EC-derived blood vessel structure and accumulation of αSMA-positive cells using a mouse lung gel implantation model. When we implanted fibrin gel supplemented with fluorescently labeled IPAH patient lung ECs on the NSG mouse lung (8–10 week old), CD31+ blood vessel formation (Supplementary Fig. 3d), which is well developed in the implanted gel supplemented with healthy control PAECs, was attenuated in the gel supplemented with IPAH patient-derived PAECs; vascular density and blood vessel length were lower by 28% and 61%, respectively in the gel supplemented with IPAH patient-derived PAECs, while Twist1 knockdown using shRNA treatment inhibited these effects and partially restored vessel structures in the gel (Fig. 5a). Supplemented IPAH patient PAECs also increased accumulation of αSMA-positive cells in the gel by 1.9-times and stimulated PDGFB expression in the gel by 1.8-times compared to those in the gel supplemented with control healthy PAECs (Fig. 5b). Twist1 knockdown in IPAH patient PAECs suppressed PDGFB expression and accumulation of αSMA-positive cells in the gel (Fig. 5b), suggesting that accumulation of αSMA-positive cells in the gel is inhibited in IPAH-patient derived PAECs by suppressing Twist 1-PDGFB signaling.
Figure 5.
Twist1 mediates accumulation of αSMA-positive cells in the fibrin gel supplemented with IPAH patient ECs and implanted on the mouse lung. (a) IF micrographs of fibrin gel supplemented with GFP-labeled PAECs derived from IPAH patients or healthy controls or in combination with treatment with control shRNA or Twist1 shRNA, and implanted on the mouse lung for 7 days showing GFP-labeled blood vessel structures in the fibrin gel. Scale bar, 25 μm. Graphs showing vessel area and length (n = 5–6, *p < 0.05). Error bars represent s.e.m. (b) IF micrographs of fibrin gel supplemented with GFP-labeled PAECs derived from IPAH patients or healthy controls or in combination with treatment with control shRNA or Twist1 shRNA, and implanted on the mouse lungs for 7 days showing GFP-labeled blood vessel lumen structure, αSMA expression and distribution, and DAPI (top) and GFP-labeled blood vessel lumen structure, PDGFB expression and distribution, and DAPI (bottom) in the fibrin gel. Scale bar, 10 μm. Graphs showing integrated fluorescent density to show expression of αSMA and PDGFB (n = 8, *p < 0.05). Error bars represent s.e.m.
Discussion
Here we have demonstrated that knockdown of endothelial Twist1 prevents hypoxia-induced DNA synthesis and migration of αSMA-positive cells by decreasing PDGFB expression (Fig. 6). Twist1 overexpression increases the expression of PDGFB in ECs, and CM collected from Twist1 overexpressing ECs induces SMC migration and DNA synthesis in vitro. Hypoxia stimulates accumulation of αSMA–positive cells in the gel supplemented with mouse and human ECs and implanted on the mouse lung and upregulates expression of PDGFB in the gel, while Twist1 knockdown in ECs attenuates the effects. The levels of Twist1 and PDGFB are higher in IPAH patient-derived PAE cells, which stimulates accumulation of αSMA–positive cells in the implanted gel, while Twist1 knockdown inhibits the effects. Endothelial Twist 1-PDGFB signaling could be one of the key signaling mechanisms in the pathogenesis of PH.
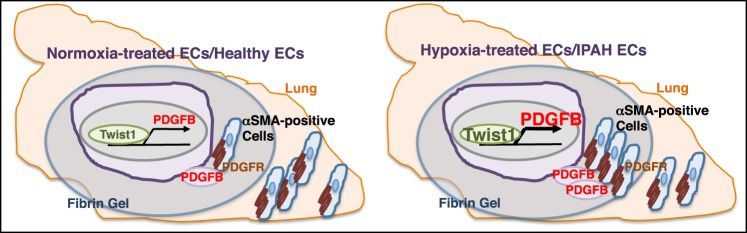
Figure 6.
Schematic illustration of accumulation of αSMA-positive cells through Twist1-PDGFB signaling. Schematic illustration demonstrating that endothelial Twist1 stimulates PDGFB expression and mediates hypoxia-induced αSMA-positive cell accumulation in the gel implanted on the mouse lungs. Endothelial Twist1 also mediates accumulation of αSMA-positive cells in the gel supplemented with human IPAH patient-derived ECs and implanted on the mouse lungs. PDGFR: PDGF receptor.
Our results reveal that CM from ECs overexpressing Twist1 or treated with hypoxia stimulates HPASMC migration and DNA synthesis by increasing PDGFB expression, while knockdown of Twist1 inhibits the hypoxia-induced stimulation of HPASMC DNA synthesis (Figs. 2 and 3). Twist1 is a bHLH transcription factor and controls expression of other angiogenic genes which contain an E-box in their promoter region (e.g.,TGFβ228, VEGFR29, Tie213, TGFβR220)30, and may regulate endothelial and PASMC behaviors in a cooperative way. In addition to PDGFB, PDGFA is also involved in PH pathology32. Other pathways known to mediate the PH pathology (e.g., eNOS42, High Mobility Group AT-hook 1 (HMGA1)22, smad20,43, PGC1α/TFAM44) may be involved in the mechanism. For example, Twist1 controls PGC1α expression in brown fat tissue and regulates cellular metabolism45, which also plays important roles in PH pathology44. Twist1 also interacts with other signaling molecules (e.g., Wnt25), which may contribute to hypoxic PH. Thus, although hypoxia increases Twist1 expression, the effects of Twist1 overexpression may be different from those of hypoxia, which also stimulates other pathways. We have reported that Twist1 Ser42 phosphorylation plays a key role in PH pathology in the hypoxia-induced mouse PH model20. Since Twist1S42A mutant construct decreases PDGFB expression in HPAE cells (not shown), Twist1 Ser42 phosphorylation may contribute to PH pathology by changing PDGFB expression as well. In fact, the hypoxia-induced changes in the levels of Twist1 are modest compared to those in PDGFB levels, suggesting the effects of post-translational modification of Twist1 (e.g., Twist1 Ser42 phosphorylation20) on PDGFB expression. Factors other than Twist1 may also be involved. It is reported that HIF1α controls PDGFB expression in breast cancer cells46. Since HIF1α controls a number of downstream signaling pathways, these other pathways could be involved in the hypoxia-induced change in PDGFB expression. Nevertheless, given that Twist1 knockdown decreased the hypoxia-induced PDGFB levels to the baseline levels (Fig. 3b), Twist1 may be one of the major molecules contributing to this mechanism.
We found that when we implanted fibrin gel supplemented with ECs isolated from B6-GFP mouse lungs to B6-mRFP mouse lungs and treated the mice with hypoxia, αSMA-stained mRFP-positive cells accumulated in the gel (Supplementary Fig. 3b). These results suggest that αSMA-positive cells in the gel seem to be recruited from host mouse lungs. The origin of these recruited αSMA-positive cells remains unclear. Given that the sub-plural area of the mouse lung, where the gel is implanted, is generally devoid of muscularized vessels, these αSMA-expressing cells may be myofibroblasts derived from fibroblasts in the host lungs47, EndMT-derived αSMA-positive cells from the host lung ECs20, or progenitor cells which differentiate into αSMA-positive cells2,48,49. Knockdown of endothelial Twist1 attenuates hypoxia-induced SMC DNA synthesis and migration in vitro and accumulation of αSMA-positive cells in the gel implanted on the mouse lungs through PDGFB signaling (Fig. 3). αSMA-positive cells (e.g., pericytes, myofibroblasts) and other lung cells (e.g., epithelial cells, immune cells)50 recruited into the gel may reciprocally interact and/or secrete angiogenic and other chemical factors, which results in altering Twist1-PDGFB signaling and indirectly affects vascular structures and αSMA-positive cell recruitment in a spatiotemporal manner. Hypoxia-induced accumulation of αSMA-positive cells may not be mediated through endothelial Twist1-PDGFB signaling but through hypoxic effects on αSMA-positive cells or other lung cells. Supplementation of differently labeled other lung cells will enable us to further understand the mechanism by which endothelial Twist1 controls vascular remodeling in the gel.
In addition to angiocrine signaling such as PDGFB, hypoxia also induces vascular structural changes through other mechanisms. For example, hypoxia induces an inflammatory response in the lung, which is critical for the later development of hypoxic PH51,52. Twist1 is involved in various inflammatory pathways13,53–55. PDGFB is also involved in the inflammatory pathways in the lung and contributes to airway remodeling in asthma56 and pulmonary fibrosis57. Thus, inhibition of Twist1 and/or PDGFB expression may attenuate hypoxic PH by inhibiting the inflammatory response to hypoxia as well. EndMT is one of the important processes of the PH pathology and may change the behaviors of surrounding cells and microenvironment (e.g., shear flow, vascular mechanics), which leads to the subsequent vascular structural changes. We have reported that EndMT and accumulation of αSMA-positive cells in the gel are stimulated by treatment with hypoxia for 3 days through Twist1 signaling20 (Fig. 3). Twist1 interacts with other EMT/EndMT genes (e.g., Snail/Slug20,22) and may contribute to hypoxic PH. Although EndMT was stimulated by treatment with hypoxia for 3 days through Twist1 signaling20 (Fig. 3), EndMT was not clearly detected in ECs derived from IPAH patients in the gel (Fig. 5b). EndMT may ensue in only a small percentage of IPAH patient-derived ECs or during the specific time point. Precise time course IHC analysis in combination with supplementation of inflammatory cells in the gel or manipulation of expression of inflammatory mediators or EndMT-related genes will clarify the mechanism.
In addition to hypoxic PH20, we have found that the levels of Twist1 increase in IPAH patient ECs. The mechanism other than hypoxia may be involved in the upregulation of Twist1 in IPAH patient ECs. It is reported that the levels of the chromatin-associated transcriptional regulator, HMGA1, increase in IPAH ECs, which induces EndMT in pulmonary hypertension; knockdown of HMGA1 inhibits EndMT gene expression induced by loss of BMPR222. Since Twist1 is involved in EndMT, HMGA1 may control Twist1 expression in IPAH patient ECs. Other signaling pathways contributing to PH pathology, including TGFβ-smad signaling20,58, TNFα-NFkB signaling59,60, IL661,62, and CD44-LOX signaling63,64 are known to induce Twist1 expression in cancer cells and may be involved in the mechanism.
We have investigated the role of Twist 1-PDGFB signaling in αSMA-positive cell accumulation using ECs isolated from IPAH patient PA with a variety of conditions that can affect endothelial signaling and angiogenic activity. We excluded the samples from >50 years old patients, which are more susceptible to COPD or pulmonary fibrosis that affect other mechanisms, and collected EC samples from the region >5 mm in diameter. However, the heterogeneity of the samples due to cardiopulmonary condition (e.g., chronic lung diseases, inflammation), obesity, sex, and type-2 bone morphogenetic protein receptor (BMPR2) mutations may impact blood vessel formation, SMC DNA synthesis and migration and the signaling pathways2. It is well known that BMPR2 mutations contribute to severity of PH phenotype for vascular remodeling (SMC accumulation, EndMT)2,17. However, due to sample availability, we did not investigate the effects of BMPR2 mutation in human IPAH patient samples in this study. Further investigation in another cohort with a larger sample size will be necessary to elucidate the mechanism of Twist1-PDGFB signaling in the PH pathology.
In summary, we have demonstrated that endothelial Twist1 controls PDGFB expression and mediates hypoxia-induced DNA synthesis of SMCs in vitro and accumulation of αSMA-positive cells in the gel implanted on the mouse lungs. Twist1 knockdown in IPAH patient-derived PAECs attenuates accumulation of αSMA-positive cells in a gel implanted on the mouse lung. These findings suggest that Twist1-PDGFB signaling may be one of the central pathways involved in the pathogenesis of PH.
Supplementary information
Acknowledgements
We thank H. Naito and N. Takakura (Osaka University, Osaka, Japan) for their technical assistance for mouse lung endothelial cell isolation. The authors have declared that no competing interests exist. This work was supported by funds from NIH R21AG054830 (to A.M., to T.M.), R01HL139638 (to A.M., to T.M.), R21AG062893 (to A.M., to T.M.), NIH R01HL142578 (to A.M., to T.M.), American Heart Association (AHA) 18TPA34170129 (to A.M.), and Medical College of Wisconsin Children’s Research Institute Pilot Innovative Research Award (to A.M., to T.M.).
Author contributions
Conceived and designed the experiments: A.M., T.M. Performed the experiments: A.M., K.H., M.M., T.M. Analyzed the data: A.M., K.H., T.M. Contributed reagents/materials/analysis tools: A.M., K.H., M.M., T.M. Wrote the paper: A.M., T.M.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Akiko Mammoto and Tadanori Mammoto.
Contributor Information
Akiko Mammoto, Email: amammoto@mcw.edu.
Tadanori Mammoto, Email: tmammoto@mcw.edu.
Supplementary information
is available for this paper at 10.1038/s41598-020-64298-5.
References
- 1.Guignabert C, et al. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest. 2015;147:529–537. doi: 10.1378/chest.14-0862. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur. Respir. Rev. 2010;19:59–63. doi: 10.1183/09059180.00007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N. Engl. J. Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 5.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J. Cell Physiol. 1996;166:495–505. doi: 10.1002/(SICI)1097-4652(199603)166:3<495::AID-JCP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Dewachter L, et al. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2006;174:1025–1033. doi: 10.1164/rccm.200602-304OC. [DOI] [PubMed] [Google Scholar]
- 8.Alastalo TP, et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Invest. 2011;121:3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, et al. Endothelial TWIST1 Promotes Pathological Ocular Angiogenesis. Invest. Ophthalmol. Vis. Sci. 2014;55:8267–8277. doi: 10.1167/iovs.14-15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, et al. The role of TWIST as a regulator in giant cell tumor of bone. J. Cell Biochem. 2011;112:2287–2295. doi: 10.1002/jcb.23149. [DOI] [PubMed] [Google Scholar]
- 11.Mironchik Y, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mammoto T, Jiang A, Jiang E, Mammoto A. The Role of Twist1 Phosphorylation in Angiogenesis and Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2016;55:633–644. doi: 10.1165/rcmb.2016-0012OC. [DOI] [PubMed] [Google Scholar]
- 13.Mammoto T, et al. Twist1 Controls Lung Vascular Permeability and Endotoxin-induced Pulmonary Edema by Altering Tie2 Expression. PLoS ONE. 2013;8:e73407. doi: 10.1371/journal.pone.0073407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoud MM, et al. TWIST1 Integrates Endothelial Responses to Flow in Vascular Dysfunction and Atherosclerosis. Circ. Res. 2016;119:450–462. doi: 10.1161/CIRCRESAHA.116.308870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranchoux B, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 16.Good RB, et al. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015;185:1850–1858. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Soubrier F, et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2013;62:D13–21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Pozharskaya V, et al. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS ONE. 2009;4:e7559. doi: 10.1371/journal.pone.0007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am. J. Respir. Cell Mol. Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 20.Mammoto T, Muyleart M, Konduri GG, Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension through TGFβ-Smad signaling. Am. J. Respir. Cell Mol. Biol. 2018;58:194–207. doi: 10.1165/rcmb.2016-0323OC. [DOI] [PubMed] [Google Scholar]
- 21.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hopper RK, et al. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation. 2016;133:1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T, et al. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L118–L126. doi: 10.1152/ajplung.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, et al. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J. Orthop. Res. 2007;25:964–971. doi: 10.1002/jor.20356. [DOI] [PubMed] [Google Scholar]
- 26.West JD, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 2014;307:C415–430. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HF, et al. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat. Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 28.Xue G, et al. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2012;2:248–259. doi: 10.1158/2159-8290.CD-11-0270. [DOI] [PubMed] [Google Scholar]
- 29.Cufi S, et al. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9:4461–4468. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Lee NK, Ham S, Roh TY, Kim SH. Twist1 is essential in maintaining mesenchymal state and tumor-initiating properties in synovial sarcoma. Cancer Lett. 2014;343:62–73. doi: 10.1016/j.canlet.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Ten Freyhaus H, et al. Genetic Ablation of PDGF-Dependent Signaling Pathways Abolishes Vascular Remodeling and Experimental Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2015;35:1236–1245. doi: 10.1161/ATVBAHA.114.304864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perros F, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 33.Schermuly RT, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammoto A, Muyleart M, Mammoto T. LRP5 in age-related changes in vascular and alveolar morphogenesis in the lung. Aging. 2019;11:89–103. doi: 10.18632/aging.101722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc. Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Mammoto, T. & Mammoto, A. Implantation of fibrin gel on mouse lung to study lung-specific angiogenesis. J. Vis. Exp.94, e52012 (2014). [DOI] [PMC free article] [PubMed]
- 38.Chen X, et al. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng. Part. A. 2010;16:585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, et al. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mammoto, T., Muyleart, M. & Mammoto, A. Endothelial YAP1 in regenerative lung growth through the angiopoietin-Tie2 pathway. Am. J. Respir. Cell. Mol. Biol.60, 117–127 (2019). [DOI] [PMC free article] [PubMed]
- 42.Frump AL, Datta A, Ghose S, West J, de Caestecker MP. Genotype-phenotype effects of Bmpr2 mutations on disease severity in mouse models of pulmonary hypertension. Pulm. Circ. 2016;6:597–607. doi: 10.1086/688930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orriols M, Gomez-Puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol. Life Sci. 2017;74:2979–2995. doi: 10.1007/s00018-017-2510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diebold I, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21:596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bos R, et al. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46:31–36. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]
- 47.Sun W, Chan SY. Pulmonary Arterial Stiffness: An Early and Pervasive Driver of Pulmonary Arterial Hypertension. Front. Med. 2018;5:204. doi: 10.3389/fmed.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passman JN, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc. Natl Acad. Sci. USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davie NJ, et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L668–678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 50.Stevens T, et al. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc. Am. Thorac. Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergadi E, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niesner U, et al. Autoregulation of Th1-mediated inflammation by twist1. J. Exp. Med. 2008;205:1889–1901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettersson AT, et al. A possible inflammatory role of twist1 in human white adipocytes. Diabetes. 2010;59:564–571. doi: 10.2337/db09-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin G, et al. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seidel P, et al. The MNK-1/eIF4E pathway as a new therapeutic pathway to target inflammation and remodelling in asthma. Cell Signal. 2016;28:1555–1562. doi: 10.1016/j.cellsig.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Sun Q, et al. PDGF-BB induces PRMT1 expression through ERK1/2 dependent STAT1 activation and regulates remodeling in primary human lung fibroblasts. Cell Signal. 2016;28:307–315. doi: 10.1016/j.cellsig.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Shiota M, et al. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012;72:5261–5272. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 59.Li CW, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, et al. Histone demethylase JARID1B regulates proliferation and migration of pulmonary arterial smooth muscle cells in mice with chronic hypoxia-induced pulmonary hypertension via nuclear factor-kappa B (NFkB) Cardiovasc. Pathol. 2018;37:8–14. doi: 10.1016/j.carpath.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Su YW, Xie TX, Sano D, Myers JN. IL-6 stabilizes Twist and enhances tumor cell motility in head and neck cancer cells through activation of casein kinase 2. PLoS ONE. 2011;6:e19412. doi: 10.1371/journal.pone.0019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savale L, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Haibi CP, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA. 2012;109:17460–17465. doi: 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bertero T, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.