Abstract
Water and nitrogen stresses are major constraints for agricultural and forest productivity. Although the effects of water scarcity or nitrogen stress on plant growth, physiology, and yield have been widely studied, few studies have assessed the combined effects of both stresses. In the present study, we investigated the effects of different nitrogen forms (NO3−N, NH4+-N, and a combination of NO3−N + NH4+-N) on antioxidant enzyme activity, osmotic regulatory substances, and nitrogen assimilation in Chinese fir (Cunninghamia lanceolata) plantlets under drought stress (induced by 10% polyethylene glycol). We found that different N ionic forms had different effects on drought-stressed plantlets. Nitrogen supply greatly increased the activities of superoxide dismutase (SOD), peroxidase (POD) and polyphenol oxidase (PPO) when plantlets were exposed to water stress. The malondialdehyde (MDA) contents significantly decreased under the NH4+ + water stress treatment. The proline (Pr) contents significantly increased in both the NO3−N and NH4+-N + water stress treatment. The nitrate reductase (NR) increased by 7.1% in the NO3− + water stress treatment, and the glutamine synthetase (GS), and the glutamate synthase (GOGAT) activity increased in all the nitrogen + water stress treatments. These results suggested that nitrogen supply could alleviate the adverse effects of drought stress on plants by enhancing antioxidant defense and improving nitrogen assimilation, while the effects on plant tolerance to drought stress varied with nitrogen ionic forms.
Subject terms: Ecology, Drought
Introduction
Drought is considered to be one of the most devastating threats to agriculture and forestry. As a result of economic development and climate change, many countries now face water scarcity and water pollution1. Drought stress hampers plant growth and physiology, biochemical processes, and productivity2. Plants respond to oxidative stress caused by water deficit by overproducing reactive oxygen species (ROS), which result in damage to biological molecules and cellular organelles3,4. Plant tolerance to abiotic stress depends largely on their tolerance to oxidative stress, i.e., the strength of their antioxidant system (SOD, POD, PPO, CAT, etc.)5. For example, Zhang et al.6 reported that the antioxidant enzyme activity in the leaves of drought-hardened potato (Solanum tuberosum L. ‘Atlantic’) seedlings was markedly higher for 7 days compared to control seedlings, but then decreased over time (7–14 days). Besides the active oxygen scavenging system, another important mechanism to adapt to drought involves an increase in osmotic adjustment compounds, such as proline, soluble sugars, and abscisic acid content7. Therefore, plants show a natural ability to reduce ROS accumulation and maintain the stability of the membrane system and thus alleviate the damage caused by drought stress.
Furthermore, nitrogen metabolism is crucial for drought tolerance, namely, ion uptake, nitrogen assimilation, amino acid, and protein synthesis. Nitrate (NO3−) assimilation involves the conversion of NO3− into nitrogen dioxide (NO2−) and then into ammonium (NH4+) through nitrate reductase (NR) in the cytosol and nitrite reductase (NiR) in the chloroplasts. A nitrate reductase is the first enzyme in nitrate assimilation and its activity levels have been shown to decrease in the leaves of many species under water stress8. Ammonium derived from the primary nitrate reduction as well as other metabolic pathways, such as root uptake, photorespiration, and amino acid catabolism, is first converted to glutamine by glutamine synthetase (GS), and then to glutamate by glutamate synthase (GOGAT)9. The sequential reactions of GS and GOGAT constitute the principle pathway for assimilating ammonia (NH4+). The capacity for nitrogen metabolism, expressed by NR, GS, GOGAT etc., has been recognized as an indicator of drought tolerance in plants10,11. However, the effects of nitrogen supply through different nitrogen forms on the N assimilation response under drought stress are not yet well understood.
Chinese fir [Cunninghamia lanceolata (Lamb.) Hook.] is one of the most important evergreen conifer species in southern China, with a planting area of over 11 million ha that occupy 15.8% of forest plantations in China and 6.5% globally12,13. This species is distributed across 17 provinces and autonomous regions, with a large geographical range from 21°31′ to 34°03′ N, and from 101°30′ to 121°53′ E. Chinese fir has suffered the effects of drought caused by the spatial and seasonal inhomogeneity of precipitation occurring frequently due to global climate change, especially associated with the subtropical high pressure in the Pacific Ocean. Drought stress usually occurs in summer and autumn, when Chinese fir is in the fast-growing period; thus, the effects of drought on the growth and survival of Chinese fir species can be devastating14. Lin15 reported that the survival rate, current annual increment of ground diameter, and current annual increment of tree height of 1-year-old Chinese fir plantations decreased by 11.5%, 54.3%, and 36.4%, respectively, under drought conditions compared to normal precipitation conditions in 2000–2002.
Nitrogen is the most limiting nutrient for tree growth and productivity; further, NH4+ and NO3− are the main inorganic nitrogen forms that can be absorbed and utilized by plants16. Currently, the declining timber yield and soil degradation of Chinese fir plantations are serious issues due to many inappropriate silvicultural practices, such as monoculture, short rotations, clear cutting, etc.17,18. These practices have resulted in soil nutrient depletion; and nitrogen deficiency is an especially important factor that severely limits sustainable productivity of Chinese fir plantations19. Until now, only a few studies have been conducted on the effects of either drought or nitrogen stress on Chinese fir antioxidant defense system or osmotic adjustment substances. For example, previous studies showed that free radicals or ROS, such as H2O2, O2−, and OH−, accumulated under drought stress, while CAT content increased and POD and SOD content decreased for Chinese fir species20,21. Li et al.22 reported that exogenous Ca2+ and ascorbic acid could reduce MDA content and protect the plasma membrane system from damage by abiotic stress. They also found that exogenous Ca2+ and ascorbic acid could increase the content of osmotic-adjustment compounds like soluble sugars and soluble proteins for Chinese fir species. However, information on the response of nitrogen-metabolism regulation in Chinese fir under drought conditions is scarce. Nitrogen addition affects plant physiological features by reducing MDA, increasing foliar free proline, and influencing antioxidant enzymes23,24. Ma et al.25 found that, as nitrogen stress increased, the NR activity and the light saturation point decreased among different Chinese fir clones. Although many studies have assessed physical responses of Chinese fir to either drought or nitrogen stress, the effects of addition of different nitrogen forms on the response of physiological characteristics to drought stress have not been thoroughly investigated. Furthermore, the responses to drought stress through altered nitrogen metabolism (nitrate and ammonia assimilation) have been scarcely reported. Thus, the physiological mechanisms of coniferous tree species in response to the combined effects of drought stress and nitrogen supply have not yet been clarified.
In view of this, we hypothesized that nitrogen supply would (1) increase antioxidant enzyme activity and the content of osmotic-adjustment-substances, and (2) activate nitrogen assimilation enzymes to enhance drought tolerance. To verify these hypotheses, we measured the MDA content and activities of the plant antioxidant enzyme system (SOD, POD, PPO), osmotic adjustment substances (proline, soluble sugars), and nitrogen assimilation activity (NR, GS, GOGAT) in Chinese fir needles under the supply of various nitrogen forms, (three types: NO3−N, NH4+-N, and a combination of both) and polyethylene glycol (PEG)-induced drought stress. The results of this study can provide scientific basis for drought tolerance evaluation and improvement of nutrient utilization efficiency (NUE) for Chinese fir species.
Results
Effects of nitrogen forms and drought stress on antioxidant enzyme activity and lipid peroxidation
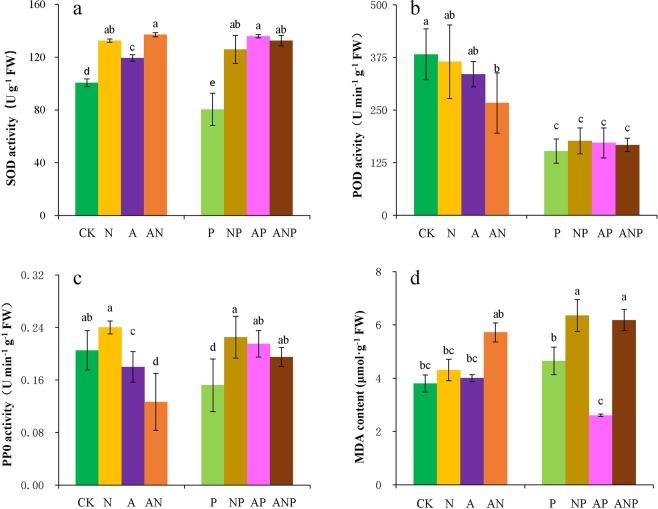
Under non-limiting water conditions, the SOD activity was significantly higher in Chinese fir plantlets under different nitrogen treatment, while the SOD activity was significantly lower by 20.0% under drought stress than in the control. However, the SOD activity was significantly higher by 56.5%, 68.7% and 64.8% in NP, AP and ANP, respectively (Fig. 1a) than under the water stress conditions. Under common water conditions, the POD activity decreased by 4.6%, 12.4%, and 30.3% in the NO3−N, NO4+-N, and NO3−N and NO4+-N combination conditions, respectively. The POD activity was also considerably lower under drought stress than in the control. However, the POD activity was slightly higher than that of the control treatment, by an average of 12.8%, under nitrogen treatment (Fig. 1b). The PPO activity increased by 17.1%% in the NO3−N treatment, and decreased by 12.2% and 38.2% in NO4+-N and the NO3−N and NO4+-N combination treatments, respectively. The PPO activity was also significantly lower, by 25.9%, under drought stress, while PPO activity significantly higher by 48.0%, 41.5% and 28.3%, respectively, under the NP, AP and ANP treatments (Fig. 1c).
Figure 1.
Effects of different nitrogen ion forms and water stress on SOD (a), POD (b), PPO (c) and MDA (d). Treatments: CK, seedlings treated normal water and basic nutrient without nitrogen; N, seedlings treated with NO3−N only; A, seedlings treated with NO4+-N only; AN, seedlings treated with NO3−N and NO4+-N in combination; P, seedlings with 10% PEG; NP, seedlings with NO3−N + 10% PEG; AP, seedlings with NO4+-N + 10% PEG; ANP, seedlings treated with NO3−N and NO4+-N in combination +10% PEG. Mean (± SD) was calculated for three replicates for each treatment. Vertical bars with different lowercase letters are significant at P < 0.05, determined by LSD test.
Under non-limiting water conditions, MDA content was all higher in the NO3−N and NO4+-N combination treatment than in the control. Water stress led to a 22.4% higher MDA content in Chinese leaf under drought stress (Figs. 1d) and 36.6% and 32.9% higher content in AP and ANP treatments than in the control, while a noteworthy significant decrement was recorded at 44.0% under the AP treatment than under drought stress. Thus, different nitrogen applications increased the activities of SOD, POD, and PPO to different degrees, and decreased MDA content under NO4+-N + water stress with respect to that under drought stress.
Effects of nitrogen forms and drought stress on osmotic adjustment substances
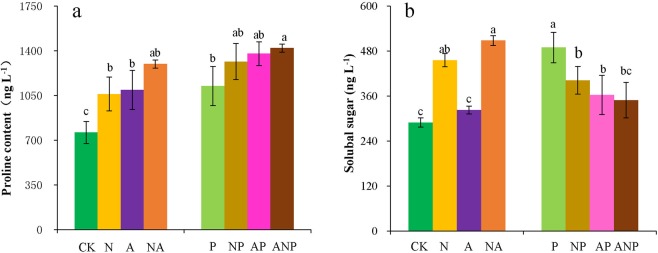
Under normal water conditions, the leaf proline contents and the sugar contents were all higher following nitrogen applications than for non-nitrogen treatments (Fig. 2). Specifically, the leaf proline contents under the NO3−N, NH4+-N, and combination treatments increased by 39.4%, 43.8% and 70.6%, respectively (Fig. 2a). The leaf sugar contents were significantly higher by 57.3% and 75.4% respectively under the NO3−N and combination treatments than under CK (Fig. 2b). Similarly, the leaf proline contents and the sugar contents of drought-stressed seedlings were considerably higher, by 47.8% and 69.0%, respectively, than in non-stressed control plants. However, the proline contents were higher by 17.0%, 22.6%, and 26.5% in NP, AP, and ANP treatments, respectively, than that in drought-stressed seedlings, while the sugar contents were significantly lower by 17.9%, 25.8%, and 28.6%, respectively.
Figure 2.
Effects of different nitrogen ion forms and water stress on Pro content (a) and soluble sugar content (b). Treatments: CK, seedlings treated normal water and basic nutrient without nitrogen; N, seedlings treated with NO3−N only; A, seedlings treated with NO4+-N only; AN, seedlings treated with NO3−N and NO4+-N in combination; P, seedlings with 10% PEG; NP, seedlings with NO3−N + 10% PEG; AP, seedlings with NO4+-N + 10% PEG; ANP, seedlings with NO3−N and NO4+-N in combination +10% PEG. Mean (±SD) was calculated for three replicates for each treatment. Vertical bars with different lowercase letters are significant at P < 0.05, determined by LSD test.
Effects of nitrogen forms and drought stress on nitrogen reduction and assimilation
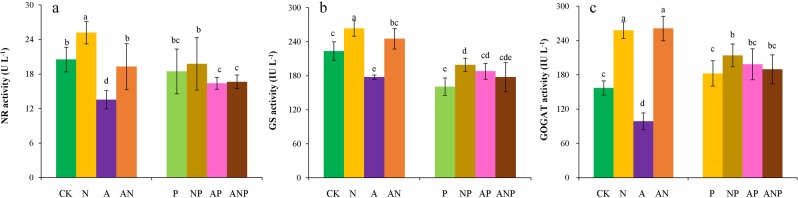
Under normal water conditions, the NR activity was significantly higher in the NO3−N treatment and lower in NH4+-N treatment. Compared to that under CK, the NR activity was lower by 10.1% under water stress. However, NO3−N treatment led to a 7.1% higher NR activity under NP treatment than under water stress (Fig. 3a). The same trend was observed for the GS activity, which was significantly higher by 17.9% in the NO3−N treatment and significantly lower by 20.5% in the NH4+-N treatment respectively than under CK. The GS activity was significantly higher by 23.8%, 16.9%, and 10.4% for NP, AP, and ANP treatments, respectively, than under water stress (Fig. 3b). In contrast, under normal water conditions, the GOGAT activity was significantly higher by 64.4% and 66.4% in the NO3−N and the combination treatments, respectively, than CK. the GOGAT activity was slightly higher under water stress than under normal water conditions. Compared to water stress, the GOGAT activity was higher by 17.2%, 8.8% and 3.9% under the NP, AP, and ANP treatment, respectively, and the differences were significant under NP treatment. Therefore, nitrogen supply, specifically the NO3−N ion, can alleviate the negative effects of drought on nitrate reduction and nitrogen assimilation in plants.
Figure 3.
Effects of different nitrogen ion forms and water stress on NR activity (a) and GS activity (b) and GOGAT activity (c). Treatments: CK, seedlings treated with normal water and basic nutrient without nitrogen; N, seedlings treated with NO3−N only; A, seedlings treated with NO4+-N only; AN, seedlings treated with NO3−N and NO4+-N in combination; P, seedlings with 10% PEG; NP, seedlings with NO3−N + 10% PEG; AP, seedlings with NO4+-N + 10% PEG; ANP, seedlings with NO3−N and NO4+-N in combination +10% PEG. Mean (±SD) was calculated for three replicates for each treatment. Vertical bars with different lowercase letters are significant at P < 0.05, determined by LSD test.
Discussion
We confirmed our first hypothesis that nitrogen supply would enhance antioxidant enzyme activity and osmotic adjustment by inducing proline accumulation to alleviate the physiological damage caused by drought stress (Figs. 1a–c and 2a). However, the effects of nitrogen varied with the nitrogen ionic form supplied. Under drought stress, plants face the risk of ROS toxicity, which may result in lipid peroxidation and oxidative damage to DNA26. Recently, it was proposed that plants have an effective ROS scavenging and signaling system, as suggested by a correlation between drought tolerance and antioxidant enzyme activities27. We found that different nitrogen ionic forms had different effects on antioxidant enzyme activities. Needle SOD activity and PPO activity significantly improved under nitrogen + drought stress treatment, but the extent of the increase varied with the nitrogen ion form supplied (Fig. 1a,c). Needle POD activity also showed higher values by supplying nitrogen under drought stress (Fig. 1b). While MDA content of Chinese fir needles decreased significantly by 44.0% under NH4+-N + drought stress (Fig. 1d). These findings indicate that nitrogen supply reduced oxidative damage to Chinese fir plantlets under drought stress; which are consistent with those of previous studies. For example, Gou et al.28 reported that foliar-applied urea could enhance SOD and POD activities and decrease MDA content of Maize (Zea mays L.) under drought conditions. Saneoka et al.29 demonstrated that high nitrogen fertilizer rates (100 and 200 kg/ha) prevented cell membrane damage and enhanced osmotic regulation in Agrostis palustris Huds. under water stress. Nutrients play important role in decreasing the adverse effects of drought in plants, through maintaining enzyme activity, charge balance and providing osmoticum. Wu et al.30 found the antioxidant activities such as SOD and catalase (CAT) were significantly enhanced, but MDA content was remarkably reduced by supplying zinc nutrient for cotton (Gossypium Hirsutum) under drought stress, which is consistent with our results.
Besides this oxygen scavenging system, the amount of osmotic adjustment compounds, such as proline, soluble sugars, and starch, may increase to improve plant tolerance to drought stress30. Some studies have reported that proline and soluble sugar, synthesized during photosynthesis, play an important role in osmoregulation, and that they increase in response to water deficits31,32. Furthermore, low molecular substances, such as soluble sugars, were superior to macromolecular starch in osmoregulation in response to drought stress33. In this study, proline content significantly increased in the NO3−N and NH4+-N + combination treatment under drought stress, while sugar content decreased in nitrogen + drought stress treatment, compared to the water stress treatments (Fig. 2a,b). Several studies have reported similar results on the effects of N supply on osmotic adjustment in response to water stress. For example, Premachandra et al.34 reported that solute concentrations (e.g., sugars and K+) greatly influenced the osmotic potential at a higher nitrogen application rate (ammonium sulfate, 300 kg ha-1) in soybean (Glycine max L.). Similarly, Saneoka et al.29 found that although osmotic potential may decrease under water stress, osmotic adjustment increased with increasing N supply level in A. palustris, thereby maintaining the water potential gradient that drives water flow vertically (upwards) and horizontally. However, Villar-Salvador et al.35 observed that high N fertilization decreased plasmalemma stability and favored higher water potential, while drought hardening increased plasmalemma stability and increased tissue non-structural carbohydrates and N concentration. They concluded that drought-hardening and N fertilization exert different effects on the physiological stress tolerance of Pinus seedlings, with drought hardening increasing stress tolerance by inducing osmotic adjustment and triggering the scavenging system, and N nutrition reducing the extent of the damaging effects by directly and indirectly promoting a wide array of biochemical processes.
The NR is the first enzyme to assimilate NO3− to NH4+. After NH4+ or NO3−, or both, are absorbed by roots, a large amount of NO3− is assimilated36. Thus, most NO3− is converted into NH4+ by NR, and NH4+ is locally assimilated to glutamine and glutamate via GS and GOGAT37, while the remaining ions are translocated to the leaves or other organs. Drought stress influences the activity of these N metabolism enzymes38,39. In our study, the NR activity was higher by 10.1% in NO3−N + water stress than that under water stress only (Fig. 3a). GS and GOGAT activity in Chinese fir needles also increased to varying degrees under nitrogen + water stress with the enhancement observed in the order NO3−N + water stress> NH4+-N + water stress> the NO3−N and NH4+-N combination treatment + water stress (Fig. 3b,c). Our study suggested that increased NO3−N supply contributed to the conversion of NO3− to NH4+ and increased NH4+ assimilation, which is consistent with the findings of previous studies. Meng et al.38 found that drought stress decreased NR and GOGAT activities in the leaves of Populus simonii seedlings, but NR activity increased in response to normal nitrogen supply (1 mM NH4NO3) under drought stress. These authors found that two ammonium transporter genes (AMTI; 2 and AMTI; 6), closely related to NH4+ uptake, were up-regulated in response to drought stress. Zaghdoud et al.40 reported that NO3− or NO3−/NH4+ co-provision alleviated the effect of salt stress regarding water balance in broccoli plants through an enhanced rate of photosynthesis and an improvement of N metabolism (NR and GS). However, Silveira et al.41 found a rapid increase in nitrate content in roots and a marked reduction in leaf NR activity in cowpea plants (Vigna unguiculata L.) under both water stress and NO3− supply (5 mM). In conclusion, the effects of nitrogen ionic forms (i.e., NO3−, NH4+ and NO3−/NH4+) on plant tolerance to drought stress may vary among species and the parameters that are consideration.
Conclusion
Generally, plants that are adapted to low pH tend to take up nitrogen in the form of ammonium (NO4+) or amino acids, whereas plants that are adapted to high pH and highly aerobic soils prefer nitrate (NO3−) uptake. Soil nitrogen deficiency and water stress are both important factors that restrict the sustainable development of Chinese fir plantations. Different nitrogen ion forms had differing effects on the enhancement of POD, SOD, PPO, GS and GOGAT activity. NH4+ led to significantly lower MDA content while NO3− led to slightly higher NR activity than that in water-stressed plantlets. Proline content significantly increased under the NO3−N and NH4+-N combination treatment. In summary, the addition of nitrogen greatly decreased the negative effects of drought stress and enhanced the drought tolerance of Chinese fir seedlings, but the enhancement effects of nitrogen varied with ion forms.
Materials and methods
Plant materials and applied treatments
The original plant materials were obtained from the seeds of the third-generation seed-orchard of Chinese fir species in Youxi National Farm, Fujian province. The seeds of each available genotype were planted in pots and the seedlings growth were evaluated. One superior Chinese fir clone (No. 7–14, propagated asexually) was chosen as the study material. Besides its growth rate, the No. 7–14 family has strong drought resistance and nitrogen absorption ability42. The average seedling height was 38.5 cm. Plantlets were grown using a water cultivation method under controlled conditions (16:8 h light: dark regime, 120 μmol m−2 s−1 photon flux; at 25 °C and 60% RH) in a growth chamber (LT-ACC400, China).
The basic nutrient solution was controlled using a modified Hoagland nutrient solution that contained K2SO4 (0.41 g L−1), Mg2SO4·7H2O (0.49 g L−1), KH2PO4 (0.136 g L−1); H3BO3 (0.286 g L−1), H2MoO4 (0.0623 g L−1), MnCl2·4H2O (0.181 g L−1), CuSO4·5H2O (0.008 g L−1), ZnSO4·7H2O (0.022 g L−1); ferric salts, FeSO4·7H2O (0.278 g L−1), and EDTA-Na2 (0.373 g L−1). Three nitrogen sources were used to provide 4.571 mM: NO3−N from Ca(NO3)2 at 0.3748 g L−1, NH4+-N from (NH4)2SO4 at 0.3016 g L−1, and the combination treatment using NO3−N and NH4+-N from Ca(NO3)2 at 0.1874 g L−1 and (NH4)2SO4 at 0.1508 g L−1. We then added nitrification inhibitor (0.01 mM Dicyandiamide, DCD) to each of these nutrient solutions. In total, eight treatments were applied: CK (normal water + basic nutrient solution without nitrogen), N (NO3−N only), A (NH4+-N only), AN (NO3−N + NH4+-N), P (10% PEG), NP (NO3−N + 10% PEG), AP (NH4+-N + 10% PEG), and ANP (NO3−N + NH4+-N + 10% PEG). After acclimation, plantlets were divided into eight treatment groups; each with three replicates.
Antioxidant enzyme activity and lipid peroxidation determination
Enzyme liquid extraction: Fresh one-year needles (~0.2 g) were homogenized in liquid nitrogen in centrifuge tubes (5 mL) for 15 min, and then phosphate buffer (4 mL, pH = 7) was added. The homogenates were centrifuged at 10,000 g for 20 min at 4 °C. The supernatants were used to determine the antioxidant enzyme activity as described below.
Superoxide dismutase activity (SOD; EC 1.15.1.1): SOD was determined by the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) according to Costa et al.42. The reaction mixture contained 0.1 mL of enzyme extract in 50 mM potassium phosphate buffer, (pH = 7.8), 0.1 mM EDTA, 50 mM methionine, 75 μM NBT, and 20 μM riboflavin. The reaction mixtures were placed under a high intensity lamp (4000 lx) for 15 min. They were then placed in the dark to stop the reaction, and the absorbance at 560 nm was recorded (Puxi Instrument, Beijing, China). One unit of SOD was defined as the amount of enzymes required to produce 50% inhibition of NBT reduction.
Peroxidase activity (POD; EC 1.11.1.7): the POD activity was determined according to Ekmekca and Terzioglu43. The reaction mixture contained 2.9 mL of 50 mM potassium phosphate buffer (pH = 6.0), 1 mL of 50 mM guaiacol, 1 mL of 50 mM H2O2, and 0.1 mL of enzyme extract. Absorbance at 470 nm was recorded.
Polyphenol oxidase activity (PPO; EC 1.14.18.1): the PPO activity was determined at 420 nm in a spectrophotometer44. The reaction mixture contained 1.5 mL of 0.02 M NaSO4 solution, 5 mM substrate, and 1.5 mL of enzyme extract. After the reaction was completed, absorbance at 420 nm was recorded every 1 min. In total, five absorbance readings were recorded. One unit of enzyme activity was defined as the amount of enzymes required to cause a rate of change of 0.001 absorption unites per min at 420 nm.
Lipid peroxidation determination: Lipid peroxidation was estimated by measurement of malondialdehyde (MDA, a product of lipid peroxidation) using thiobarbituric acid (TBA) according to Hasanuzzaman et al.45. The reaction mixture was homogenized in a centrifuge tube containing 2.5 mL of 0.5% TBA and 1.5 mL of enzyme extract. The mixture was placed in boiling water for 15 min and centrifuged for 10 min at 4000 rpm after cooling. The supernatant was measured and absorbance recorded at 450 nm, 532 nm, and 600 nm. Results were expressed as μmol g-1 on a fresh weight (FW) basis.
Osmotic adjustment substances
Enzyme liquid extraction: Chinese fir needle samples (0.1 g) were ground in liquid nitrogen and dissolved in 0.9 mL phosphate buffer (pH = 7.2). The mixtures were centrifuged for 10 min at 10,000 g, and the supernatants used for determination of osmotic-adjustment substances.
Proline content: Proline was measured according to Bates et al.46. Briefly, the supernatant was mixed with acid ninhydrin with glacial acetic acid and phosphoric acid. This mixture was incubated in a boiling-water bath for 1 h. Cooling toluene was then added. After chromophore containing toluene was produced, absorbance was read at 520 nm.
Soluble sugars: Soluble sugars were determined by the anthrone method47. Reaction mixtures contained 1 mL extract, 1 mL distilled water, 0.5 mL mixed reagent (1 g anthrone + 50 mL ethyl acetate), and 5 mL H2SO4 (98%). The mixtures were heated in a boiling-water bath for 1 min. After cooling, absorbance was recorded at 630 nm.
Nitrogen metabolism
Nitrate reductase activity (EC 1.6.6.1): the NR activity was determined according to Silveira et al.41. Samples (0.2 g) of 7 mm length were placed in vials of ice – cold incubation medium, consisting of 100 mM K-phosphate buffer (pH 7.5), 50 mM KNO3, and 1% (v/v) isopropanol. Tissues were vacuum-infiltrated for 2 min at -67 kPa, and then incubated in water in the dark for 30 min at 30 °C. After incubation, the concentration of nitrite released into the medium was determined by measuring absorbance at 540 nm.
GS activity (EC 6.3.1.2): Enzyme liquid extraction was performed following the method for osmotic adjustment substances. GS activity was determined by the hydroxamate biosynthetic method with the following reaction mixture: Tris-HCl buffer (pH = 7.0), 200 μL 300 mM sodium glutamate (pH = 7.0), 200 μL 30 mM ATP (pH = 7.0), 200 μL 500 mM MgSO4, and 200 μL 1000 mM hydroxylamine hydrochloride neutralized with 1000 mM HCl and 500 μL of enzyme extract. The mixtures were incubated at 30 °C for 30 min. After brown complex formation, absorbance was recorded at 540 nm.
GOGAT activity (EC 1.4.7.1): The mixture contained 25 mM Tris-HCl buffer (pH 7.6), 0.4 mL 20 mM L-glutamine, 0.05 mL 0.1 M 2-oxoglutarate, 0.1 mL 10 mM KCl, 0.2 mL 3 mM NADH, and 0.5 mL of enzyme extract. Absorbance was read at 340 nm.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to determine significant treatment effects, followed by the least significant difference test (LSD) for separate the means. The data are means ± SE. Differences at P ≤ 0.05 were regarded as significant. The software SPSS Statistical Package (SPSS 12.0, SPSS Ins., IL, USA) was used to perform the statistical analysis.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (Grant No. 31800532), Major Special Project of Industry-University-Research, Cooperation of Fujian Province (Grant No. 2012N0010), and the open project of Fujian Provincial Colleges and University Engineering Research Center of Plantation Sustainable Management (PSM-2017002). We thank B.W. Dong and B.Y. Chen for their help during the experiment. We also thank Editage [http://online.editage.cn/] for English language editing.
Author contributions
S.Z. Lin and S.B. Li designed and supervised the study process. M. Sun and S.P. Wu carried out the experiment. G.C. Ding analysed the data and prepared the figures. L.L. Zhou and S.D.A.D wrote and edited the manuscript. All authors commented on the draft and approved the final submission of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mishra AK, Singh VP. A review of drought concepts. J Hydrol. 2010;391:202–216. doi: 10.1016/j.jhydrol.2010.07.012. [DOI] [Google Scholar]
- 2.Cao Y, Luo Q, Tian Y, Meng F. Physiological and proteomic analyses of the drought stress response in Amygdalusmira (Koehne) Yüet Lu roots. BMC Plant Biol. 2017;17:53–69. doi: 10.1186/s12870-017-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faize M, et al. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- 4.Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants. 2015;7:plv069. doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasanuzzaman, M., Hossain, M. A., da Silva, J. A. T. & Fujita, M. Plant responses and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M, eds. Crop stress and its management: perspectivesand strategies. Germany: Springer, 261–316 (2012).
- 6.Zhang SH, Xu XF, Sun YM, Zhang JL, Li CZ. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J Integr Agr. 2018;17:336–347. doi: 10.1016/S2095-3119(17)61758-1. [DOI] [Google Scholar]
- 7.Fang YJ, Xiong LZ. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fresneau C, Ghashghaie J, Cornic G. Drought effect on nitrate reductase and sucrose-phosphate synthases activities in wheat (Triticum durum L.): role of leaf internal CO2. J Exp Bot. 2007;58:2983–2992. doi: 10.1093/jxb/erm150. [DOI] [PubMed] [Google Scholar]
- 9.Ireland, R. J. & Lea, P.J. The enzymes of glutamine, glutamate, asparagine andaspartate metabolism (ed. Singh, B. K.). Plant Amino Acids: Biochemistry and Biotechnology. Marcel Dekker, New York, 49–109 (1999).
- 10.Oaks A. Evidence for deamination by glutamate dehydrogenase in higher plants: reply. Can. J Bot. 1995;73:1116–1117. doi: 10.1139/b95-121. [DOI] [Google Scholar]
- 11.Nagy Z, et al. Metabolic indicators of drought stress tolerance in wheat: Glutamine synthetase isoenzymes and Rubisco. Plant Physiol Biochem. 2013;67:48–54. doi: 10.1016/j.plaphy.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Hu ZH, et al. Effects of harvest residue management on soil carbon and nitrogen processes in a Chinese fir plantation. For Ecol Manag. 2014;326:163–170. doi: 10.1016/j.foreco.2014.04.023. [DOI] [Google Scholar]
- 13.State Forestry Administration of the People’s Republic of China Forest resources in China: 773 The 8th National Forest Inventory, http://211.167.243.162:8085/8/book/jiankuang/index.html 774. (2014)
- 14.Sun, X. M., Wen, X. F., Yu, G. R., Liu, Y. F. & Liu, Q. J. Effects of subtropical seasonal drought on carbon sequestration in Qianyanzhou plantation ecosystem. Sci China Ser D36, 103–110 (in Chinese) (2006).
- 15.Lin, Y. L. Effects of the 2003 drought on the growth of 1- to 3-year-old Chinese fir (Cunninghamia Lanceolata) plantaion. Jour of Fujian Forestry Sci and Tec31, 31–34 (in Chinese) (2004).
- 16.Carvalho PAD, Oliveira LEMD, Sodek L, Carvalho JN. Nitrogen metabolism in the roots of rubber tree (Hevea brasiliensis) plants suwith nitrate or ammonium as nitrogen source during hypoxia. Aust J Crop Sci. 2015;9:1278–1285. [Google Scholar]
- 17.Zhou LL, et al. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ Sci Pollut Res. 2016;23:1–16. doi: 10.1007/s11356-015-5714-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou LL, et al. Biomass production, nutrient cycling and distribution in age-sequence Chinese fir (Cunninghamia lanceolate) plantations in subtropical China. J Forestry Res. 2016;27:357–368. doi: 10.1007/s11676-015-0167-0. [DOI] [Google Scholar]
- 19.Xu X, Timmer VR. Growth and nitrogen nutrition of Chinese fir seedlings exposed to nutrient loading and fertilization. Plant Soil. 1999;216:83–91. doi: 10.1023/A:1004733714217. [DOI] [Google Scholar]
- 20.Su, M. Y. & Fan, M. Q. Effect of osmotic stress and calcium treatment on membrane-lipid peroxidation and protective enzyme in Chinese fir seedling. For Res13, 391–396. (in Chinese) (2000).
- 21.Liu, W. X. et al. The effect of PEG-6000 simulated drought stress on the physiological and biochemistry indexes in Cunninghamia lanceolata family. ForEnviron Sci34, 19–24 (in Chinese) (2018).
- 22.Li, S. B., Ding, G. C., Guo, Z. J., Li, K. & Lin, S. Z. Effects of physiological characteristics of excellent Chinese fir clones and the role of calcium and ascorbic acid under water stress. Anhui Agri Sci Bull20, 17–20 (in Chinese) (2014).
- 23.Nakaji T, Fukami M, Dokiya Y, Izuta T. Effects of high nitrogen load on growth, photosynthesis andnutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees. 2001;15:453–461. doi: 10.1007/s00468-001-0130-x. [DOI] [Google Scholar]
- 24.Yao XQ, Liu Q. Changes in morphological, photosynthetic and physiological responses of MonoMaple seedlings to enhanced UV-B and to nitrogen addition. Plant Growth Regul. 2006;50:165–177. doi: 10.1007/s10725-006-9116-4. [DOI] [Google Scholar]
- 25.Ma, X. Q., Liu, A. Q., Huang, B. L. & Chen, Y. L. Study on selection on high-nitrogen-efficiency-genotypes of Chinese fir clones. Scientia Silvae Sinicae6, 53–57 (in Chinese) (2002).
- 26.Chutipaijit S. Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chil J Agr Res. 2016;76:455–462. doi: 10.4067/S0718-58392016000400009. [DOI] [Google Scholar]
- 27.Uzilday BI, Turkan AH, Sekmen R, Ozgur Karakaya HC. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Science. 2012;182:59–70. doi: 10.1016/j.plantsci.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Gou W, et al. Exogenous application of urea and a urease inhibitor improves drought stress tolerance in maize (Zea mays L.) J Plant Res. 2017;130:599–609. doi: 10.1007/s10265-017-0933-5. [DOI] [PubMed] [Google Scholar]
- 29.Saneoka H, Moghaieb REA, Premachandra GS, Fujita K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ Exp Bot. 2004;52:131–138. doi: 10.1016/j.envexpbot.2004.01.011. [DOI] [Google Scholar]
- 30.Wu S, et al. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium Hirsutum) Acta Physiol Plant. 2015;37:167. doi: 10.1007/s11738-015-1919-3. [DOI] [Google Scholar]
- 31.Monreal JA, et al. Proline content of sugar beet storage roots: response to water deficit and nitrogen fertilization at field conditions. Environ Exp Bot. 2007;60:257–267. doi: 10.1016/j.envexpbot.2006.11.002. [DOI] [Google Scholar]
- 32.Ahmed CB, Rouina BB, Sensoy S, Boukhris M, Abdallah FB. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive culivars under contrasting water availability regimes. Environ Exp Bot. 2009;67:345–352. doi: 10.1016/j.envexpbot.2009.07.006. [DOI] [Google Scholar]
- 33.Praxedes SC, DaMattaa FM, Loureiro ME, Ferrão MAG, Cordeiro AT. Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ Exp Bot. 2006;56:263–273. doi: 10.1016/j.envexpbot.2005.02.008. [DOI] [Google Scholar]
- 34.Premachandra GS, Saneoka H, Ogata S. Cell membrane stability, an indicator of drought tolerance as effected by applied nitrogen in soyabean. J Agr Sci. 1990;115:63–66. doi: 10.1017/S0021859600073925. [DOI] [Google Scholar]
- 35.Villar-Salvador P, Peñuelas JL, Jacobs DF. Nitrogen nutrition and drought hardening exert opposite effects on the stress tolerance of Pinus pinea L. seedlings. Tree Physiol. 2013;33:221–232. doi: 10.1093/treephys/tps133. [DOI] [PubMed] [Google Scholar]
- 36.Xu GH, Fan XR, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63:153–182. doi: 10.1146/annurev-arplant-042811-105532. [DOI] [PubMed] [Google Scholar]
- 37.Huang L, et al. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol Bioch. 2018;127:185–193. doi: 10.1016/j.plaphy.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Meng S, Zhang CX, Su L, Li YM, Zhao Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ Exp Bot. 2016;123:78–87. doi: 10.1016/j.envexpbot.2015.11.005. [DOI] [Google Scholar]
- 39.Huang L, et al. Ammonium uptake increases in response to PEG-induced drought stress in Malus hupehensis Rehd. Environ Exp Bot. 2018;151:32–42. doi: 10.1016/j.envexpbot.2018.04.007. [DOI] [Google Scholar]
- 40.Zaghdoud C, Carvajal M, Ferchichi A, Martínez-Ballesta MC. Water balance and N-metabolism in broccoli (Brassica oleracea L. var. Italica) plants depending on nitrogen source under salt stress and elevated CO2. Sci Total Environ. 2016;571:763–771. doi: 10.1016/j.scitotenv.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 41.Silveira JAG, Costa RCL, Oliveira JTA. Drought-induced effects and recovery of nitrate assimilation and nodule activity in cowpea plants inoculated with Bradyrhizobium spp. under moderate nitrate level. Braz J Microbiol. 2001;32:187–194. doi: 10.1590/S1517-83822001000300005. [DOI] [Google Scholar]
- 42.Li, S. B. et al. Effects of different nitrogen forms on nutrient uptake and distribution of Cunninghamia lanceolata plantlets under drought stress. J Plant Nutri and Ferti26(1): 152–162 (in Chinese) (2020).
- 43.Ekmekci Y, Terzioglu S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic Biochem Physiol. 2005;83:69–81. doi: 10.1016/j.pestbp.2005.03.012. [DOI] [Google Scholar]
- 44.Singh HP, Ravindranath SD. Occurrence and distribution of PPO activity in floral organs of some standard and local cultivars of Tea. J Sci Food Agric. 1994;64:117–120. doi: 10.1002/jsfa.2740640117. [DOI] [Google Scholar]
- 45.Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353–365. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- 46.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 47.Li, H. S. Principles and Techniques of Plant Physiology and Biochemistry Experiments. Higher Education Press, Beijing (2000).