Abstract
Background
Since 1979, mortality from hepatocellular cancer (HCC) has doubled in the United States (US). Lifesaving drugs, prohibitively expensive for some, were approved and marketed to treat hepatitis C virus (HCV), a major risk factor for HCC, beginning in 1997. After the prior introduction of other lifesaving innovations, including active retroviral drug therapy for human immunodeficiency virus and surfactant for respiratory distress syndrome of the newborn, racial inequalities in their mortalities increased in the US. In this descriptive study, we explored racial inequalities in mortality from HCC before and after licensure of HCV drugs in the US.
Methods
The US Centers for Disease Control and Prevention Wide-ranging ONline Data for Epidemiologic Research (WONDER) were used to describe HCC mortality rates from 1979 to 2016 in those 55 years of age and older, because they suffer the largest disease burden. Joinpoint regression was used to analyze trends. To estimate excess deaths, we applied White age-sex-specific rates to corresponding Black populations.
Findings
From 1979 to 1998, racial inequalities in mortality from HCC in the US were declining but from 1998 to 2016 racial inequalities steadily increased. From 1998 to 2016, of the 16,770 deaths from HCC among Blacks, the excess relative to Whites increased from 27.8% to 45.4%, and the trends were more prominent in men. Concurrently, racial inequalities in mortality decreased for major risk factors for HCC, including alcohol, obesity and diabetes.
Interpretation
These descriptive data, useful to formulate but not test hypotheses, demonstrate decreasing racial inequalities in mortality from HCC which were followed by increases after introduction of lifesaving drugs for HCV in the US. Among many plausible hypotheses generated are social side effects, including unequal accessibility, acceptability and/or utilization. Analytic epidemiological studies designed a priori to do so are necessary to test these and other hypotheses.
Keywords: Hepatitis C, Racial inequalities, Lifesaving drugs, Hepatocellular cancer
Research in context panel.
Evidence before this study
Previous reports note increases in racial inequalities in mortality following licensure of lifesaving drugs for human immunodeficiency virus disease and respiratory distress syndrome of the newborn in the United States (US).
Added value of this study
This descriptive study shows that declining racial inequalities in mortality from hepatocellular cancer in the US began to steadily increase one year following the licensure of drugs for hepatitis C virus.
Implications of all the available evidence
These data pose potential major clinical, public health and research challenges. Among the many hypotheses formulated are social side effects, including unequal accessibility, acceptability and/or utilization.
Alt-text: Unlabelled box
1. Introduction
Hepatocellular cancer (HCC) is a major and increasing clinical and public health problem, and is the second most common cause of mortality from cancer [1]. Risk factors include hepatitis C virus (HCV) infection, obesity, diabetes, non-alcoholic fatty liver disease, and excessive alcohol use [2].
In the United States (US), mortality from HCC has been increasing at the highest rate of all malignancies in both men and women [3]. Further, HCC is unequally distributed by race as older Blacks have significantly higher incidence and mortality rates than Whites [4].
The HCV was identified in 1989, and by 1997, effective drugs had become available. Such drugs are lifesaving but prohibitively expensive for some [5,6]. In the US, after the prior introduction of lifesaving drugs, including active retroviral drug therapy for human immunodeficiency virus (HIV) and surfactant for respiratory distress syndrome (RDS), there were increases in racial inequalities in their mortalities [[7], [8], [9]]. In this descriptive study, we explored racial inequalities in mortality from HCC before and after licensure of lifesaving drugs for HCV in the US.
2. Methods
The US Centers for Disease Control and Prevention Wide-ranging ONline Data for Epidemiologic Research (WONDER) afforded the opportunity to obtain mortality data, including overall age-adjusted and age-specific rates by person, place, and time. HCC mortality rates were based on the underlying cause of death using International Classification of Diseases (ICD) codes 155.0 (primary liver cancer, ICD 9th Edition 1979–1998) [10] or B22.0 (liver cell carcinoma, ICD 10th Edition, 1999–2016) from 1979 to 2016 [11]. Further details on coding are presented in Supplementary Table 2.
We chose to explore those age 55 and over, because they account for 83% of all deaths from HCC [11]. The Joinpoint Regression Program [12], [13] (National Cancer Institute software) was used to analyze trends in mortality rates and Black:White mortality rate ratios (MRR's) over time using its Bayesian Information Criterion model selection method. The numbers of excess deaths in Blacks were estimated by applying White age-sex-specific rates to corresponding Black populations. Finally, a percentage change calculator [14] was used to describe race-specific mortality rates from 1998 to 2016.
This research was classified as exempt by the Institutional Review Board of the Baylor College of Medicine.
3. Results
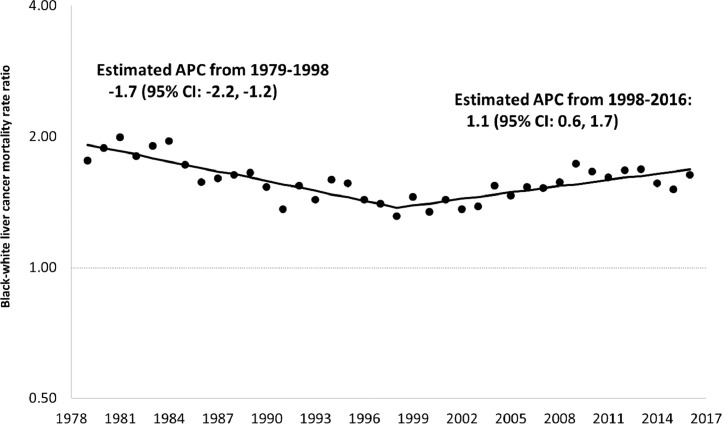
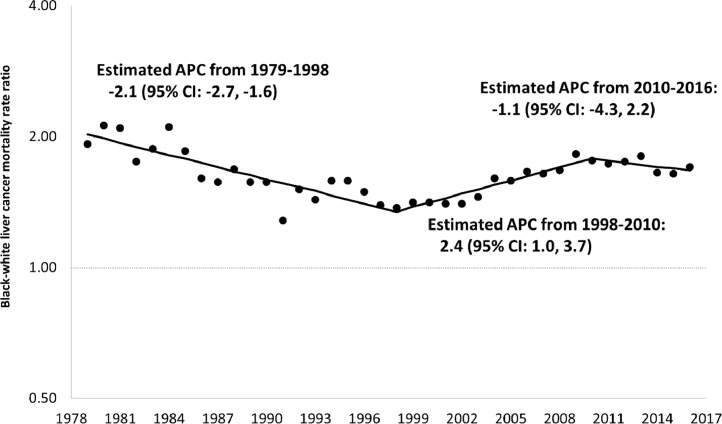
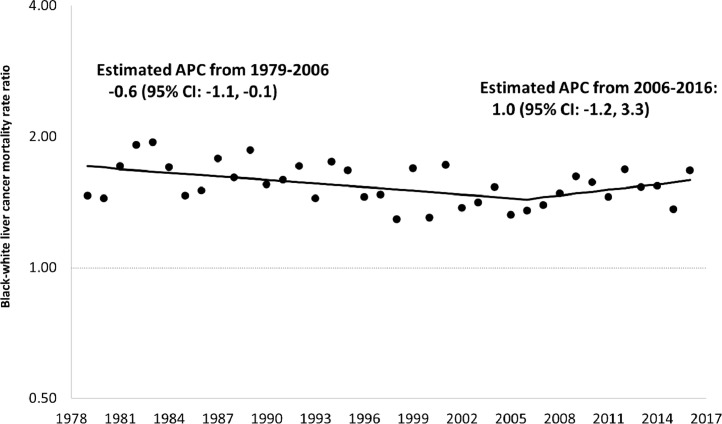
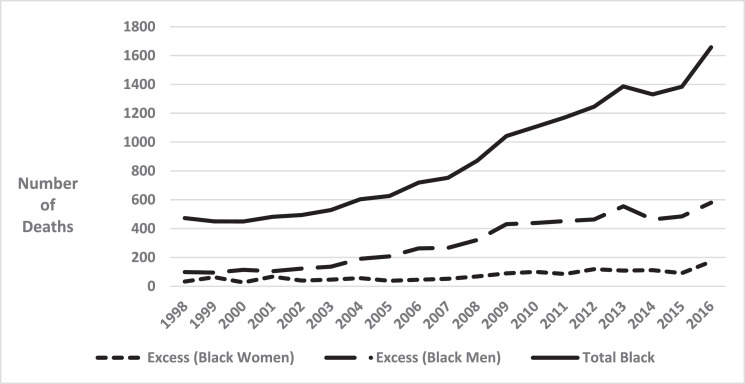
Between 1979 and 1998, among those 55 years of age and older, racial inequalities in mortality from HCC appeared to be steadily declining but appeared to steadily increase between 1998 and 2016. The apparent increase was greater among Blacks than among Whites. Specifically, the Black rate increased from 9.4 per 100,000 in 1998 to 16.7 per 100,000 in 2016, an increase of 77.7%, while the corresponding values for Whites were 7.2 to 10.3, an increase of 43.1%. (Supplementary Figure 1 shows all rates from 1979 to 2016.) Black rates for persons 55+ years of age increased by 1.7% per year from 1979–1997 and by 4.2% per year from 2000–2016. In contrast, corresponding White rates increased by 3.5% per year from 1979–1990, and then increased by 2.0% per year from 1990–2016. Fig. 1 shows that Black:White MRR's in this age group began to increase in 1998 (Supplementary Figure 2). Fig. 2, Fig. 3 show that increased inequalities were more prominent among men. Table 1 shows that Black:White MRR's for alcohol, obesity, as well as diabetes mellitus declined from the late 1980′s through 2016. Sex-specific trends showed similar inequalities (Supplementary Table 1). Fig. 4 shows a steady increase in excess deaths from HCC among Black men and women ages 55 years and older from 1998 to 2016. In 1998, of 473 deaths from HCC among Blacks there would have been 131 (27.8%) fewer if White age-sex-specific rates had applied to the corresponding Black populations. By 2016, this excess had increased to 45.4% (751/1657). From 1998 to 2016, there were 7195 excess deaths from HCC among Blacks (5684 among men and 1411 among women).
Fig. 1.
Joinpoint regression analysis of trends in annual percent change (APC) for Black-White Mortality Rate Ratios in Primary Malignant Hepatocellular Cancer Among Persons 55+ Years. US. 1979–2016.
Fig. 2.
Joinpoint regression analysis of trends in annual percent change (APC) for Black-White mortality rate ratios in primary malignant hepatocellular cancer among Men 55+ Years. US. 1979–2016.
Fig. 3.
Joinpoint regression analysis of trends in annual percent change (APC) for Black-White mortality rate ratios in primary malignant hepatocellular cancer among women 55+ Years. US. 1979–2016.
Table 1.
Joinpoint Regression Analyses. Annual Percent Changes (APC) for Black:White Mortality Rate Ratios for Diabetes Mellitus, Obesity, and Causes of Death Specifically Related to Alcohol Among Persons Ages 55 to 85+ Years. United States of America. 1979–2016.
| Segment | Lower Endpoint | Upper Endpoint | APC | |
|---|---|---|---|---|
| Black:White Mortality Rate Ratios, Diabetes Mellitus | 1 | 1979 | 1987 | 2.1 |
| 2 | 1987 | 1997 | 0.6 | |
| 3 | 1997 | 2016 | −0.8 | |
| Black:White Mortality Rate Ratios, Obesity | 1 | 1979 | 1987 | 3.9 |
| 2 | 1987 | 2016 | −2.3 | |
| Black-White Mortality Rate Ratios, Causes of Death Specifically Related to Alcohol | 1 | 1979 | 1991 | 1.3 |
| 2 | 1991 | 2010 | −4.2 | |
| 3 | 2010 | 2016 | −1.9 |
Fig. 4.
Excess black deaths from hepatocellular carcinoma relative to whites among black women and black men, and total black deaths from hepatocellular carcinoma. USA. 1998–2016.
4. Discussion
These descriptive data demonstrate decreasing racial inequalities in HCC mortality before and increasing racial inequalities after the introduction of lifesaving drugs for HCV in the US.
Initial Food and Drug Administration (FDA) licensure occurred in 1997 (interferon alfacon-1, which was sometimes used in combination with ribavirin), and 1998 (Ribavirin in combination with interferon alfa-2b, and for those who had relapsed following interferon therapy, Rebetol and Intron) [5]. Recently, the evolution of more drugs of life saving benefit for HCC had progressed to clinical claims of cures [15]. The present data show that Black:White MRR's from HCC changed from declining from 1979 to 1998 to steadily increasing thereafter from 1998 to 2016.
In addition to the inability to test hypotheses from these descriptive data, several additional limitations merit mention. Specifically, these data may be restricted in generalizability to the US and those ages 55 and older. With respect to the former, it is tempting to speculate that these findings may be related, at least in part, to mistrust of the health care system [16,17], decreases in adherence, and responses to drugs or even, in theory, biological differences [18,19]. It is certainly plausible that decreases in adherence in Blacks leads to lower efficacy [19]. Thus, the lower efficacy of HCV drugs among Blacks than Whites in a largely male population of US veterans [18], may be due, at least in part, to lower adherence among Blacks [19]. With respect to mistrust, this may be especially prominent among older Black men in the US, possibly as a consequence, at least in part, of withholding potentially lifesaving drugs from Black men as part of the US Public Health Service Study of Syphilis at Tuskegee [16]. Mistrust has also been important in reducing African American engagement with HIV treatment programs in the US [17]. Finally, differential access to care and other social benefits may play a role. Additional limitations of the data include reliance on underlying cause of death. This may lead to misclassification, but data are available to support the validity of standard underlying cause of death data for surveillance of trends in mortality from HCC [20]. The data also are based on deaths whose cell type is specified as due to HCC. Cell type was specified on 63% of the deaths but the distributions were virtually identical in Blacks and Whites [11]. As another consequence of using death certificates, there are no data concerning access to anti-hepatitis C treatment for HCV or the proportion of treated individuals who have HCV infection. Our assumptions are that HCV infection is a major risk factor for HCC and that the distributions of HCV by race were stable during the observation period. Further, data were not available on alcohol consumption, obesity, or diabetes, so it was not possible to control for confounding or explore effect modification during the several decades when significant HCV transmission occurred [21]. Nonetheless, the present data show declines in mortality attributable to major risk factors for HCC within populations 55+ years of age. In addition, the largest increases in MRR's for HCC and its risk factors occurred for HCV. (Supplementary Table 3 and Supplementary Fig. 3 through 8).
Despite these and other possible limitations, we believe the most plausible interpretation of these descriptive data to be that racial inequalities in mortality from HCC were decreasing but then increased following licensure of lifesaving drugs for HCV in the US. These findings about possible unintended consequences of the introduction of lifesaving drugs and HCC mortality in the US, are consistent with previous reports concerning HIV and RDS [[7], [8], [9]]. Analytic epidemiological studies designed a priori to do so are necessary to test the many plausible hypotheses generated by these descriptive data. These include, but are not limited to, the possibilities that widening racial inequalities following licensure of lifesaving innovations may have resulted, at least in part, from unintended social side effects such as unequal accessibility, acceptability and/or utilization. Tests of these hypotheses might lead to a focus on the possibilities of improved product safety and health licensing programs [22]. While the current data are limited in their ability to address these potentially important clinical and public health issues, it is clear that an important priority should be to decrease racial inequalities in mortality following the introduction of life saving drugs.
Declaration Competing of Interest
RSL, MCM, JLS, SJG, MHA, BAH, RJZ report no conflicts.
Acknowledgments
Acknowledgments
This project received no external funding. There are no additional acknowledgements.
CHH reports
My sources of financial support are as follows:
Research and teaching
I am funded at 61% effort by the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU).
My outside activities are funded to Charles H. Hennekens, LLC and include the following:
Data and Safety Monitoring Boards (DSMBs)
I serve as an independent scientist in an advisory role to investigators and sponsors as Chair of 7 DSMBS:
Amgen: Denosumab (Pediatric Osteoporosis and Osteogenesis Imperfecta)
Amgen: Erenumab (Migraine)
Amgen: Evolocumab (CVD)
Amgen: Romosozumab (Osteoporosis)
British Heart Foundation and Cadila: Polypill (CVD)
Dalcor: Dal-GeneE (CVD)
Regeneron: REGN475 (Pain)
Consultancies:
I serve as an independent scientist in an advisory role to
Collaborative Institutional Training Initiative (CIC)
Legal counsel Pfizer (Lipids)
United States (U.S.) Food and Drug Administration (Special Government Employee)
UpToDate
Miscellaneous:
I receive royalties for authorship or editorship of three textbooks.
I receive royalties as co-inventor on patents concerning inflammatory markers and cardiovascular disease which are held by Brigham and Women's Hospital.
I have an investment management relationship with The West-Bacon Group within SunTrust Investment Services who has discretionary investment authority.
I do not own any common or preferred stock in any pharmaceutical or medical device company.
Aurhorship
RSL conceived and designed the work, performed the literature search, acquired and performed data analyses and interpretation, designed the figures, drafted and revised the work critically for important intellectual content and contributed to the final version of the manuscript. He agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MCM made substantial contribution to the main conceptual ideas and drafting the manuscript. JLS aided with development of the statistical methodology, data management, data analysis, drafting of the first draft of the Methods, and providing critical edits to the initial and subsequent manuscript drafts. SJG, MHA, BAH, and RJZ assisted with data interpretation, manuscript writing and contributing to critical edits to subsequent drafts. CHH contributed to data analyses, interpretation of the findings as well as drafting and redrafting numerous revisions of the manuscript. All authors discussed the results, contributed to and approved the final manuscript
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100350.
Appendix. Supplementary materials
References
- 1.London W.T., Petrick J.L., MCGlunn K.A. Schottenfeld and fraumeni cancer epidemiology and prevention. 4th Ed. Oxford University Press; New York: 2018. Liver cancer. Chapter 33 in thun MJ, (Lead editor), linet MS, cerhan JR, haiman CD and schottenfeld D (Co-Editors) and landgren am (Project manager) pp. 635–659. [Google Scholar]
- 2.Lafaro K.J., Demirjian A.N., Pawlik T.M. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24(1):1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson A.B., Eheman C.R., Altekruse S.F., Ward J.W., Jemal A., Sherman R.L., Henley S.J., Holtzman D., Lake A., Noone A.-.M., Anderson R.N., Ma J., Ly K.N., Cronin K.A., Penberthy L., Kohler B.A. Annual report to the nation on the status of cancer, 1975–2012. Featur Increas Incidence Liver Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse S.F., Henley S.J., Cucinelli J.E., McGlynn K.A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hepatitis Central. Medications to treat hepatitis c – a timeline. Available at URL: https://www.hepatitiscentral.com/medications-to-treat-hepatitis-c-a-timeline/Accessed May 26, 2019 and March 22, 2020.
- 6.Kockaya G., Kose A., Yenilmez F.B., Ozdemir O., Kucuksayrac E. Cost-effectiveness analysis of oral anti-viral drugs used for treatment of chronic hepatitis B in Turkey. Cost Eff Resour Alloc. 2015;13:21. doi: 10.1186/s12962-015-0046-8. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisbie W.P., Song S.-.E., Powers D.A., Street J.A. The increasing racial disparity in infant mortality: respiratory distress syndrome and other causes. Demography. 2004;41(4):773–800. doi: 10.1353/dem.2004.0030. [DOI] [PubMed] [Google Scholar]
- 8.Rubin M.S., Golen C.G., Link B.G. Examination of inequalities in HIV/AIDS mortality in the united states from a fundamental cause perspective. Am J Public Health. 2010;100(6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine R.S., Rust G., Pisu M., Agboto V., Baltrus P., Briggs N.C., Zoorob R., Juarez P., Hull P., Goldzweig I., Hennekens C.H. Increased black:white disparities in mortality following lifesaving innovations: a possible consequence of us federal laws. Am J Public Health. 2010 Nov;100(11):2176–2184. doi: 10.2105/AJPH.2009.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National center for health statistics. Compressed Mortality File 1979-1998. CDC WONDER On-line Database, compiled from Compressed Mortality File CMF 196801988, Series 20, No. 2A, 2000 and CMF 1989-1998, Series 20, No. 2E, 2004. Accessed at http://wonder.cdc.gov/cmf-icd9. html April 2019-March2020.
- 11.Centers for Disease Control and Prevention, National center for health statistics. Compressed Mortality File 1999-2016 on CDC WONDER Online Database, released June 2017. Data are from the Compressed Mortality File 1999–2016 Series 20 No. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/cmf-idc10.html April 2019-March2020.
- 12.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. correction: 2001;20:655. [DOI] [PubMed] [Google Scholar]
- 13.Joinpoint Regression Program, Version 4.6.0.0 - April 2018; Statistical methodology and applications branch, Surveillance Research Program, National Cancer Institute.
- 14.Anon. Percentage change calculator. Available at URL: http://www.percentage-change-calculator.com/calculate.php. Accessed June14, 2019.
- 15.Weitzel C., Hepatitis C. treatment – yesterday, tomorrow and …. Med Monatsschr Pharm. 2017;40(4):147–150. [PubMed] [Google Scholar]
- 16.Alsan M., Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133(1):407–455. doi: 10.1093/qje/qjx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaston G.B., Alleyne-Green B. The impact of african americans’ beliefs about hiv medical care on treatment adherence: a systematic review and recommendations for interventions. AIDS Behav. 2013;17(1):31–40. doi: 10.1007/s10461-012-0323-x. [DOI] [PubMed] [Google Scholar]
- 18.Su F., Green P.K., Berry K., Ioannou G.N. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65:426–438. doi: 10.1002/hep.28901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benhammou J.N., Dong T.S., May F.P., Kawamoto J., Dixit R., Jackson S., Dixit V., Bhattacharya D., Han S.B., Pisegna J.R. Race affects SVR12 in a large and ethnically diverse hepatitis C-infected patient population following treatment with direct-acting antivirals: analysis of a single-center department of veterans affairs cohort. Pharmacol Res Perspect. 2018;6(2):e00379. doi: 10.1002/prp2.379. eCollection 2018 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polednak A.P. Using cancer registries to assess the accuracy of primary liver or intrahepatic bile duct cancer as the underlying cause of death, 1999-2010. J Registry Manag. 2013;40(4):168–175. [PubMed] [Google Scholar]
- 21.Pinheiro P.S., Callahan K.E., Jones P.D., Morris C., Ransdell J.M., Kwon D., Brown C.P., Kobetz E.N. Liver cancer: a leading cause of cancer death in the united states and the role of the 1945-1965 birth cohort by ethnicity. JHEP Rep. 2019 doi: 10.1016/j.jhepr.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roose-Snyder B., Doyle M.K. The global health licensing program: a new model for humanitarian licensing at the university level. Am J Law Med Ethics. 2009;35:281–309. doi: 10.1177/009885880903500203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.