Summary
Ultrasound stimulation has recently emerged as a non-invasive method for modulating brain activity in animal and human studies with healthy subjects. Whether brain diseases such as Alzheimer's disease, epilepsy, and depression can be treated using ultrasound stimulation still needs to be explored. Recent studies have reported that ultrasound stimulation suppressed epileptic seizures in a rodent model of epilepsy. These findings raise the crucial question of whether ultrasound stimulation can inhibit seizures in non-human primates with epilepsy. Here, we addressed this critical question. We confirmed that ultrasound stimulation significantly reduced the frequency of seizures in acute epileptic monkeys. Furthermore, the results showed that the number and duration of seizures were reduced, whereas the inter-seizure interval was increased after ultrasound stimulation. Besides, no significant brain tissue damage was observed by T2-weighted MR imaging. Our results are of great importance for future clinical applications of ultrasound neuromodulation in patients with epilepsy.
Subject Areas: Ultrasound Technology, Medical Imaging, Neuroscience
Graphical Abstract
Highlights
-
•
Ultrasound stimulation can inhibit seizures in non-human primates with epilepsy
-
•
Ultrasound stimulation reduces the number and duration of seizures
-
•
Ultrasound stimulation is a safe, noninvasive therapeutic method for epilepsy
Ultrasound Technology; Medical Imaging; Neuroscience
Introduction
Epilepsy is one of the most prevalent neurological disorders characterized by recurrent seizures resulting from excessive excitation or inadequate inhibition of neurons (Pavlov et al., 2013, Blumcke, 2017). Neuromodulation techniques have gained widespread attention owing to their therapeutic utility for epilepsy. They used physical means to modulate neuronal activity, thereby decreasing the frequency or duration of seizures (Theodore and Fisher, 2004).
Ultrasound neuromodulation has gained global attention in recent years owing to its bimodal modulatory effects with exquisite spatial specificity and depth penetration. The evidence from animal and human studies with healthy subjects illustrates that ultrasound can penetrate the skull to the specific brain regions causing behavioral change and improving sensory discrimination abilities (Tufail et al., 2010, Legon et al., 2014, Folloni et al., 2019, Fouragnan et al., 2019). Recent studies have demonstrated that ultrasound stimulation can inhibit the epileptic seizures in a rodent model of epilepsy (Hakimova et al., 2015, Li et al., 2019). Min et al. showed that low-intensity, pulsed ultrasound sonication suppressed the number of epileptic signal bursts using the acute epilepsy model in the rat (Min et al., 2011). Also, Hakimova et al. indicated that ultrasound stimulation effectively inhibited acute seizure activity, including status epilepticus, and subsequent recurrent seizures in the chronic period in a kainate-induced mouse model of mesial temporal lobe epilepsy (Hakimova et al., 2015). Recently, Li et al. reported that low-intensity ultrasound could effectively modulate nonlinear dynamics in acute epileptic mice (Li et al., 2019). These findings suggested a potential role for ultrasound in the treatment of epilepsy, but it has not yet been tested whether ultrasound stimulation can inhibit seizures in nonhuman primates with epilepsy.
We aimed to determine whether ultrasound stimulation was capable of functionally modulating brain activity in non-human primates with epilepsy. The effectiveness of ultrasound neuromodulation was identified by a penicillin-induced epilepsy model in non-human primates (Lin et al., 2020). The results indicated that the number of seizures was significantly reduced, whereas the inter-seizure interval was increased after ultrasound stimulation. The present study suggested that ultrasound may offer a non-invasive method for the treatment of epilepsy.
Results
Examination of the Effect of Ultrasound Parameters on Epileptic Seizures
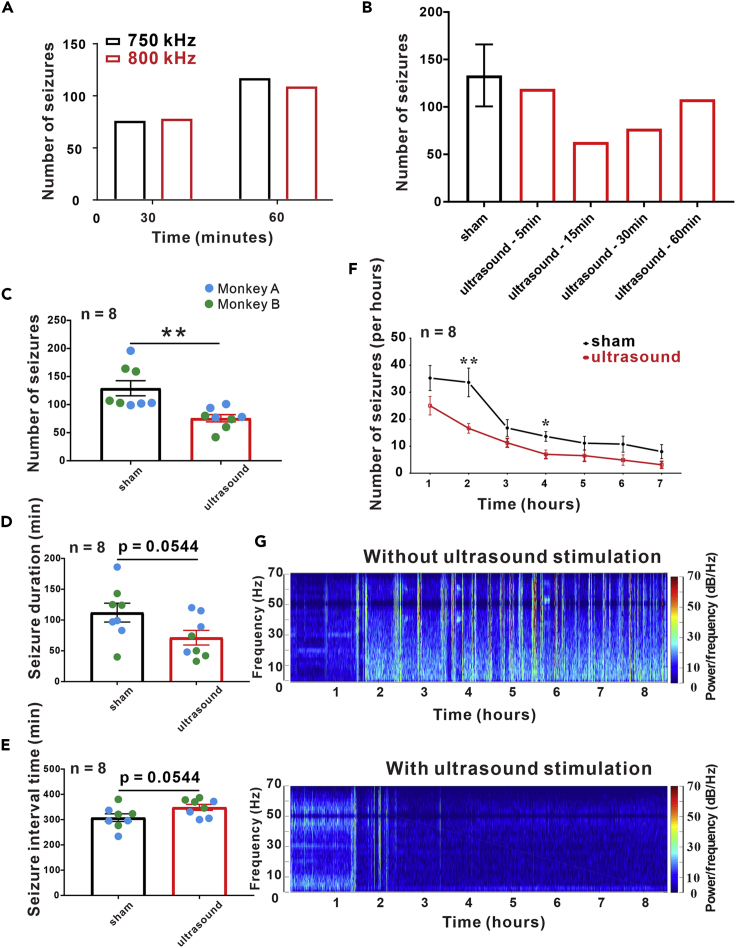
The sonication parameters were selected based on real-time monitoring of behavior and electroencephalograph (EEG). Ultrasound waves with different frequencies and durations were delivered to the prefrontal motor cortex (Figure 1). We found that ultrasound stimulation at a frequency of 800 kHz, a pulse repetition frequency (PRF) of 500 Hz, a duty cycle of 36%, and an acoustic pressure of 1.74 MPa reduced the number of seizures compared with ultrasound stimulation at a frequency of 750 kHz (Figure 2A). In addition, the number of seizures was reduced when ultrasound was delivered at a frequency of 800 kHz, a PRF of 500 Hz, and an acoustic pressure of 1.74 MPa for 15 min (Figure 2B). Therefore, a frequency of 800 kHz and a duration of 15 min were used as the ultrasound parameters in subsequent experiments.
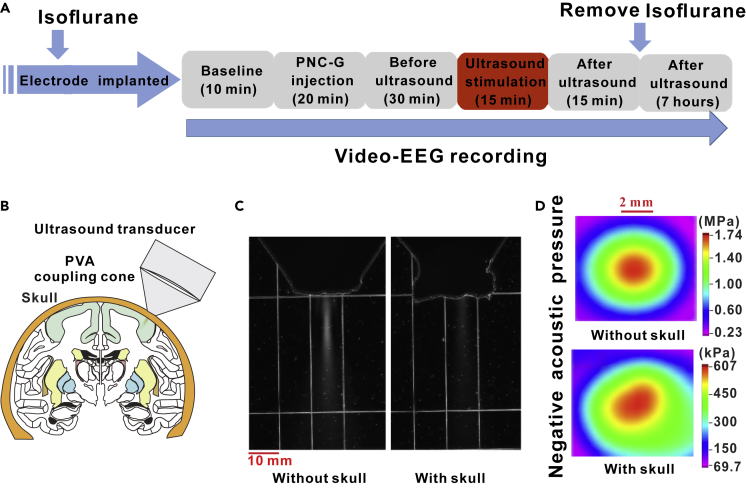
Figure 1.
Schematic of the Ultrasound Neuromodulation System
(A) The experimental process. (B) Ultrasound transducer was placed on the scalp and fixed to the mechanical arm. The coupling cone was filled with PVA phantom. (C) Acoustic field distributions in longitudinal plane without and with mouse monkey skull measured by the OptiSon Ultrasound Beam Analyzer (Onda, USA). Scale bar, 10 mm. (D) Acoustic pressure distribution in axial plane without and with mouse monkey skull measured by a calibrated hydrophone. Scale bar, 2mm.
Figure 2.
Ultrasound Parameters Selection and Video-EEG within 7 h after Ultrasound Stimulation
Ultrasound transducers with a frequency of 750 kHz and 800 kHz were used to stimulate for 30 min and 60 min. By observing the total number of epileptic seizures, we found that the two transducers had the same effect on epileptic EEG. B. An 800 kHz ultrasound transducer was used to stimulate epileptic monkeys with difference time, and under the action of different ultrasonic stimulation time, the number of seizures was as follow: sham (133.3 ± 16.36), 5 min (119), 15 min (63), 30 min (77) and 60 min (108). We found that 15 min ultrasound stimulation had an obvious inhibition effect. C. The total number of epileptic seizures was significantly reduced after ultrasound stimulation (sham: 129.1 ± 13.42, ultrasound: 75.75 ± 6.527, n = 8, independent-sample t-test, p < 0.01). D. The duration of epileptic seizures was decreased after ultrasound stimulation (sham: 112.1 ± 15.33, ultrasound: 71.38 ± 11.9, n = 8, independent-sample t-test, p = 0.0544). E. The inter-seizure interval was longer with ultrasound stimulation than sham stimulation (sham: 307.9 ± 15.33, ultrasound: 348.6 ± 11.9, n = 8, independent-sample t-test, p = 0.0544). F. The frequency of epileptic seizures per hour after 15 min of ultrasound stimulation. The number of seizures gradually decreased in both groups as time progressed. 1st hour (sham: 35.25 ± 4.636, ultrasound: 25 ± 3.423, n = 8, independent-sample t-test, p = 0.1265, 2nd hour (sham: 33.625 ± 5.305, ultrasound: 16.625 ± 1.802, n = 8, independent-sample t-test, p = 0.0085), 3rd hour (sham: 16.750 ± 3.098, ultrasound: 11.250 ± 1.623, n = 8, independent-sample t-test, p = 0.0889), 4th hour (sham: 13.625 ± 1.812, ultrasound: 7.000 ± 1.604, n = 8, independent-sample t-test, p = 0.0318), 5th hour (sham: 11.125 ± 2.539, ultrasound: 6.500 ± 2.104, n = 8, independent-sample t-test, p = 0.2506), 6th hour (sham: 10.750 ± 2.975, ultrasound: 4.875 ± 1.922, n = 8, independent-sample t-test, p = 0.1633), 7th hour (sham: 8.000 ± 2.619, ultrasound: 3.125 ± 1.274, n = 8, independent-sample t-test, p = 0.1218). G. The total number of seizures and the EEG power density with time in two groups. Data are represented as mean ± sem.
Ultrasound Neuromodulation Inhibits Behavioral Seizures
After penicillin injection, all monkeys were monitored for behavioral seizures by continuous video-EEG recording. The total seizure counts for 7 h (sham: 129.1 ± 13.42, ultrasound: 75.75 ± 6.527, t test, p = 0.003) were significantly reduced after 15 min of ultrasound treatment (Figure 2C). Seizure monkeys were randomly selected for ultrasound stimulation; the result revealed that the monkeys in the ultrasound stimulation group had a shorter seizure duration (sham: 112.1 ± 15.33 min, ultrasound: 71.38 ± 11.9 min, t test, p = 0.0544), as shown in Figure 2D. Figure 2E indicated that the mean interval between seizures was 307.9 ± 15.33 min in the sham group and 348.6 ± 11.9 min in the stimulation group (p = 0.0544). In addition, we observed that the mean number of seizures per hour in the ultrasound stimulation group was lower than that in the sham stimulation group (Figures 2F, 2G, and S1). These results showed a trend in the suppression of acute seizures in non-human primates by ultrasound stimulation.
The Safety of Ultrasound Neuromodulation
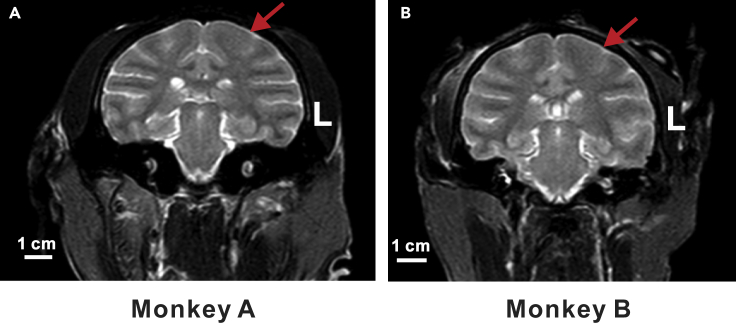
To evaluate the safety of ultrasound neuromodulation, we visualized the temperature change on the surface of the skull during ultrasound stimulation using a thermal infrared imager (R300, NEC Avio, Tokyo, Japan). After 15 min of ultrasound stimulation, the temperature rise was approximately 0.3°C (Figure S2). In addition, T2-weighted MR imaging showed that there was no tissue damage or bleeding after ultrasound stimulation (Figures 3A and 3B, Videos S1 and S2).
Figure 3.
MRI images of monkeys stimulated by ultrasound.
MRI images of monkeys stimulated by ultrasound. T2-weighted MR imaging was performed after ultrasound stimulation. The red arrows indicated where the stimulation was applied to. No pathological damage was found in each monkey after ultrasound stimulation. Scale bar, 1 cm.
Discussion
This study demonstrated that noninvasive ultrasound stimulation could inhibit acute seizures in monkeys. Video-EEG recordings from the epileptic foci tend to show that ultrasound neuromodulation reduced the frequency and duration of seizures and increased the inter-seizure interval in a penicillin-induced epilepsy nonhuman primate model.
Epilepsy is a prevalent neurological disorder resulting in disruptive seizures and is often associated with pharmaco-resistance. Neuromodulation techniques have recently been employed to modulate aberrant neuronal activity and decrease the frequency or duration of seizures. These techniques employ physical means to modulate neuronal activity, thereby decreasing the frequency or duration of seizures (Liebetanz et al., 2006, Krook-Magnuson et al., 2013, Salanova et al., 2015, Bauer et al., 2016, Bauer et al., 2017). Compared with deep brain stimulation and optogenetics, ultrasound can noninvasively penetrate the skull to reversibly modulate neuronal activity and does not require the implantation of an electrode or optical source (Li and Cook, 2018, Deffieux et al., 2013, Wang et al., 2017). Ultrasound neuromodulation has a higher spatial resolution and offers deeper tissue penetration than non-invasive neuromodulation methods, such as transcranial magnetic stimulation and transcranial direct current stimulation (Bystritsky et al., 2011, Folloni et al., 2019, Fouragnan et al., 2019). In this study, we found that ultrasound could noninvasively stimulate the prefrontal motor cortex and inhibit behavioral seizures in monkeys. Overall, low-intensity ultrasound neuromodulation is a promising noninvasive brain stimulation tool that appears to have neuromodulatory effects associated with behavioral changes.
In this study, we found that the inhibitory effect could last for 7 h after 15 min of ultrasound stimulation (Figure 2). In addition, Davide Folloni et al. indicated that 40-s of ultrasound stimulation could cause brain activity of macaque monkeys for more than 1 h (Folloni et al., 2019). These suggest that ultrasound may offer possible non-invasive treatment of epilepsy.
The mechanism by which ultrasound inhibits seizures was not examined in this study. Most authors champion nonthermal mechanical mechanisms of ultrasound neuromodulation. Recently, we reported that ultrasound could open the Escherichia coli mechanosensitive channel of large conductance (MscL) to control neuronal activities (Ye et al., 2018). Moreover, Huang et al. indicated that the therapeutic mechanism of ultrasound neuromodulation could possibly be attributed to promoted brain-derived neurotrophic factor (BDNF) expression (Huang et al., 2017). In addition, we found that ultrasound conferred neuroprotection in Parkinson's disease mice (Zhou et al., 2019a, Zhou et al., 2019b). An important study on the mechanism of ultrasound to suppress epileptic seizures is confirmed by our recent research (Lin et al., 2020). We used patch-clamp to record brain slices of patients with epilepsy and found that ultrasound stimulation can inhibit neuronal excitability in brain slices from epileptic patients, and the inhibition efficiency is more than 65%. In addition, we observed increased expression of c-Fos protein in GABAergic neurons, suggesting that ultrasound stimulation may enhance GABAergic neuron activity and increase the inhibitory postsynaptic inputs. But, owing to the different ultrasound parameters used in two studies, the potential mechanism of ultrasound stimulation for treatment of epilepsy still needs to be studied.
Magnetic resonance imaging showed that ultrasound neuromodulation did not cause any tissue damage, which may suggest that ultrasound is a safe neuromodulation tool. Previous studies have shown that ultrasound was able to mitigate focal cerebral ischemia in rats (Guo et al., 2015, Li et al., 2017), reduce essential tremors in rats (Sharabi et al., 2019), and modulate brain function in humans (Monti et al., 2016). Our recent studies have indicated that ultrasound stimulation can improve motor function in Parkinson's disease model mice (Zhou et al., 2019a, Zhou et al., 2019b). In a study of 54 cases of ultrasound regulating the central nervous system, only two had ultrasound-related injuries (Blackmore et al., 2019). Another study on magnetic resonance acoustic radiation force imaging also pointed out that localized brain regions did not cause tissue damage after ultrasound stimulation (Gaur et al., 2020). Therefore, ultrasound may be a safe, noninvasive therapeutic method for the modulation of neurological disorders, including epilepsy and Parkinson's disease.
Limitations of Study
Our research also has several limitations that should be addressed in the future. First, there were only two animals used in our study; to further verify the role and mechanism of ultrasound neuromodulation technology in non-human primate epilepsy models, we will need to increase the number of animals in the future. Second, from the current experimental results, the ultrasound duration time plays a significant role in the effect of ultrasound to inhibit seizures. We should focus on more point-in-time of ultrasound in the future. Different combinations of ultrasonic parameters were also crucial. Third, the acute epilepsy model was used in our experiments. This model is helpful for us to study the role of ultrasound neuromodulation in epilepsy. However, chronic human temporal epilepsy is more common in human diseases. The other models were studied in the next study. In addition, to reduce the attenuation of the skull, the array ultrasound transducer with low frequency will be developed to deliver ultrasound energy to the targeted region.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The work was supported by the National Natural Science Foundation of China, China (Grant No. 81527901, 11774371, 11574341, 11674347, 11904380, 81671193). Natural Science Foundation of Guangdong Province, China (Grant No. 2017A030313879); Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence Fund, China (No. 2019024); Guangdong grant “Key technologies for treatment of brain disorders” (No. 2018B030332001, No. 2018B030331001) and The Guangdong Provincial Clinical Medical Centre for Neurosurgery, China (No. 2013B020400005). CAS Key Laboratory of Health Informatics Fund, China (2011DP173015).
Author Contributions
J.Z., L.N., L.M., and H.Z. designed experiments. W.M., Y.W., X.H., Z.L., Y.Q., C.T., and J.Z. conducted the experiments. J.Z., Y.C., and Y.G. designed and performed epilepsy surgery. J.Z., L.N., L.M., T.Y., and H.Z. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101066.
Contributor Information
Lili Niu, Email: ll.niu@siat.ac.cn.
Yanwu Guo, Email: dguoyanwu@163.com.
Hairong Zheng, Email: hr.zheng@siat.ac.cn.
Data and Code Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplemental materials.
Supplemental Information
References
- Bauer P.R., De Goede A.A., Ter Braack E.M., Van Putten M.J., Gill R.D., Sander J.W. Transcranial magnetic stimulation as a biomarker for epilepsy. Brain. 2017;140:e18. doi: 10.1093/brain/aww345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Baier H., Baumgartner C., Bohlmann K., Fauser S., Graf W., Hillenbrand B., Hirsch M., Last C., Lerche H. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02) Brain Stimul. 2016;9:356–363. doi: 10.1016/j.brs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Blackmore J., Shrivastava S., Sallet J., Butler C.R., Cleveland R.O. Ultrasound neuromodulation: a review of results, mechanisms and safety. Ultrasound Med. Biol. 2019;45:1509–1536. doi: 10.1016/j.ultrasmedbio.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumcke I. Histopathological findings in brain tissue obtained during epilepsy surgery. N. Engl. J. Med. 2017;377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- Bystritsky A., Korb A., S., Douglas P., K., Cohen M., S., Melega W., P., Mulgaonkar A., P., Antonio D., Min B.K., Yoo S.S. A review of low-intensity focused ultrasound pulsation. Brain Stimulation. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Deffieux T., Younan Y., Wattiez N., Tanter M., Pouget P., Aubry J.F. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 2013;23:2430–2433. doi: 10.1016/j.cub.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Folloni D., Verhagen L., Mars R.B., Fouragnan E., Constans C., Aubry J.F., Rushworth M.F.S., Sallet J. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. 2019;101:1109–1116.e5. doi: 10.1016/j.neuron.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouragnan E.F., Chau B.K.H., Folloni D., Kolling N., Verhagen L., Klein-Flugge M., Tankelevitch L., Papageorgiou G.K., Aubry J.F., Sallet J., Rushworth M.F.S. The macaque anterior cingulate cortex translates counterfactual choice value into actual behavioral change. Nat. Neurosci. 2019;22:797–808. doi: 10.1038/s41593-019-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur P., Casey K.M., Kubanek J., Li N., Mohammadjavadi M., Saenz Y., Glover G.H., Bouley D.M., Pauly K.B. Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep. Brain Stimulation. 2020;13:804–814. doi: 10.1016/j.brs.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Li H., Lv Y., Lu H., Niu J., Sun J., Yang G.Y., Ren C., Tong S. Pulsed transcranial ultrasound stimulation immediately after the ischemic brain injury is neuroprotective. IEEE Trans. Biomed. Eng. 2015;62:2352–2357. doi: 10.1109/TBME.2015.2427339. [DOI] [PubMed] [Google Scholar]
- Hakimova H., Kim S., Chu K., Lee S.K., Jeong B., Jeon D. Ultrasound stimulation inhibits recurrent seizures and improves behavioral outcome in an experimental model of mesial temporal lobe epilepsy. Epilepsy Behav. 2015;49:26–32. doi: 10.1016/j.yebeh.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Huang S.L., Chang C.W., Lee Y.H., Yang F.Y. Protective effect of low-intensity pulsed ultrasound on memory impairment and brain damage in a rat model of vascular dementia. Radiology. 2017;282:113–122. doi: 10.1148/radiol.2016160095. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E., Armstrong C., Oijala M., Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W., Sato T.F., Opitz A., Mueller J., Barbour A., Williams A., Tyler W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014;17:322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- Li M., C.H., Cook M., J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59:273–290. doi: 10.1111/epi.13964. [DOI] [PubMed] [Google Scholar]
- Li H., Sun J., Zhang D., Omire-Mayor D., Lewin P.A., Tong S. Low-intensity (400 mW/cm(2), 500 kHz) pulsed transcranial ultrasound preconditioning may mitigate focal cerebral ischemia in rats. Brain Stimul. 2017;10:695–702. doi: 10.1016/j.brs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Li X., Yang H., Yan J., Wang X., Li X., Yuan Y. Low-intensity pulsed ultrasound stimulation modulates the nonlinear dynamics of local field potentials in temporal lobe epilepsy. Front. Neurosci. 2019;13:287. doi: 10.3389/fnins.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D., Klinker F., Hering D., Koch R., Nitsche M.A., Potschka H., Loscher W., Paulus W., Tergau F. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- Lin Z., M L, Zou J., Zhou W., Huang X., Xue S., Bian T., Yuan T., Niu L., Guo Y., Zheng H. Noninvasive ultrasonic neuromodulation of neuronal excitability for treatment of epilepsy. Theranostics. 2020 doi: 10.7150/thno.40520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.K., Bystritsky A., Jung K.I., Fischer K., Zhang Y., Maeng L.S., Park S.I., Chung Y.A., Jolesz F.A., Yoo S.S. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 2011;12:23. doi: 10.1186/1471-2202-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M.M., Schnakers C., Korb A.S., Bystritsky A., Vespa P.M. Non-invasive ultrasonic thalamic stimulation in disorders of consciousness after severe brain injury: a first-in-man report. Brain Stimul. 2016;9:940–941. doi: 10.1016/j.brs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Pavlov I., Kaila K., Kullmann D., M., Miles R. Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions? The Journal of physiology. 2013;519:765–774. doi: 10.1113/jphysiol.2012.237958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M., Labar D., Sperling M.R., Sharan A., Sandok E. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi S., Daniels D., Last D., Guez D., Zivli Z., Castel D., Levy Y., Volovick A., Grinfeld J., Rachmilevich I. Non-thermal focused ultrasound induced reversible reduction of essential tremor in a rat model. Brain Stimul. 2019;12:1–8. doi: 10.1016/j.brs.2018.08.014. [DOI] [PubMed] [Google Scholar]
- Theodore W.H., Fisher R.S. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Tufail Y., Matyushov A., Baldwin N., Tauchmann M.L., Georges J., Yoshihiro A., Tillery S.I., Tyler W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu C., Xu Z., Ji C., Liang J., Wang Y., Chen B., Wu X., Gao F., Wang S., Guo Y., Li X., Luo J., Duan S., Chen Z. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:92–105. doi: 10.1016/j.neuron.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Ye J., Tang S., Meng L., Li X., Wen X., Chen S., Niu L., Li X., Qiu W., Hu H. Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett. 2018;18:4148–4155. doi: 10.1021/acs.nanolett.8b00935. [DOI] [PubMed] [Google Scholar]
- Zhou H., Niu L., Meng L., Lin Z., Zou J., Xia X., Huang X., Zhou W., Bian T., Zheng H. Noninvasive ultrasound deep brain stimulation for the treatment of Parkinson's disease model mouse. Research (Wash D C) 2019;2019:1748489. doi: 10.34133/2019/1748489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Niu L., Xia X., Lin Z., Liu X., Su M., Guo R., Meng L., Zheng H. Wearable ultrasound improves motor function in an MPTP mouse model of Parkinson's disease. IEEE Trans. Biomed. Eng. 2019;66:3006–3013. doi: 10.1109/TBME.2019.2899631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplemental materials.