Abstract
Coniochaeta sp. strain 2T2.1 is a key member of a microbial consortium that degrades lignocellulosic biomass. Due to its ecological niche and ability to also grow in pure culture on wheat straw, protocols for transformation and antibiotic selection of the strain were established. Hygromycin was found to be a reliable selectable transformation marker, and the mammalian codon-optimized green fluorescent protein was expressed and used to visualize fluorescence in transformed cells of strain 2T2.1.
Keywords: transformation, protoplast, GFP, fungi, biomass
Introduction
Coniochaeta are ascomycetes that inhabit soil and decaying plant matter [1]. Coniochaeta species exhibit mycelial growth, but in liquid culture generally have a yeast-like morphology due to the formation of conidia on reduced conidiophores [2]. Members of the genus are potential sources of hydrolytic enzymes [3–5], and Coniochaeta isolates also function in bioconversion of furanic compounds [6–8] and have capacity for production of secondary metabolites [9–11]. Relevant to degradation of lignocellulosic biomass, Coniochaeta sp. strain 2T2.1 is the fungal member of a microbial consortium capable of growing on raw wheat straw [6]. The strain is also capable of growing alone on biomass, as shown in Figure 1.
Figure 1:

Brightfield microscopic image of C. sp. 2T2.1 growing in association with ground wheat straw.
The genome of C. sp. 2T2.1 was recently sequenced in an effort to identify genes involved in the degradation of lignocellulosic materials. Due to its ecological niche as part of a mixed fungal–bacterial consortium and its potential for production of secondary metabolites, the strain is of interest as a target for genetic engineering. To that end, a protocol for transformation and antibiotic selection of Coniochaeta sp. 2T2. 1 was developed and green fluorescent protein (GFP) was expressed in the transformed strain.
Materials and methods
Strains, plasmids and growth conditions
Coniochaeta sp. 2T2.1 was isolated from soil as part of a microbial consortium growing on raw wheat straw [6]. pCNS43 [12] is an Escherichia coli–fungal shuttle vector carrying the E. coli hygromycin B phosphotransferase gene (hph) [24]. pRS410 and pRS418 (addgene.org plasmids # 11258 and 11256, respectively) carry the KanMX and NatMX resistance markers for G418 and nourseothricin resistance, respectively, in the yeast centromere (CEN) plasmid backbone. pJQ7 has the mammalian codon-optimized gfp gene (hrgfp) and hph for selection of fungal transformants. Coniochaeta was cultured in YPD medium which contained, per liter, 20 g glucose, 10 g Bacto yeast extract, 20 g Bacto peptone, and for solid medium, 15 g Bacto agar (Becton, Dickinson, Sparks, MD, USA).
Cultures for determination of antibiotic sensitivities were incubated in 100 µl volume at 30°C and 950 rpm in humidified microtiter plates in a MB100–4A orbital microplate thermo-shaker (Allsheng Instruments, Hangzhou, Zhejiang, China). Density was measured using a PowerWave XS2 plate reader (0. 3125 cm path length) and Gen5 v.1.11 software (BioTek Instruments, Inc., Winooski, VT, USA). Cultures were inoculated at a density of 0.05–0.1, and final density was measured after 24 h. Glucose was filter-sterilized separately and added to media after it was sterilized by autoclaving. Glucose and antibiotics were purchased from Sigma-Aldrich (St Louis, MO, USA).
For growth on wheat straw, strain 2T2.1 was cultured at 30°C with aeration by shaking in defined mineral medium [25 mM each KH2PO4 and Na2HPO4, 0.1% (w/v) (NH4)2SO4, 0.1% Hutner mineral base [13] (final pH 6.8)]. Wheat straw [1% (w/v)] was added prior to autoclaving the medium. A cell pellet was collected by centrifugation (7000 g for 5 min) and washed with an equal volume of mineral medium to remove loosely associated extracellular fungal cells. Cells were photographed using a BX51 microscope (Olympus Life Science, Waltham, MA, USA).
Transformation of Coniochaeta sp. 2T2.1
Fungal protoplasts were prepared from cells cultured to log phase of growth (∼4–6 h) in 25 ml YPD medium at 30°C, with aeration by shaking at 200 rpm. Cells were collected by centrifugation (7000 g for 10–15 min at 4°C), suspended to a density (absorbance at 600 nm) of 0.5 (1.0 cm path length) and washed twice with an equal volume of protoplasting buffer (0.5 M sorbitol, 20 mM KH2P04, pH 6.4). Pellets were suspended in the same buffer containing 1% (v/v) β-mercaptoethanol and incubated at 30°C for 45 min. Cell pellets were again collected by centrifugation and resuspended in 5 ml protoplasting buffer containing 20–160 µg/ml cell wall lysing enzymes (L1412, Sigma-Aldrich) and incubated for 0.5–6 h at 30°C. Formation of protoplasts was monitored via light microscopy. Protoplasts were harvested by centrifugation at 21000 g for 5 min at 4°C, washed twice with an equal volume of STC buffer (1.0 M sorbitol, 10 mM Tris pH 7.5, 50 mM CaCl2) and resuspended in 0.6 mL STC.
A mixture containing 0.2 mL of protoplasts, 50 µl STC buffer containing 25% w/v PEG8000 and 1.0 µg plasmid DNA in water was incubated on ice for 20 min. An additional 2 ml of STC buffer containing 25% (w/v) PEG8000 was added, with incubation at room temperature for 20 min. Regeneration buffer (4 ml of 1 M sorbitol, 0.1% yeast extract, 0.1% K2HPO4, 0.05% MgSO4) was added and the mixture was incubated overnight at 30°C with shaking. Transformants were selected by plating onto solid YPD medium containing the relevant selective antibiotic (100 µg/ml hygromycin, G418 or nourseothricin). For transformation of pJQ7, colonies arising after incubation for 3–4 days at 30°C were also assessed for fluorescence. Transformation of C. sp. 2T2.1 was also verified by the presence, as detected by PCR, of hrgfp and/or hph using oligonucleotide primers 5′-GCAC TTTTTGCAGTACTAACCGCAG3′ and 5′-AGGCTAAGGGTCCAGTT GTAGACG3′ for detection of the hrgfp gene and 5′-CGTCTGTC GAGAAGTTTC3′ and 5′-CATTGTTGGAGCCGAAATC3′ for amplification of the hph gene. polymerase chain reaction (PCR) reaction mixtures typically contained a portion of a hygromycin-resistant colony as template, with Phusion HF DNA polymerase and reagents (New England Biolabs, Ipswich, MA, USA) under the manufacturer’s recommended conditions. Transformation of four separate protoplast preparations was carried out for each condition tested.
GFP detection and quantitation
GFP fluorescence intensity per cell was measured using an image cytometer [Cellometer® Vision CBA analysis system (Nexcelom Bioscience LLC, Lawrence, MA, USA)]. Fluorescence images were obtained from 20 μl cells excited using an LED light source and filter set (optics module VB-535–402, Nexcelom Bioscience LLC) for GFP excitation (475 nm) and emission (535 nm) during a 2 s exposure. Data generated from the image cytometer were analyzed using FCS Express Cytometry software (DeNovo Software, Glendale, CA, USA). Mean GFP fluorescence intensity per cell was determined by counting approximately 3000 cells per replicate. Fluorescence was measured from three independent cultures for each transformant.
Results and discussion
To establish a transformation protocol, selective agents were investigated for use with C. sp. strain 2T2.1. The strain grows in defined mineral medium without the need for supplementation beyond provision of any of a variety of carbon sources, along with a source of nitrogen. It has native ability to grow using 10 mM acetamide as a nitrogen source (data not shown), which rules out the use of acetamidase activity encoded by the Aspergillus nidulans amdS gene [14] as a selectable transformation marker. Consequently, microbial inhibitors were tested for use as selective agents. Benomyl, hygromycin, nourseothricin, and G418 were found to inhibit growth of the fungal strain. Benomyl is an inhibitor of tubulin polymerization [15]. The minimum inhibitory concentration for benomyl against 2T2.1 was 1.0 µg/ml, with some reduction of growth observed at 0.5 µg/ml. The activity of benomyl at low concentration against strain 2T2.1 is consistent with previous results which demonstrated the fungicidal action of benomyl in actively growing cells [16]. Hygromycin is a protein synthesis inhibitor that binds the small ribosomal subunit and interferes with translation [17]. Growth of C. sp. 2T2.1 was inhibited by 50 µg/ml hygromycin, an aminoglycoside antibiotic, in liquid YPD medium. Resistance to hygromycin is conferred by the hph gene-encoding hygromycin phosphotransferase [18]. Nourseothricin is a broad-spectrum protein synthesis inhibitor that induces mRNA miscoding [19]. G418, an aminoglycoside, inhibits the elongation step of protein synthesis [20]. Resistance to nourseothricin is conferred by nourseothricin acetyltransferase [19], whereas resistance to G418 is conferred by aminoglycoside 3‘-phosphotransferase. Growth of C. sp. 2T2.1 was inhibited by ∼50 µg/ml of G418 and nourseothricin; however, considerable variability in the effective concentration was observed for both G418 and nourseothricin in numerous replicates (data not shown). These experiments showed that the hygB (conferring hygromycin resistance) and possibly KanMX (G418 resistance), and NatMX (nourseothricin resistance) markers have potential utility as dominant selectable markers in C. species 2T2.1.
In solid YPD medium, supplementation with hygromycin at 100 µg/ml was found to reliably suppress growth of the strain. Hygromycin has also been used as a selective agent for Coniochaeta ligniaria [16]. Accordingly, pCSN43 [12], which confers hygromycin resistance via the hph gene, was used to establish the transformability of strain 2T2.1 protoplast preparations. Transformation with pRS410 and PRS418 encoding resistance to G418 and nourseothricin, respectively, resulted in no colonies on solid YPD medium containing those antibiotics at 100 µg/ml.
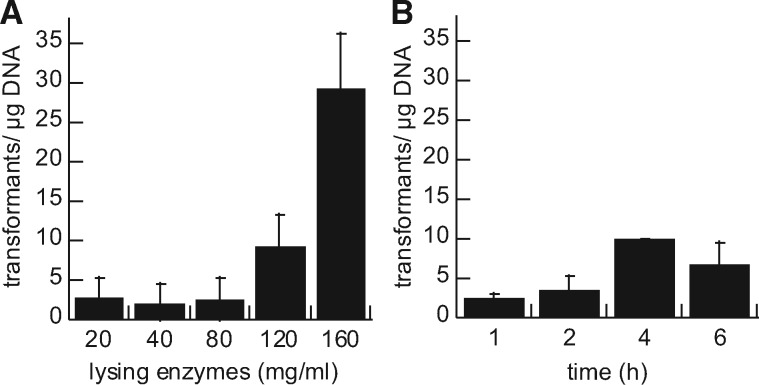
Transformation of C. sp. strain 2T2.1 required optimization of the cell wall lysing enzymes used to form protoplasts. Preparation of fungal protoplasts entails treating cells with a mixture of lytic enzymes in order to digest cell wall polymers and render the cells amenable to uptake of purified DNA. The concentration and incubation time of the lytic enzyme mixture, which comprises β-glucanase, cellulase, protease, and chitinase activities, were varied and transformation efficiency in C. sp. strain 2T2.1 was measured. Increased concentration (up to 160 mg/ml lysing enzyme cocktail) and to some extent increased incubation time both contributed to consistent formation of protoplasts (data not shown) and successful transformation (Figure 2). Further increase in the concentration of lysing enzyme (320 mg/ml) for preparation of protoplasts yielded no transformants, presumably due to cell lysis during protoplasting formation. In comparison, a related ascomycete, C. ligniaria NRRL30616, was previously transformed at low frequency using 20 mg/ml lysing enzymes for 60 min [16].
Figure 2:
Effect of lysing enzymes (A) concentration and (B) incubation time on C. sp. 2T2.1 transformation efficiency. (A) Varied concentrations of lysing enzymes were incubated for 60 min; (B) lysing enzymes (80 mg/ml) were incubated for varying times during protoplast formation. Error bars indicate standard deviation of three to five replicates.
The stability of pCSN43-transformed strains was determined by nonselective serial subculture. Cultured cells were transferred at 24- to 48-h intervals in liquid YPD medium without selective antibiotic. At each serial subculture, cells were also plated onto solid YPD medium. Individual colonies arising on plates without hygromycin were then replica-plated onto media with and without hygromycin to determine the persistence of the transforming element. Over six serial subcultures, 97 ± 1.2% of colonies retained the hygromycin-resistance phenotype, indicating the stability of the hph gene in the transformed strains.
After successful transformation of a plasmid conferring hygromycin resistance into strain 2T2.1, the strain was also transformed with pJQ7, in which expression of the mammalian codon-optimized version of Renilla reniformis GFP (hrGFP) is driven by the constitutive TEF promoter and followed by the CYC1 terminator sequence [21]. Previously, the R. reniformis hrGFP was shown to be well-expressed in the oleaginous yeast Yarrowia lipolytica [22] and in a single C. ligniaria transformant [21], and here it was also found to be useful for expression in C. sp. 2T2.1 (Figure 3). A fluorescent Coniochaeta strain may be useful for manipulating a reconstituting version of a microbial consortium.
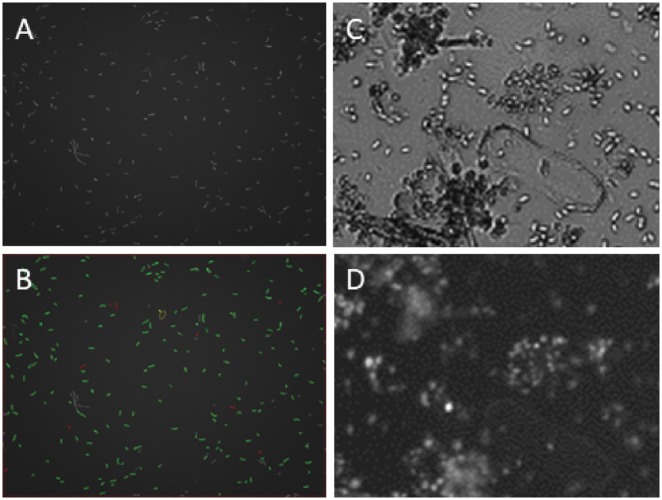
Figure 3:
C. sp. 2T2.1 transformed with pJQ7. (A) Fluorescence image of cells grown in YPD medium; (B) same field as A, with green outlines showing cells counted as fluorescent and red outlines showing nonfluorescing cells; (C) brightfield image of cells grown in liquid mineral medium containing ground wheat straw as carbon source and (D) same field as C; fluorescence image.
Coniochaeta sp. 2T2.1, transformed with pJQ7, was also examined for stability of fluorescence by comparing fluorescence after growth with and without selective antibiotic. Individual colonies arising from a single transformant were subcultured at 24- to 48-h intervals in liquid YPD medium with or without hygromycin as selective agent. As described above for hygromycin resistance, fluorescence of cells grown in nonselective medium also remained stable as compared with medium containing hygromycin (Figure 4). However, the fluorescence intensity varied among individual transformants of the strain (Figure 5). In 20 selected hygromycin-resistant isolates, confirmed by PCR to carry both hph (hygromycin resistance) and gfp sequences, the fluorescence intensity varied from as low as 1.77 ± 0.02 to as high as 13.3 ± 2.7 relative fluorescence units (rfu). In comparison, untransformed (nonfluorescent) strain 2T2.1 exhibited 1.76 ± 0.12 rfu. If the transforming DNA was integrated in the chromosome of 2T2.1, then it is possible that cis-acting elements of the fungal chromosome affect the range of possible expression outcomes. This finding would also be relevant for expression of, for example, heterologous enzymes in the strain.
Figure 4:

Stability of fluorescence in a C. sp. 2T2.1 pJQ7 transformant. Median fluorescence is shown for cells cultured with hygromycin (black bars) or without hygromycin (gray bars). Error bars indicate standard deviation for four replicates in subcultures 1 through 4 and two replicates in subcultures 5 and 6.
Figure 5:
Variation in fluorescence intensity among C. sp. strain 2T2.1 transformants expressing GFP. Individual transformed colonies were cultured in YPD containing hygromycin for measurement. Bars indicate standard deviation of three cultures for each transformant. Asteriks indicate untransformed negative control.
Coniochaeta sp. strain 2T2.1 has the capacity to grow in pure culture on wheat straw and is a key member of a microbial consortium that degrades lignocellulosic biomass (Figures 1 and 3). The strain also has potential use as a platform for biotechnological applications and consolidated bioprocessing [6]. The ability to transform C. sp. strain 2T2.1 is thus of interest for its potential use as a platform and for examining it as a member of a mixed microbial consortium. Availability of a GFP-tagged strain could enable work to reconstitute and manipulate a simplified consortium capable of hydrolyzing lignocellulosic biomass and metabolizing associated degradation products [23].
Previously, a strain of C. ligniaria (NRRL30616) was transformed with a gene-encoding GFP as a facile method for monitoring fungal growth in pretreated biomass hydrolysates [21]. Work presented here extends to the mixed microbial consortium member C. sp. 2T2.1. The strain was investigated for sensitivity to commonly used selective antibiotics, and a method of protoplast formation and transformation was developed. Use of hygromycin as a selectable transformation marker was established, and the mammalian-optimized GFP was expressed. Work described here establishes the preliminary groundwork for engineering the fungal member of a microbial consortium, toward investigating the interactions that occur between bacterial and fungal species and for use in engineering a strain capable of degradation of lignocellulose and toxic furanic compounds.
Acknowledgements
The authors thank J.D. van Elsas for Coniochaeta sp. strain 2T2.1.
Author contributions
N.N.N. devised the project, conducted experiments and prepared the manuscript. R.E.H. and S.E.F. conducted experiments and edited the manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer
Conflict of interest statement: The authors declare no conflict of interest. No external funding was used for this project.
References
- 1. Weber E. The Lecythophora-Coniochaeta complex I. Morphological studies on Lecythophora species isolated from Picea abies. Nova Hedw 2002;74:159–85. [Google Scholar]
- 2. Damm U, Fourie PH, Crous PW. Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 2010;24:60–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brossi MJL, Jiménez DJ, Cortes-Tolalpa L, et al. Soil-derived microbial consortia enriched with different plant biomass reveal distinct players acting in lignocellulose degradation. Microb Ecol 2015;71:616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. López MJ, Vargas-García MC, Suárez-Estrella F, et al. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: application for a lignocellulosic substrate treatment. Enzyme Microbial Technol 2007;40:794–800. [Google Scholar]
- 5. Ravindran A, Adav SS, Sze SK. Characterization of extracellular lignocellulolytic enzymes of Coniochaeta sp. during corn stover bioconversion. Process Biochem 2012;47:2440–8. [Google Scholar]
- 6. Jiménez DJ, Korenblum E, van Elsas JD. Novel multispecies microbial consortia involved in lignocellulose and 5-hydroxymethylfurfural bioconversion. Appl Microbiol Biotechnol 2014;98:2789–803. [DOI] [PubMed] [Google Scholar]
- 7. López MJ, Nichols NN, Dien BS, et al. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl Microbiol Biotechnol 2004;64:125–31. [DOI] [PubMed] [Google Scholar]
- 8. Nichols NN, Sharma LN, Mowery RA, et al. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzyme Microbial Technol 2008;42:624–30. [Google Scholar]
- 9. Hirokawa K, Gomi K, Kajiyama N. Molecular cloning and expression of novel fructosyl peptide oxidases and their application for the measurement of glycated protein. Biochem Biophys Res Commun 2003;7:104–11. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Niu S, Liu S, et al. The first naturally occurring thiepinols and thienol from an endolichenic fungus Coniochaeta sp. Org Lett 2010;12:5081–3. [DOI] [PubMed] [Google Scholar]
- 11. Segeth MP, Bonnefoy A, Brönstrup M, Knauf M, Schummer D, Toti L, Vértesy L, Wetzel-Raynal MC, Wink J, Seibert G. Coniosetin, a novel tetramic acid antibiotic from Coniochaeta ellipsoidea DSM 13856. J Antibiot 2003;56:114–122. [DOI] [PubMed] [Google Scholar]
- 12. Staben C, Jensen B, Singer M, et al. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl 1989;36:79–81. [Google Scholar]
- 13. Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Phillips GB (1981) Manual of Methods for General Bacteriology, American Society for Microbiology, Washington, DC. [Google Scholar]
- 14. Hynes MJ, Corrick CM, King JA. Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol 1983;3:1430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holloman DW, Butters JA, Barker H. Tubulins: a target for antifungal agents. In: Bentley PH, O’Hanlon PJ (eds), Antiinfectives: Recent Advances in Chemistry and Structure–activity Relationships, Chapter 11. London: Royal Society of Chemistry. [Google Scholar]
- 16. Nichols NN, Szynkarek MP, Skory CD, Gorsich SW, López MJ, Guisado GM, Nichols WA. Transformation and electrophoretic karyotyping of Coniochaeta ligniaria NRRL30616. Curr Genet 2011;57:169–175. [DOI] [PubMed] [Google Scholar]
- 17. Cabañas MJ, Vázquez D, Modolell J. Dual interference of hygromycin B with ribosomal translocation and with aminoacyltRNA recognition. Eur J Biochem 1978;87:21–7. [DOI] [PubMed] [Google Scholar]
- 18. Rao RN, Allen NE, Hobbs JN Jr, et al. Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli. Antimicrob Agents Chemother 1983;24:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDade HC, Cox GM. A new dominant selectable marker for use in Cryptococcus neoformans. Med Mycol 2001;39:151–4. [DOI] [PubMed] [Google Scholar]
- 20. Bar-Nun S, Shneyour Y, Bechmann JS. G-418, an elongation inhibitor of 80 S ribosomes. Biochim Biophys Acta 1983;741:123–7. [DOI] [PubMed] [Google Scholar]
- 21. Nichols NN, Quarterman JC, Frazer SE. Use of green fluorescent protein to monitor fungal growth in biomass hydrolysate. Biology Methods Protocols 2018;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blazeck J, Liu L, Redden H, Alper H. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Appl Environ Microbiol 2011;77:7905–7914 doi:10.1128/AEM.05763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trifonova R, Postma J, Verstappen FWA, et al. Removal of phytotoxic compounds from torrefied grass fibres by plant-beneficial microorganisms. FEMS Microbiol Ecol 2008;66:158–66. [DOI] [PubMed] [Google Scholar]
- 24. Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 1983;25:179–88. [DOI] [PubMed] [Google Scholar]