Abstract
Characteristics of the home language environment, independent of socioeconomic background, may account for disparities in early language abilities. Past studies have reported links between the quantity of language input within the home and differences in brain function during early childhood. The current study examined associations between home language input and EEG brain activity in a socioeconomically diverse sample of 6- to 12-month-old infants (N = 94). Replicating past studies, a positive correlation was found between measures of socioeconomic status and language input. Examining links between language input and brain activity, analyses yielded a negative association, with children who heard more adult words in the home demonstrating reduced EEG beta power (13–19 Hz) in the parietal region. Exploratory analyses revealed a significant interaction between language input and the amount of chaos and disorganization in the home. Specifically, among children living in high-chaos households, children who heard more adult words tended to have reduced EEG activity. Among children living in low-chaos homes, there was no link between adult word count and children’s EEG activity. These findings demonstrate the importance of the early home environment context in shaping neurocognitive trajectories.
Keywords: EEG, Socioeconomic status, Language, Chaos, Infancy
1. Introduction
Early experience has a profound impact on developing language skills. At birth, newborns show a preference for their mother’s voice (DeCasper and Fifer, 1980) and the language or languages spoken by their mother during pregnancy (Byers-Heinlein et al., 2010; Moon et al., 1993). By the time infants reach their first birthday, their brains have undergone significant experience-dependent reorganization, commonly referred to as perceptual narrowing (Werker and Tees, 1984) or neural commitment (Kuhl, 2004). Whereas neonates have a capacity to support learning any of the world’s languages, between 6 and 12-months of age there is a substantial decline in perceptual sensitivity to speech sounds not present in the infant’s environment. During this first year of life, infants attune to the properties of the language or languages heard within their proximal environment – scaffolding language acquisition and setting the stage for subsequent language abilities.
Considerable variation in language skills is observed across childhood, and early language ability is one of the best predictors of school readiness and later academic achievement (Burchinal et al., 2016; Hoff, 2013). Some of these differences can be traced, in part, to socioeconomic disparities in language input within the home. By kindergarten, children from higher socioeconomic status (SES) homes outperform their age-matched peers from lower SES households on standardized measures of language skills (Lee and Burkam, 2002), with children living in poverty scoring an estimated 14.8 months behind the national average on receptive vocabulary by the age of 5 (Layzer and Price, 2008). These socioeconomic disparities in language skills may already be present during infancy. In a sample of 179 infants, Noble et al. (2015) reported significant SES differences in language skills emerging between 15- and 21-months of age, with higher parental education associated with higher receptive and expressive language scores (Noble et al., 2015). This result was consistent with past studies demonstrating SES disparities in early language skills by the age of 2 (Fernald et al., 2013; Hoff, 2003; Rowe and Goldin-Meadow, 2009).
Research using electroencephalography (EEG) as a measure of cortical function during infancy and childhood have measured baseline or resting EEG power to examine individual differences in cognitive development (Anderson and Perone, 2018). In typically developing children there is a developmental decrease in EEG power of low-frequency rhythms (e.g., delta and theta) and an increase in high-frequency rhythms (e.g., beta and gamma) across age (Marshall et al., 2002; Matousek and Peterson, 1973). Relative to typically developing children, children with learning or attention disorders often demonstrate higher levels of low-frequency power and lower levels of high-frequency power (Barry et al., 2003). This atypical EEG profile has also been found in children who were previously institutionalized (Marshall et al., 2002; Vanderwert et al., 2016) and children growing up in economically disadvantaged environments (Harmony et al., 1990; Otero, 1994; Otero et al., 2003; Tomalski et al., 2013).
Socioeconomic differences in brain structure and function relate to numerous language outcomes during childhood (see Brito and Noble, 2014) and several studies have reported associations between SES and brain function using EEG. Maguire & Schneider (2019) report differences in resting EEG by household income within a sample of forty-five 8 to 15-year-olds. In their study, results indicated that children from lower income households exhibited more low-frequency theta power (4–8 Hz) and less high-frequency alpha power (9–12 Hz) compared to their higher income peers. A study examining baseline EEG differences during infancy found that 6- to 9-month-olds from lower SES households demonstrated reduced low-gamma EEG power (21–30 Hz) in frontal brain regions, compared to infants from higher SES homes (Tomalski et al., 2013). In children, gamma power increases across age (Takano and Ogawa, 1998; Uhlhaas et al., 2010), and differences in frontal gamma power have been related to cognitive differences in toddlers (Benasich et al., 2008) and preschoolers (Gou et al., 2011). Within a sample of 63 toddlers between the ages of 16 and 36 months, gamma power (31–50 Hz) was linked to concurrent language skills (Benasich et al., 2008) and gamma power during toddlerhood predicted later language abilities (Gou et al., 2011).
In a study of 66 full-term infants, Brito et al. (2016) reported no significant associations between SES factors and EEG power at birth, suggesting that socioeconomic disparities in brain function may not emerge until after the perinatal period. Individual differences in neonatal low-gamma power (24–35 Hz) in the parietal region, however, did prospectively predict receptive language scores at 15-months of age. In a separate analysis (N = 129), controlling for a host of covariates including SES, results yielded significant correlations between neonatal EEG (13–36 Hz in frontal polar, temporal, and parietal brain regions) and socioemotional skills at 24–36 months measured by the Brief Infant-Toddler Social Emotional Assessment (Brito et al., 2019). These findings suggest that high-frequency EEG activity may be a marker for concurrent or future cognitive skills.
Outside of distal factors like SES, direct characteristics of the home environment may account for disparities in early language abilities. Some studies have reported that children from lower-SES households experience less child-directed speech and engage in fewer turn-taking conversations relative to their higher-SES peers (Gilkerson et al., 2017; Hoff, 2003, 2006; Huttenlocher et al., 2010; Johnson et al., 2017; Rowe, 2008). But even after controlling for SES, the quantity and quality of language input has been found to be significantly associated with language abilities (Rowe, 2012; Weisleder and Fernald, 2013). In a sample of 2-year-olds, quality of maternal speech fully explained differences in expressive vocabulary growth between children from lower vs. higher SES families (Hoff, 2003). Similarly, the quality of the home environment, but not SES, predicted 9-month phonemic discrimination ability, even after controlling for 9-month language skills (Melvin et al., 2017). Finally, past literature has also reported that chaotic or disorganized home environments may be negatively associated with early receptive and expressive language abilities during toddlerhood (Vernon-Feagans et al., 2012) and communication skills during infancy (Wachs and Chan, 1986). Observing 12-month-olds and their mothers in their homes, Wachs and Chan (1986) found that aspects of the physical environment, including the number of people in the home and regular suppertime, contributed to variability in infant communication skills independent of SES and social environmental factors (i.e., adult vocalizations, responses to child vocalizations).
There is also growing neuroimaging evidence that early language input is related to both brain structure and function, and that neural architecture and activity may mediate associations between the home language environment and developing language skills. Romeo et al. (2018a) collected home audio recordings of 36 four-to-six-year-old children and reported significant positive associations between the number of conversational turns (back-and-forth interactions between adult and child) and activation in Broca’s area during a story-listening fMRI task. Broca’s area activation also mediated links between conversational turns and child language scores. In a recent study with 5-to-9-year-olds, Merz et al. (2019) found that both adult word count (the number of adult words heard by the child) and conversational turns were significantly related to gray matter morphometry, with children who experienced more language input having greater cortical surface area in the left perisylvian region, an area highly involved in language comprehension and production (Schlaggar and McCandliss, 2007). Additionally, language input was indirectly associated with children’s reading scores via left perisylvian cortical surface area. Together, these studies suggest a direct relation between language exposure within the home and subsequent brain development in language-related areas.
Support for the neural mechanisms underlying links between the home language environment and language processing skills has also been demonstrated during infancy. Garcia-Sierra et al. (2011) recruited 37 infants (11-to14-months of age) to examine correlations between the amount of language input in the home and neural responses reflective of speech discrimination (MMN: mismatch negativity). Roughly half of the participants were only exposed to English at home (English monolingual) and the other half exposed to both English and Spanish within the home (Spanish-English bilingual). For both groups, the number of adult words was correlated with child MMN responses to language-specific sounds. When groups were stratified by language input (high vs. low adult word count), monolingual infants with high language input demonstrated more robust negative mismatch response (nMMR), indicating stronger neural commitment to the native language (Kuhl and Rivera-Gaxiola, 2008), compared to monolingual infants with low language input. Within the bilingual group, more language input was associated with progress towards neural commitment, but even bilingual infants with high language input did not show an nMMR response reflective of full neural commitment. Bilingual caregivers do not speak more to their infants than monolingual caregivers; therefore bilingual infants must receive reduced input from each of their languages – which may explain differences in neural commitment timelines between the two language groups (Garcia-Sierra et al., 2011). Overall, these findings demonstrate the importance of early language input for brain activation patterns related to language processing.
1.1. Present study
Prior research has reported associations among family SES, neural mechanisms underlying language processing, and developing language skills during early childhood. The current study examines these correlations in a socioeconomically diverse sample of 6- to 12-month-old infants. There were three main research questions addressed in the present study. First, are there SES-related differences in home language input during the first year of life? Replicating past studies, we hypothesized that both maternal education and family income-to-needs would be associated with measures of home language input. Second, are home language input measures related to infant neural activity? Based on past research (Benasich et al., 2008; Brito et al., 2016; Tomalski et al., 2013), we hypothesized that both adult word count and conversational turns would be correlated with higher-frequency EEG power (13–30 Hz). And finally, if baseline EEG is related to home language input, does this resting state brain activity mediate any associations between SES and language skills? Exploratory analyses also examined the extent to which home chaos and disorganization moderated the association between language input and resting state brain activity.
2. Methods
2.1. Participants
Our sample included 94 infants (M age = 9.15 months, SD = 2.45; 61 males) and their caregivers recruited from community events and flyers within New York City. To be included in the study, infants had to be born at or after 36 weeks of gestation, have no history of or signs of developmental delay, and be between 5 and 13 months of age at the time of testing. Participants were invited to participate when they were 6- (n = 32), 9- (n = 31), or 12- (n = 31) months of age, see Table 1 for a summary of socio-demographic information and Supplemental Table 1 for a breakdown by ages.
Table 1.
Overall Sample demographics (N = 94).
| Mean (SD; Range) or N (%) | |
|---|---|
| Child Age (months) | 9.15 (2.4; 5.6–12.9) |
| Child Sex | |
| Male | 61 (64.9 %) |
| Female | 33 (35.1 %) |
| Child Race | |
| White | 14 (14.9 %) |
| Black | 15 (16 %) |
| Asian/Pacific Islander | 2 (2.2 %) |
| Mixed (Two or More) | 43 (45.7 %) |
| Did not provide response | 20 (21.3 %) |
| Bilingual | 34 (36.2 %) |
| Maternal Education (years) | 15.18 (3.8; 6–22) |
| Mothers with less than college degree | 46 (48.9 %) |
| Income-To-Needs | 3.91 (4.4; 0–25.3) |
| Families below the poverty line | 29 (32.6 %) |
2.2. Measures
2.2.1. Parental-report measures
The caregiver was asked to complete a questionnaire assessing infant age, sex, race/ethnicity, and other family demographics including maternal education, annual household income, and the number of adults and children in the household. Income-to-needs was calculated by dividing the household income by the poverty threshold for that specific family size. For example, for a family of four with two adults and two children making $60,000/year, their income-to-needs would be 2.36 – making a little more than two times the 2018 poverty threshold for a family of that size of $25,465. As income was positively skewed, the natural log transformed values were included in all models. An estimate of the number of languages the child was consistently exposed to was reported by the caregiver; children who heard at least 25 % of another language on a daily basis were categorized as bilingual.
2.2.2. Home confusion and disorganization
The Confusion, Hubbub, and Order Scale (CHAOS) scale is a 15-item questionnaire designed to assess the level of confusion and disorganization in the child’s home environment. The questionnaire is filled out by parents using a 4-point scoring system (1 = “Very much like your own home” to 4 = “Not at all like your own home”) and items assess the extent to which the daily home environment is characterized by lack of routine, confusion, and noise. Seven items reflect routines and organization (e.g., “The atmosphere in our home is calm”) and eight items reflect disorganization (e.g., “It’s a real zoo in our home”). The routines and organization items are reverse coded before adding the total number of items – higher scores represent more disorganized, confused, and noisy home environments. Thirteen families did not fill out this measure. This measure has been validated with observational measures of home disorganization and parenting (Cronbach’s α = .79) and shows stability across a year (r = .74). Additionally, this instrument has been reported to measure differences in the child’s environment independent of socioeconomic status (Matheny et al., 1995).
2.2.3. Language measure
The Preschool Language Scale (PLS-5) is a standardized language measure that has been normed from birth through age six (Zimmerman and Castilleja, 2005). This measure assesses infant receptive and expressive language through a series of interactive items designed to elicit language skills. The Auditory Comprehension subscale assesses a child’s ability to understand and respond to spoken language, whereas the Expressive Communication subscale assesses a child’s ability to verbally express their needs and respond to questions. Infants sat next to their parent on the floor of a well-lit room. Parents were instructed to not aid the infant on any of the assessment questions, unless specifically instructed to do so. Eleven infants did not complete the PLS assessment due to infant fussiness (n = 4), experimenter error (n = 5), or opt-out by caregiver (n = 2). Standardized sores for both subscales were used in the following analysis.
2.2.4. Home language input
To measure the home language environment, caregivers were given a Language Environment Analysis (LENA) digital language processor (DLP) and two specially designed t-shirts to take home with them after the lab visit. The LENA system (LENA Research Foundation, Boulder, CO) is an automated vocalization analysis device that can audio-record the child’s language environment for up to 16 hours. Twenty-two infants did not have LENA data due to caregivers refusing to participate (n = 3), not returning the DLP (n = 9), or returning DLP without data (n = 10). Strong reliability and validity of the LENA speech identification algorithms has been reported, with over 75 % accuracy for both adult and child speech (Gilkerson et al., 2017). LENA has also been validated for Spanish-speakers (Weisleder and Fernald, 2013).
The parent was instructed to have the child wear the DLP within the shirt pocket for one full day when the typical caregivers were present, within 2 weeks of the lab visit. The average number of days between lab visit and LENA home recording was 11.15 days. Once the DLP was returned, the recording was uploaded to a computer and analyzed by the LENA software. The software derives three primary measures: adult word count (number of words spoken near the child), child vocalizations (defined as a speech segment of any length surrounded by 300 milliseconds or more of non-speech or silence), and conversational turns (number of reciprocal vocalizations by an adult and the target child within 5 seconds). Any recordings less than 5 hours were excluded from analysis (range = 5.75–16 hours). As in past studies (Romeo et al., 2018a, b), per-participant highest hourly totals of adult words, child vocalizations, and conversational turns were extracted separately for analyses. Highest counts for each measure ensured that different recording lengths did not bias results. LENA language counts were not normally distributed; therefore, square root transformation was conducted to normalize the data.
2.3. EEG collection and analysis
During EEG acquisition, infants were seated on their caregiver’s laps and watched a video of engaging but non-social stimuli (e.g., spinning wheel, bubbles). An experimenter sat next to the infant and blew bubbles if the infant became fussy during the 5-minute baseline. EEGs from 18 infants were unable to be recorded due to infant fussiness (n = 14), computer error (n = 3), or experimenter error (n = 1). The number of recorded EEGs were fairly evenly distributed at 6 (n = 27), 9 (n = 26), and 12 (n = 23) months. EEG was recorded using a 28-channel HydroCel Geodesic Sensor Net (Electrical Geodesic, Inc., Eugene, OR) and on a GEM 100 amplifier (Electrical Geodesic, Inc., Eugene, OR; EB NEURO S.p.A., Firenze, Italy). The GEM system is a high input-impedance system, and electrode impedances were kept below 50 kΩ whenever possible. The sampling rate was 250 Hz and data were online referenced to the vertex (Cz) electrode.
EEG was analyzed using the EEGLAB toolbox (Delorme & Makeig, 2004) and custom-written MATLAB scripts (The MathWorks, Natick, MA). Data were high-pass filtered at 0.3 Hz and low-pass filtered at 50 Hz. Bad channels were identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). Consistent with the approach used by other developmental studies (e.g., Debnath et al., 2019), ocular artifacts and generic noise removal were completed by creating a copy of the dataset and performing independent component analysis (ICA) on the copied dataset. This copied dataset was high-pass filtered at 1-Hz and segmented into arbitrary 1000 ms epochs. Epochs were removed from this copied dataset if the amplitude was +/− 1000 μV or if power in the 20–40 Hz band (after Fourier analysis) was greater than 30 dB (Harrewijn et al., 2019). Additionally, if more than 20 % of the epochs in a given channel were removed, that channel was excluded from both the ICA-copied dataset and the original dataset (Debnath et al., 2019; Troller-Renfree et al., 2016). If a child had more than six electrodes (> 20 %) deemed globally bad, that child was then removed from all future processing (N = 8). Then, ICA (Comon, 1994; Makeig et al., 1997) was performed on the copied dataset and the ICA weights were copied back to the original continuous dataset (high-pass filtered at 0.3 Hz). The ADJUST toolbox (Mognon et al., 2011) was used to automatically identify artifactual independent components (ICs) in the original dataset, and, as is common in developmental work, ICs were also visually inspected (Buzzell et al., 2019; Debnath et al., 2019). All ICs identified as artifactual were removed from the data. Then, data were epoched in segments of 2 s with 50 % (1 s) overlap (Levin et al., 2018; Pierce et al., 2019; Tomalski et al., 2013). Similar to other infant work (Debnath et al., 2019), epochs where any of the frontal electrodes (electrode numbers 1, 2, 11, 12, 27) exceeded a voltage threshold of +/−100 μV were automatically rejected to ensure ocular artifacts were removed. For the remaining epochs, remaining bad channels were identified if the electrode exceeded a voltage threshold of +/−100 μV. Finally, all rejected channels were interpolated using a spherical spline (Perrin et al., 1989). Epochs were rejected when more than 20 % of channels were interpolated (Harrewijn et al., 2019). Participants needed to have at least 10 artifact-free epochs (DeBoer et al., 2007) to be included in the analysis (removed N = 7). The average number of used epochs was 176.42 (SD = 125.98, range: 12–452). Finally, remaining data were re-referenced to an average reference.
A Fast Fourier Transformation (FFT) with a 2-second Hamming window was applied to the epoched data. Consistent with other infant studies (Tomalski et al., 2013), spectral power (μV2) was computed for theta (3–5 Hz), alpha (6–9 Hz), beta (13–19 Hz), and two gamma frequency ranges (21–30 Hz, 31–45 Hz). Similar to past studies (Brito et al., 2019; St. John et al., 2016; Tomalski et al., 2013), average absolute power was computed separately for each hemisphere across electrodes in region specific groupings: Frontal (electrodes 3, 4, 11, 12), Central (electrodes 5, 6), Parietal (electrodes 7, 8, 15, 16), Temporal (electrodes 13, 14), and Occipital (electrodes 9, 10) (see Supplemental Fig. 1 for diagram). There were no significant differences in absolute power by hemisphere, region, or hemisphere*region interactions for theta, alpha, low-gamma or high gamma (p’s > .05); for these frequency bands all included electrodes were averaged to create whole-brain measures of theta, alpha, low-gamma, and high-gamma. Significant differences in power by region, but not hemisphere, were present for the beta frequency range. Therefore, subsequent analyses will examine EEG power separately in Frontal, Parietal, Temporal, and Occipital regions within the beta frequency range. Here we report absolute power values, since relative power data yielded congruent but non-significant results, as there were no major differences in the low frequency bands across participants within these analyses. Confirmatory analyses examined beta and low-gamma frequencies only. All EEG power values included in subsequent models were natural log transformed to correct for non-normality (Gabard-Durnam et al., 2019; St. John et al., 2016; Missana et al., 2014; Pierce et al., 2019).
2.4. Planned statistical analyses
Descriptive statistics and preliminary analyses were conducted in SPSS (Version 25). Subsequent regression analyses were performed in R (R Core Team, 2013). These separate multiple regressions included (1) SES (income-to-needs and/or maternal education) as the independent variable and home language environment (LENA) values as the dependent variables, (2) SES as the independent variable and Preschool Language Scale (PLS) scores as the dependent variable, (3) SES as the independent variables and EEG power (separate regressions for each brain region within 13–19 Hz and whole-brain for 20–30 Hz), and (4) separate regressions for LENA values as the independent variables and EEG power (within 13–19 Hz and whole-brain for 20–30 Hz) as the dependent variables. In all subsequent analyses, infant age and infant sex were included as covariates. For all regression analyses, the sample size was maximized (N = 94) using Full Information Maximum Likelihood Estimation to account for missing data, which were missing at random (Little’s MCAR: χ2 = 56.59, p = .92). Preliminary analyses found no differences between children classified as monolingual or bilingual on any measure of interest including age (p = .51), maternal education (p = .77), family ITN (p = .79), home language environment (Adult Word Count: p = .80; Conversational Turn Count: p = .90; Child Vocalization Count: p = .07), PLS language scores (Auditory Comprehension: p = .53; Expressive Communication: p = .06) or whole-brain EEG power values in any frequency (p’s > .15). A categorical measure of bilingualism was added as a covariate to all analyses but results were nearly identical with or without this variable. Therefore, the bilingualism variable was dropped from subsequent analyses.
3. Results
3.1. Higher family income associated with greater home language input
Bivariate correlations yielded significant associations among both family income-to-needs (ITN) and maternal education and home language exposure measures, with higher SES related to greater adult word count (ITN: r = .46, p < .001, n = 58; ED: r = .43, p < .001, n = 62) and conversational turns (ITN: r = .33, p = .011, n = 58; ED: r = .34, p = .005, n = 62), see Table 2. When both maternal education and family ITN were put into the same model as predictors, with infant age and sex as covariates, only family ITN was significantly associated with hourly adult word count (β = 4.58, p = .009, adjusted R2 = .27) and passed FDR correction, see Table 3. When controlling for covariates, there were no significant associations between SES variables and conversational turns or child vocalizations. There were also no significant associations between SES measures and PLS language scores (p’s > .31) or LENA measures (Table 3) and PLS language scores (p’s > .45), see Supplemental Table 2.
Table 2.
Zero-Order Correlations.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Family ITN | – | ||||||
| 2. Maternal ED | .67*** | – | |||||
| 3. Hourly adult words | .46*** | .43*** | – | ||||
| 4. Hourly conversational turns | .33** | .34** | .63*** | – | |||
| 5. Hourly child vocalizations | .06 | .10 | .13 | .41*** | – | ||
| 6. Auditory Comprehension | −.01 | .02 | −.03 | .18 | −.01 | – | |
| 7. Expressive Communication | .08 | .14 | .14 | .08 | .01 | .60*** | – |
*p < .05, **p < .01, ***p < .001. Family ITN and LENA measures are transformed variables.
Table 3.
Associations between SES and language outcomes.
| Adult Word Count (AWC) | Conversational Turns (CTC) | Child Vocalizations (CVC) | PLS Auditory Scores | PLS Expressive Scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | |
| Age | −1.05 | 0.67 | 0.12 | −0.11 | 0.70 | 0.24 | 0.01 | 0.15 | 0.93 | 0.01 | 0.45 | 0.81 | 0.01 | 0.04 | 0.87 |
| Sex | 4.01 | 3.35 | 0.23 | 0.07 | 0.48 | 0.15 | −2.47 | 0.74 | 0.001 | −0.11 | 0.23 | 0.62 | 0.15 | 0.22 | 0.49 |
| Maternal ED | 0.46 | 0.57 | 0.81 | 0.08 | 0.08 | 0.31 | 0.12 | 0.13 | 0.33 | 0.02 | 0.04 | 0.70 | 0.04 | 0.04 | 0.30 |
| Family ITN | 4.58 | 1.75 | 0.009 | 0.38 | 0.26 | 0.14 | 0.12 | 0.40 | 0.76 | −0.04 | 0.11 | 0.75 | −0.04 | 0.12 | 0.71 |
| Adj. R2 | 0.27 | 0.13 | 0.13 | 0.006 | .022 | ||||||||||
3.2. Examining links between SES and EEG power
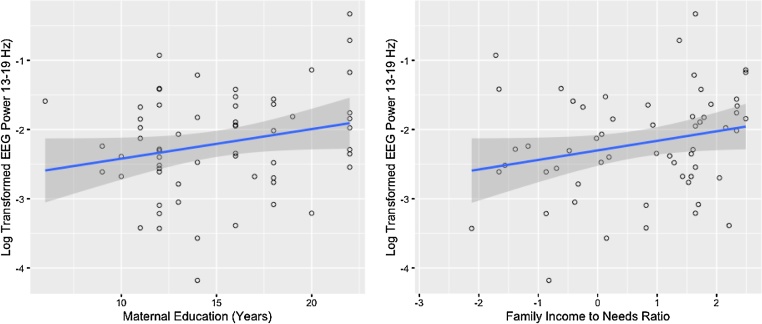
Both maternal education and family ITN were significantly associated with beta EEG power in the temporal region (ED: β = 0.05, p = .025, adjusted R2 = .06; ITN: β = 0.14, p = .048, adjusted R2 = .03), with higher SES associated with higher EEG power (Fig. 1), but neither result passed FDR correction, see Supplemental Table 3.
Fig. 1.
Correlations between SES and beta EEG power in the temporal region (ITN: n = 58, ED: n = 60). Family ITN is natural log transformed (ln ITN of 0 = at the poverty line). Associations not significant after FDR correction.
3.3. Home language exposure negatively associated with EEG
Next, associations between home language exposure and EEG were assessed. As family ITN was significantly correlated with LENA measures, ITN was also added as a covariate in regression models. Analyses yielded significant FDR-corrected associations between adult word count and beta EEG power in the parietal region (β = −0.019, p = .01, adjusted R2 = .17), with no significant interactions between adult word count and family ITN (p = .78). There were no significant associations between EEG power values and conversational turns (p’s > .15) or child vocalizations (p’s > .14), see Table 4.
Table 4.
Associations between LENA outcomes and EEG Power.
| Frontal Beta | Temporal Beta | Parietal Beta | Occipital Beta | Whole-Brain Low-Gamma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | |
| Age | 0.08 | 0.04 | 0.02 | 0.03 | 0.04 | 0.54 | 0.02 | 0.04 | 0.65 | 0.02 | 0.03 | 0.59 | 0.01 | 0.04 | 0.81 |
| Sex | −0.16 | 0.16 | 0.34 | −0.22 | 0.19 | 0.24 | −0.20 | 0.16 | 0.21 | −0.30 | 0.15 | 0.05 | −0.14 | 0.17 | 0.41 |
| Family ITN | 0.03 | 0.08 | 0.65 | 0.11 | 0.09 | 0.24 | 0.11 | 0.08 | 0.16 | 0.04 | 0.07 | 0.57 | 0.06 | 0.08 | 0.46 |
| AWC | −0.01 | 0.01 | 0.55 | 0.01 | .01 | 0.52 | −0.02 | 0.01 | 0.01 | −0.01 | 0.01 | 0.35 | −0.01 | 0.01 | 0.25 |
| Adj. R2 | 0.102 | 0.045 | 0.171 | 0.077 | 0.063 | ||||||||||
| B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | B | SE B | p | |
| Age | 0.08 | 0.04 | 0.02 | 0.02 | 0.04 | 0.64 | 0.02 | 0.04 | 0.50 | 0.03 | 0.03 | 0.31 | 0.02 | 0.04 | 0.55 |
| Sex | −0.18 | 0.16 | 0.28 | −0.21 | 0.19 | 0.27 | −0.26 | 0.17 | 0.12 | −0.31 | 0.15 | 0.05 | −0.16 | 0.17 | 0.36 |
| Family ITN | 0.02 | 0.07 | 0.74 | 0.13 | 0.08 | 0.09 | 0.05 | 0.08 | 0.50 | −0.02 | 0.07 | 0.78 | 0.002 | 0.07 | 0.98 |
| CTC | −0.03 | 0.06 | 0.64 | 0.009 | 0.07 | 0.89 | −0.08 | 0.06 | 0.15 | 0.03 | 0.06 | 0.59 | 0.005 | 0.06 | 0.94 |
| Adj. R2 | 0.095 | 0.034 | 0.077 | 0.051 | 0.023 | ||||||||||
| B | SE B | p | B | SE B | P | B | SE B | p | B | SE B | p | B | SE B | p | |
| Age | 0.08 | 0.03 | 0.02 | 0.02 | 0.04 | 0.64 | 0.04 | 0.03 | 0.30 | 0.03 | 0.03 | 0.31 | 0.02 | 0.04 | 0.55 |
| Sex | −0.28 | 0.19 | 0.14 | −0.19 | 0.23 | 0.40 | −0.34 | 0.20 | 0.10 | −0.17 | 0.18 | 0.35 | −0.18 | 0.21 | 0.40 |
| Family ITN | 0.04 | 0.07 | 0.60 | 0.14 | 0.08 | 0.07 | 0.02 | 0.07 | 0.77 | −0.03 | 0.06 | 0.61 | 0.008 | 0.07 | 0.91 |
| CVC | −0.05 | 0.04 | 0.26 | 0.008 | 0.05 | 0.88 | −0.04 | 0.05 | 0.36 | 0.06 | 0.04 | 0.14 | −0.01 | 0.05 | 0.91 |
| Adj. R2 | 0.124 | 0.036 | 0.052 | 0.133 | 0.024 | ||||||||||
Note: AWC = adult word count, CTC = conversational turns, CVC = child vocalizations.
3.4. Post-hoc analyses: links between home language input and EEG moderated by home environment
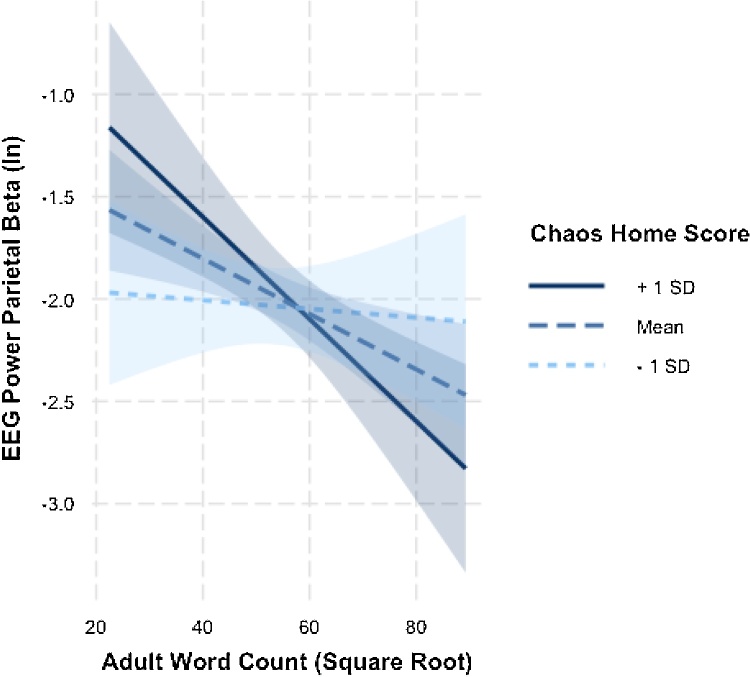
The negative coefficient between adult word count and EEG power suggests that a higher number of adult words heard by the child was related to lower EEG power. This was unexpected, and we thus sought to further understand this negative relationship within post-hoc analyses. It is possible that more chaotic households contribute negatively to child development (Evans, 2006; Martin et al., 2012); therefore, scores on the Confusion, Hubbub, and Order Scale (CHAOS) were examined in relation to adult word count and EEG power. CHAOS scores did not differ by infant age (p = .42) or sex (p = .51), but were moderately correlated with both SES measures (ITN: r = −.34, p = .002, n = 77; ED: r = −.31 p = .005, n = 81). Controlling for all covariates of interest including ITN and maternal education, there was no main effect of CHAOS score (p = .94) on infant EEG. However, a significant interaction between adult word count and CHAOS score emerged in relation to EEG beta power in the parietal region (β =−0.006, p = .04, adjusted R2 = .31), see Supplemental Table 4. Probing the interaction using simple slopes analysis, the association between higher adult word count and reduced infant EEG beta power was not significant for families with low levels of chaos in the home (t = −0.21, p = .84) and was only significant for families with higher chaos in the home (t = −2.28, p = .03), see Fig. 2.
Fig. 2.
Simple slopes analysis examining interaction between CHAOS home score and Adult Word Count on EEG Power.
3.5. Other exploratory analyses
Because older infants have had greater cumulative exposure to language, it is plausible that the association between adult word count and infant neural activity would be stronger among older infants. We therefore examined whether infant age interacted with adult word count in predicting EEG beta power in the parietal region. Indeed, this analysis yielded a significant interaction (β = −0.007, p = .006, adjusted R2 = .32). To explore these age-related differences in associations between adult word count and EEG power, separate regressions were conducted for each age group. There were no significant associations between adult word count and EEG beta power in the parietal region at 6-months (p = .91). However, significant associations between adult word count and EEG beta activity in the parietal region were found at both 9-months (β = −0.037, p < .001, adjusted R2 = .58) and 12-months (β =−0.033, p < .001, adjusted R2 = .63). Across the whole sample, infant age did not correlate with any other independent variable or covariate (i.e., income-to-needs, maternal education, adult word count, conversational turns, child vocalizations, CHAOS scores).
Additional exploratory regression analyses examining other EEG frequencies (theta, alpha, and high-gamma) in relation to SES, LENA, and PLS scores were conducted and yielded no other significant associations. There were also no significant associations between EEG power values of interest and Preschool Language Scale (PLS) scores for either auditory comprehension or expressive communication (p’s > .13). As there were also no correlations between SES and PLS language scores, mediation analyses examining links between SES, EEG power, and PLS scores were not conducted.
4. Discussion
The results from the current study replicate previous research examining links between family SES and home language input, and also extend previous findings to include measures of brain activity in the first year of life. As in past studies (Gilkerson et al., 2017; Hoff, 2003, 2006; Hoff-Ginsberg, 1998; Huttenlocher et al., 2010; Rowe, 2008), family socioeconomic background was positively associated with both adult word count and conversational turns. Controlling for covariates, family ITN in this sample was a stronger predictor of adult word count in the home than maternal education. When controlling for covariates, there were no significant associations between SES variables and conversational turns or child vocalizations. With respect to our second research question, we found that home language input was related to EEG power, but this relation was in an unexpected negative direction – children who heard more adult words in the home had reduced EEG beta power in the parietal region. Post-hoc analyses explored this association in relation to the amount of chaos and disorganization within the home. Results indicated that it was only among high-chaos households that higher adult word counts were associated with reduced infant EEG power. Finally, we found no significant associations between SES and language skills (PLS auditory and expressive scores), and therefore did not examine any mediation pathways for these variables.
It was not entirely surprising that our SES variables did not correlate with early language skills measured by the Preschool Language Scale. Socioeconomic differences in language have more consistently been reported within the second year of life (Fernald et al., 2013; Hoff, 2003; Noble et al., 2015), with one prior study finding no differences at 9 months, but demonstrable disparities in receptive language by 15 months and disparities in expressive language by 21 months of age (Noble et al., 2015). Melvin et al. (2017) reported null findings between phonetic discrimination (a foundational language skill) and SES in a sample of 9-month-olds. Similar to the current study, however, phonetic discrimination at 9-months was related to the home language environment as assessed by the IT-HOME (Caldwell and Bradley, 1984).
There are two possibilities that could account for the null association between SES and language skill within the current study. First, it is possible that distal socioeconomic characteristics may not be sensitive enough to account for differences in early linguistic skills; whereas measures of the home environment, reflecting more proximal individual differences in language experience, may be more reliable. A second possibility is that behavioral measures of infant language more accurately reflect true skill levels as children age. This is in line with our exploratory findings reporting stronger associations between the home language environment and EEG among older infants. Future work using both behavioral and neural (e.g., ERP: event-related potentials) measures of infant language skills could help differentiate between these varying accounts.
Associations between home language input and brain activity were specific to the beta frequency in the parietal region. Beta frequency is most commonly associated with sensorimotor activity (Hari and Salmelin, 1997), but increasingly has been reported in relation to higher-order cognitive functioning including memory (Hanslmayr et al., 2016) and language processing (Weiss and Mueller, 2012). In a sample of 129 newborns, Brito et al. (2019) found links between higher-frequency oscillations, including beta, and potential risk for autism spectrum disorder at age 2, with reduced beta EEG power associated with increased risk. The present study’s significant findings in the parietal region are in line with past results demonstrating associations between parietal activity and infant language development, including aspects of semantic generalization (Friedrich et al., 2015), auditory comprehension (Brito et al., 2016), and word discrimination (Mills et al., 1997). These links between the parietal lobe and language processing may be mediated by mechanisms of attention (Behrmann et al., 2004) – left parietal activation has been correlated with infant responding to joint attention (RJA) at 14 months (Mundy et al., 2000) and RJA between 6- to 18-months has been positively related to individual differences in vocabulary acquisition at age 2 (Morales et al., 2000). Future research should examine associations how early attentional processes may moderate correlations between home language input and brain activity during infancy.
While not a main research question in this study, household chaos has been investigated in prior research in relation to child development, and has been shown to negatively influence language development. Chronic environmental noise (Maxwell and Evans, 2000; Song et al., 2011), household crowding (Evans, 2006; Evans et al., 1999; Maxwell, 2003), and lack of routines or disorganization (Johnson et al., 2008; Martin et al., 2012) have all been associated with poorer performance on early language assessments, even after controlling for SES (Wachs and Chan, 1986). It has been suggested that chaotic households may provide children with too much stimulation or that children may not be able to process language efficiently because of the many distractions within their environment (Evans et al., 1999). Other studies point to more proximal interactions with caregivers. In a large sample of infants (N = 1292) from predominantly low-income households, Vernon-Feagans et al. (2012) reported that household disorganization was negatively related to receptive and expressive language skills at 36 months, and that parenting partially mediated this association. This was in line with previous research reporting higher degrees of chaos in the home associated with reduced parental responsiveness to infants (Corapci and Wachs, 2002; Matheny et al., 1995; Wachs and Camli, 1991). Caregivers living in households of increased chaos report higher levels of parenting stress and symptoms relating to depression (Corapci and Wachs, 2002; Wachs and Chan, 1986); these disorganized environments may influence a caregiver’s time and ability to provide optimal interactions. A more thorough investigation of links among household chaos, parenting, and brain activity is needed in future analyses to better understand these mechanisms.
Past studies have examined brain activity in relation to household chaos within composites of chronic family stress (e.g., Chen et al., 2015), but this is the first study, to our knowledge, that has reported links between language input and EEG activity, during the first year of life. Nonetheless, there are several limitations within the current study. First, while the present study had a fairly large sample overall, the relatively small number of infants at each age group limits our interpretation of age-related effects. Replicating these effects in a larger sample, with more infants in each age group, is a necessary next step to strengthen confidence in these preliminary findings. Additionally, our study uses a cross-sectional design, but multiple measures of home language input over time would contribute to a better understanding of changes in parent-infant verbal interactions in relation to early brain activity. Secondly, although LENA measures of adult-child speech offer a wealth of naturalistic language data within the home, the data is limited to measures of quantity over quality. Capturing qualitative properties of language (i.e., structure, complexity, and meaning) in relation to brain function would be ideal in future studies. Furthermore, the effect sizes for regression models examining SES, home language input, and EEG power were relatively small. Multiple measures of the home environment together, like language input and household chaos which explained significantly more of the variance in EEG power, are needed. Finally, our diverse sample of families included a relatively large proportion of infants exposed to two languages within the home (36.2 %). Even though no differences were found between children classified as monolingual or bilingual on any measure of interest, because past studies have reported differences in neural commitment timelines in relation to bilingualism (Garcia-Sierra et al., 2011), future studies should examine how language input in both languages, independently and jointly, contribute to individual differences in brain function.
The first year of life is a sensitive time period for language learning. Characterizing how qualities of the home environment and parent-infant interactions shape the developing brain is crucial in understanding the pathways between early language abilities and later academic achievement. Increasing both the amount and diversity of language interactions within the home can positively influence language development, regardless of SES. The current findings suggest that the situational context in which those language interactions occur are worth exploring.
Declaration of Competing Interest
No conflict of interests to declare
Acknowledgements
We are grateful to all the families who participated in this research and to Samantha Melvin, Elsa Obus, Mayra Lemus-Rangle, Cynthia Wiltshire, Erin Holahan, and Gabrielle Lipson for their invaluable help in collecting and coding data. This study was supported by the Robert Wood Johnson Foundation Seed Grant, Sackler Parent-Infant Project Fellowship, and NICHDR00-HD086255 to NHB and Columbia Presidential Scholars in Society and Neuroscience (PSSN) Seed Grant to KGN and WPF.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100780.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Anderson A.J., Perone S. Developmental change in the resting state electroencephalogram: insights into cognition and the brain. Brain Cogn. 2018;126:40–52. doi: 10.1016/j.bandc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114(2):171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Geng J.J., Shomstein S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004;14(2):212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Benasich A.A., Gou Z., Choudhury N., Harris K.D. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav. Brain Res. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8:276. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Fifer W.P., Myers M.M., Elliott A.J., Noble K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cogn. Neurosci. 2016;19:144–151. doi: 10.1016/j.dcn.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Elliott A.J., Isler J.R., Rodriguez C., Friedrich C., Shuffrey L.C., Fifer W.P. Neonatal EEG linked to individual differences in socioemotional outcomes and autism risk in toddlers. Dev. Psychobiol. 2019 doi: 10.1002/dev.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchinal M.R., Pace A., Alper R., Hirsh-Pasek K., Golinkoff R.M. Early language outshines other predictors of academic and social trajectories in elementary school. Administration for Children and Families (ACF) National Research Conference on Early Childhood; Washington, DC; 2016. [Google Scholar]
- Buzzell G.A., Barker T.V., Troller-Renfree S.V., Bernat E.M., Bowers M.E., Morales S. Adolescent cognitive control, theta oscillations, and social observation. NeuroImage. 2019;198:13–30. doi: 10.1016/j.neuroimage.2019.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers-Heinlein K., Burns T.C., Werker J.F. The roots of bilingualism in newborns. Psychol. Sci. 2010;21(3):343–348. doi: 10.1177/0956797609360758. [DOI] [PubMed] [Google Scholar]
- Caldwell B.M., Bradley R.H. University of Arkansas at Little Rock; Little Rock: 1984. Home Observation for Measurement of the Environment. [Google Scholar]
- Chen N., Bell M.A., Deater-Deckard K. Maternal frontal EEG asymmetry and chronic stressors moderate the link between child conduct problems and maternal negativity. Soc. Dev. 2015;24(2):323–340. doi: 10.1111/sode.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comon P. Independent component analysis, a new concept? Signal Processing. 1994;36(3):287–314. doi: 10.1016/0165-1684(94)90029-9. [DOI] [Google Scholar]
- Corapci F., Wachs T.D. Does parental mood or efficacy mediate the influence of environmental chaos upon parenting behavior? Merrill. Q. 2002:182–201. [Google Scholar]
- Debnath R., Salo V.C., Buzzell G.A., Yoo K.H., Fox N.A. Mu rhythm desynchronization is specific to action execution and observation: evidence from time-frequency and connectivity analysis. NeuroImage. 2019;184:496–507. doi: 10.1016/j.neuroimage.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T., Scott L.S., Nelson C.A. Methods for acquiring and analyzing infant event-related potentials. Infant EEG Event-Related Potentials. 2007;500:5–37. [Google Scholar]
- DeCasper A.J., Fifer W.P. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Evans G.W. Child development and the physical environment. Annu. Rev. Psychol. 2006;57:423–451. doi: 10.1146/annurev.psych.57.102904.190057. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Maxwell L.E., Hart B. Parental language and verbal responsiveness to children in crowded homes. Dev. Psychol. 1999;35(4):1020–1023. doi: 10.1037//0012-1649.35.4.1020. https://www.ncbi.nlm.nih.gov/pubmed/10442870 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Fernald A., Marchman V.A., Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci. 2013;16(2):234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M., Wilhelm I., Born J., Friederici A.D. Generalization of word meanings during infant sleep. Nat. Commun. 2015;6:6004. doi: 10.1038/ncomms7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Wilkinson C., Kapur K., Tager-Flusberg H., Levin A.R., Nelson C.A. Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat. Commun. 2019;10(1):1–12. doi: 10.1038/s41467-019-12202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sierra A., Rivera-Gaxiola M., Percaccio C.R., Conboy B.T., Romo H., Klarman L. Bilingual language learning: an ERP study relating early brain responses to speech, language input, and later word production. J. Phon. 2011;39(4):546–557. doi: 10.1016/j.wocn.2011.07.002. [DOI] [Google Scholar]
- Gilkerson J., Richards J.A., Warren S.F., Montgomery J.K., Greenwood C.R., Kimbrough Oller D. Mapping the early language environment using all-day recordings and automated analysis. Am. J. Speech Lang. Pathol. 2017;26(2):248–265. doi: 10.1044/2016_AJSLP-15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z., Choudhury N., Benasich A.A. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav. Brain Res. 2011;220(2):263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Staresina B.P., Bowman H. Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum. Trends Neurosci. 2016;39(1):16–25. doi: 10.1016/j.tins.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmony T., Marosi E., de León A.E.D., Becker J., Fernández T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalography and clinical Neurophysiology. 1990;75(6):482–491. doi: 10.1016/0013-4694(90)90135-7. [DOI] [PubMed] [Google Scholar]
- Hari R., Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20(1):44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Harrewijn A., Buzzell G.A., Debnath R., Leibenluft E., Pine D.S., Fox N.A. Frontal alpha asymmetry moderates the relations between behavioral inhibition and social-effect ERN. Biol. Psychol. 2019;141:10–16. doi: 10.1016/j.biopsycho.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. https://www.ncbi.nlm.nih.gov/pubmed/14552403 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hoff E. How social contexts support and shape language development. Dev. Rev.: DR. 2006;26(1):55–88. doi: 10.1016/j.dr.2005.11.002. [DOI] [Google Scholar]
- Hoff E. Interpreting the early language trajectories of children from low-SES and language minority homes: implications for closing achievement gaps. Dev. Psychol. 2013;49(1):4–14. doi: 10.1037/a0027238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff-Ginsberg E. The relation of birth order and socioeconomic status to children’s language experience and language development. Appl. Psycholinguist. 1998;19(4):603–629. doi: 10.1017/S0142716400010389. [DOI] [Google Scholar]
- Huttenlocher J., Waterfall H., Vasilyeva M., Vevea J., Hedges L.V. Sources of variability in children’s language growth. Cogn. Psychol. 2010;61(4):343–365. doi: 10.1016/j.cogpsych.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.D., Martin A., Brooks-Gunn J., Petrill S.A. Order in the house! associations among household chaos, the home literacy environment, maternal reading ability, and children’s early reading. Merrill. Q. 2008;54(4):445–472. doi: 10.1353/mpq.0.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.J., Avineri N., Johnson D.C. Exposing gaps in/between discourses of linguistic deficits. Int. Multiling. Res. J. 2017;11(1):5–22. doi: 10.1080/19313152.2016.1258185. [DOI] [Google Scholar]
- Kuhl P.K. Early language acquisition: cracking the speech code. Nature reviews. Neuroscience. 2004;5(11):831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl P., Rivera-Gaxiola M. Neural substrates of language acquisition. Annu. Rev. Neurosci. 2008;31:511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- Layzer J., Price C. US Department of Health and Human Services; Washington, DC: 2008. Closing the Gap in the School Readiness of Low-income Children.http://www.nccp.org/projects/files/event_download_152.pdf Retrieved from. [Google Scholar]
- Lee V.E., Burkam D.T. 2002. Inequality at the Starting Gate: Social Background Differences in Achievement as Children Begin School.https://eric.ed.gov/?id=ED470551 Retrieved from. [Google Scholar]
- Levin A.R., Méndez Leal A.S., Gabard-Durnam L.J., O’Leary H.M. BEAPP: the batch electroencephalography automated processing platform. Front. Neurosci. 2018;12:513. doi: 10.3389/fnins.2018.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S., Jung T.-P., Bell A.J., Ghahremani D., Sejnowski T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. 1997;94(20):10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Martin A., Razza R., Brooks-Gunn J. Specifying the links between household chaos and preschool children’s development. Early Child Dev. Care. 2012;182(10):1247–1263. doi: 10.1080/03004430.2011.605522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M.J., Schneider J.M. Socioeconomic status related differences in resting state EEG activity correspond to differences in vocabulary and working memory in grade school. Brain Cogn. 2019;137:103619. doi: 10.1016/j.bandc.2019.103619. [DOI] [PubMed] [Google Scholar]
- Matheny A.P., Wachs T.D., Ludwig J.L., Phillips K. Bringing order out of chaos: psychometric characteristics of the confusion, hubbub, and order scale. J. Appl. Dev. Psychol. 1995;16(3):429–444. doi: 10.1016/0193-3973(95)90028-4. [DOI] [Google Scholar]
- Matousek M., Peterson I. Frequency analysis of the EEC in normal children and adolescents. In: Kelloway P., Peterson I., editors. Automation of clinical electroencephalography. Raven Press; New York: 1973. pp. 75–102. [Google Scholar]
- Maxwell L.E. Home and school density effects on elementary school children: the role of spatial density. Environ. Behav. 2003;35(4):566–578. doi: 10.1177/0013916503035004007. [DOI] [Google Scholar]
- Maxwell L.E., Evans G.W. The effects of noise on pre-school children’s pre-reading skills. J. Environ. Psychol. 2000;20(1):91–97. doi: 10.1006/jevp.1999.0144. [DOI] [Google Scholar]
- Melvin S.A., Brito N.H., Mack L.J., Engelhardt L.E. Home environment, but not socioeconomic status, is linked to differences in early phonetic perception ability. Infancy. 2017 doi: 10.1111/infa.12145. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzz E.C., Maskus Elaine A., Melvin Samantha A., Xiaofu He, Noble Kimberly G. Socioeconomic disparities in language input are associated with children’s language‐related brain structure and reading skills. Child Dev. 2019 doi: 10.1111/cdev.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D.L., Coffey‐Corina S., Neville H.J. Language comprehension and cerebral specialization from 13 to 20 months. Dev. Neuropsychol. 1997;13(3):397–445. [Google Scholar]
- Missana M., Grigutsch M., Grossmann T. Developmental and individual differences in the neural processing of dynamic expressions of pain and anger. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48(2):229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Moon C., Cooper R.P., Fifer W.P. Two-day-olds prefer their native language. Infant Behav. Dev. 1993;16(4):495–500. http://infantstudies-psych.sites.olt.ubc.ca/files/2015/03/Moon-et-al.-1993.pdf Retrieved from. [Google Scholar]
- Morales M., Mundy P., Delgado C.E.F., Yale M., Neal R., Schwartz H.K. Gaze following, temperament, and language development in 6-month-olds: a replication and extension. Infant Behav. Dev. 2000;23(2):231–236. doi: 10.1016/S0163-6383(01)00038-8. [DOI] [Google Scholar]
- Mundy P., Card J., Fox N. EEG correlates of the development of infant joint attention skills. Dev. Psychobiol. 2000;36(4):325–338. [PubMed] [Google Scholar]
- Noble K.G., Engelhardt L.E., Brito N.H., Mack L.J., Nail E.J., Angal J. Socioeconomic disparities in neurocognitive development in the first two years of life. Dev. Psychobiol. 2015;57(5):535–551. doi: 10.1002/dev.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Otero G.A. EEG spectral analysis in children with sociocultural handicaps. Int J Neurosci. 1994;79(3–4):213–220. doi: 10.3109/00207459408986082. [DOI] [PubMed] [Google Scholar]
- Otero G.A., Pliego-Rivero F.B., Fernández T., Ricardo J.E.E.G. EEG development in children with sociocultural disadvantages: a follow-up study. Clin Neurophysiol. 2003;114(10):1918–1925. doi: 10.1016/s1388-2457(03)00173-1. [DOI] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pierce L.J., Thompson B.L., Gharib A., Schlueter L., Reilly E., Valdes V., Roberts S., Conroy K., Levitt P., Nelson C.A. Association of perceived maternal stress during the perinatal period with electroencephalography patterns in 2-month-old infants. JAMA Pediatr. 2019;173(6):561–570. doi: 10.1001/jamapediatrics.2019.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Leonard J.A., Robinson S.T. Beyond the 30-million-word gap: children’s conversational exposure is associated with language-related brain function. Psychological. 2018 doi: 10.1177/0956797617742725. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R.R., Segaran J., Leonard J.A., Robinson S.T., West M.R., Mackey A.P. Language exposure relates to structural neural connectivity in childhood. J. Neurosci. 2018;38(36):7870–7877. doi: 10.1523/JNEUROSCI.0484-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M.L. Child-directed speech: relation to socioeconomic status, knowledge of child development and child vocabulary skill. J. Child Lang. 2008;35(1):185–205. doi: 10.1017/s0305000907008343. https://www.ncbi.nlm.nih.gov/pubmed/18300434 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Rowe M.L. A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Dev. 2012;83(5):1762–1774. doi: 10.1111/j.1467-8624.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M.L., Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science. 2009;323(5916):951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Song J.H., Skoe E., Banai K., Kraus N. Perception of speech in noise: neural correlates. J. Cogn. Neurosci. 2011;23(9):2268–2279. doi: 10.1162/jocn.2010.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John A.M.S., Kao K., Choksi M., Liederman J., Grieve P.G. Variation in infant EEG power across social and nonsocial contexts. J. Exp. Child Psychol. 2016;152:106–122. doi: 10.1016/j.jecp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Takano T., Ogawa T. Characterization of developmental changes in EEG‐gamma band activity during childhood using the autoregressive model. Pediatr. Int. 1998;40(5):446–452. doi: 10.1111/j.1442-200x.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Team, R. C. (2013). R: A language and environment for statistical computing. Chicago.
- Tomalski P., Moore D.G., Ribeiro H., Axelsson E.L., Murphy E., Karmiloff-Smith A. Socioeconomic status and functional brain development--associations in early infancy. Dev. Sci. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S., Nelson C.A., Zeanah C.H., Fox N.A. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. J. Child Psychol. Psychiatry. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Rodriguez E., Rotarska-Jagiela A., Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Vanderwert R.E., Zeanah C.H., Fox N.A., Nelson III C.A. Normalization of EEG activity among previously institutionalized children placed into foster care: a 12-year follow-up of the Bucharest early intervention project. Dev. Cogn. Neurosci. 2016;17:68–75. doi: 10.1016/j.dcn.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L., Garrett-Peters P., Willoughby M., Mills-Koonce R., The Family Life Project Key Investigators Chaos, poverty, and parenting: predictors of early language development. Early Child. Res. Q. 2012;27(3):339–351. doi: 10.1016/j.ecresq.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs T.D., Camli O. Do ecological or individual characteristics mediate the influence of the physical environment upon maternal behavior. J. Environ. Psychol. 1991;11(3):249–264. doi: 10.1016/S0272-4944(05)80186-0. [DOI] [Google Scholar]
- Wachs T.D., Chan A. Specificity of environmental action, as seen in environmental correlates of infants’ communication performance. Child Dev. 1986;57(6):1464–1474. doi: 10.2307/1130424. [DOI] [Google Scholar]
- Weisleder A., Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychol. Sci. 2013;24(11):2143–2152. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Mueller H.M. “Too many betas do not spoil the broth”: the role of Beta brain oscillations in language processing. Front. Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J.F., Tees R.C. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behav. Dev. 1984;7(1):49–63. doi: 10.1016/S0163-6383(84)80022-3. [DOI] [Google Scholar]
- Zimmerman I.L., Castilleja N.F. The role of a language scale for infant and preschool assessment. Ment. Retard. Dev. Disabil. Res. Rev. 2005;11(3):238–246. doi: 10.1002/mrdd.20078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.