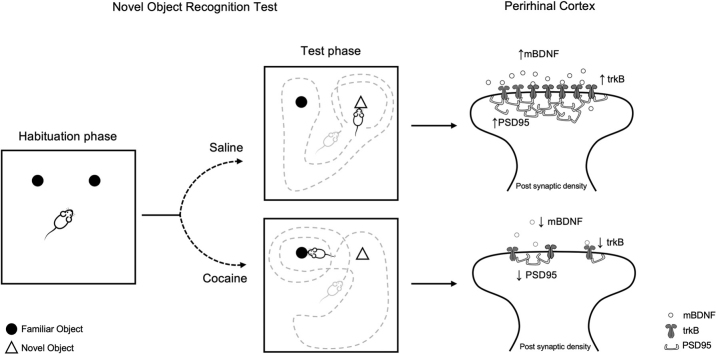
Graphical abstract
Keywords: Cocaine, Adolescence, Perirhinal cortex, BDNF
Abstract
The perirhinal cortex (PrhC) is critical for object recognition memory; however, information regarding the molecular mechanisms underlying this type of memory following repeated exposure to drugs of abuse during adolescence is unknown. To this end, adolescent or adult rats were exposed to cocaine from postnatal day (PND) 28 to PND 42 or PND 63 to PND 77, respectively. Two weeks later, rats were subjected to the cognitive test named Novel Object Recognition (NOR) test. We found that adolescent, but not adult, cocaine exposure caused a significant impairment in the NOR test, independently from changes in the stress response system. In adolescent saline-treated rats, NOR test up-regulated BDNF and its downstream signaling whereas a downregulation of the same pathway was observed in cocaine-treated rats together with a reduction of Arc/Arg3.1 and PSD95 expression, indicating reduced pro-cognitive structural adaptations in the PrhC. Of note, cocaine-treated adult rats correctly performed in the NOR test indicating intact recognition memory mechanisms, despite a significant cocaine-induced reduction of BDNF levels in the PrhC, suggesting that recognition memory is heavily dependent on BDNF during adolescence whereas during adulthood other mechanisms come into play.
1. Introduction
Adolescents are highly vulnerable to external stimuli such as, for instance, exposure to drugs of abuse. Such higher sensitivity seems to rely, primarily, upon the fact that young people are more prone to the rewarding effects of drugs and feel less their adverse effects (Casey et al., 2008; Spear, 2000). During adolescence, the brain is still maturing and interfering with its correct development may be detrimental. We have previously shown that developmental exposure to cocaine alters the homeostasis of the medial prefrontal cortex, a brain region that is still developing during adolescence and whose main function is to integrate information from various cortical and subcortical brain regions to mediate cognition (Caballero et al., 2016). Interestingly, both single (Caffino et al., 2018b, 2017b; Giannotti et al., 2015) and repeated (Caffino et al., 2018a, 2015a, 2015b, Giannotti et al., 2016, 2013) administration of cocaine during adolescence have shown to alter cortical homeostasis. Our work has been strengthened by elegant work from Gourley’s lab showing that cocaine exposure during adolescence has long-term effects on decision making, via enduring alterations of dendrite structure in the orbitofrontal cortex (Gourley et al., 2012; DePoy et al., 2017). Of note, in humans, cocaine use impairs cognition (Bolla et al., 2003; Verdejo-García et al., 2007), an effect presumably contributing to the perpetuation of drug abuse (Torregrossa et al., 2011). In order to test the effect of exposure to cocaine during adolescence on rats cognitive abilities, we employed the Novel Object Recognition (NOR) test, i.e. a simple and ethologically relevant, cognitively demanding test based on the discrimination between novel and familiar objects (Bevins and Besheer, 2006). In a recent work, Rubino’s group has elegantly shown that adolescent THC exposure causes cognitive deficits in the NOR test (Prini et al., 2018). Similarly, significant deficits in recognition memory following alcohol consumption have been shown in adolescent rats (Marco et al., 2017). These lines of evidence point to exposure to drugs of abuse as crucial for the manifestation of cognitive deficits in adolescent rats and to the NOR test as a rapid, non-invasive test to inform about cognition. However, so far, no information is available on the effects caused by cocaine in the adolescent rat and its effect on recognition memory.
The discrimination ability, that is crucial for recognition memory, seems to heavily rely on a specific region of the temporal lobe, i.e. the perirhinal cortex (PrhC) (Winters, 2004). A recent elegant paper has strengthened such role since methamphetamine-induced memory deficit were reverted by chemogenetic activation of PrhC (Peters et al., 2018). The role of several neurotransmitters in the mechanisms subserving different functions of the PrhC has been widely studied (Miranda and Bekinschtein, 2018); however recent evidence shed light on the role of the neurotrophin BDNF and its high affinity receptor trkB in this brain region (Bekinschtein et al., 2008; Romero-Granados et al., 2010), since interfering with perirhinal BDNF impairs recognition memory in rats (Seoane et al., 2011) and because BDNF-stimulated intracellular signaling is critical for consolidation of recognition memory (Callaghan and Kelly, 2012). For these reasons, we have investigated BDNF-mediated signaling in the PrhC together with the analysis of some partners of BDNF in the modulation of synaptic plasticity such as the immediate early gene Arc/Arg3.1, which has been previously shown to be modulated by object recognition in PrhC (Miranda et al., 2017) and altered by cocaine exposure (Caffino et al., 2014; Fumagalli et al., 2009, 2006). Since Arc/Arg3.1 is a well-accepted index of neuronal activity but it also plays a structural role in the synapse (Bramham et al., 2008), we decided to investigate also the expression of a critical determinant of the post-synaptic density such as the post-synaptic density protein 95 (PSD95), which also has been shown to be altered by exposure to cocaine in adolescent rats (Caffino et al., 2017b).
Based on the results mentioned above, we hypothesize that repeated exposure to cocaine during adolescence would impact on rat’s cognitive abilities through changes in BDNF expression in the PrhC; further, we were also interested in investigating potential differences between adolescent and adult rats. To this end, adolescent or adult rats were injected daily with cocaine (5 mg/kg/day, subcutaneously) from post-natal day (PND) 28 to PND 42 (adolescent rats) or from PND 63 to PND 77; after two weeks of drug withdrawal, rats were exposed to the NOR test and sacrificed immediately after the end of the test itself. Our analyses were undertaken in two different cell preparations, i.e. the whole homogenate and the post-synaptic density, i.e. the location where synaptic activity takes place, in order to dissect the effect of adolescent cocaine exposure on BDNF protein translation from the availability of the neurotrophin at synaptic sites.
2. Material and methods
2.1. Subjects
The adolescent Sprague Dawley male rats used in this study were obtained from Charles River (Calco, Italy) and housed under standard conditions of temperature and humidity under artificial light (from 07:00 to 19:00 h). A maximum of two male siblings was taken from each litter in order to reduce “litter effects” (Chapman and Stern, 1978). Twenty-nine male rats were treated subcutaneously with cocaine (5 mg/kg/day) (Space Import Export srl, Milan, Italy) or saline from postnatal day (PND) 28 to PND 42, a period that roughly approximates adolescence in humans (Collins and Izenwasser, 2004). Cocaine was always administered at the same time in the morning (11:00 am), in order to avoid any stress due to unpredictability of the treatment.
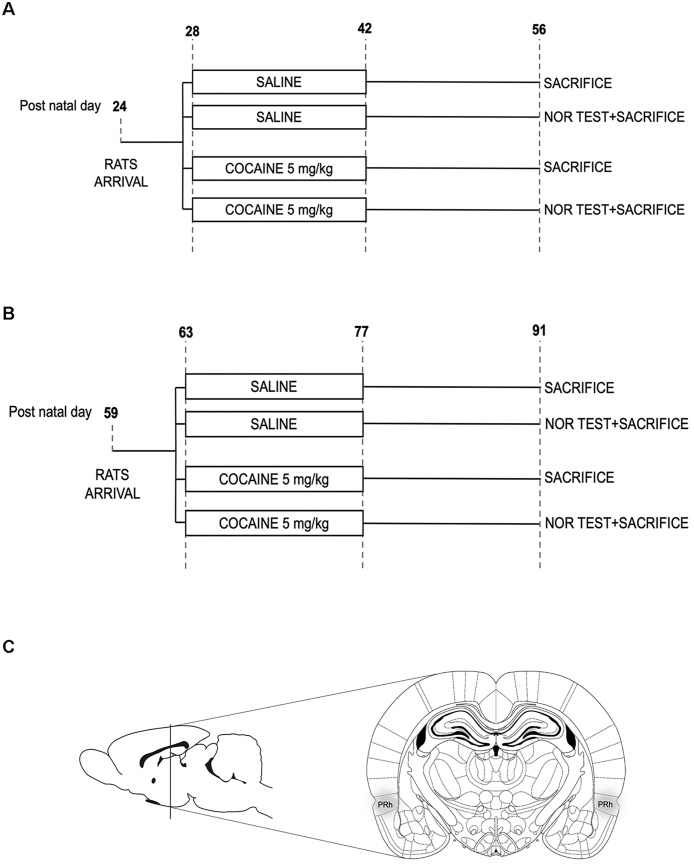
Animals were assigned to four experimental groups. Group 1 and group 2 were formed by rats exposed to saline from PND 28 to 42: rats in group 1 were then sacrificed on PND 56 without any further manipulation (n = 6) whereas rats in group 2 were sacrificed on PND 56 immediately after the NOR test (n = 8); group 3 and group 4 were formed by rats exposed to cocaine from PND 28–42; rats from group 3 were then sacrificed on PND 56 without any further manipulation (n = 7) whereas rats from group were sacrificed on PND 56 immediately after the NOR (n = 8) (Fig. 1a).
Fig. 1.
Schematic representation of the experimental procedures and specific coordinates of Perirhinal cortex (PrhC).
Panel a shows the experimental paradigm used for adolescent animals. Animals were treated with cocaine (5 mg/kg/day) or saline during adolescence [postnatal day (PND) 28-PND 42]. Two weeks after the end of the adolescent treatment, animals were subjected to novel object recognition (NOR) test and sacrificed immediately after the end of the test. Panel b shows the experimental paradigm used for adult animals. Animals were treated with cocaine (5 mg/kg/day) or saline from PND 63 to PND 77]. Two weeks after the end of the treatment, animals were subjected to NOR test and sacrificed immediately after the end of the test. Panel c shows the dissection of the PrhC. This procedure was undertaken according to the coordinates indicated by the atlas of Paxinos and Watson, 2005 (please see the Material and Methods section). The PrhC was then stored at −80 °C until processing.
After decapitation, whole brains were frozen on dry ice for micro-dissection procedure. Trunk blood from each rat was collected in heparinized tubes for quantification of corticosterone plasma levels.
Bilateral punches of perirhinal cortex (PrhC) (from Bregma −3.00 mm to Bregma −9.36 mm, Fig. 1c) were microdissected according to Giannotti et al. (2016) with minor modifications. Briefly, the frozen brains were acclimatized for 1 h in the cryostat at the temperature of −15 °C before being sliced. Coronal slices of 220 μm were placed on histological slides (4–5 brain section per slide) and a microdissection needle of 1 mm of inner diameter was used to dissect the PrhC. The dissected tissue pellets were blown out of the needle in centrifuge tubes, immediately frozen on dry ice and stored at −80 °C until being processed for molecular analysis.
To investigate whether repeated cocaine administration could similarly affect the performance in the NOR test in adult animals, we incorporated 25 Sprague Dawley male rats (6–7/group) that were treated subcutaneously with saline or cocaine (5 mg/kg/day) from PND 63 to PND 77, and then exposed to the NOR test 2 weeks after the end of the treatment, i.e. on PND 91 (Fig. 1b).
Of note, all the animals used in these experiments (both adolescents and adults) have been handled starting from two days after arrival and before being exposed to the repeated treatment with cocaine. In particular, adolescent rats were left undisturbed in their home cages till PND 56, two weeks after the end of the treatment, when the response to the NOR test was evaluated whereas adult rats were left undisturbed in their home cages until PND 91 before exposure to the NOR test. Manipulation was performed in order to avoid any potential bias due to stress-related effects.
Procedures involving animals were conducted at the Department of Pharmacological and Biomolecular Sciences, which adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D.lgs 26/2014; Authorization n.19/2008-A issued March 6, 2008 by Ministry of Health); the NIH Guide for the Care and Use of Laboratory Animals (2011 edition) and EU directives and guidelines (EEC Council Directive 2010/63/UE). All efforts were made to minimize animal suffering and to keep the lowest number of animals used. The experiments have been reported in compliance with the ARRIVE guidelines.
2.2. Novel object recognition (NOR) test
Both adolescent and adult rats performed the NOR test 2 weeks after the last cocaine exposure. This test takes advantage of the innate tendency of rodents to explore a novel more than a familiar object. At their first visit, rats were naive to the NOR apparatus, which consists of a Plexiglas open-field box (60 × 60 × 60 cm), situated in a dimly illuminated room. Each rat performed each test individually. Rats go through different steps for the NOR test: first, a familiarization phase of 5 min, during which the rat is placed into a squared arena with two identical previously unseen objects, located in two non-opposite corners of the arena; then, an inter-trial interval of 3 min during which the animal is moved back to its home cage to reinforce the previously seen scenario, then, rats return to the arena for the test phase, which lasts 5 min. Of note, both familiar and the novel objects showed different color, shape and material, to facilitate rats to distinguish them. Both familiar vs. novel objects and object placement were counterbalanced. The arena and the objects were cleaned with 0.1 % acetic acid between every phase. The time animals spent exploring or showing interest (sniffing, snout directed to the object), either for the familiar object (TF) or for the novel one (TN) was taken by two independent investigators, blind to the experimental design. A total of 2 adolescent animals were removed from the study because they failed to successfully explore the two objects (more than 10 s) during the familiarization phase. During the test phase, we measured two different indexes: 1) the recognition index, considered as a measure of approach to both objects, was calculated as follows: the time spent investigating the familiar or the novel object relative to the total object investigation [RIF = TF/(TN + TF); RIN = TN/(TN + TF)] and 2) the discrimination index, that was calculated as the time spent on the novel object compared to the time spent on the familiar object over the total time of exploration of both the novel and the familiar object: (TN-TF)/(TN+TF). Locomotor activity was evaluated as number of passages (count) among the four quadrants in which the arena was divided during test session.
2.3. Preparation of protein extracts and western blot analyses
Proteins from unilateral punches of the PrhC, counterbalanced across hemispheres, were extracted as previously described with minor modifications (Caffino et al., 2018b). Briefly, PrhC was homogenized in a teflon-glass potter in cold 0.32 M sucrose buffer pH 7.4 containing 1 mM HEPES, 1 mM MgCl2, 1 mM NaHCO3 and 0.1 mM PMSF, in presence of commercial cocktails of protease (Roche, Monza, Italy) and phosphatase (Sigma-Aldrich, Milan, Italy) inhibitors and an aliquot of each homogenate was then sonicated. The remaining homogenate was centrifuged at 13,000 g for 15 min obtaining a pellet. This pellet was resuspended in buffer containing 75 mM KCl and 1% Triton X-100 and centrifuged at 100,000 g for 1 h. The resulting pellet, referred as postsynaptic density (PSD) or Triton X-100 insoluble fraction (TIF), was homogenized in a glass–glass potter in 20 mM HEPES, protease and phosphatase inhibitors and stored at −20 °C in presence of glycerol 30 %. Total proteins have been measured in the total homogenate and in the TIF fraction according to the Bradford Protein Assay procedure (Bio-Rad Laboratories, Italy), using bovine serum albumin as calibration standard.
Equal amounts of proteins of the homogenate (6 ug) and of TIF fraction (5 ug) were run on criterion TGX precast gels (Bio-Rad Laboratories) under reducing conditions and electrophoretically transferred onto nitrocellulose membrane (Bio-Rad Laboratories). Blots were blocked 1 h at room temperature with I-Block solution (Life Technologies Italia, Italy) in TBS + 0.1 % Tween-20 buffer and incubated with antibodies against the proteins of interest.
The conditions of the primary antibodies were the following: anti mBDNF (1:500, Icosagen, Estonia); anti total trkB (1:500, Cell Signaling Technology Inc., USA); anti phospho-Akt S473 (1:1000, Cell Signaling Technology); anti total Akt (1:1000, Cell Signaling Technology); anti phospho-ERK2 T185/Y187 (1:1000, Cell Signaling Technology); anti total ERK2 (1:5000, Cell Signaling Technology); anti PSD95 (1:4000, Cell Signaling Technology), anti Arc/Arg3.1 (1:500, BD Transduction Laboratories, San Jose, CA, USA) and anti β-Actin (1:10000, Sigma-Aldrich). Results were standardized using β-actin as the control protein, which was detected by evaluating the band density at 43 kDa. Immunocomplexes were visualized by chemiluminescence using the Chemidoc MP Imaging System (Bio-Rad Laboratories). Gels were run 3 times each and the results represent the average from 3 different western blots.
2.4. RNA preparation and real-time polymerase chain reaction
Total RNA of unilateral punches of the PrhC, counterbalanced across hemispheres, was isolated by single step guanidinium isothiocyanate/phenol extraction using PureZol RNA isolation reagent (Bio-Rad Laboratories) according to the manufacturer’s instructions and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for real-time reverse transcription polymerase chain reaction (real time RT-PCR) to assess mRNA levels, as previously described (Caffino et al., 2018c). Briefly, an aliquot of each sample was treated with DNase to avoid DNA contamination. RNA was analyzed by TaqMan qRT-PCR instrument (CFX384 real time system, Bio-Rad Laboratories) using the iScriptTM one-step RT-PCR kit for probes (Bio-Rad Laboratories). Samples were run in 384 wells formats in triplicate as multiplexed reactions. Thermal cycling was initiated with an incubation at 50 °C for 10 min (RNA retrotranscription) and then at 95 °C for 5 min (TaqMan polymerase activation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95 °C for 10 s to enable the melting process and then for 30 s at 60 °C for the annealing and extension reaction. Data were analyzed with the comparative threshold cycle (ΔΔCt) method using either β-actin as reference gene. The primer efficiencies were experimentally set up for each couple of primers. Primers and probes sequences are shown below:
total BDNF: forward primer 5’-AAGTCTGCATTACATTCCTCGA-3’, reverse primer 5’-GTTTTCTGAAAGAGGGACAGTTTAT-3’, probe 5’-TGTGGTTTGTTGCCGTTGCCAAG-3’;
Arc/Arg3.1: forward primer 5’-GGTGGGTGGCTCTGAAGAAT-3’, reverse primer 5’-ACTCCACCCAGTTCTTCACC -3’, probe 5’-GATCCAGAACCACATGAATGGG-3’;
β-Actin: forward primer 5’-CACTTTCTACAATGAGCTGCG-3’, reverse primer 5’-CTGGATGGCTACGTACATGG-3’, probe 5’-TCTGGGTCATCTTTTCACGGTTGGC-3’.
2.5. Analysis of plasma corticosterone levels
Plasma was separated by centrifugation (3000 g for 20 min) and corticosterone levels were determined by an enzyme-linked immunosorbent assay (ELISA) using a commercial kit, according to the manufacturers' instructions (IBL, Hamburg, Germany).
2.6. Data analysis and statistics
Data were collected in individual animals (independent determinations) and are presented as means and standard errors. The recognition index measured in the NOR test were analyzed by three-way analysis of variance (ANOVA), with cocaine treatment (saline vs cocaine), NOR test (tested vs. non tested) and period of treatment (treatment during adolescence vs. treatment during adulthood) as independent variables. When dictated by relevant interaction terms, Tukey’s multiple comparisons test was used to characterize differences among individual groups of rats. The discrimination index measured in the NOR test and the molecular changes produced by cocaine treatment and NOR test alone as well as by their combination were analyzed using a two-way ANOVA, with adolescent cocaine treatment and NOR test as independent variables. When dictated by relevant interaction terms, Tukey’s multiple comparisons test was used to characterize differences among individual groups of rats. However, when no interaction between cocaine treatment and NOR test was observed, only the main effects were reported. Subjects were eliminated from the final dataset if their data deviated from the mean by 2 SDs. Prism 6.0 (GraphPad) was used to analyze all the data. Significance for all tests was assumed at p < 0.05.
3. Results
Fig. 1a and b illustrate the experimental paradigm used in our experiments. Adolescent (Fig. 1a) or adult (Fig. 1b) rats were chronically treated with cocaine or saline; two weeks after the end of treatment, rats were tested for memory retention in the NOR and sacrificed immediately after the test (please see materials and methods section for further details). PrhC was dissected through punching as depicted in Fig. 1c.
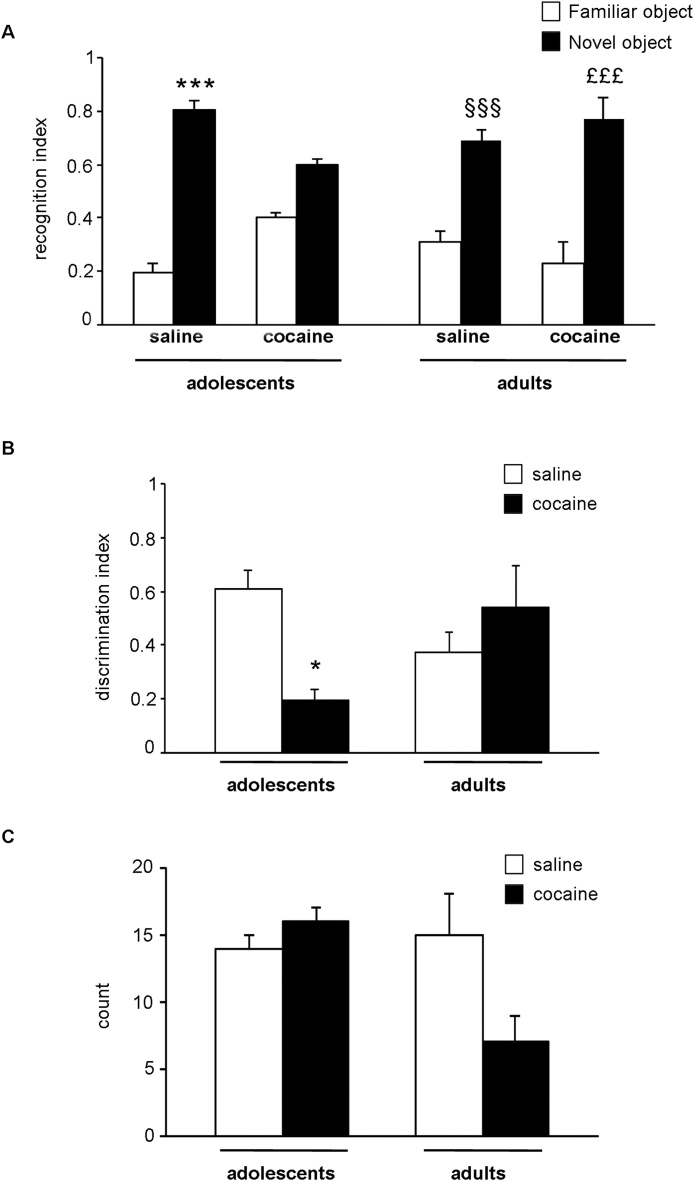
Fig. 2a shows the recognition index (RI) of NOR test, calculated as the time spent investigating the novel object relative to the total object investigation, performed in adolescent or adult saline- or cocaine-exposed animals. Three-way ANOVA of the RI revealed a novelty effect (F(1,48) = 154.08, p < 0.0001) and a cocaine treatment × novelty effect × age (F(1,48) = 17.263, p = 0.00013). Considering individual multiple comparisons, adolescent saline-treated rats explored longer the novel object vs. the familiar (+0.61, p < 0.0001; Tukey HSD test), as expected. Interestingly, two weeks of cocaine-withdrawal in adolescent rats caused an impairment in the exploration of the familiar object in respect to the novel one (+0,198, p = 0.1005; Tukey HSD test). Notably, in adult animals the cognitive performance calculated as RI is maintained in both saline- (+0.377 vs novel object, p < 0.001; Tukey HSD test) and cocaine-treated animals (+0.539 vs novel object, p < 0.001; Tukey HSD test).
Fig. 2.
Effect of 2-week withdrawal on memory retention in the novel object recognition (NOR) test in rats with a previous history of cocaine (5 mg/kg) or saline administration during adolescence or adulthood.
Panel a: recognition index. Recognition index was calculated as familiar or novel object exploration time/(familiar object + novel object exploration time) in cocaine- or saline-exposed adolescent animals at PND 56 (left) or in cocaine- or saline-exposed adult animals at PND 91 (right).
***p < 0.001 vs. time spent by adolescent saline-treated rats exploring the familiar object; §§§p < 0.001 vs. time spent by adult saline-treated rats exploring the familiar object; £££p < 0.001 vs. time spent by adult cocaine-treated rats exploring the familiar object (three-way ANOVA followed by Tukey HSD test).
Panel b: discrimination index. Discrimination index was calculated as (novel object–familiar object exploration time)/(familiar object + novel object exploration time) in cocaine- or saline-exposed adolescent animals at PND 56 (left) or in cocaine- or saline-exposed adult animals at PND 91 (right).
*p < 0.05 vs. DI of adolescent saline-treated rats (two-way ANOVA followed by Tukey HSD test).
Panel c: Locomotor activity. Locomotor activity was evaluated as number of passages (count) among the four quadrants in which was divided the arena during test session at PND 56 (left) or at PND 91 (right).
Behavioral analyses were done by two independent investigators who were blinded to the experimental design. Histograms represent the mean ± SEM of 6–8 rats per group.
Fig. 2 panel b shows the discrimination index (DI) calculated as the time spent exploring the novel object in comparison to the familiar one, related to the total time of exploration. Two-way ANOVA of the DI revealed a significant treatment × age interaction (F(1,24) = 8.631, p = 0.0072). Considering individual multiple comparisons, two weeks of withdrawal from developmental cocaine exposure showed a reduced DI indicating a reduced exploration time of the novel object compared to the saline group (−0.4109, p = 0.0396; Tukey HSD test). No changes were instead observed in the DI measured at PND 91 in cocaine-exposed animals during adulthood (+0.1641, p = 0.6138; Tukey HSD test).
As shown in Fig. 2 panel c, no changes were observed in the locomotor activity of both saline and cocaine-treated groups measured during NOR test at PND 56 and at PND 91 (treatment: F(1,24) = 1.326, p = 0.261; age: F(1,24) = 2.962, p = 0.098; cocaine treatment × age: F(1,24) = 3.840, p = 0.061; two-way ANOVA).
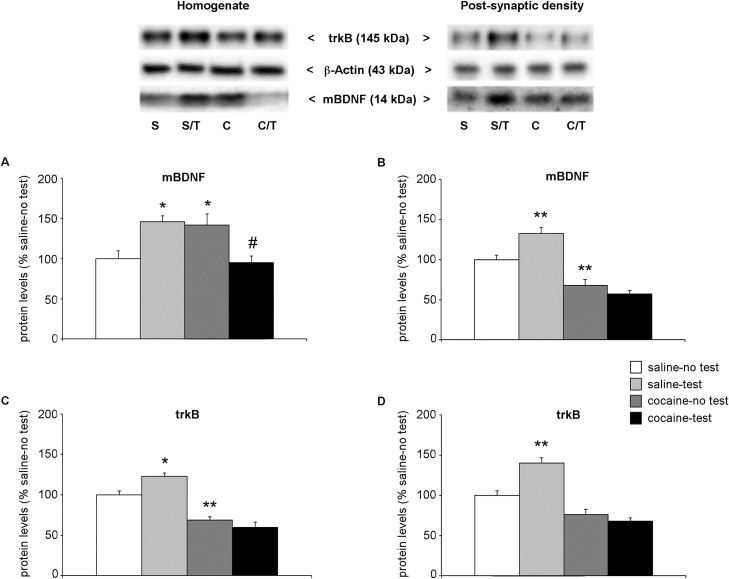
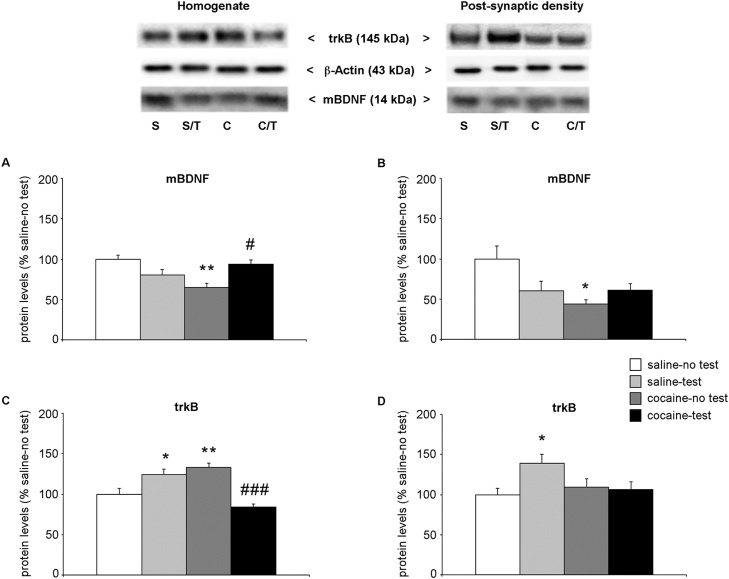
In an attempt to find a mechanism that could underlie the memory deficit observed in adolescent rats after two weeks of withdrawal, we analyzed the dynamic regulation of the neurotrophin BDNF in the PrhC in response to NOR test. Fig. 3 panel A shows the protein levels of the mature form of the neurotrophin BDNF (mBDNF) in the whole homogenate of PrhC. Two-way ANOVA of mBDNF revealed a main treatment × test effect, (F(1,20) = 20.77, p = 0.0002), whereas no effect of NOR test (F(1,20) = 0.006, p = 0.941) or cocaine treatment (F(1,20) = 0.673, p = 0.184) was observed. Focusing on individual effects, we observed that: 1) two weeks of withdrawal caused a significant increase in BDNF protein levels (+42 % vs. saline-no test rats, p = 0.039; Tukey HSD test) and 2) the NOR test increased BDNF protein levels in saline-treated rats (+46 % vs saline-no test, p = 0.023; Tukey HSD test) whereas the neurotrophin protein levels were decreased in cocaine-treated rats (−47 % vs. cocaine-no test, p = 0.018; Tukey HSD test). In the post-synaptic density of PrhC, two-way ANOVA of mBDNF levels showed a significant effect of treatment (F(1,21) = 79.61, p < 0.0001), a treatment × test interaction (F(1,21) = 13.24, p = 0.0015) with no effect of the NOR test (F(1,21) = 3.255, p = 0.087; Fig. 3b). Further intergroup subtesting revealed that, at variance from the effects in the whole homogenate, repeated exposure to cocaine reduced mBDNF levels in the post-synaptic density (−32 % vs. saline-no test, p = 0.007; Tukey HSD test). Interestingly, it also appears that cocaine has influenced the response to the cognitive test. In fact, NOR test caused an increase of BNDF levels in saline-treated rats (+33 % vs. saline-no test, p = 0.006; Tukey HSD test), while no changes were found in cocaine-treated rats (−11 % vs. cocaine-no test, p = 0.5597; Tukey HSD test).
Fig. 3.
Effect of 2-week withdrawal from cocaine exposure during adolescence on mBDNF and trkB protein levels in the PrhC.
Rats were treated with cocaine or saline from PND 28 to PND 42. Two weeks after the end of treatment (PND 56), rats were tested for NOR and sacrificed immediately after the test. mBDNF protein levels (14 kDa) are shown in the whole homogenate (panel a) and in the post-synaptic density fraction (panel b) of the PrhC. TrkB protein levels (145 kDa) are shown in the whole homogenate (panel c) and in the post-synaptic density fraction (panel d) of the PrhC.
In the upper panel, representative immunoblots are shown for mBDNF, trkB and β-Actin proteins in the homogenate and PSD of PrhC.
Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of six to seven rats per group. *p < 0.05, **p < 0.01 vs. saline-no test rats; #p < 0.05 vs. cocaine- no test (two-way ANOVA followed by Tukey HSD test).
S = saline-no test; S/T = saline-test; C = cocaine-no test; C/T = cocaine-test.
We next measured the protein levels of trkB, the high affinity BDNF receptor. In the whole homogenate of PrhC, two-way ANOVA of trkB showed a significant effect of treatment (F(1,20) = 77.06, p < 0.0001) and of treatment × test interaction (F(1,20) = 9.066, p = 0.007; Fig. 3c). Comparing individual experimental groups, we found a significant reduction of trkB expression in cocaine-treated rats (−31 % vs. saline-no test rats, p = 0.003; Tukey HSD test). Furthermore, NOR test enhanced trkB levels in saline-treated rats (+23 %, p = 0.037; Tukey HSD test) but not in cocaine-treated rats (−9% vs. cocaine-no test, p = 0.608; Tukey HSD test). Similarly in the post-synaptic density, two-way ANOVA of trkB showed a significant effect of NOR test (F(1,21) = 4.714, p = 0.0415), treatment (F(1,21) = 41.44, p < 0.0001) and of the interaction between treatment and test (F(1,21) = 10.49, p = 0.0039) (Fig. 3d). Subsequent post hoc analysis revealed that adolescent exposure to cocaine influenced the response to the NOR test, as shown by the NOR-induced increase in trkB protein levels in saline-treated rats (+40 % vs. saline-no test, p = 0.0059; Tukey HSD test), effect that is not observed in cocaine-treated rats that performed the test (−8 % vs. cocaine-test, p = 0.867; Tukey HSD test).
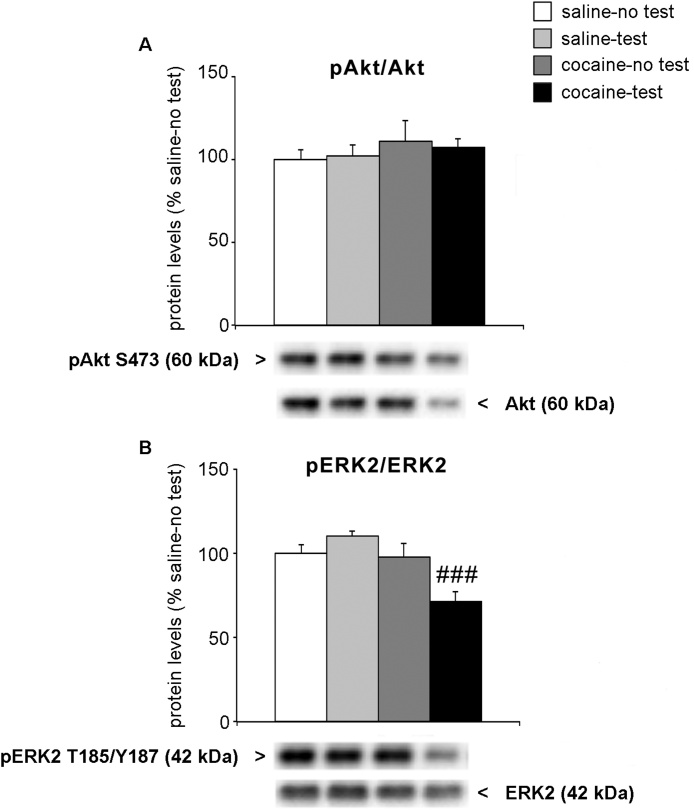
Next, we evaluated the activation of BDNF downstream effectors Akt and ERK2 in the whole homogenate of the PrhC, expressed as a ratio between the phosphorylated and the total levels of protein. We first focused our attention on Akt pathway because we have previously shown the activation of this pathway brought about by cocaine withdrawal following long-term adolescent administration of the drug (Giannotti et al., 2014). Two-way ANOVA of Akt activation revealed no significant effect (NOR test: F(1,21) = 0.001, p = 0.9752; treatment: F(1,21) = 0.9201, p = 0.3484; treatment × test: F(1,21) = 0.093, p = 0.764; Fig. 4a). Fig. 4 panel B shows the levels of ERK2 phosphorylation and expression in the whole homogenate. Two-way ANOVA revealed a significant effect of the NOR test (F(1,21) = 9.116, p = 0.0065), of the treatment (F(1,21) = 5.907, p = 0.0241) and a treatment × test interaction (F(1,21) = 12.43, p = 0.002). Interestingly, while no effect was observed in saline-treated rats that performed the NOR test (+10 % vs. saline-no test, p = 0.9847; Tukey HSD test), the activation of ERK2 was reduced in cocaine-treated rats exposed to the NOR test (−27 %, p = 0.0006 vs. cocaine-no test; Tukey HSD test).
Fig. 4.
Effect of 2-week withdrawal from cocaine exposure during adolescence on BDNF-mediated signaling pathways in the PrhC.
Rats were treated with cocaine or saline from PND 28 to PND 42. Two weeks after the end of treatment (PND 56), rats were tested for NOR and sacrificed immediately after the test. Panel a shows the activation of Akt (expressed as a ratio: pAkt S473/Akt 60 kDa, panel a) and of ERK2 (expressed as a ratio: pERK2 T185/Y187/ERK2 42 kDa, panel b) protein levels are shown in the whole homogenate of the PrhC. Below each graph, representative immunoblots are shown for pAkt S473, Akt, pERK2 T185/Y187 and ERK2 proteins in the homogenate of PrhC.
Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of six to seven rats per group. ###p < 0.001 vs. cocaine-no test (two-way ANOVA followed by Tukey HSD test).
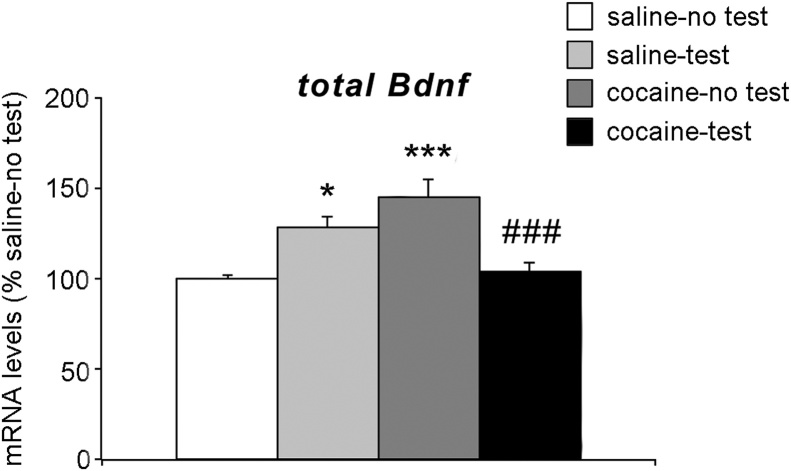
Fig. 5 shows total Bdnf mRNA levels measured in the PrhC. Two-way ANOVA showed a significant effect of treatment × test interaction (F(1,21) = 29.85, p < 0.0001) with no effect of the NOR test (F(1,21) = 1.208, p = 0.284) and of treatment per se (F(1,21) = 3.015, p = 0.097). Further intergroup sub-testing revealed that repeated exposure to cocaine enhanced total Bdnf mRNA levels (+45 % vs saline-no test, p = 0.0003; Tukey HSD test). Interestingly, NOR test provoked an opposite response in the Bdnf mRNA levels in saline- or cocaine-treated animals. More specifically, Bdnf mRNA levels were increased in saline-treated rats (+28 % vs saline-no test, p = 0.03; Tukey HSD test), whereas they were reduced in cocaine-treated rats (−41 % vs cocaine-no test, p = 0.0006; Tukey HSD test).
Fig. 5.
Effect of 2-week withdrawal from cocaine exposure during adolescence on total BDNF mRNA levels in the PrhC.
Rats were treated with cocaine or saline from PND 28 to PND 42. Two weeks after the end of treatment (PND 56), rats were tested for NOR and sacrificed immediately after the test.
Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of six to seven rats per group. *p < 0.05, ***p < 0.001 vs. saline-no test rats; ###p < 0.001 vs. cocaine-no test (two-way ANOVA followed by Tukey HSD test).
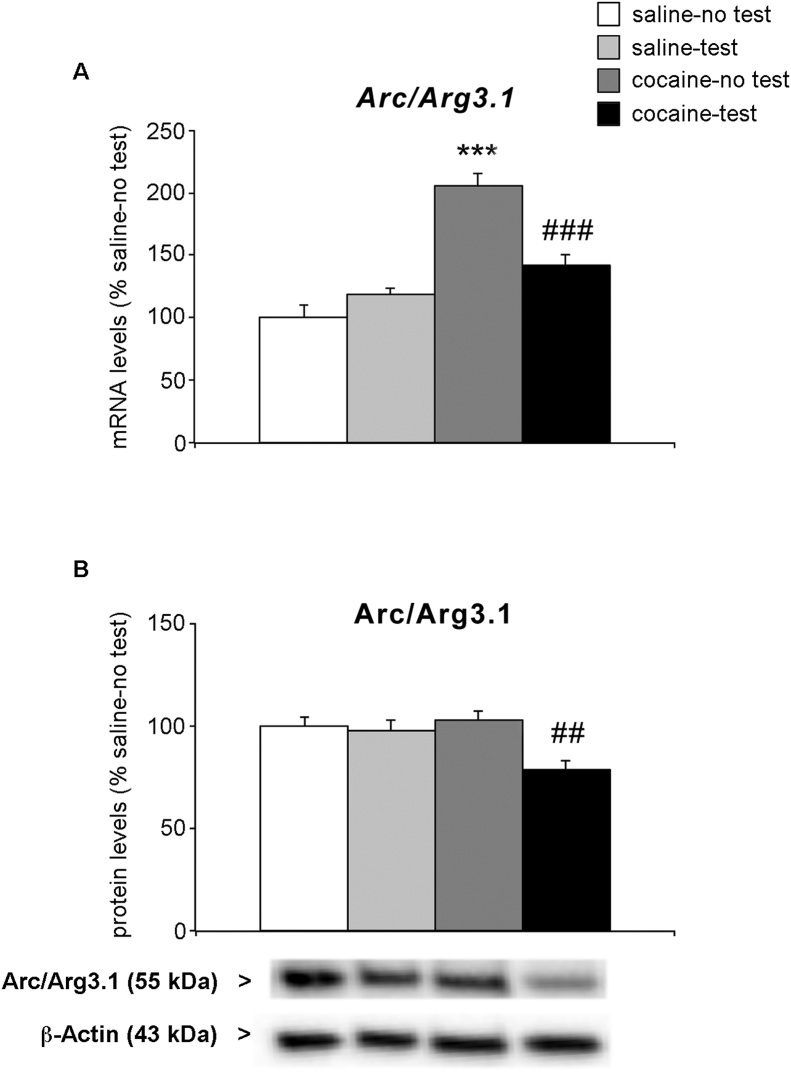
The next step was to investigate whether the observed changes could be due, at least partially, to reduced neuronal activity, which is a feature of long-term exposure to drugs of abuse (Goldstein and Volkow, 2011; Jentsch and Taylor, 1999). To this end, we evaluated the expression of the activity regulated cytoskeleton-associated protein (Arc/Arg3.1), which is considered a valid marker of neuronal activity (Bramham et al., 2008). Two-way ANOVA analysis of Arc/Arg3.1 mRNA levels revealed a significant effect of the NOR test (F(1,21) = 6.604, p = 0.0179), of the treatment (F(1,21) = 50.96, p < 0.0001) and of treatment × test interaction (F(1,21) = 21.75, p = 0.0001) (Fig. 6a). Examining individual comparisons, two weeks of withdrawal from long-term cocaine exposure during adolescence markedly increased Arc/Arg3.1 expression (+105 % vs. saline-no test, p < 0.0001; Tukey HSD test). Interestingly, NOR test induced a reduction in the Arc/Arg3.1 mRNA levels only in cocaine-treated animals (−63 % vs. cocaine-no test, p = 0.0002; Tukey HSD test). The transcriptional changes of Arc/Arg3.1 induced by the combination between cocaine exposure and the performance in the NOR test resulted in significant alterations in its protein levels, as shown in Fig. 6b. In the whole homogenate of the PrhC, we found a significant effect of NOR test (F(1,21) = 10.09, p = 0.0045) and of the treatment × test interaction (F(1,21) = 6.629, p = 0.0177). Comparing individual experimental groups, we found that, while no changes of Arc/Arg3.1 protein levels were observed in cocaine-treated rats (+3% vs. saline-no test, p = 0.9596; Tukey HSD test), its levels are significantly reduced in the PrhC of cocaine-treated rats exposed to the test (−24 % vs cocaine-no test, p = 0.0024; Tukey HSD test).
Fig. 6.
Effect of 2-week withdrawal from cocaine exposure during adolescence on Arc/Arg3.1 expression in the PrhC.
Rats were treated with cocaine or saline from PND 28 to PND 42. Two weeks after the end of treatment (PND 56), rats were tested for NOR and sacrificed immediately after the test. Arc/Arg3.1 mRNA levels of the PrhC were shown in panel a. Arc/Arg3.1 protein levels were shown in the whole homogenate, panel b, of the PrhC. Below the graph, representative immunoblots are shown for Arc/Arg3.1 and β-Actin proteins in the homogenate of PrhC.
Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of six to seven rats per group. ***p < 0.001 vs. saline-no test rats; ##p < 0.01, ###p < 0.001 vs. cocaine-no test (two-way ANOVA followed by Tukey HSD test).
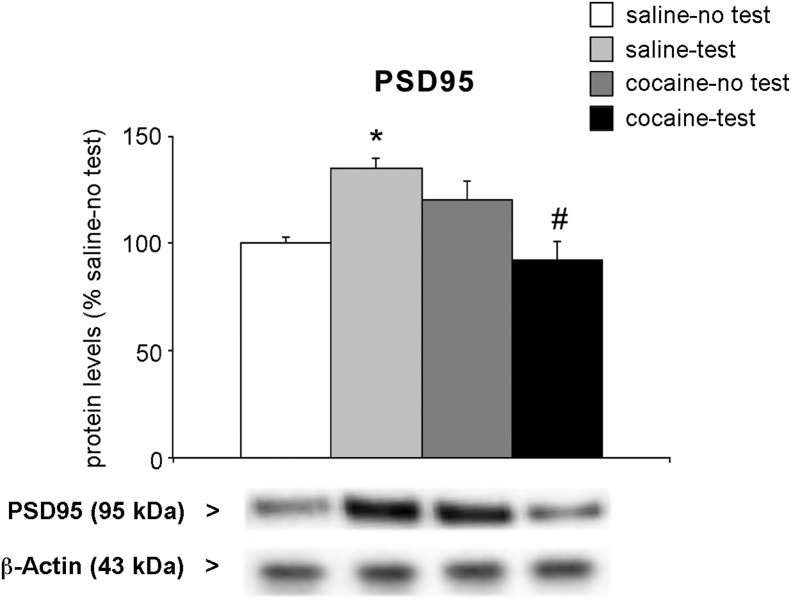
We decided to investigate the levels of post-synaptic density protein (PSD95), a well-established marker of postsynaptic density integrity and stability (Berry et al., 2018) that has also been shown to be modulated by exposure to cocaine during development (Caffino et al., 2017a, 2015b). Two-way ANOVA of PSD95 levels measured in the post-synaptic density fraction revealed a main effect of the interaction between treatment × test (F (1,21) = 19.23, p = 0.0003), whereas no effect of treatment (F (1,21) = 2.546, p = 0.1255) or NOR test (F(1,21) = 0.2037, p = 0.6564) were observed (Fig. 7). Interestingly, adolescent exposure to cocaine has altered the response to the test. In fact, while NOR test caused an increase in PSD95 levels in saline-treated rats (+35 % vs. saline-no test, p = 0.0146; Tukey HSD test), NOR test reduced PSD95 expression in cocaine-treated animals (−28 % vs. cocaine-no test, p = 0.0453; Tukey HSD test).
Fig. 7.
Effect of 2-week withdrawal from cocaine exposure during adolescence on PSD95 protein levels in the PrhC.
Rats were treated with cocaine or saline from PND 28 to PND 42. Two weeks after the end of treatment (PND 56), rats were tested for NOR and sacrificed immediately after the test. PSD95 protein levels were shown in the post-synaptic density fraction of the PRh. Below the graph, representative immunoblots are shown for PSD95 and β-Actin proteins in the PSD fraction of PrhC.
Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of six to seven rats per group. *p < 0.05 vs. saline-no test rats; #p < 0.05 vs. cocaine- no test (two-way ANOVA followed by Tukey HSD test).
In order to investigate whether there was a correlation between recognition memory and the herein investigated molecular markers in the PrhC of rats after 2 weeks of withdrawal from developmental exposure to cocaine, we performed a Pearson’s product–moment correlation analysis. In particular, Pearson’s product–moment correlation was generated to correlate DI, calculated as (novel object–familiar object exploration time)/(familiar object + novel object exploration time), with molecular results obtained from mBDNF and trkB analysis in the homogenate and mBDNF, trkB PSD95 analysis in the post synaptic density fraction. Table 1 shows a significant positive correlation between the DI and the expression of the molecular results (for detailed statistical data, please refer to Table 1).
Table 1.
Pearson’s product–moment correlation analyses between discrimination index and BDNF-related molecular targets of interest.
| total BDNF | mBDNF | trkB | mBDNF | trkB | PSD95 | ||
|---|---|---|---|---|---|---|---|
| Homogenate | Homogenate | PSD | PSD | PSD | |||
| Discrimination Index | r | 0.7037 | 0.6058 | 0.7485 | 0.7224 | 0.7117 | 0.7126 |
| P value | 0.0073** | 0.0368* | 0.0051** | 0.0053** | 0.0064** | 0.0063** |
r = pearson correlation coefficient.
p<0.05.
p<0.01.
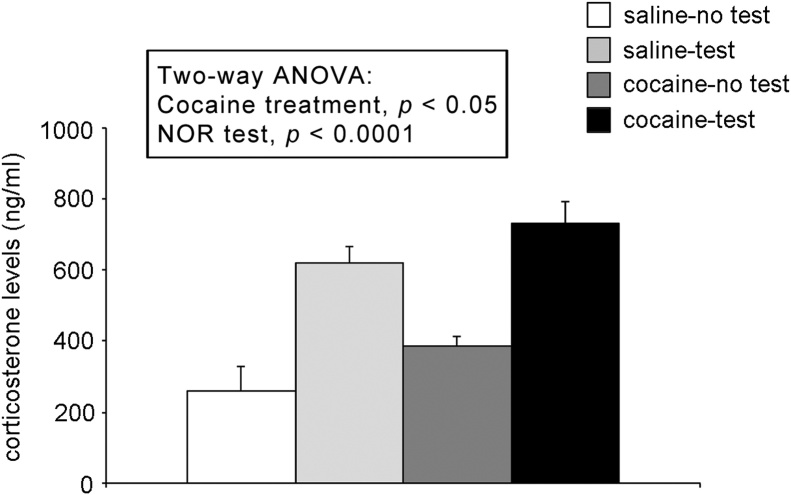
Next, we measured the circulating levels of corticosterone to investigate whether the cognitive deficit observed in cocaine-treated rats could be due to a different release of corticosterone in response to the NOR test. Two-way ANOVA revealed an effect of NOR test (F(1,21) = 41.38, p < 0.0001) and of treatment (F(1,21) = 4.75, p = 0.041), but no treatment × NOR interaction (F(1,21) = 0.019, p = 0.892, two-way ANOVA, Fig. 8).
Fig. 8.
Effect of 2-week withdrawal from cocaine exposure during adolescence on the levels of circulating corticosterone (expressed in ng/mL) measured at PND 56. Histograms represent the mean ± SEM of 6–7 rats per group.
Last, we decided to investigate the BDNF system also in adult animals. To this end, adult rats (PND 63) were exposed to cocaine for two weeks, until PND 77 and, after two weeks of abstinence, were exposed to the NOR test (PND 91). Two-way ANOVA of mBDNF in the whole homogenate of PrhC revealed a main treatment × test effect, (F(1,21) = 16.98, p = 0.0005), whereas no effect of NOR test (F(1,21) = 0.5724, p = 0.4577) or cocaine treatment (F(1,21) = 2.827, p = 0.1075) was observed (Fig. 9a). Repeated exposure to cocaine during adulthood reduced mBDNF levels (-35 % vs saline-no test, p = 0.0052; Tukey HSD test), while NOR test increased the neurotrophin levels only in cocaine-exposed rats (+29 % vs cocaine-no test, p = 0.0205; Tukey HSD test). In the post-synaptic density, two-way ANOVA of mBDNF levels showed a significant effect of treatment (F(1,19) = 6.814, p = 0.0172), a treatment × test interaction (F(1,19) = 5.295, p = 0.0329) with no effect of the NOR test (F(1,19) = 0.1613, p = 0.6925; Fig. 9b). Further intergroup subtesting revealed that, akin to the whole homogenate, repeated exposure to cocaine reduced mBDNF levels in the post-synaptic density (−56 % vs. saline-no test, p = 0.0205; Tukey HSD test). However in adult animals, it also appears that cocaine exposure has not influenced the response to the cognitive test (+17 %% vs. cocaine-test, p = 0.597; Tukey HSD test).
Fig. 9.
Effect of 2-week withdrawal from cocaine exposure during adulthood on mBDNF and trkB protein levels in the PrhC.
Rats were treated with cocaine or saline from PND 63 to PND 77. Two weeks after the end of treatment (PND 91), rats were tested for NOR and sacrificed immediately after the test. mBDNF protein levels (14 kDa) are shown in the whole homogenate (panel a) and in the post-synaptic density fraction (panel b) of the PrhC. TrkB protein levels (145 kDa) are shown in the whole homogenate (panel c) and in the post-synaptic density fraction (panel d) of the PrhC. In the upper panel, representative immunoblots are shown for mBDNF, trkB and β-Actin proteins in the homogenate and PSD of PrhC. Results are expressed as percentages of saline-treated rats not exposed to NOR (saline-no test). Histograms represent the mean ± SEM of five to seven rats per group. *p < 0.05, **p < 0.01 vs. saline-no test rats; #p < 0.05, ###p < 0.001 vs. cocaine- no test (two-way ANOVA followed by Tukey HSD test).
S = saline-no test; S/T = saline-test; C = cocaine-no test; C/T = cocaine-test.
The analysis of trkB protein levels in the whole homogenate and in the post-synaptic density of adult animals revealed a significant treatment × test interaction (homogenate: F(1,19) = 34.52, p < 0.0001, Fig. 9c; PSD: F(1,21) = 4.579, p = 0.0443; Fig. 9d). Interestingly, post-hoc comparisons of the whole homogenate data revealed that exposure to cocaine during adulthood increased trkB expression (+33 % vs. saline-no test, p = 0.0089; Tukey HSD test) and altered the response to the test. In fact, while NOR test caused an increase in trkB levels in saline-treated rats (+24 % vs. saline-no test, p = 0.0385; Tukey HSD test), NOR test reduced trkB expression in cocaine-treated animals (−49 % vs. cocaine-test, p = 0.0001; Tukey HSD test; Fig. 9c). In the post-synaptic density, cocaine exposure did not alter trkB expression (+9% vs. cocaine-test, p = 0.9285; Tukey HSD test), whereas NOR test caused an increase of trkB levels in saline-treated rats (+39 % vs. saline-no test, p = 0.0407; Tukey HSD test) and no changes were found in cocaine-treated rats (-3% vs. cocaine-no test, p = 0.9966; Tukey HSD test; Fig. 9d).
4. Discussion
The Novel Object Recognition (NOR) test, a simple behavioral test that is based on the innate curiosity of the rat to explore novelty (Bevins and Besheer, 2006), was used to investigate the cognitive abilities of rats exposed to cocaine during adolescence or adulthood. After 2 weeks of withdrawal, cocaine-treated adolescent rats explored the novel object significantly less when compared to saline-treated rats. Interestingly, adult rats exposed to the same experimental paradigm (i.e. same dosage of cocaine, duration of treatment as well as length of withdrawal) did not show any cognitive deficit in the NOR test. Our results indicate that 2-weeks withdrawal from exposure to cocaine during adolescence, but not adulthood, has impaired the response of the rat to a cognitively demanding test. Notably, the evidence that both saline- and cocaine-treated rats showed a similar increase of circulating corticosterone as well as a similar locomotor activity suggests that the cognitive performance is neither influenced by emotional- nor by motor-related factors. Indeed, we have recently shown an opposite behavioral effect in the NOR test of adolescent rats exposed to cocaine (Caffino et al., 2017a): however, significant differences such as the higher dose of cocaine (20 mg/kg vs. 5 mg/kg) and the much shorter time elapsed from the end of treatment (24 h vs. 14 days) may contribute to explain the behavioral difference observed in our previous manuscript. The present findings suggest that the duration of the drug-free period is critical for the cognitive performance in the NOR test.
In an attempt to find a mechanism that could explain, at least partially, the cognitive deficit observed in adolescent cocaine-treated rats, we investigated the expression of the neurotrophin BDNF and its dependent signaling in the PrhC since evidence exists that reduced PrhC expression of BDNF impairs recognition memory in rats (Callaghan and Kelly, 2012; Seoane et al., 2011). Analyses were undertaken both in the whole homogenate and post-synaptic density of PrhC in order to get critical information on BDNF translation and its availability at synaptic sites, respectively. Before the exposure to the cognitive test, we found that basal BDNF protein levels were markedly increased in the whole homogenate while significantly reduced in the post-synaptic density of cocaine-withdrawn rats. These data reveal a dysregulated compartimentalization of the neurotrophin as a result of cocaine treatment and indicate that, at the beginning of the familiarization phase of the NOR test, the availability of BDNF at active synaptic sites is reduced in the PrhC of cocaine-treated rats, a deficiency that may contribute to the cognitive impairment. This concept is further reinforced by the evidence that saline-treated rats exposed to the NOR test showed an increase of BDNF expression both in the whole homogenate and post-synaptic density, indicating that BDNF recruitment is critical for an optimal NOR test performance; conversely, the lack of such neurotrophin increase in both fractions of the PrhC in cocaine-treated rats suggests that repeated cocaine treatment has significantly impaired such recruitment.
Notably, the analysis of Bdnf transcription revealed an expression profile that fully overlaps with BDNF translation observed in the whole homogenate suggesting that the discrepancy in mBDNF protein expression in both fractions may be due to reduced local translation at dendrites, caused by cocaine treatment. Alternatively, the possibility exists that the increase in Bdnf mRNA and protein levels in the whole homogenate may represent a compensatory upregulation of the neurotrophin to offset the reduced local synthesis in the post-synaptic density.
The analysis of the downstream BDNF effectors revealed a similar dysregulation. In fact, drug withdrawal reduced the expression of BDNF receptor trkB, an effect that influences the response to the cognitive test: after the NOR test, trkB synaptic localization is increased in saline-, but not in cocaine-treated-rats, confirming the lack of BDNF-trkB pathway activation at active synaptic sites, since trkB can be considered an index of activation upon neurotrophin release (Saarelainen et al., 2003). Such changes were accompanied by a downregulation of activation of ERK2 in cocaine-treated rats performing the NOR test. Overall, these results indicate a dysregulation of the BDNF-ERK2 pathway, which may hold functional implications since it is known that such postsynaptic signaling is necessary for both functional and structural long-term potentiation (Harward et al., 2016). Further, the observation that Arc/Arg3.1 expression is reduced in cocaine-treated rats performing the NOR test is in line with previous evidence showing that BDNF and Arc/Arg3.1 act in concert in the recognition memory processes in the PrhC (Miranda et al., 2017).
Among the different functions of BDNF, the neurotrophin plays a critical role in synaptic pruning, maturation or maintenance of mature synapses (Gao et al., 2009) acting in concert with PSD95, which is critical for the regulation of dendritic spine density, structure and stability (Berry et al., 2018; Kim and Sheng, 2004). Of note, saline-treated rats display an increase of PSD95 expression following the test performance, which might be indicative of more synaptic connections brought about by the NOR test. Conversely, PSD95 expression is significantly reduced in cocaine-treated rats performing the test, indicating that 2-weeks withdrawal has offset the ability to promote pro-cognitive, dynamic structural adaptations in the PrhC and adding a further potential explanation for the cognitive impairment. Although correlational analyses do not necessarily indicate involvement in mechanisms, the positive within-animal correlation observed in the PrhC between the discrimination index and the expression of BDNF, its high affinity receptor and PSD95 suggests an increased dependence of cognitive deficit on molecular and structural adaptations in the PrhC of adolescent rats.
As mentioned above, adult rats exposed to cocaine did not show any deficit in the NOR test indicating intact recognition memory mechanisms. Of note, in these rats, BDNF expression was significantly reduced in both homogenate and post-synaptic density implying that, in adult rats, other mechanisms may come into play to sustain the cognitive performance. Such difference from adolescent animals is further confirmed by the fact that, in saline-treated animals, BDNF expression is not increased but trkB expression is nevertheless up-regulated, suggesting that the neurotrophin high affinity receptor might be transactivated, as previously suggested (Lee and Chao, 2001), to compensate for the BDNF deficit. These data once again highlight the difference existing between adolescent and adult rats and further point to the higher vulnerability of adolescent rat, which lacks crucial compensatory mechanisms presumably due to the fact that the cortex is still maturing during adolescence.
5. Conclusions
Our findings indicate that developmental exposure to cocaine results in impaired recognition memory associated with altered compartmentalization of BDNF in the PrhC, providing a mechanistic ground for the memory deficit. The evidence that recognition memory is not impaired in adult rats reinforces the need to expand our understanding of how exposure to psychostimulants during adolescence may cause disordered brain development, an effect that may not appear until long after drug discontinuation.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgements
This research was supported by grants from the Dipartimento delle Politiche Antidroga (Rome, Italy), through the ERANID Grant “STANDUP” awarded to FF and from MIUR Progetto Eccellenza.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100789.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Bekinschtein P., Cammarota M., Katche C., Slipczuk L., Rossato J.I., Goldin A., Izquierdo I., Medina J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. 2008 doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K.P., Nedivi E., Sciences C. Spine dynamics_ are they all the same. Neuron. 2018;96:43–55. doi: 10.1002/chem.200400090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins R.A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory.”. Nat. Protoc. 2006 doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bolla K.I., Eldreth D.A., London E.D., Kiehl K.A., Mouratidis M., Contoreggi C., Matochik J.A., Kurian V., Cadet J.L., Kimes A.S., Funderburk F.R., Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003 doi: 10.1016/S1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C.R., Worley P.F., Moore M.J., Guzowski J.F. The immediate early gene Arc/Arg3.1: regulation, mechanisms, and function. J. Neurosci. 2008 doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Granberg R., Tseng K.Y. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino L., Giannotti G., Malpighi C., Racagni G., Filip M., Fumagalli F. Long-term abstinence from developmental cocaine exposure alters Arc/Arg3.1 modulation in the rat medial prefrontal cortex. Neurotox. Res. 2014;26:299–306. doi: 10.1007/s12640-014-9472-1. [DOI] [PubMed] [Google Scholar]
- Caffino L., Calabrese F., Giannotti G., Barbon A., Verheij M.M.M., Racagni G., Fumagalli F. Stress rapidly dysregulates the glutamatergic synapse in the prefrontal cortex of cocaine-withdrawn adolescent rats. Addict. Biol. 2015;20:158–169. doi: 10.1111/adb.12089. [DOI] [PubMed] [Google Scholar]
- Caffino L., Giannotti G., Malpighi C., Racagni G., Fumagalli F. Short-term withdrawal from developmental exposure to cocaine activates the glucocorticoid receptor and alters spine dynamics. Eur. Neuropsychopharmacol. 2015;25:1832–1841. doi: 10.1016/j.euroneuro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Caffino L., Giannotti G., Mottarlini F., Racagni G., Fumagalli F. Developmental exposure to cocaine dynamically dysregulates cortical Arc/Arg3.1 modulation in response to a challenge. Neurotox. Res. 2017 doi: 10.1007/s12640-016-9683-8. [DOI] [PubMed] [Google Scholar]
- Caffino L., Giannotti G., Racagni G., Fumagalli F. A single cocaine exposure disrupts actin dynamics in the cortico-accumbal pathway of adolescent rats: modulation by a second cocaine injection. Psychopharmacol. (Berl.) 2017;234:1217–1222. doi: 10.1007/s00213-017-4559-z. [DOI] [PubMed] [Google Scholar]
- Caffino L., Giannotti G., Messa G., Mottarlini F., Fumagalli F. Repeated cocaine exposure dysregulates BDNF expression and signaling in the mesocorticolimbic pathway of the adolescent rat. World J. Biol. Psychiatry. 2018 doi: 10.1080/15622975.2018.1433328. [DOI] [PubMed] [Google Scholar]
- Caffino L., Messa G., Fumagalli F. A single cocaine administration alters dendritic spine morphology and impairs glutamate receptor synaptic retention in the medial prefrontal cortex of adolescent rats. Neuropharmacology. 2018;140:209–216. doi: 10.1016/j.neuropharm.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Caffino L., Verheij M.M.M., Que L., Guo C., Homberg J.R., Fumagalli F. Increased cocaine self-administration in rats lacking the serotonin transporter: a role for glutamatergic signaling in the habenula. Addict. Biol. 2018 doi: 10.1111/adb.12673. [DOI] [PubMed] [Google Scholar]
- Callaghan C.K., Kelly Á.M. Differential BDNF signaling in dentate gyrus and perirhinal cortex during consolidation of recognition memory in the rat. Hippocampus. 2012 doi: 10.1002/hipo.22033. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008 doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R.H., Stern J.M. Maternal stress and pituitary–adrenal manipulations during pregnancy in rats: effects on morphology and sexual behavior of male offspring. J. Comp. Physiol. Psychol. 1978;92:1074–1083. doi: 10.1037/h0077509. [DOI] [PubMed] [Google Scholar]
- Collins S.L., Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. Adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- DePoy L.M., Zimmermann K.S., Marvar P.J., Gourley S.L. Induction and blockade of adolescent cocaine-induced habits. Biol. Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F., Bedogni F., Frasca A., Di Pasquale L., Racagni G., Riva M.A. Corticostriatal up-regulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Mol. Pharmacol. 2006;70:1726–1734. doi: 10.1124/mol.106.026302. [DOI] [PubMed] [Google Scholar]
- Fumagalli F., Caffino L., Racagni G., Riva M.A. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009;19:402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Gao X., Smith G.M., Chen J. Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Giannotti G., Caffino L., Calabrese F., Racagni G., Fumagalli F. Dynamic modulation of basic Fibroblast Growth Factor (FGF-2) expression in the rat brain following repeated exposure to cocaine during adolescence. Psychopharmacol. (Berl.) 2013;225:553–560. doi: 10.1007/s00213-012-2840-8. [DOI] [PubMed] [Google Scholar]
- Giannotti G., Caffino L., Calabrese F., Racagni G., Riva M.A., Fumagalli F. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int. J. Neuropsychopharmacol. 2014;17:625–634. doi: 10.1017/S1461145713001454. [DOI] [PubMed] [Google Scholar]
- Giannotti G., Caffino L., Malpighi C., Melfi S., Racagni G., Fumagalli F. A single exposure to cocaine during development elicits regionally-selective changes in basal basic Fibroblast Growth Factor (FGF-2) gene expression and alters the trophic response to a second injection. Psychopharmacol. (Berl.) 2015;232:713–719. doi: 10.1007/s00213-014-3708-x. [DOI] [PubMed] [Google Scholar]
- Giannotti G., Caffino L., Mottarlini F., Racagni G., Fumagalli F. Region-specific effects of developmental exposure to cocaine on fibroblast growth factor-2 expression in the rat brain. Psychopharmacol. (Berl.) 2016;233:2699–2704. doi: 10.1007/s00213-016-4315-9. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011 doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley S.L., Olevska A., Warren M.S., Taylor J.R., Koleske A.J. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J. Neurosci. 2012 doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harward S.C., Hedrick N.G., Hall C.E., Parra-Bueno P., Milner T.A., Pan E., Laviv T., Hempstead B.L., Yasuda R., McNamara J.O. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016 doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch J.D., Taylor J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol. (Berl.) 1999 doi: 10.1007/PL00005483. [DOI] [PubMed] [Google Scholar]
- Kim E., Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004 doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Lee F.S., Chao M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. 2001 doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E.M., Peñasco S., Hernández M.-D., Gil A., Borcel E., Moya M., Giné E., López-Moreno J.A., Guerri C., López-Gallardo M., Rodríguez de Fonseca F. Long-term effects of intermittent adolescent alcohol exposure in male and female rats. Front. Behav. Neurosci. 2017 doi: 10.3389/fnbeh.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Bekinschtein P. Plasticity mechanisms of memory consolidation and reconsolidation in the perirhinal cortex. Neuroscience. 2018 doi: 10.1016/j.neuroscience.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Miranda M., Kent B., Facundo Morici J., Gallo F., Weisstaub N.V., Saksida L.M., Bussey T.J., Bekinschtein P. Molecular mechanisms in perirhinal cortex selectively necessary for discrimination of overlapping memories, but independent of memory persistence. eneuro. 2017 doi: 10.1523/ENEURO.0293-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Sco M.D., Reichel C.M. Chemogenetic activation of the perirhinal cortex reverses methamphetamine-induced memory deficits and reduces relapse. Learn. Mem. 2018:410–416. doi: 10.1101/lm.046797.117.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prini P., Rusconi F., Zamberletti E., Gabaglio M., Penna F., Fasano M., Battaglioli E., Parolaro D., Rubino T. Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. J. Psychiatry Neurosci. 2018 doi: 10.1503/jpn.170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Granados R., Fontán-Lozano Á., Delgado-García J.M., Carrión Á.M. From learning to forgetting: behavioral, circuitry, and molecular properties define the different functional states of the recognition memory trace. Hippocampus. 2010 doi: 10.1002/hipo.20669. [DOI] [PubMed] [Google Scholar]
- Saarelainen T., Hendolin P., Lucas G., Koponen E., Sairanen M., MacDonald E., Agerman K., Haapasalo A., Nawa H., Aloyz R., Ernfors P., Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003 doi: 10.1523/JNEUROSCI.23-01-00349.2003. https://doi.org/23/1/349 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A., Tinsley C.J., Brown M.W. Interfering with perirhinal brain-derived neurotrophic factor expression impairs recognition memory in rats. Hippocampus. 2011 doi: 10.1002/hipo.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000 doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Torregrossa M.M., Corlett P.R., Taylor J.R. Aberrant learning and memory in addiction. Neurobiol. Learn. Mem. 2011 doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A.J., Perales J.C., Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict. Behav. 2007 doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Winters B.D. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 2004 doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.