“These are dark times, there is no denying. Our world has perhaps faced no greater threat than it does today.” This iconic quote from an acclaimed J. K. Rowling novel series (39) resembles the current worldwide fight against COVID-19. COVID-19 (alias 2019-nCov), a novel disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in December 2019 from Wuhan, China, and has since spread like wildfire to more than 200 countries and territories. On January 30, 2020, the outbreak was declared a Public Health Emergency of International Concern. As of April 6, 2020, there are over 1.2 million confirmed cases worldwide with near 68,000 associated deaths (47). In the United States alone, 330,891 positive cases and 8,910 mortalities have been reported to date (8). It is petrifying that the number of confirmed cases and deaths day-to-day are continuously escalating, which foreshadows that SARS-CoV-2 is not going down without a fight. This prompts a critical question: What existing or innovative measures need to be taken to win the COVID-19 battle? As one strategy, society has been asked to engineer a “social vaccination,” which involves restricting social gatherings, minimizing public appearances, and implementing a “rule-of-thumb” to keep at least 6 feet apart from others. This tactic is appropriate as SARS-CoV-2 is extremely contagious by transmitting via human-to-human respiratory droplets; intriguingly, it has been demonstrated that the virus can be found in the feces, suggesting that the fecal route may be another mode of transmission (18, 46). Moreover, many individuals may have been infected with SARS-CoV-2 but do not exhibit typical symptoms, making those folks potential asymptomatic carriers (28). For others infected with SARS-CoV-2, they can exhibit symptoms of acute respiratory disease or pneumonia (28).
Clinicians and researchers are hard at work to uncover therapeutics to treat COVID-19. This includes targeting the receptor binding-domain on the spike (S) protein layer on the “corona” or halo of SARS-CoV-2, which mediates virus fusion and entry into host epithelial cells lining mucosal surfaces such as the lungs and intestine (35, 43, 45, 48). Because angiotensin converting enzyme 2 (ACE2) is the receptor for the S protein to mediate viral invasion, soluble ACE2 might be a potential therapeutic, which is supported by previous reports demonstrating that it can inhibit infection of the SARS-CoV-2 relative, severe acute respiratory syndrome coronavirus (SARS-CoV) (5, 42). In a similar manner, there is the concept of repurposing known antiviral agents (29) and redirecting existing antibiotics that were originally designed against bacterial infections (i.e., teicoplanin, antibiotic that treats Staphylococcal infections) (3) to thwart SARS-CoV-2. One such agent that recently gained substantial societal and media attention is the antimalarial drug hydroxychloroquine, which has been shown to inhibit SARS-CoV-2 infection in in vitro settings (30) and reduce viral load, in combination with azithromycin, for COVID-19 patients in an open-label nonrandomized clinical trial (16). However, it must be emphasized that the therapeutic potential of hydroxychloroquine is still controversial and begs for further investigation. Aside from repurposing existing therapies, there is an increasing demand for immunotherapy, driven by immunoinformatics and comparison homology sequencing with SARS-CoV to narrow down epitopes as potential candidates to design a peptide vaccine against SARS-CoV-2 (1, 4, 7, 19). Another approach that has been proposed is to passively immunize newly infected patients with IgG antibodies collected from patients that have recovered from COVID-19 (24).
Unfortunately, for the time being, there is no effective vaccine or alternative treatment available for COVID-19. Despite the ongoing efforts, we must be aware of the potential limitations and consequences of some current conventional strategies to fight this pathogen. For one, would conventional approaches be able to keep up with SARS-CoV-2, which is rapidly evolving and acquiring new single nucleotide mutations (33) that allow it to escape antiviral drugs? It is imperative to also acknowledge that there is no clinical, effective antiviral treatment against coronaviruses, like SARS-CoV and MERS-CoV (12), which further predicts that antiviral approaches may not be the most viable against SARS-CoV-2 to treat COVID-19 disease. There is also a high risk of antibody-dependent enhancement, which can assist SARS-CoV-2 virulence through noncanonical viral-receptor-dependent pathways. Prior studies on other coronaviruses inform us that upon antibody-based drug therapy, the S protein on coronaviruses can undergo a conformational change and enter host cells through the Fc region of IgG (33). Such pitfalls inherent in conventional treatments argue in favor of consideration for more novel “outside the box,” nonconventional therapies to abate COVID-19.
The fact that many people infected with SARS-CoV-2 will clear the virus without even developing symptoms suggests that the immune system may hold the key to defeat this virus. While surely this involves adaptive immunity and suggests vaccination will play a central role in ameliorating this pandemic, we would like to propose harnessing innate immunity as a potential approach to immediately combat COVID-19. Specifically, we suggest immunomodulation through activation of the innate immune sensor toll-like receptor 5 (TLR5), as one innovative approach to fight COVID-19. TLR5 is an extracellular pattern recognition receptor that recognizes flagellin, a structural protein of flagellum found in motile Gram-positive and Gram-negative bacteria (21). We hypothesize that flagellin could act as a trojan horse “danger” signal, which favorably tricks” the host into thinking that immune responses are required to subdue a “bacterial” infection but instead triggers antiviral responses to strike SARS-CoV-2. In one of our recent studies, we serendipitously discovered that flagellin-mediated TLR5 activation on dendritic cells induces interleukin (IL)-22, whereupon this cytokine production was able to immediately eliminate rotavirus (RV) infection in immune-sufficient and immunocompromised mice (50). We discovered that flagellin also elicited NLR family CARD domain containing 4 (NLRC4)-dependent IL-18 production to promote RV clearance (50). It is noteworthy that this effective and efficient antiviral response of flagellin was independent of interferon (IFN) responses (50). Considering that coronaviruses are capable of hijacking type I IFN antiviral responses through structural and nonstructural proteins (9), utilizing the flagellin-TLR5 axis could provide an effective loophole to target and eliminate SARS-CoV-2. Ironically, it was recently found that flagellin is also capable of inducing TLR5-mediated production of IFN-β and subsequent activation of type I IFN responses (26), which presents a potential avenue to restore antiviral immune defenses that are impaired during coronavirus infections.
Aside from our studies, there are multiple reports that delineate the antiviral capability of flagellin against other infections, particularly for viruses that, like SARS-CoV-2, replicate in epithelial cells. For one, prophylactic and therapeutic flagellin administration reduces viral load in the lungs of mice infected with influenza A virus (17). In other investigations, the coadministration of flagellin with inactivated influenza virus induced influenza-specific IgA and IgG titers, which highlights the potential of flagellin as a potent mucosal adjuvant (13, 41). Similarly, oral administration of flagellin with the trivalent inactivated influenza vaccine was critically important for antibody production, where titers were significantly reduced in TLR5-deficient mice (34). Besides promoting antibody production, flagellin is important for the maturation of lung dendritic cells (14), inhibition of epithelial apoptosis (44), production of IL-17C cells (36), and induction of cathelicidin-dependent antimicrobial responses (49), all of which coincide with the essential nature of flagellin’s mucosal adjuvant activity. It is noteworthy that flagellin was found to be very effective in activating neonatal lung antigen-presenting cells (40). Furthermore, flagellin-TLR5 activation was observed to protect mice from invasive pneumonia through early clearance of Pseudomonas aeruginosa in respiratory epithelial cells (2, 32). These studies support the concept that flagellin or pharmacological TLR5 agonists (i.e., entolimod, currently under clinical trials) can be redirected as a potent therapeutic to eliminate SARS-CoV-2.
In company with immunomodulation of TLR5, we also posit the idea of modulating neutrophil antimicrobial responses through use of host-derived nucleases as a nonconventional method to combat COVID-19. COVID-19 patients are reported to have elevated neutrophil levels (31, 38). Hypothetically, it is plausible that elevated neutrophil abundance is associated with increased reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), both of which are potent antimicrobial defenses; yet inappropriate levels of these neutrophil-derived products can inflict tissue damage. This tissue damage could contribute to a “proinflammatory cytokine storm” initiated by macrophages and monocytes infiltrating the damaged tissue (15, 23, 37). This inflammatory response occurring at a late stage of infection can cause aggravated respiratory/cardiovascular problems. As a countermeasure, deoxyribonuclease I (DNase I) can work to promote the clearance of overproduced NETs and, thus, minimize unwarranted neutrophil-mediated collateral damage (25). This concept is supported by the report that DNase I treatment reduced NET-induced airway obstruction during respiratory syncytial virus infection (10). Furthermore, DNase I has been proven to reduce the human hepatitis B virus (HBV) genome copy number through catabolism of the DNA genome (20).
While modulation of TLR5 signaling via flagellin and regulation of neutrophilic immune responses via DNase I represent new avenues to fight against COVID-19, there are some potential risks as expected with any therapeutic approach. Exposure of flagellin has been reported to support lentiviral pseudovirus attachment on lung epithelial cells via TLR5 activation of NF-κB signaling (6). Hence, it is plausible that TLR5 signaling could advance SARS-CoV-2 virulence, and TLR5-mediated proinflammatory responses could inflict unwarranted damage. Thus, alternatively, combinations of IL-18 and IL-22, which mimic the antiviral efficacy of flagellin but lack its potential toxicity, may be a possible therapeutic option. In any case, the lack of immune responses exhibited in the early stage of coronavirus infections may suggest that TLR5 agonists could be most valuable in the early phase of infection, whereas the media-hyped chloroquine could be most useful against late-stage infection since chloroquine is an inhibitor of nucleic acid recognizing TLR-mediated inflammatory responses (27). Regardless, the benefits or costs of TLR5 activation would still expand the mechanistic knowledge on COVID-19 pathogenesis and direct us toward appropriate therapeutic targets at the pertinent viral stage. In line with TLR5, degradation of NETs through host nucleases might have potential risks, as such intervention has been reported as a fuel source for certain pathogens, such as Hemophilus influenzae (11). Hence, if SARS-CoV-2 utilizes a similar strategy, this could also advance its pathogenesis. However, no article to date has associated coronaviruses with the utilization of NET degradation via host nucleases for virulence; therefore, studying this relationship could provide one of two novel findings: 1) an unexplored, yet new, mechanism for coronavirus virulence or 2) a potential therapeutic (i.e., DNase I) to defeat COVID-19 through regulation of aberrant innate immune responses.
Considering that SARS-CoV-2 infection exhibits an “early stage” (lack of host antiviral immune responses) and a late stage (cytokine storm), the appropriate time to administer either flagellin or DNase I is critical since they are targeting different aspects of viral infection. There are also other criteria to address whether a therapeutic “significantly increases the risk (or decreases the acceptability of the risk) associated with the use of a drug product,” such as route of administration, dosage level, and dosage form (22). Additionally, it is critical to determine the half-life and potential drug-to-drug interaction if giving a combinatorial therapy. Based on our previous studies of flagellin protection against rotavirus infection, we hypothesize that flagellin would be most potent within the first 48 h of infection, as this will allow for early intervention to boost antiviral responses and block virulence. Comparatively, DNase I would be most effective toward the late stage of infection (after 72 h of first symptom appearance) to minimize neutrophil/NET-mediated pulmonary and cardiovascular damage. In general, many unanswered questions need to be tested in animal models that reflect human disease.
Overall, our “2 cents” to fight against the enemy of SARS-CoV-2 and, thus, COVID-19 disease, is immunomodulation of hijacked host innate immunity (as summarized in Fig. 1). It is important to acknowledge that eliciting TLR5 signaling or restricting inappropriate neutrophil responses are only two options out of the multitude of possibilities that can be directed as nonconventional methods to alleviate COVID-19. We hope that this editorial triggers alternative outlook therapies in the COVID-19 battle, which could later be used as platforms for preventing existing and novel viral infections. Stay safe and stay healthy.
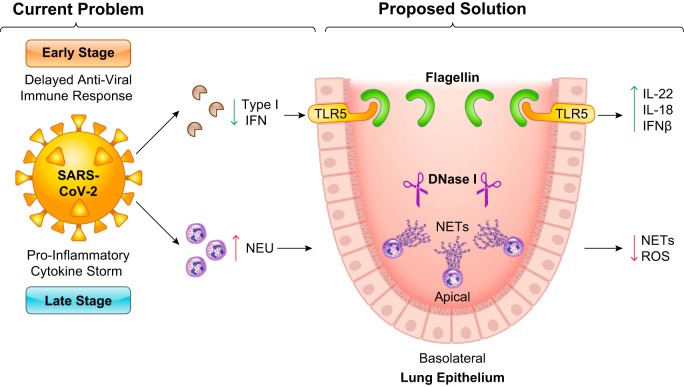
Fig. 1.
Innate immunity may be the key to defeat severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In the early stage of infection, coronaviruses are capable of inhibiting host type I interferon (IFN) antiviral immune defenses. We propose to counteract this by utilizing flagellin to activate Toll-like receptor 5 (TLR5). TLR5 can induce the production of cytokines (i.e., IL-22, IL-18) and IFN-β, which may restore the impaired immune responses. In the late stage of infection, there is a proinflammatory cytokine storm initiated by innate immune cells like neutrophils (NEU). Theoretically, this can result in inappropriate levels of neutrophil extracellular traps (NETs) and reactive oxygen species (ROS) that cause unwarranted collateral damage. Deoxyribonuclease I (DNase I)-mediated degradation of NETs could provide a therapeutic avenue to suppress excess injury.
GRANTS
M. Vijay-Kumar is supported by National Institutes of Health (NIH) R01Grant CA-219144. B. Joe is supported by NIH R01 Grant HL-143082. S. Chattopadhyay is supported by NIH Grant AA-026017, the Medical Research Society, and the University of Toledo College of Medicine and Life Sciences. P. Saha is supported by the Crohn's and Colitis Foundation Research Fellowship Award 522820.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.G. and P.S. prepared figure; R.M.G. and M.V.-K. drafted manuscript; R.M.G., P.S., B.S.Y., S.C., A.T.G., B.J., and M.V.-K. edited and revised manuscript; R.M.G., P.S., B.S.Y., S.C., A.T.G., B.J., and M.V.-K. approved final version of manuscript.
REFERENCES
- 1.Ahmed SF, Quadeer AA, McKay MR. Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses 12: 254, 2020. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anas AA, van Lieshout MH, Claushuis TA, de Vos AF, Florquin S, de Boer OJ, Hou B, Van’t Veer C, van der Poll T. Lung epithelial MyD88 drives early pulmonary clearance of Pseudomonas aeruginosa by a flagellin dependent mechanism. Am J Physiol Lung Cell Mol Physiol 311: L219–L228, 2016. doi: 10.1152/ajplung.00078.2016. [DOI] [PubMed] [Google Scholar]
- 3.Baron SA, Devaux C, Colson P, Raoult D, Rolain JM. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents: 105944, 2020. doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J Med Virol 92: 495–500, 2020. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 134: 543–545, 2020. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 6.Benedikz EK, Bailey D, Cook CNL, Gonçalves-Carneiro D, Buckner MMC, Blair JMA, Wells TJ, Fletcher NF, Goodall M, Flores-Langarica A, Kingsley RA, Madsen J, Teeling J, Johnston SL, MacLennan CA, Balfe P, Henderson IR, Piddock LJV, Cunningham AF, McKeating JA. Bacterial flagellin promotes viral entry via an NF-kB and Toll Like Receptor 5 dependent pathway. Sci Rep 9: 7903, 2019. doi: 10.1038/s41598-019-44263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol 92: 618–631, 2020. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Center for Disease Control and Prevention Coronavirus Disease 2019. (COVID-19): Cases in U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [April 6, 2020].
- 9.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529–539, 2017. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortjens B, de Jong R, Bonsing JG, van Woensel JBM, Antonis AFG, Bem RA. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 73: 578–580, 2018. doi: 10.1136/thoraxjnl-2017-210289. [DOI] [PubMed] [Google Scholar]
- 11.de Buhr N, Bonilla MC, Pfeiffer J, Akhdar S, Schwennen C, Kahl BC, Waldmann KH, Valentin-Weigand P, Hennig-Pauka I, von Köckritz-Blickwede M. Degraded neutrophil extracellular traps promote the growth of Actinobacillus pleuropneumoniae. Cell Death Dis 10: 657, 2019. doi: 10.1038/s41419-019-1895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14: 523–534, 2016. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faham A, Altin JG. Antigen-containing liposomes engrafted with flagellin-related peptides are effective vaccines that can induce potent antitumor immunity and immunotherapeutic effect. J Immunol 185: 1744–1754, 2010. doi: 10.4049/jimmunol.1000027. [DOI] [PubMed] [Google Scholar]
- 14.Fougeron D, Van Maele L, Songhet P, Cayet D, Hot D, Van Rooijen N, Mollenkopf HJ, Hardt WD, Benecke AG, Sirard JC. Indirect Toll-like receptor 5-mediated activation of conventional dendritic cells promotes the mucosal adjuvant activity of flagellin in the respiratory tract. Vaccine 33: 3331–3341, 2015. doi: 10.1016/j.vaccine.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect 9: 558–570, 2020. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents: 105949, 2020. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Georgel AF, Cayet D, Pizzorno A, Rosa-Calatrava M, Paget C, Sencio V, Dubuisson J, Trottein F, Sirard JC, Carnoy C. Toll-like receptor 5 agonist flagellin reduces influenza A virus replication independently of type I interferon and interleukin 22 and improves antiviral efficacy of oseltamivir. Antiviral Res 168: 28–35, 2019. doi: 10.1016/j.antiviral.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, Fricchione MJ, Chugh RK, Walblay KA, Ahmed NS, Stoecker WC, Hasan NF, Burdsall DP, Reese HE, Wallace M, Wang C, Moeller D, Korpics J, Novosad SA, Benowitz I, Jacobs MW, Dasari VS, Patel MT, Kauerauf J, Charles EM, Ezike NO, Chu V, Midgley CM, Rolfes MA, Gerber SI, Lu X, Lindstrom S, Verani JR, Layden JE; Illinois COVID-19 Investigation Team . First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395: 1137–1144, 2020. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 27: 671–680.e2, 2020. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallez C, Li X, Suspène R, Thiers V, Bouzidi MS, M Dorobantu C, Lucansky V, Wain-Hobson S, Gaudin R, Vartanian JP. Hypoxia-induced human deoxyribonuclease I is a cellular restriction factor of hepatitis B virus. Nat Microbiol 4: 1196–1207, 2019. doi: 10.1038/s41564-019-0405-x. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 22.Holbein ME. Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators. J Investig Med 57: 688–694, 2009. doi: 10.2310/JIM.0b013e3181afdb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawhara S. Could Intravenous Immunoglobulin Collected from Recovered Coronavirus Patients Protect against COVID-19 and Strengthen the Immune System of New Patients? Int J Mol Sci 21: 2272, 2020. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, Kluge S, Panzer U, Mizuta R, Mannherz HG, Kitamura D, Herrmann M, Napirei M, Fuchs TA. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358: 1202–1206, 2017. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Park A, Huh JW, You G, Jung DJ, Song M, Lee HK, Kim YM. Flagellin-Stimulated Production of Interferon-β Promotes Anti-Flagellin IgG2c and IgA Responses. Mol Cells 43: 251–263, 2020. doi: 10.14348/molcells.2020.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 186: 4794–4804, 2011. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 28.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect, 2020. [In press]. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 19: 149–150, 2020. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6: 16, 2020. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, and Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020. [In press]. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muñoz-Wolf N, Rial A, Fougeron D, Tabareau J, Sirard JC, Chabalgoity JA. Sublingual flagellin protects against acute pneumococcal pneumonia in a TLR5-dependent and NLRC4-independent fashion. Future Microbiol 11: 1167–1177, 2016. doi: 10.2217/fmb-2016-0045. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TM, Zhang Y, Pandolfi PP. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res 30: 189–190, 2020. doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41: 478–492, 2014. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11: 1620, 2020. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeifer P, Voss M, Wonnenberg B, Hellberg J, Seiler F, Lepper PM, Bischoff M, Langer F, Schäfers HJ, Menger MD, Bals R, Beisswenger C. IL-17C is a mediator of respiratory epithelial innate immune response. Am J Respir Cell Mol Biol 48: 415–421, 2013. doi: 10.1165/rcmb.2012-0232OC. [DOI] [PubMed] [Google Scholar]
- 37.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38: 1–9, 2020. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 38.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis: ciaa248, 2020. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowling JK. Harry Potter and the Deathly Hallows. United Kingdom: Bloomsbury Publishing, 2007. [Google Scholar]
- 40.Sharma P, Levy O, Dowling DJ. The TLR5 Agonist Flagellin Shapes Phenotypical and Functional Activation of Lung Mucosal Antigen Presenting Cells in Neonatal Mice. Front Immunol 11: 171, 2020. doi: 10.3389/fimmu.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skountzou I, Martin MP, Wang B, Ye L, Koutsonanos D, Weldon W, Jacob J, Compans RW. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine 28: 4103–4112, 2010. doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun P, Lu X, Xu C, Wang Y, Sun W, Xi J. CD-sACE2 Inclusion Compounds: An Effective Treatment for Corona Virus Disease 2019 (COVID-19). J Med Virol, 2020. [In press]. doi: 10.1002/jmv.25804. [DOI] [PubMed] [Google Scholar]
- 43.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol, 2020. [In press]. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol 169: 1686–1700, 2006. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181: 281–292.e6, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA, 2020. [In press]. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization Coronavirus disease 2019. (COVID-19): Situation Report-77 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200406-sitrep-77-covid-19.pdf?sfvrsn=21d1e632_2 [April 6, 2020].
- 48.Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, Ying T, Liu S, Shi Z, Jiang S, Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol, 2020. [In press]. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol 185: 1142–1149, 2010. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, Dermody TS, Ouyang W, Williams IR, Vijay-Kumar M, Gewirtz AT. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 346: 861–865, 2014. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]