1. Introduction
Glycogen storage diseases (GSDs) are rare disorders affecting the metabolism of glycogen that takes place mainly in liver (hepatic GSDs) and in muscle (muscle GSDs). More than 14 disorders are known [1] that affect the mobilization of energy stores in liver or muscle. In certain GSDs, such as GSD type III (amylo-1,6-glucosidase (EC 3.2.1.33; EC 2.4.1.25) deficiency, Cori-Forbes disease, OMIM #232400), both tissues are affected.
Among these disorders, GSD type II (acid α-glucosidase (EC 3.2.1.20) deficiency, Pompe disease, PD, OMIM #232300) is the only lysosomal GSD, resulting in lysosomal glycogen accumulation [2] in many tissues, but mainly cardiac and skeletal muscle. The enzyme is encoded by the GAA gene (localized in 17q25.3). This disorder is transmitted as an autosomal recessive trait and classified as two subtypes, based on the age of presentation, rate of progression, and the presence of cardiac disease. The classic infantile form (infantile-onset PD, IOPD) was first described clinically in 1932; signs are present before 6 months of age and include cardiomegaly, and often severe hypotonia. Without treatment, death usually occurs within 2 years [3]. In some cases (non-classic IOPD), the onset of symptoms is delayed by a few months. Late-onset PD (LOPD) may occur in late infancy, childhood (juvenile form) or adulthood, and is typically associated with a residual enzyme activity in cultured skin fibroblasts and muscle. Clinical signs may be moderate although the heart is usually spared. Patients exhibit a progressive limb-girdle myopathy, and a life-threatening pulmonary insufficiency that occurs in the later stages of the disease [4].
The prevalence of LOPD has been reported to be 1/69,927 in France [5]. The prevalence of IOPD is about 1/15,000 to 1/138,000, but is more frequent in some populations such as the Buschinengue ethnic group from French Guiana, which has a predicted incidence of 1 in 1727 [6].
The diagnosis of PD is based on enzymatic activity measurement in various tissues such as dried blood spots, leuco/lymphocytes, cultured skin fibroblasts and muscle. However, the presence of pseudodeficiency alleles pose a challenge, as these result in in vitro low enzyme activity, but not in substrate accumulation in vivo [7,8].
Classic IOPD is associated with two ‘severe’ pathogenic variants on the GAA gene. Among patients with LOPD, 68–90% of Caucasian patients are reported to carry the leaky c.-32-13T>G splice variant (IVS1-13T>G in intron 1 [[9], [10], [11]]) mainly in a heterozygous state, associated with a second pathogenic variant on the GAA gene. This “protective” variant affects a splicing site, allowing the synthesis of a small amount of functional enzyme and is always associated with LOPD [5]. Thus, in adults, the severity of the disease is correlated with the severity of the second pathogenic variant. Missense variants are generally associated with a less severe phenotype than other types of variants (stop codon, variants affecting consensus splice sites, deletions, insertions), unless they affect the catalytic site.
Several therapies have been developed in order to correct the enzyme deficiency. Enzyme replacement therapy (ERT, Lumizyme® in the USA, also named Myozyme® in other countries, Sanofi-Genzyme Corporation, Cambridge, MA) was first approved in 2006. In IOPD, the ERT treatment improves the cardiomyopathy, resulting in a much better outcome compared with untreated patients [12,13]. The patients who express “cross-reacting immune material” (CRIM-positive) generally have a better clinical outcome than those who are CRIM-negative. For CRIM negative patient immunomodulative treatment is often used in conjunction with ERT to suppress the immune response against the infused protein [14]. Most patients with LOPD benefit from ERT, which improves walking distance and stabilizes the pulmonary function [15]. New treatments such as NeoGAA® (Sanofi Genzyme Corporation, Cambridge, MA, USA) or combination of ERT with N-deoxynojirimicine (Miglustat®, Idorsia Pharmaceuticals Ltd., Allschwil, Switzerland) are under investigation [16].
There is a need for biomarkers that can be useful for the diagnosis and the follow-up of these patients. Clinical (heart volume) and biological (muscle glycogen content, plasma CPK, ALT, AST levels) parameters have been reported as biomarkers, but they are either invasive or nonspecific.
In 1974, Hallgren et al. [17] discovered large quantities of a urinary tetrahexose oligosaccharide (Glcα1-6Glcα1-4Glcα1-4Glc) called tetraglucose (Glc4). This tetrasaccharide, resulting from the amylolytic degradation of glycogen, is present in small quantities in normal urine, but in larger quantities in the urine of pregnant women [18]. It is also reported to be increased in several pathological conditions associated with increased storage or turnover of glycogen: GSD II, GSD III (amylo-1,6-deficiency, OMIM #232400) and GSD VI (liver phosphorylase deficiency, Hers disease, OMIM #232700), but also Duchenne muscular dystrophy, muscle trauma, pancreatitis and cancers [19,21]. Although not specific, Glc4 is thus a candidate biomarker for GSD II diagnosis and monitoring. Glc4 is reported to be well correlated with muscular glycogen content in quadriceps biopsies of patients with IOPD [22]. Urinary Glc4 normalized to creatinine concentration is helpful for the diagnosis and follow-up of patients with IOPD [22]. One study showed that urinary Glc4 has high sensitivity (94%) and specificity (84%) for PD [21] and allows differentiation PD from pseudo-PD in symptomatic patients.
A number of methods have been developed for Glc4 analysis, including one method that analyzed butyl-4-aminobenzoate (BAB) derivative of Glc4 using reverse phase HPLC with UV detection [23]. Manwaring et al. [7] reported in 2012 a HPLC method with electrochemical detection; with this method, the results of control patients were below the detection limit. The advent of tandem mass spectrometry (MS/MS) allowed Glc4 analysis to become more easily accessible to laboratories involved in the diagnosis and follow-up of GSDs. Glc4 (or its derivatives) analysis by LC-MS/MS methods are susceptible to potential interference from isomers, such as maltotetraose (M4), which share the same mass transitions as Glc4. However, one study reported that Glc4 is the major component of the total urine hexose tetrasaccharides (>92%) [24]. Thus MS/MS methods that do not separate hexose tetrasaccharide isomers are adequate for urinary Glc4 analysis. Different methods have been reported, either with 1-phenyl-3-methyl-5-pyrazolone [25] or BAB [20] derivatization of Glc4, followed by LC-MS/MS measurement. The method in use in our lab is that described by Young et al. (BAB derivative [20,22]) who developed a HPLC-MS/MS assay with a 13C6-Glc4 internal standard (IS) synthesized in-house using an enzymatic method [20]. Although a stable-isotope IS is now commercially available (TRC, Toronto, Canada), very few reports about Glc4 measurement have been published, most of them concerning the diagnosis and ERT monitoring of patients with IOPD. In 2012, Sluiter et al. [26] published a MS/MS method without derivatization, using acarbose as IS.
We report here our experience in measuring urinary Glc4 by LC-MS/MS over the period 2006–2017 in GSD II patients (IOPD and LOPD, before and after ERT), as well as in GSD III patients, and controls.
2. Materials and methods
2.1. Reagents
Glc4 (d-glucopyranosyl maltotriose, Glcα1-6Glcα1-4Glcα1-4Glc) was from TRC, Toronto, Canada). 13C6 hexose tetrasaccharide internal standard (6C13-labeled mixture of Glc4 and isomaltose-isomaltose, Glcα1-6Glcα1-4Glcα1-6Glc, IS) was a generous gift of Dr. S. Young (Duke Biochemical Genetics Laboratory, Durham, NC, USA). Sodium cyanoborohydride (NaBH3CN) and butyl-4-aminobenzoate (BAB) were from Sigma-Aldrich® (Saint Quentin-Fallavier, France). Acetonitrile (ACN) for HPLC, methanol and acetic acid were from Carlo-Erba (Val de Reuil, France). HPLC grade water was from Ecotainer Aqua B.Braun© (Melsungen, Germany). Bond Elut C18 50 mg, 1 mL solid phase extraction (SPE) columns were used with a Vac Elut 20 system, all from Varian (Agilent, Santa Clara, CA). Reverse phase LC was performed on a Uptisphere 3BioP2 C18 column (100 × 2.1 mm, 3 μm, ref. UP3BP2#10QS, Interchrom©, Interchim, Montluçon, France).
2.2. Patients
Between 2006 and 2017, nearly 2700 urine samples were referred to our laboratory for Glc4 measurement (Table 1 suppl).
Table 1.
Genotype of the untreated GSD II patients. For details, see text § 2.2.2.
| c.-32-13T>G + del ex 18 |
c.-32-13T>G + known severe |
c.-32-13T>G + severe missense |
c.-32-13T>G + other |
Without c.-32-13T>G |
Complete genotype |
Only one variant |
Genotype unknown |
Total |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1a | Group 1b | Group 1c | Group 1d | Group 2 | ||||||
| IOPD (20%) | ||||||||||
| Classic | 33a | 32 | 1 | 5 | 38 | |||||
| Non-Classic | 8 | 8 | 2 | 10 | ||||||
| LOPD (80%) | ||||||||||
| Juvenile | 3 | 1 | 1 | 1 | 10 | 16 | 1 | 17 | ||
| Adult | 22 | 59 | 27 | 46b | 17c | 165 | 6 | 4 | 175 | |
| IOPD + LOPD | 221 | 240 | ||||||||
Including 1 patient with only del ex 18 identified.
Including 4 patients with only the c.-32-13T>G variant identified.
Including 2 patients with only one variant identified (del ex 18 or c.1193delT).
2.2.1. Control patients
Urine samples from 195 patients for which the GSD II diagnosis was excluded because of a normal α-glucosidase activity or for which urinary screening of other metabolic diseases was negative were used as controls for establishing age-specific reference ranges (Table 1 suppl).
2.2.2. GSD II patients
All GSD II patients (n = 240, classic IOPD n = 38, non-classic IOPD n = 10; LOPD juvenile form with onset of symptoms <16 years n = 17, adult form with onset of symptoms ≥ 16 years n = 175) were diagnosed on the basis of a demonstrated lysosomal acid α-glucosidase deficiency using classic fluorimetric methods either in dried blood spots [27] or in lymphocytes/fibroblasts/muscle biopsy [28,29]. Two GAA gene variants were identified in 221 patients (Table 1); in 9 other cases, only one GAA alteration could be identified. All variants, present in the Pompe Disease Mutation Database (http://www.pompevariantdatabase.nl/pompe_mutations_list.php?orderby=aMut_ID1, 2019, September 24th) were reported as pathogenic (very severe, severe, less severe, potentially less severe, potentially mild [30]) except two (one genetic variant of unknown significance (GVUS), one not pathogenic). Eight GAA variants were absent from this database. In 10 cases, the genotype was unknown.
Patients with cardiac involvement were classified as classic IOPD (n = 48) when the symptoms started before the age of 6 months (n = 38, 33 genotyped), and non-classic IOPD (late-infantile) when onset was after 6 months of age (n = 10, 8 genotyped). The CRIM status was not available for a majority of patients; thus this information was not included in our study.
LOPD cohort (n = 192; juvenile: n = 17, 16 genotyped; adult: n = 175, 171 genotyped) were divided into 5 groups, according to the presence of the c.-32-13T>G variant, and the severity of the second gene variant identified. This was established according to the August 2019 edition of the Pompe Disease Mutation Database. Several missense variants that were also present in patients with classic IOPD of our cohort were grouped separately. The subgroups are as follows (Table 1):
-
i)
Group 1a: patients with the c.-32-13T>G variant in association with the very severe c.2481+102_2646+31del variant (del ex 18, p.Gly828_Asn882del, n = 25);
-
ii)
Group 1b: patients with the c.-32-13T>G variant in association with a known severe/very severe variant (including stop codon, insertion and deletion leading to frame shift, variants affecting consensus splice sites, n = 60);
-
iii)
Group 1c: patients with the c.-32-13T>G variant in association with a severe missense variant (n = 28). This group included p.Gly219Arg (n = 7) and p.Arg224Trp (n = 2) missense variants, that were also found in a homozygous state in 2 patients with classic IOPD in our cohort. It also included five other missense variants (p.Cys103Gly, p.Asp489Asn, p.Arg600His, p.Gly643Arg and p.Arg725Trp) identified in one or more patient with classic IOPD in our cohort (n = 19);
-
iv)
Group 1d: patients with the c.-32-13T>G variant in association with another type of variant (n = 47). Among these, one patient seemed to be homozygous for the c.-32-13T>G variant (the allele segregation could not be verified). This group contains the two patients with variants reported as GVUS or not pathogenic, as well as those with the GAA variants absent from the Pompe Disease Mutation Database. For four other patients, only the c.-32-13T>G variant was found, the second one being unknown;
-
v)
Group 2: patients not carrying the c.-32-13T>G common late-onset variant (n = 27).
A majority of the patients with LOPD, but only a few patients with IOPD in this study are included in the French national PD registry (created in 2004 and qualified in 2008).
2.2.3. GSD III patients
All GSD III patients (n = 57) were diagnosed on the basis of a deficient amylo-1,6-glucosidase activity in leukocytes or fibroblasts [31], and/or by demonstration of two deleterious alterations in the AGL gene (localized in 1p31.2 [32]). For most of these patients an increased glycogen content in erythrocytes [33] was demonstrated.
2.3. Samples
GSD II urine samples (Table 1 suppl) were received either at the time of GSD II diagnosis, just before starting ERT (n = 332), or after starting ERT (n = 2168). Urine samples (n = 137) were also received from 57 patients for the diagnosis or follow-up of GSD III (mainly from Pr Pascal Laforêt, Hôpital Raymond Poincaré, Garches, Assistance Publique des Hôpitaux de Paris, France).
Urinary creatinine concentration (creat, in mmol/L) was determined for each urine sample, either with a kinetic, automated Jaffé method (ABX diagnostics, Montpellier, France; samples before 2007), or with an enzymatic method on an Architect c16000 (Abbott laboratories, Wiesbaden, Germany; samples after 2007).
2.4. Sample preparation
Calibration points with increasing Glc4 standard concentrations (0, 2.5, 5, 10, 20, 50, 75, 100, 150 and 200 μmol/L) were prepared in control urine and stored in 100 μL aliquots at −20 °C for maximum 2 years. High level internal quality control (QC) was a urine sample containing a high Glc4 concentration. For external QC, a samples exchange scheme was conducted twice a year, the Duke Biochemical Genetics Laboratory (Durham, NC, USA).
Glc4 measurement method is derived from that previously reported by Young et al. [20]. In brief, a derivatization solution containing BAB (152 mmol/L), NaBH3CN (400 mmol/L) and acetic acid (5.3% v/v) was prepared in methanol. Twenty μL of centrifuged urine (or standards or controls) were mixed with 20 μL of 50 μmol/L IS solution and 70 μL of the derivatization solution, in a tightly closed vial, and incubated 1 h at 80 °C. After cooling to room temperature, 500 μL of ACN/water 15:85 (v/v) were added to each vial. C18 SPE columns were used to remove excess reagent from samples. SPE columns were primed with 500 μL methanol, 500 μL water then 200 μL ACN/water 15:85 (v/v), loaded with derivatized sample, rinsed twice with 500 μL ACN/water (15:85, v/v), and eluted with 500 μL ACN/water 30:70 (v/v). After drying under nitrogen at 45 °C, the samples were reconstituted with 500 μL mobile phase (methanol 80% v/v) for injection (10 μL injected).
2.5. LC-MS/MS
Samples were injected onto a C18 column (Uptisphere 3BioP2, 3 μm, 100 mm × 2.1 mm, ref. UP3BP2#10QS, Interchrom©, Interchim, Montluçon, France) and separated using isocratic elution with methanol:H2O 80:20 v/v, at 400 μL/min. The total analysis time was 5 min. The column was maintained at 40 °C. Retention time of Glc4 and the IS was 1.3 min.
LC-MS/MS was performed using a binary LC pump system (LC20AD) and an auto-sampler (SIL20AC) (Prominence Liquid Chromatograph, Shimadzu©, Kyoto, Japan) coupled either to an API 3200 (before April 2014) or an API 4500 (after April 2014) from Applied Biosystems© (Concord, Canada) operated in the multiple reaction mode and equipped with a TurboIonSpray source heated at 550 °C. Nitrogen was used as curtain and collision gas. Common MS/MS parameters were as follow: mode: positive; gas flows (in arbitrary units): curtain gas: 20; source gas 1: 25; source gas 2: 60; collision activated dissociation gas: 8; resolution of Q1 and Q3: unit; ion spray voltage: 4500 V, entrance potential: 10 V, cell exit potential: 8 V. Transitions 866.4 > 509.1 (for Glc4) and 872.4 > 509.1 (for IS) were monitored with a declustering potential of 90 V and a collision energy of 67 V.
2.6. Quantification
Peak integration was performed with the Analyst software (version 1.6.2, Applied Biosystems©; smoothing width: 3 points). Glc4 concentrations were calculated using the Glc4 area/IS area ratio, and the external calibration curve (linear through zero). Glc4 concentrations were normalized to the urinary creatinine (creat) concentration and expressed in mmol/mol creat.
2.7. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v 21.0.0.0 (SPSS Hong Kong Headquarters,1804, Westlands Centre, Westlands Road,Quarry Bay, HK). Non-parametric tests were chosen because of the small group size and the non-normal distribution of variables.
2.8. Method validation
Intra- and inter-run imprecision were evaluated by replicate analyses of QCs (intra-run: n = 10; inter-run n = 110 over 27 runs). Limit of detection (LOD) and limit of quantification (LOQ) were determined by analysing dilutions of a standard solution, until the signal was not detectable (signal/noise ratio < 3 for LOD and < 10 for LOQ). Linearity was evaluated up to 1000 μmol/L.
3. Results
3.1. MS/MS performance of the method - validation
Intra-run imprecision was 8.0% (mean: 399 μmol/L, n = 10). Long term inter-run precision was 8.8% (mean 232 μmol/L, n = 40). LOD and LOQ were determined to be 0.31 and 0.62 μmol/L, respectively. All physiologically measured concentrations were above the LOQ; thus Glc4 is measurable even in control urines with a good sensitivity. The method was linear to at least until 1000 μmol/L (r2 = 0.991). External QC between gave results in good accordance with those of the other laboratory (data not shown).
3.2. Reference values
Reference intervals according to age are shown in Table 2. The 90th percentile was chosen as the cut-off value for each age group. No sex difference was observed (results not shown). Results show that urinary Glc4 normalized to creatinine decreases with age
Table 2.
Reference range of urinary Glc4 in mmol/mol creatinine.
| N | Median [25th - 75th percentile] | Min – max | Threshold (90th percentile) | |
|---|---|---|---|---|
| 0 - < 2 months | 25 | 6.6 [4.4–15.5] | 0.6–47.1 | 23.6 |
| 2 months - < 1 year | 36 | 3.7 [2.8–6.2] | 0.1–22.5 | 9.8 |
| 1 - < 4 years | 30 | 1.7 [1.2–2.6] | 0.1–22.5 | 7.9 |
| 4 - < 20 years | 52 | 1.1 [0.7–1.6] | 0.1–9.2 | 2.50 |
| ≥ 20 years | 52 | 0.95 [0.6–1.2] | 0.1–9.4 | 1.95 |
| TOTAL | 195 | |||
The threshold was established as the 90th percentile of the values of the control population. N: number of samples. SD: standard deviation.
3.3. Samples
Among the nearly 2700 samples received in our laboratory for a Glc4 measurement, 2.2% were from patients determined to be unaffected with GSD II, 11.8% from patients with GSD II either at the time of diagnosis or later before receiving ERT, 80.6% for patients with GSD II receiving ERT, and 5.4% for GSD III diagnosis or follow-up (Table 1 suppl).
3.4. GSD II untreated patients
Glc4 was measured in 332 urine samples from 174 patients affected with GSD II at the time of diagnosis or before ERT. The median age at first Glc4 measurement was 0.14 years for classic IOPD, 0.9 years for non-classic IOPD, 13.6 years for juvenile LOPD and 49.4 years for adult LOPD (Table 1 suppl).
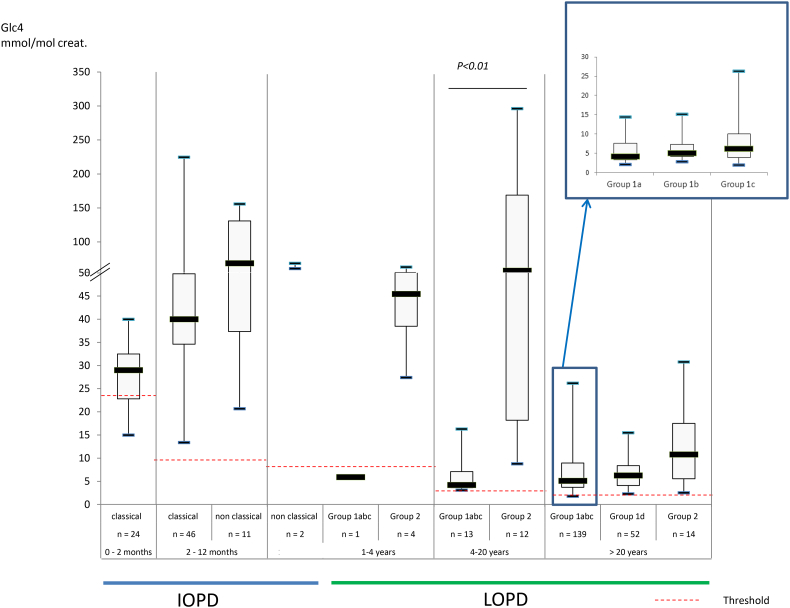
Results for patients with IOPD (classic form: 35 patients; 70 samples; non-classic form: 8 patients; 13 samples) are presented in Fig. 1a and Fig. 2 (see also Table 2 suppl). Results for patients with LOPD (juvenile form: 14 patients, 27 samples; adult form: n = 117 patients, 222 samples) are presented in Fig. 1b and Fig. 2 (see also Table 3 suppl), according to the genotype (see 2.2.2).
Fig. 1.
Results of urinary Glc4 in untreated PD patients: Black line: threshold. (a) Patients with IOPD: classic form (35 patients, 70 samples), non classic form (8 patients, 13 samples). (b) Patients with LOPD: juvenile form (27 samples, 14 patients), adult form (222 samples, 117 patients). Logarithmic scale.
Fig. 2.
Urinary Glc4 levels in untreated patients with PD compared with the age matched threshold.
Dotted line: threshold.
As shown in Fig. 1a and 1b, a majority of the untreated patients with GSD II have increased urinary Glc4 above the reference intervals (threshold). Patients affected with IOPD have generally high Glc4 levels (median elevation: 4.0–5.8× above the threshold), except for those diagnosed at a very early age (<2 months). For 13 patients diagnosed <2 months of age, the median elevation was 1.2× above the threshold, and 5 of these patients had normal Glc4 (14% of classic IOPD cases, Fig. 1a suppl). We observed that Glc4 was also elevated in a majority of adult patients with LOPD; a small number of adults had a normal level (see 4.2.1.2). Although the concentrations are lower than in LOPD, the relative increase is comparable to that in IOPD (2.6–5.5× the threshold). Juvenile patients with LOPD (aged 4–20 years) have clearly increased Glc4 (23× the threshold). Some severely affected patients diagnosed at a more advanced disease state have marked increases in Glc4.
3.5. GSD III patients
Glc4 was increased in all patients affected with GSD III. Glc4 values are higher than in GSD II for the same age class (p < .001, patients >20 years, Fig. 2 suppl). However, four patients (6 samples) exhibited Glc4 levels slightly above the threshold.
4. Discussion
The MS/MS method had sufficient sensitivity to quantify Glc4 in all control urines, even in dilute samples with low creatinine values. As demonstrated, the method is linear in the range of the standards (0–200 μmol/L), and the range of measurement can be extended to 1000 μmol/L.
4.1. Reference range
It is noticeable that the distribution of control results is large in each age range, and that many control patients present with high Glc4 levels (high standard deviation). This has been previously observed [21]. High values are observed particularly when the creatinine level is very low (results not shown), a situation that currently occurs in very young babies. Thus, the 90th percentile was chosen as threshold for age range control values, in order to minimize the number of false negative results. These results are in good accordance with those reported by Young et al. [21]. Patients' results must be interpreted according to age.
4.2. GSD II patients
As our laboratory is the only centre performing urinary Glc4 measurement in France, our GSD II cohort probably represents the GSD II population in France during this period. Most of the diagnoses (n = 241) are LOPD (adult 73%; juvenile 7%) while IOPD represents the remaining patients (20%; including classic form: 79% and non-classic form 21%).
The genotype was known in 221 out of the 230 GSD II patients tested (9 with only one variant identified). 85.6% patients with LOPD carry the c.-32-13T>G splice site variant (adult: 90%; juvenile 37.5%), while this variant was absent in all the 44 genotyped IOPD cases.
4.2.1. Untreated GSD II patients
Among the 240 GSD II patients, 51 had a Glc4 measurement only before ERT for different reasons: either the patient had never benefitted from ERT because of being too severely affected at the time of diagnosis (patients with IOPD), the patient refused ERT (patients with LOPD), or the patient was treated after the year 2017. Conversely, 66 patients had no Glc4 level before ERT: either the sampling was missed before starting ERT, or in most LOPD cases, the diagnosis was made before 2006, and the patient started ERT prior to the availability of testing. The results of the remaining 123 patients were informative for studying the impact of ERT on the Glc4 level.
4.2.1.1. Baseline Glc4 in patients with IOPD
The observation that patients with classic IOPD diagnosed before two months of age have normal or mildly increased Glc4 levels, is similar to that reported in a cohort of patients with IOPD diagnosed through newborn screening [8]. In our cohort, two patients were diagnosed prenatally or in the neonatal period because of a positive family history, and were asymptomatic at the time of testing (one positive prenatal diagnosis, one diagnosed at birth because of an affected sibling). Three other patients were diagnosed at a young age because of an early onset of symptoms. Notably, the Glc4 level of these 5 patients was only slightly below the threshold. Patients with IOPD older than two months at the time of diagnosis have much higher Glc4 levels (median: 4.0 x the threshold). Thus, Glc4 may have limitations as a biomarker for the diagnosis of GSD II at an early stage of the disease (in accordance with [21]). Three patients with classic IOPD were diagnosed later than 6 months, and had elevated Glc4; two of them were recent immigrant patients diagnosed at the time of their arrival in France with a very advanced stage of the disease, and thus could not benefit from ERT. The cohort of patients with non-classic IOPD had higher Glc4, compared with the classic IOPD cohort (median: 5.8 fold the threshold versus 4.0). However, the non-classic cohort presented later and patients were older at the time of testing (median: 0.9 years versus 0.43). Thus it seems that urinary Glc4 increases with age in IOPD, representing the glycogen accumulation in parallel with the spontaneous evolution of the disease. The calculated sensitivity of urinary Glc4 for IOPD was 0.93.
4.2.1.2. Untreated patients with LOPD
4.2.1.2.1. Patients with juvenile LOPD
Among patients with LOPD (n = 192), several who presented in late infancy with muscle symptoms, but without cardiac involvement, were classified as juvenile LOPD (n = 17). Six out of the 16 genotyped juvenile patients had the c.-32-13T>G common variant. These untreated juvenile patients exhibited a high excretion of Glc4, especially when the c-32-13T>G variant is absent (4–20 years, 23.0 fold the threshold versus 2.3 when present, p < .01, Table 3 suppl).
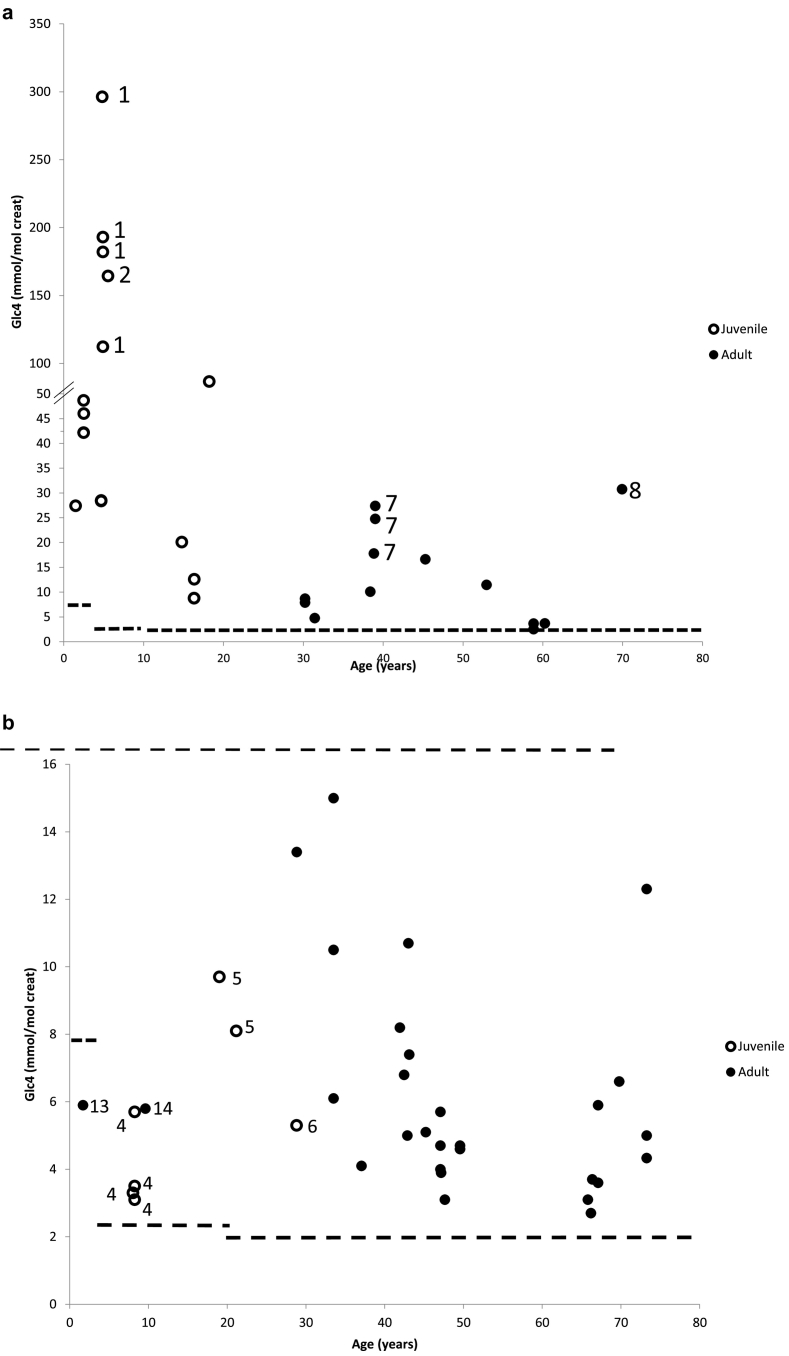
o Glc4 is higher when the disease is more severe
Two patients presented exceptionally high Glc4 levels at the time of analysis. Patient 1 (Fig. 3a, 4 samples) presented at a few months of age with significant hepatomegaly, but was not diagnosed until 4 years and 10 months, at which time, he had a severe respiratory insufficiency, severe myopathy, and he died at 5 years. His younger brother, diagnosed at 2.5 years, had more moderate Glc4 elevations (about 5-fold the threshold). Patient 2 (Fig. 3a) was diagnosed in 2001 at 14 months with myopathy; Glc4 was first evaluated in 2005 when she was 5 years old, just before starting ERT; at that time, the disease had progressed and she had tetraplegia and tracheotomy. Thus these two patients were severely affected, showing that the Glc4 level likely correlates with the severity of the disease at least in young patients.
Number of patients and samples:
Fig. 3.
Results of urinary Glc4 in untreated patients with LOPD according to genotype. Patients with LOPD were divided into groups according to the presence of the c.-32-13T>G variant, and to the nature of the second variant (see 2.2.2). (a) Patients not carrying the c.-32-13T>G variant (group 2), (b) patients carrying the c.-32-13T>G variant associated with del ex 18 (group 1a), (c) patients carrying the c.-32-13T>G variant associated with a severe variant (group 1b and 1c), (d) patients carrying the c.-32-13T>G variant associated with another variant (group 1d)".
Numbers on the figures correspond to patients described in the text (Section 4.2.1.2)
o The c.-32-13T>G variant can be present in juvenile form
The c.-32-13T>G variant was observed in 3 juvenile patients with onset of symptoms at a young age. In patient 3 (Fig. 3c), it was associated with a very severe variant (c.525delT, myalgia appeared at 6 years). In patients 4 and 5 it was associated with del ex 18. These patients were diagnosed because of their symptoms at 8 and 14 years that included cramps and fatigability (Fig. 3b). Patient 6, the brother of patient 5 presented at 24 years with cramps, and digestive troubles). All 4 had elevated CK levels.
4.2.1.2.2. Patients with adult LOPD
o Classification according to genotype
Adult LOPD results have been interpreted according to the genotype of the patient. The c.-32-13T>G variant is present in 86% of our patients with LOPD (90% of adult patients).
Due to the high c.-32-13T>G variant frequency (calculated carrier frequency 1/154 in Dutch population [34] and 1/184 in the Genome Aggregation Database [35]), the number of patients who are homozygous for the c.-32-13T>G variant should be much higher than observed. We diagnosed only one patient probably homozygous for this variant. Likely either most of the homozygous patients are underdiagnosed or they are asymptomatic [5].
We evaluated the severity of the second variant according to the Erasmus Pompe Center Database, and categorized the LOPD cohort presenting the c.-32-13T>G variant into four groups, based on the severity of the second variant (see 2.2.2). We observed that the second variant was severe in 70% of cases: group 1a, del ex 18, 16%; group 1b, known severe variants, 37%; group c, potentially severe missense variants, 17%. This latter group was defined because of the presence of these variants in patients with classic IOPD. Patients in group 1d (association with other variants) accounted for 30% of cases.
o Glc4 is higher in the absence of the c.-32-13T>G variant
Urinary Glc4 adult patients with LOPD (> 20 years) ranged from 1.8 to 31 mmol/mol creat (reference range < 1.95). It was increased in all adult patients with LOPD (n = 132, 249 samples) except three (4 samples). The calculated sensitivity of Glc4 for LOPD was 0.98.
Our results show that the Glc4 level of patients without the c.-32-13T>G variant is about 10 fold (4–20 years, median 23 fold the threshold vs 2.3, Table 3 suppl) or two fold (> 20 years; median 5.5 fold the threshold vs 2.6 / 3.2) higher than that of patients carrying one c.-32-13T>G variant allele. Moreover, the patients are younger (4–20 years, median age 5.2 vs 12.4; >20 years, median age 42.2 years vs 49.4–54.1). Thus, in the presence of the c.-32-13T>G variant, there is less increase in urinary Glc4. However, among the different classes of variant associated with the c.-32-13T>G, patients carrying a severe variant (group 1a and 1b) have higher Glc4 levels (5.1 and 6.2 fold the threshold, Table 3 suppl) than those carrying a severe missense variant (group 1c, 4.2 fold the threshold). Thus these results corroborate that even if found in classic IOPD, some missense variants could possibly be less deleterious than variants classified as severe (Pompe Disease Mutation Database).
o Glc4 is higher when the disease is more severe
Two adult patients had particularly high Glc4 levels. Patient 7 (Fig. 3a, p.Arg224Trp and p.Arg412Ser) was diagnosed at 22 years, and Glc4 was highly elevated at 39 (17.8, 27.4 and 24.8 mmol/mol creat, N < 1.95) when the patient experienced a worsening of symptoms (tetraparesia, severe myopathy). Patient 8 was diagnosed at 46 years with two severe variants (Fig. 3a, del ex 18 and p.Arg725Trp), but Glc4 was not assessed until 70 years of age (31 mmol/mol creat, N < 1.95), again when aggravation occurred (tetraparesia and invasive ventilation) and ERT was hence initiated.
o Glc4 can be normal in some patients
These three patients (Fig. 3c) carried the c.-32-13T>G variant and another severe variant. Patient 9 (heterozygous for c.525delT) had a level of 1.8 mmol/mol creat (2 samples). The urines were sampled after perfusing the patient in preparation for the first Myozyme® treatment. We have observed that sampling urine after a few minutes of perfusion considerably lowers the Glc4 concentration (results not shown). Thus, Glc4 measurement requires a urine sampling before placing the patient under perfusion, which is particularly important for the follow-up of ERT. Several other results collected under inappropriate conditions were excluded of the study. Patient 10 had a Glc4 level of 1.9 mmol/mol creat before ERT and 1 year after ERT (1.5 mmol/mol creat). This patient carried the c.-32-13T>G variant as well as the severe c.2460 duplication leading to a frameshift p.Gly821Trpfs*63 protein. In this case, no reason was found to explain the low Glc4 level. Patient 11 (heterozygous for c.3G>A, p.Met1?) had a Glc4 level of 1.9 mmol/mol creat while three other pre-ERT samples were mildly elevated (2.1 to 5.4 mmol/mol creat), demonstrating variability in Glc4 excretion. Thus, in all cases results are only slightly under the threshold.
o Glc4 in adult LOPD form diagnosed in infancy
Due to the fortuitous detection of elevated CK levels after various clinical symptoms, three other patients were diagnosed at a very young age; they carried the c.-32-13T>G variant associated either with the severe p.Gly643Arg missense variant (patient 12: 7.6 years, 7.1 mmol/mol, N < 2.5, Fig. 3c), or with del ex18 (patient 13: 1.6 year, 5.9 mmol/mol, N < 7.9; patient 14: 9 years, 5.8 mmol/mol, N < 2.5, Fig. 3b). In these three cases, the clinical symptoms (serious stroke episodes at 1 and 4 months, recurrent ORL infections, or else) were not linked to GSD II and these patients could be considered as asymptomatic LOPD. It is likely that GSD II symptoms would present at a later age. The Glc4 level was normal in the youngest patient, suggesting that when the diagnosis is established in asymptomatic patients, the urinary Glc4 is not always helpful for diagnosis.
Thus, as a whole, Glc4 is elevated in most GSD II patients, with a degree of variability that seems to be related to the age at diagnosis, extent of glycogen accumulation, absence of the c.-32-13T>G variant, and to the stage of the disease [21].
4.2.2. GSD II treated patients
To evaluate if urinary Glc4 is a good biomarker for the follow-up of PD patients, an important number of parameters must be taken into account: age of patient, phenotype, age at the time of diagnosis, age when starting the treatment, number of years of treatment, kind of treatment, genotype etc.… this important work should be published separately. Very little is published about the follow-up of PD patients, and first publications state that Glc4 may have a prognostic role in IOPD [22,41]. We agree with this. However, nothing is published in this field concerning LOPD.
4.3. GSD III patients
It has long been known, that erythrocyte glycogen content is strikingly elevated in GSD III patients [36,37]. A more moderate elevation has also been described in several, but not all, types of GSD IX (phosphorylase kinase deficiency, OMIM #300798, OMIM #172490, OMIM #172471; [38]). In parallel, urinary Glc4 has also been found to be markedly increased in GSD III [23,39] and in some patients with GSD IX ([40], thin layer chromatography). Thus measuring urinary Glc4 in GSDs could be of interest.
Our results show that the Glc4 level was increased in all the GSD III patients (57 patients, 137 samples). It was highly increased in a majority, and to a greater extent than in GSD II patients (Fig. 2 suppl), especially for patients older than 20 years (p ≤.001). However four patients (6 samples) exhibited less elevated urinary Glc4. Interestingly, one of these patients also had a normal erythrocyte glycogen content. These results are comparable with the report of one GSD III patient (out of 6) with very moderately increased erythrocyte glycogen content [36] and with four other French patients of the same family with a normal erythrocyte glycogen content (results not shown). Thus, there are some GSD III patients for whom the erythrocyte glycogen content and/or the urinary Glc4 can be normal or slightly elevated. Correlation with the GSD IIIb subtype (affecting liver only) could be envisaged, but the four patients of our study presented muscular symptoms. We also know that erythrocyte glycogen content is always within reference limits in GSD I, a disorder in which there is increased liver glycogen. The reason for which the glycogen/Glc4 accumulation is not evidenced in these cases is not known. However, like erythrocyte glycogen content, urinary Glc4 is sensitive a biomarker of GSD III.
5. Conclusion
Beside the glycogen concentration in muscle, urinary Glc4 is the only available biochemical biomarker of disease burden for GSD II. This biomarker is easily measurable in urine by MS/MS.
Although not specific to GSD II, and not completely sensitive in all cases, this biomarker supports a diagnosis of GSD II and can be very helpful in difficult cases (only one GAA variant, uncertain enzyme deficiency, early diagnosis, asymptomatic patients, etc…). The degree of elevation appears to correlate with the phenotype (IOPD or LOPD), the age at diagnosis, extent of glycogen accumulation, presence of the c.-32-13 T > G variant, severity of the pathogenic variants, and to the stage of the disease.
Urinary Glc4 is also importantly increased in most GSD III patients. Further statistical analyses may help to determine if Glc4 is also a good biomarker for monitoring of specific therapies. This is important for the future, as several new treatments are under investigation.
Contribution of the authors
Monique Piraud: conception, design, manuscript writing; Magali Pettazzoni: statistical analysis, manuscript correction; Marie de Antonio: statistical analysis; Roseline Froissart: manuscript correction; Christine Saban: manuscript correction; Brigitte Chabrol: manuscript correction; Sarah Young: manuscript correction; Pascal Laforêt: manuscript correction.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2020.100583.
Appendix A. Supplementary data
Supplementary figure
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary material
References
- 1.Di Mauro S., Spiegel R. Progress and problems in muscle glycogenoses. Acta Myol. 2011;30:96–102. [PMC free article] [PubMed] [Google Scholar]
- 2.Hers H.G. alpha-Glucosidase deficiency in generalized glycogen storage disease (Pompe’s disease) Biochem. J. 1963;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishnani P.S., Hwu W.L., Mandel H., Nicolino M., Yong F., Corzo D. Infantile-onset Pompe disease natural history study group. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006;148(5):671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Hagemans M.L., Winkel L.P., Van Doorn P.A., Hop W.J., Loonen M.C., Reuser A.J., Van der Ploeg A.T. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128(Pt 3):671–677. doi: 10.1093/brain/awh384. [DOI] [PubMed] [Google Scholar]
- 5.Semplicini C., Letard P., De Antonio M., Taouagh N., Perniconi B., Bouhour F., Echaniz-Laguna A., Orlikowski D., Sacconi S., Salort-Campana E., Solé G., Zagnoli F., Hamroun D., Froissart R., Caillaud C., Laforêt P., French Pompe Study Group Late-onset Pompe disease in France: molecular features and epidemiology from a nationwide study. J. Inherit. Metab. Dis. 2018;41:937–946. doi: 10.1007/s10545-018-0243-7. [DOI] [PubMed] [Google Scholar]
- 6.Elenga N., Verloes A., Mrsic Y., Basurko C., Schaub R., Cuadro-Alvarez E., Kom-Tchameni R., Carles G., Lambert V., Boukhari R., Fahrasmane A., Jolivet A., Nacher M., Benoist J.F. Incidence of infantile Pompe disease in the Maroon population of French Guiana. BMJ Paediatr. Open. 2018;9(1) doi: 10.1136/bmjpo-2017-000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manwaring V., Prunty H., Bainbridge K., Burke D., Finnegan N., Franses R., Lam A., Vellodi A., Heales S. Urine analysis of glucose tetrasaccharide by HPLC; a useful marker for the investigation of patients with Pompe and other glycogen storage diseases. J. Inherit. Metab. Dis. 2012;35:311–316. doi: 10.1007/s10545-011-9360-2. Erratum in: J Inherit Metab Dis. 35:369. [DOI] [PubMed] [Google Scholar]
- 8.Chien Y.H., Goldstein J.L., Hwu W.L., Smith P.B., Lee N.C., Chiang S.C., Tolun A.A., Zhang H., Vaisnins A.E., Millington D.S., Kishnani P.S., Young S.P. Baseline urinary glucose tetrasaccharide concentrations in patients with infantile- and late-onset Pompe disease identified by newborn screening. JIMD Rep. 2015;19:67–73. doi: 10.1007/8904_2014_366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raben N., Nichols R.C., Martiniuk F., Plotz P.H. A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II) Hum. Mol. Genet. 1996;5:995–1000. doi: 10.1093/hmg/5.7.995. [DOI] [PubMed] [Google Scholar]
- 10.Dardis A., Zann I., Zampieri S., Stuani C., Pianta A., Romanello M., Baralle F.E., Bembi B., Buratti E. Functional characterization of the common c.-32-13T>G mutation of GAA gene: Identification of potential therapeutic agents. Nucleic Acids Res. 2014;42:1291–1302. doi: 10.1093/nar/gkt987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rairikar M.V., Case L.E., Bailey L.A., Kazi Z.B., Desai A.K., Berrier K.L., Coats J., Gandy R., Quinones R., Kishnani P.S. Insight into the phenotype of infants with Pompe disease identified by newborn screening with the common c.-32-13T>G “late-onset” GAA variant. Mol. Genet. Metab. 2017;122:99–107. doi: 10.1016/j.ymgme.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolino M., Byrne B., Wraith J.E., Leslie N., Mandel H., Freyer D.R., Arnold G.L., Pivnick E.K., Ottinger C.J., Robinson P.H., Loo J.C., Smitka M., Jardine P., Tato L., Chabrol B., McCandless S., Kimura S., Mehta L., Bali D., Skrinar A., Morgan C., Rangachari L., Corzo D., Kishnani P.S. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- 13.Chien Y.H., Lee N.C., Thurberg B.L., Chiang S.C., Zhang X.K., Keutzer J., Huang A.C., Wu M.H., Huang P.H., Tsai F.J., Chen Y.T., Hwu W.L. Pompe disease in infants: Improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124(6):e1116–e1125. doi: 10.1542/peds.2008-3667. [DOI] [PubMed] [Google Scholar]
- 14.Kazi Z.B., Desai A.K., Berrier K.L., Troxler R.B., Wang R.Y., Abdul-Rahman O.A., Tanpaiboon P., Mendelsohn N.J., Herskovitz E., Kronn D., Inbar-Feigenberg M., Ward-Melver C., Polan M., Gupta P., Rosenberg A.S., Kishnani P.S. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI Insight. 2017;2(16) doi: 10.1172/jci.insight.94328. pii: 94328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlaar L., Hogrel J.Y., Perniconi B., Kruijshaar M.E., Rizopoulos D., Taouagh N., Canal A., Brusse E., van Doorn P.A., van der Ploeg A.T., Laforêt P., van der Beek N.A.M.E. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 2019 doi: 10.1212/WNL.0000000000008441. 2019 Oct 16. Pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alonzo D., De Fenza M., Porto C., Iacono R., Huebecker M., Cobucci-Ponzano B., Priestman D.A., Platt F., Parenti G., Moracci M., Palumbo G., Guaragna A. N-Butyl-l-deoxynojirimycin (l-NBDNJ): Synthesis of an allosteric enhancer of α-glucosidase activity for the treatment of Pompe disease. J. Med. Chem. 2017;60(23):9462–9469. doi: 10.1021/acs.jmedchem.7b00646. [DOI] [PubMed] [Google Scholar]
- 17.Hallgren P., Hansson G., Henriksson K.G., Häger A., Lundblad A., Svensson S. Increased excretion of a glucose-containing tetrasaccharide in the urine of a patient with glycogen storage disease type II (Pompe’s disease) Eur. J. Clin. Investig. 1974;4:429–433. doi: 10.1111/j.1365-2362.1974.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 18.Hallgren P., Lindberg B.S., Lundblad A. Quantitation of some urinary oligosaccharides during pregnancy and lactation. J. Biol. Chem. 1977;252:1034–1040. [PubMed] [Google Scholar]
- 19.Lennartson G., Lundblad A., Sjöblad S., Svensson S., Ockerman P.A. Quantitation of a urinary tetrasaccharide by gas chromatography and mass spectrometry. Biomed. Mass Spectrom. 1976;3:51–54. doi: 10.1002/bms.1200030202. [DOI] [PubMed] [Google Scholar]
- 20.Young S.P., Stevens R.D., An Y., Chen Y.T., Millington D.S. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Anal. Biochem. 2003;316:175–180. doi: 10.1016/s0003-2697(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 21.Young S.P., Piraud M., Goldstein J.L., Zhang H., Rehder C., Laforet P., Kishnani P.S., Millington D.S., Bashir M.R., Bali D.S. Assessing disease severity in Pompe disease: the roles of a urinary glucose tetrasaccharide biomarker and imaging techniques. Am. J. Med. Genet. C Semin. Med. Genet. 2012;160C:50–58. doi: 10.1002/ajmg.c.31320. [DOI] [PubMed] [Google Scholar]
- 22.Young S.P., Zhang H., Corzo D., Thurberg B.L., Bali D., Kishnani P.S., Millington D.S. Long-term monitoring of patients with infantile-onset Pompe disease on enzyme replacement therapy using a urinary glucose tetrasaccharide biomarker. Genet. Med. 2009;11:536–541. doi: 10.1097/GIM.0b013e3181a87867. [DOI] [PubMed] [Google Scholar]
- 23.An Y., Young S.P., Hillman S.L., Van Hove J.L., Chen Y.T., Millington D.S. Liquid chromatographic assay for a glucose tetrasaccharide, a putative biomarker for the diagnosis of Pompe disease. Anal. Biochem. 2000;287:136–143. doi: 10.1006/abio.2000.4838. [DOI] [PubMed] [Google Scholar]
- 24.An Y., Young S.P., Kishnani P.S., Millington D.S., Amalfitano A., Corz D., Chen Y.T. Glucose tetrasaccharide as a biomarker for monitoring the therapeutic response to enzyme replacement therapy for Pompe disease. Mol. Genet. Metab. 2005;85:247–254. doi: 10.1016/j.ymgme.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Rozaklis T., Ramsay S.L., Whitfield P.D., Ranieri E., Hopwood J.J., Meikle P.J. Determination of oligosaccharides in Pompe disease by electrospray ionization tandem mass spectrometry. Clin. Chem. 2002;48:131–139. [PubMed] [Google Scholar]
- 26.Sluiter W., van den Bosch J.C., Goudriaan D.A., van Gelder C.M., de Vries J.M., Huijmans J.G., Reuser A.J., van der Ploeg A.T., Ruijter G.J. Rapid ultraperformance liquid chromatography-tandem mass spectrometry assay for a characteristic glycogen-derived tetrasaccharide in Pompe disease and other glycogen storage diseases. Clin. Chem. 2012;58:1139–1147. doi: 10.1373/clinchem.2011.178319. [DOI] [PubMed] [Google Scholar]
- 27.Chamoles N.A., Niizawa G., Blanco M., Gaggioli D., Casentini C. Glycogen storage disease type II: Enzymatic screening in dried blood spots on filter paper. Clin. Chim. Acta. 2004;347:97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Salafsky I.S., Nadler H.L. A fluorometric assay of alpha-glucosidase and its application in the study of Pompe’s disease. J. Lab. Clin. Med. 1973;81:450–454. [PubMed] [Google Scholar]
- 29.Bienvenu J., Mathieu M., Collombel C., Baltassat P., Divry P., Dorche C., Cotte J. Immunochemical study of acid alpha-1,4-glucosidase in 7 patients with type II glycogenosis. Pédiatrie. 1979;34:659–676. [PubMed] [Google Scholar]
- 30.Kroos M., Pomponio R.J., van Vliet L., Palmer R.E., Phipps M., Van der Helm R., Halley D., Reuser A., GAA Database Consortium Update of the Pompe disease mutation database with 107 sequence variants and a format for severity rating. Hum. Mutat. 2008;29(6):E13–E26. doi: 10.1002/humu.20745. [DOI] [PubMed] [Google Scholar]
- 31.Brown D.H., Waindle L.M., Brown B.I. The apparent activity in vivo of the lysosomal pathway of glycogen catabolism in cultured human skin fibroblasts from patients with type III glycogen storage disease. J. Biol. Chem. 1978;253(14):5005–5011. [PubMed] [Google Scholar]
- 32.Sentner C.P., Hoogeveen I.J., Weinstein D.A., Santer R., Murphy E., McKiernan P.J., Steuerwald U., Beauchamp N.J., Taybert J., Laforêt P., Petit F.M., Hubert A., Labrune P., Smit G.P.A., Derks T.G.J. Glycogen storage disease type III: Diagnosis, genotype, management, clinical course and outcome. J. Inherit. Metab. Dis. 2016;39:697–704. doi: 10.1007/s10545-016-9932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hers H.G. Glycogen storage disease. Adv. Metab. Disord. 1964;13:1–44. doi: 10.1016/b978-1-4831-6748-0.50006-9. [DOI] [PubMed] [Google Scholar]
- 34.Ausems M.G., Verbiest J., Hermans M.P., Kroos M.A., Beemer F.A., Wokke J.H., Sandkuijl L.A., Reuser A.J., van der Ploeg A.T. Frequency of glycogen storage disease type II in the Netherlands: Implications for diagnosis and genetic counselling. Eur. J. Hum. Genet. 1999;7(6):713–716. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- 35.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Tukiainen T., Birnbaum D.P., Kosmicki J.A., Duncan L.E., Estrada K., Zhao F., Zou J., Pierce-Hoffman E., Berghout J., Cooper D.N., Deflaux N., DePristo M., Do R., Flannick J., Fromer M., Gauthier L., Goldstein J., Gupta N., Howrigan D., Kiezun A., Kurki M.I., Moonshine A.L., Natarajan P., Orozco L., Peloso G.M., Poplin R., Rivas M.A., Ruano-Rubio V., Rose S.A., Ruderfer D.M., Shakir K., Stenson P.D., Stevens C., Thomas B.P., Tiao G., Tusie-Luna M.T., Weisburd B., Won H.H., Yu D., Altshuler D.M., Ardissino D., Boehnke M., Danesh J., Donnelly S., Elosua R., Florez J.C., Gabriel S.B., Getz G., Glatt S.J., Hultman C.M., Kathiresan S., Laakso M., McCarroll S., McCarthy M.I., McGovern D., McPherson R., Neale B.M., Palotie A., Purcell S.M., Saleheen D., Scharf J.M., Sklar P., Sullivan P.F., Tuomilehto J., Tsuang M.T., Watkins H.C., Wilson J.G., Daly M.J., MacArthur D.G., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;18(536(7616)):285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidbury J.B., Cornblath M., Fisher J., House E. Glycogen in erythrocytes of patients with glycogen storage disease. Pediatrics. 1961;27:103–111. [Google Scholar]
- 37.Maire I., Baussan C., Moatti N., Mathieu M., Lemonnier A. Biochemical diagnosis of hepatic glycogen storage diseases: 20 years French experience. Clin. Biochem. 1991;24:169–178. doi: 10.1016/0009-9120(91)90511-c. [DOI] [PubMed] [Google Scholar]
- 38.Davit-Spraul A., Piraud M., Dobbelaere D., Valayannopoulos V., Labrune P., Habes D., Bernard O., Jacquemin E., Baussan C. Liver glycogen storage diseases due to phosphorylase system deficiencies: Diagnosis thanks to non invasive blood enzymatic and molecular studies. Mol. Genet. Metab. 2011;104:137–143. doi: 10.1016/j.ymgme.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Halaby C.A., Young S.P., Austin S., Stefanescu E., Bali D., Clinton L.K., Smith B., Pendyal S., Upadia J., Schooler G.R., Mavis A.M., Kishnani P.S. Liver fibrosis during clinical ascertainment of glycogen storage disease type III: A need for improved and systematic monitoring. Genet. Med. 2019 doi: 10.1038/s41436-019-0561-7. [DOI] [PubMed] [Google Scholar]
- 40.Morava E., Wortmann S.B., van Essen H.Z., Liebrand van Sambeek R., Wevers R., van Diggelen O.P. Biochemical characteristics and increased tetraglucoside excretion in patients with phosphorylase kinase deficiency. J. Inherit. Metab. Dis. 2005;28:703–706. doi: 10.1007/s10545-005-0095-9. [DOI] [PubMed] [Google Scholar]
- 41.Prunty H., Manwaring V., Carey T., Harvey K., Burke D., Lukovic B., Khair U., Cleary M., Davison J., Guilder L., Heales S. Urinary glucose tetrasaccharide: A useful prognostic biomarker for Pompe disease? J. Inherit. Metab. Dis. 2019;42(Suppl. 1):460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary material