Abstract
Objective
Glucagon-like peptide-1 is a nutrient-sensitive hormone secreted from enteroendocrine L cells within the small and large bowel. Although GLP-1 levels rise rapidly in response to food ingestion, the greatest density of L cells is localized to the distal small bowel and colon. Here, we assessed the importance of the distal gut in the acute L cell response to diverse secretagogues.
Methods
Circulating levels of glucose and plasma GLP-1 were measured in response to the administration of L cell secretagogues in wild-type mice and in mice with (1) genetic reduction of Gcg expression throughout the small bowel and large bowel (GcgGut−/-) and (2) selective reduction of Gcg expression in the distal gut (GcgDistalGut−/-).
Results
The acute GLP-1 response to olive oil or arginine administration was markedly diminished in GcgGut−/- but preserved in GcgDistalGut−/- mice. In contrast, the increase in plasma GLP-1 levels following the administration of the GPR119 agonist AR231453, or the melanocortin-4 receptor (MC4R) agonist LY2112688, was markedly diminished in the GcgDistalGut−/- mice. The GLP-1 response to LPS was also markedly attenuated in the GcgGut−/- mice and remained submaximal in the GcgDistalGut−/- mice. Doses of metformin sufficient to lower glucose and increase GLP-1 levels in the GcgGut+/+ mice retained their glucoregulatory activity, yet they failed to increase GLP-1 levels in the GcgGut−/- mice. Surprisingly, the actions of metformin to increase plasma GLP-1 levels were substantially attenuated in the GcgDistalGut−/- mice.
Conclusion
These findings further establish the importance of the proximal gut for the acute response to nutrient-related GLP-1 secretagogues. In contrast, we identify essential contributions of the distal gut to (i) the rapid induction of circulating GLP-1 levels in response to pharmacological selective agonism of G-protein-coupled receptors, (ii) the increased GLP-1 levels following the activation of Toll-Like Receptors with LPS, and iii) the acute GLP-1 response to metformin. Collectively, these results reveal that distal gut Gcg + endocrine cells are rapid responders to structurally and functionally diverse GLP-1 secretagogues.
Keywords: Peptides, Intestine, Pancreas, Diabetes, Obesity, Metformin, Inflammation
Graphical abstract
Highlights
-
•
Distal gut Gcg expression is dispensable for GLP-1 response to olive oil and arginine.
-
•
GLP-1 response to GPR119 and MCR4 agonism requires distal gut Gcg expression.
-
•
The maximal GLP-1 response to lipopolysaccharide requires distal gut Gcg expression.
-
•
The distal gut is a substantial source of GLP-1 release in response to metformin.
1. Introduction
The gastrointestinal enteroendocrine system contains dozens of phenotypically distinct plurihormonal cell types linking signals from ingested nutrients and bacterial metabolites to the control of peptide hormone secretion [1,2]. Among the best characterized enteroendocrine cells, the L cell, notable for the synthesis and secretion of multiple proglucagon-derived peptides (PGDPs), has received considerable scrutiny. Indeed, posttranslational processing of proglucagon in the small intestine and large intestine yields glicentin, oxyntomodulin, GLP-1 and GLP-2 [3,4], PGDPs with roles in the regulation of gut motility, mucosal integrity, nutrient absorption, the control of appetite, and nutrient assimilation [5]. Moreover, the clinical development of GLP-1R agonists for the treatment of diabetes and obesity and a GLP-2R agonist for the therapy of intestinal failure [6] has heightened interest in understanding the translational biology of gut PGDP secretion and action.
The analysis of the location and distribution of L cells suggests an increasing gradient of the L cell number and PGDP content from the proximal gut to the distal gut, with the highest levels of Gcg mRNA transcripts and PGDPs being detected within the terminal ileum and colon [[7], [8], [9]]. Paradoxically, however, plasma levels of gut PGDPs, exemplified by GLP-1, increase within minutes of food ingestion, timing inconsistent with the notion that ingested complex macronutrients would be enzymatically digested and transported to the distal gut. Accordingly, several competing theories have evolved to reconcile these observations. First, considerable evidence, largely from preclinical studies, supports the existence of a proximal-distal gut axis, whereby neural or hormonal signals are rapidly conveyed to distal gut L cells enabling GLP-1 secretion [10]. A second hypothesis invokes the functional importance of proximal gut L cells in the jejunum as sufficient to generate a rapid initial rise in GLP-1 secretion accounting for increased circulating GLP-1 levels within minutes of food intake [9,11,12].
More recently, the putative importance of pancreatic islet GLP-1 has received renewed attention. Although the levels of processed bioactive GLP-1 are very low in the normal mouse and human pancreas [13], islets examined ex vivo secrete GLP-1 [14]. Moreover, the development of diabetes and/or pancreatic injury has been associated with increased expression of prohormone convertase-1 (Pcsk1) in islet α-cells, accompanied by the enhanced biosynthesis and liberation of bioactive islet GLP-1 [15]. Strikingly, mice with selective reactivation of Gcg expression in the pancreas reveal an important glucoregulatory role for islet glucagon and/or GLP-1 production [16], rekindling interest in the physiological and pathological circumstances under which the pancreatic islets may represent an important source of glucoregulatory PGDPs, including GLP-1.
To better understand the relative role of the proximal gut and the distal gut in the generation of circulating GLP-1, we recently generated lines of mice with substantial elimination of Gcg expression in both the small intestine and large intestine (GcgGut−/-) or more selective loss of distal gut Gcg expression in the terminal ileum and colon (GcgDistalGut−/-) [13]. The analysis of these mice reinforced the importance of the gut as the predominant source of circulating GLP-1. Unexpectedly, circulating levels of GLP-1 were also lower in the fasting state, and glucose tolerance was impaired in GcgDistalGut−/- mice [13], prompting questions about the relative contributions of the proximal and distal gut to the control of GLP-1 levels in the interprandial state and following nutrient ingestion. Here, we examined the contribution of distal gut Gcg expression to the acute response to ingested nutrients such as the amino acid arginine and olive oil, as well as pharmacological administration of an oral GPR119 agonist and parenteral administration of a melanocortin 4 receptor (MC4R) agonist, lipopolysaccharide (LPS), and metformin. Our findings reveal the unexpected importance of the distal gut Gcg system for the rapid initial response to functionally diverse L cell secretagogues.
2. Materials and methods
2.1. Animals
All studies were conducted in accordance with protocols approved by the Sinai Health System and The Centre for Phenogenomics (TCP, Toronto, ON, Canada). In vivo studies were performed predominantly in adult male mice beginning at 12 weeks old. As we did not observe sex-specific differences in secretagogue responses, in some cases, littermate-matched female mice were also used as appropriately noted in Figure Legends. The mice were housed in groups of up to five in microisolator cages in a pathogen-free facility on a 12/12 light–dark cycle. All animals had ad libitum access to irradiated rodent chow (18% kcal from fat, Harlan Teklad, Mississauga, ON, Canada) and sterile water unless otherwise noted.
GcgGut−/-, GcgDistalGut−/-, and their littermate control +/+ mice were generated and genotyped as described previously [13,16]. Following the conclusion of experiments, Gcg knockdown was assessed in segments of the gut and pancreas to verify the expression and to remove animals with unintended germline deletion, as described previously [13].
2.2. Acute in vivo studies
To assess rapid plasma GLP-1 responses to specified secretory agents (Table 1), mice were subjected to acute experiments to detect peak plasma GLP-1 levels independent of normal food intake. Mice were fasted overnight (∼16 h) in wire-bottom cages to minimize the ingestion of feces and bedding, with normal access to water. After the fasting period, mice were given a single bolus of the secretagogue by either oral gavage or intraperitoneal injection. Blood glucose was measured using a glucometer (Aviva glucometer, Accu-Chek, Roche, Toronto, ON, Canada), and blood was collected in lithium-heparin-coated capillary Microvette tubes (Sarstedt, Inc.) at the specified times, including at time 0, immediately before secretagogue treatment. The blood was quickly mixed with 10% TED (vol/vol) (5,000 KIU/mL aprotinin) (Sigma A6279, CAS #9087-70-1), 1.2 mg/mL EDTA, and 0.1 nmol/L diprotin A, (Sigma D3822, CAS #90614-45-5). Plasma was then isolated by centrifugation and then stored at −80 °C until subsequent hormone analysis.
Table 1.
GLP-1 secretagogues.
| Treatment | Description | Route of Admin | Vehicle | Dose | Manufacturer (CAS#) |
|---|---|---|---|---|---|
| Olive oil | Macronutrient | Oral | None | 200 μL | Sigma O1514 (8000-25-0) |
| l-Arginine | Amino acid | Oral | Water | 2 g/kg | Sigma A5006 (74-79-3) |
| AR231453 | GPR119 agonist | Oral | 80% Polyethyleneglycol-400, 10% Tween-80, 10% ethanol |
10 mg/kg | PEG 400, Sigma P3265 (25322-68-3) Tween-80 Sigma P4780 (9005-65-6) Abcam (CAS# 733750-99-7) |
| LY2112688 | Melanocortin-4 receptor (MC4R) peptide agonist | Intraperitoneal | PBS | 3 mg/kg | Bachem (819048-44-7) |
| Lipopolysaccharide O55:B5, O111:B4 |
Inflammatory stimulus | Intraperitoneal | PBS | 1 mg/kg | Sigma L2880 and L3024 |
| Metformin | Common diabetes drug | Oral | Water | 50 and 150 mg/kg | MP Biomedicals, Solon OH, (1115-70-4) |
2.3. Glucose tolerance tests
Prior to testing, mice were fasted overnight (∼16 h). Using a triple-crossover study design, all mice were randomized to receive an oral gavage of water (vehicle), 50 mg/kg metformin, or 150 mg/kg metformin. Treatments were switched for subsequent tests, occurring 2 weeks apart for sufficient recovery/washout, so that all animals received all treatments. Sixty minutes after treatment, a glucose bolus was provided. A dose of 2 g/kg d-glucose in water (Sigma, catalog# G8270) was used for oral glucose tolerance tests (oGTTs), and 1.5 g/kg d-glucose in PBS was used for intraperitoneal glucose tolerance tests (ipGTTs). Blood glucose was monitored at specified time points, and ∼60 μL of the whole blood was collected via tail vein at time 0 (relative to glucose administration), 15 min, and 60 min and mixed with 10% TED. Plasma was separated and stored at −80 °C for subsequent hormone analysis.
2.4. Hormone assays
Plasma samples frozen at −80 °C were thawed on ice on the day of hormone analyses. Insulin was measured using the Mouse Ultrasensitive Insulin ELISA (Alpco, 80-INSMSU-E01, 5 μL volume). Total GLP-1 was measured using V-PLEX GLP-1 Total Kit (Mesoscale Discovery, K1503PD, 25 uL volume). Unknowns were extrapolated from standard curves run in duplicate, according to provided protocols.
2.5. Data analysis
All graphs were produced and data analyzed using GraphPad Prism 7.0e. All graphical values are presented as mean ± SD. Statistical significance was calculated using either a two-tailed t-test or ANOVA with paired Tukey's multiple comparison test, where appropriate. A P value < 0.05 was considered statistically significant.
3. Results
To elucidate the importance of the distal gut in acute GLP-1 secretion, we studied GcgGut−/- and GcgDistalGut-/- mice and their respective wild-type littermate controls. GcgGut−/- mice were generated by crossing Gcgflox/flox mice with Vil-Cre mice and exhibit markedly reduced Gcg expression in both small bowel and large bowel [13]. GcgDistalGut−/- mice were generated by crossing Gcgflox/flox mice with Cdx2-Cre mice and display substantial attenuation of Gcg expression in the distal ileum and colon [13]. For all experiments, wild-type, Gcgflox/flox, Cdx2-Cre, or Vil-Cre littermates were pooled and studied as controls. To assess the consequences of reduced Gcg expression on the secretory capacity of gut L cells, we focused on GLP-1 due to its metabolic importance and the simultaneous availability of sensitive validated assays for the detection of circulating GLP-1 in mice [17].
3.1. Distal Gut Gcg expression is dispensable for nutrient-stimulated increments in plasma GLP-1
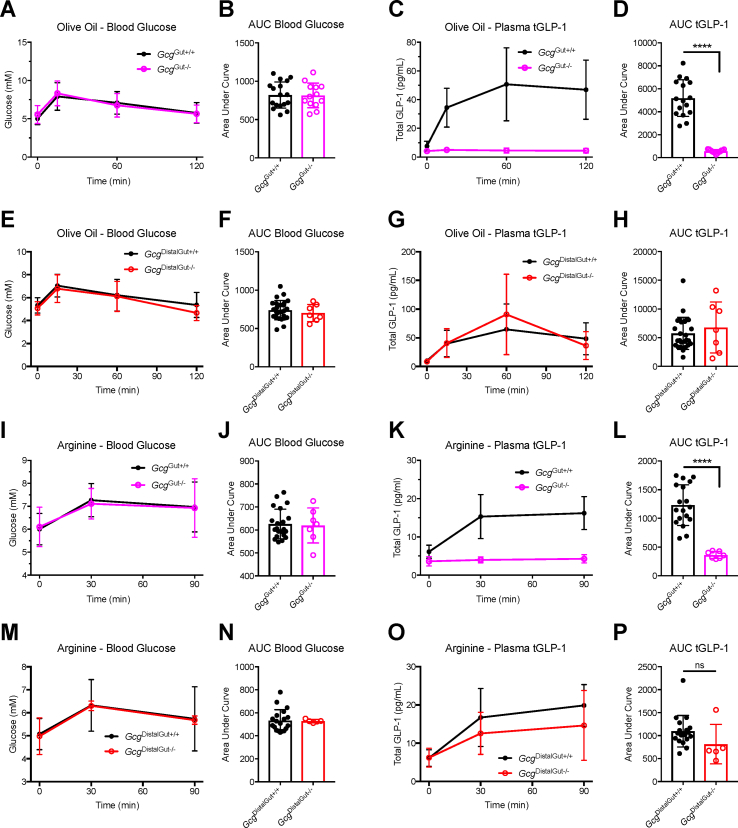
Prior studies determined that the secretion of GLP-1 following oral glucose challenge originates predominantly from the proximal gut [13]. We then assessed the GLP-1 levels in response to olive oil or arginine, known as stimulators of PGDP secretion [17]. Olive oil administered by oral gavage produced a modest rise in blood glucose excursion, consistent with a modest stress response, which was similar across all genotypes (Figure 1A,B, E, and F). In contrast to robust plasma GLP-1 excursions in GcgGut+/+ mice, GcgGut−/- mice failed to increase the levels of plasma total GLP-1 (tGLP-1) after olive oil (Figure 1C,D). Conversely, plasma GLP-1 levels were not different in GcgDistalGut+/+ versus GcgDistalGut−/- mice (Figure 1G,H), revealing that distal gut Gcg expression is not required for acute increments in plasma GLP-1 levels after lipid ingestion.
Figure 1.
Distal gut Gcg expression is not required for nutrient-stimulated plasma GLP-1 excursion.GcgGut−/-, GcgDistalGut−/-, and littermate control mice were given an oral gavage of olive oil (200 μL) or arginine (2 g/kg BW) at time zero, with subsequent monitoring of blood glucose and total GLP-1 (tGLP-1) levels. (A, B) Time course of blood glucose levels during olive oil stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (C, D) Time course of total tGLP-1 levels during olive oil stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (E, F) Time course of blood glucose levels during olive oil stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. (G, H) Time course of total tGLP-1 levels during olive oil stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. (I, J) Time course of blood glucose levels during arginine stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (K, L) Time course of total tGLP-1 levels during arginine stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (M, N) Time course of blood glucose levels during arginine stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. (O, P) Time course of total tGLP-1 levels during arginine stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. GcgGut+/+, n = 17–21 (males); GcgGut−/-, n = 7–13 (males); GcgDistalGut+/+, n = 19–26 (males + females); GcgDistalGut−/-, n = 5–7 (males + females). Statistical significance was determined using the two-tailed t-test. ∗∗∗∗P < 0.0001.
We next challenged mice with oral arginine, an amino acid known to potently stimulate GLP-1 secretion [18]. Blood glucose levels rose modestly following arginine gavage in all genotypes (Figure 1I, J, M, and N). Notably, GcgGut−/- mice exhibited a markedly reduced plasma GLP-1 excursions in response to arginine (Figure 1K and L). In contrast, maximal GLP-1 levels were not different after arginine administration in GcgDistalGut+/+ versus GcgDistalGut−/- mice (Figure 1 O, P). Taken together with prior studies of glucose-stimulated GLP-1 secretion [13], oral nutrient ingestion, as exemplified by glucose, olive oil, and arginine, stimulates GLP-1 predominantly through L cells residing in the proximal murine gut.
3.2. Distal Gut Gcg expression is required for GPR119 and MC4R agonist-stimulated increases in plasma GLP-1 levels
We next examined the intestinal sites important for the transduction of GLP-1 secretory signals pursuant to the activation of two L-cell-associated G-protein-coupled receptors (GPCRs). GPR119 is activated by multiple derivatives of dietary fatty acids and regulates metabolism in part via the stimulation of incretin secretion [[19], [20], [21]]. Oral gavage of the GPR119 agonist, AR231453, had no meaningful effect on the glycemic excursion in GcgDistalGut+/+ versus GcgDistalGut−/- mice (Figure 2A,B). Surprisingly, the rise in plasma GLP-1 levels after oral AR231453 was markedly attenuated in GcgDistalGut−/- mice, implicating the importance of the distal gut for maximal L cell responses to acute GPR119 agonism (Figure 2C,D).
Figure 2.
Distal gut Gcg expression is required for acute GPR119- and MC4R-stimulated increases in plasma GLP-1.GcgDistalGut+/+ and GcgDistalGut−/- mice were treated with either an oral gavage of the GPR119 agonist, AR231453 (10 mg/kg BW), vehicle alone, or an intraperitoneal injection of the MC4R agonist, LY2112688 (3 mg/kg BW), at time zero. Blood glucose and total GLP-1 (tGLP-1) were measured at the indicated time points. (A, B) Time course of blood glucose levels during AR231453 stimulus and corresponding AUC analysis. (C, D) Time course of total tGLP-1 levels during AR231453 stimulus and corresponding AUC analysis. (E, F) Time course of blood glucose levels during LY2112688 stimulus and corresponding AUC analysis. (G, H) Time course of total tGLP-1 levels during LY2112688 stimulus and corresponding AUC analysis. For AR231453 studies: GcgDistalGut+/+ AR231453, n = 14 (males + females); GcgDistalGut+/+ Vehicle, n = 5 (males + females); GcgDistalGut−/- AR231453, n = 5 (males + females). For LY2112688 studies: GcgDistalGut+/+, n = 19 (males + females); GcgDistalGut−/-, n = 5 (males + females). Statistical significance was determined using one-way ANOVA (panels B, D) or two-tailed t-test (panels F, H). ∗∗P < 0.01; ∗∗∗P < 0.001.
The MC4R is a GPCR expressed primarily in the brain, yet MC4R has also been detected outside the central nervous system, including within mouse and human L cells [22]. Intraperitoneal injection of the MC4R-selective agonist LY2112688 had little impact on blood glucose levels in GcgDistalGut+/+ and GcgDistalGut−/- mice (Figure 2E,F). Plasma GLP-1 levels were increased following LY2112688 in GcgDistalGut+/+ mice, yet there was no GLP-1 response in GcgDistalGut−/- mice (Figure 2G,H). Hence, maximal GLP-1 excursions to either GPR119 or MC4R agonism require Gcg expression within the distal gut.
3.3. LPS requires distal Gut Gcg expression for maximal increases in plasma GLP-1
There is considerable evidence that links the administration of bacteria-derived LPS to the augmentation of L cell GLP-1 secretion in mice and humans [23,24]. Intraperitoneal injection of LPS produced a modest reduction in blood glucose levels 3 h after treatment to a similar extent in all genotypes tested (Figure 3A,B, E, and F). The increase in plasma GLP-1 after LPS was clearly dependent on gut Gcg expression as it was virtually extinguished in GcgGut−/- mice (Figure 3C,D). Intriguingly, the increase in plasma GLP-1 in LPS-treated mice remained blunted in GcgDistalGut−/- mice (Figure 3G,H). These results suggest that LPS-mediated GLP-1 secretion originates from the gut, with a component of the response arising from the distal gut.
Figure 3.
LPS requires distal gut Gcg expression for maximal increases in plasma GLP-1. Adult GcgGut−/-, GcgDistalGut−/-, and littermate control mice were subjected to acute systemic inflammation with a single intraperitoneal injection of lipopolysaccharide (LPS, 1 mg/kg BW) given at time zero. Blood glucose and total GLP-1 (tGLP-1) were monitored at the start and 3 h later. (A) Time course of blood glucose levels during LPS stimulus, and (B) blood glucose levels at the 3 h time point after LPS treatment in GcgGut+/+ and GcgGut−/- mice. (C) Time course of total tGLP-1 levels during LPS stimulus and (D) total tGLP-1 levels at the 3 h time point after LPS treatment in GcgGut+/+ and GcgGut−/- mice. (E) Time course of blood glucose levels during LPS stimulus, and (F) blood glucose levels at the 3 h time point after LPS treatment in GcgDistalGut+/+ and GcgDistalGut−/- mice. (G) Time course of total tGLP-1 levels during LPS stimulus and (H) total tGLP-1 levels at the 3 h time point after LPS treatment in GcgDistalGut+/+ and GcgDistalGut−/- mice. GcgGut+/+, n = 9 (males); GcgGut−/-, n = 6 (males); GcgDistalGut+/+, n = 26 (males + females); GcgDistalGut−/-, n = 7-11 (males + females). Statistical significance was determined using the two-tailed t-test. ∗∗P < 0.01; ∗∗∗∗P < 0.0001.
3.4. Distal Gut Gcg expression is essential for acute metformin-induced rises in plasma GLP-1
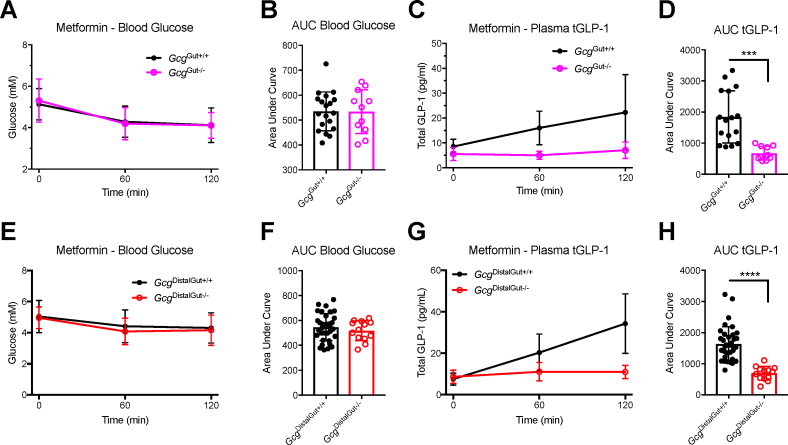
Among the many actions of metformin that may contribute to its glucoregulatory properties is the enhancement of gut GLP-1 secretion [[25], [26], [27]]. Oral metformin administration at a dose of 150 mg/kg produced a small reduction in blood glucose that was similar across genotypes (Figure 4A,B, E, and F). Notably, metformin failed to increase plasma GLP-1 levels in GcgGut−/- mice (Figure 4C,D). Unexpectedly, metformin also did not increase plasma GLP-1 levels in GcgDistalGut−/- mice (Figure 4G,H). Hence, distal gut Gcg expression is required for the acute metformin-induced increment in plasma GLP-1.
Figure 4.
Distal gut Gcg expression is essential for acute metformin-induced rises in plasma GLP-1. Blood glucose and total GLP-1 (tGLP-1) levels in GcgGut−/-, GcgDistalGut−/-, and littermate control mice following oral gavage of metformin (150 mg/kg BW). (A, B) Time course of blood glucose levels during metformin stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (C, D) Time course of total tGLP-1 levels during metformin stimulus and corresponding AUC analysis in GcgGut+/+ and GcgGut−/- mice. (E, F) Time course of blood glucose levels during metformin stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. (G, H) Time course of total tGLP-1 levels during metformin stimulus and corresponding AUC analysis in GcgDistalGut+/+ and GcgDistalGut−/- mice. GcgGut+/+, n = 19 (males); GcgGut−/-, n = 11 (males); GcgDistalGut+/+, n = 35 (males + females); GcgDistalGut−/-, n = 13 (males + females). Statistical significance was determined using the two-tailed t-test. ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
3.5. Gut Gcg expression and increases in plasma GLP-1 are not required for metformin-mediated glucoregulation
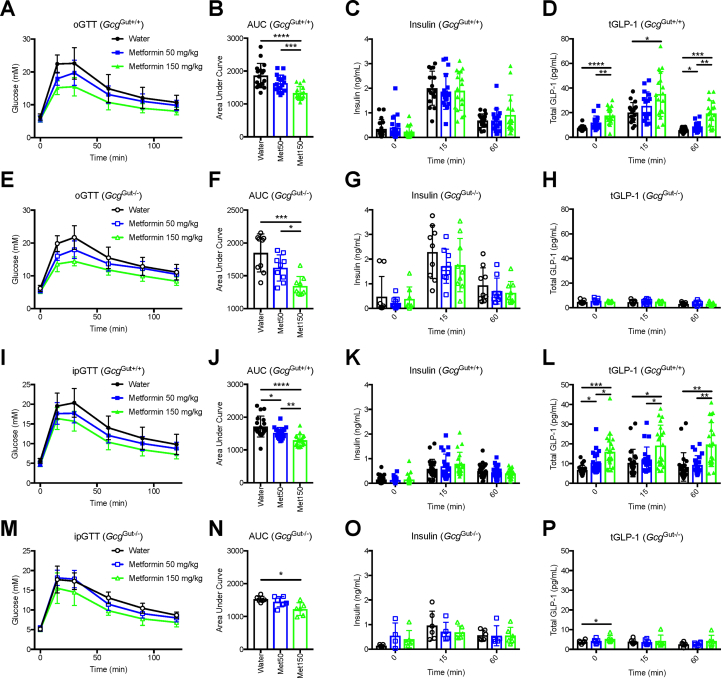
To determine if a metformin-induced increment in plasma GLP-1 was required for glucoregulation, we treated GcgGut+/+ and GcgGut−/- mice with oral metformin at doses of 50 and 150 mg/kg 60 min prior to an oral (oGTT) and intraperitoneal glucose tolerance tests (ipGTT). In the context of an oGTT, a dose-dependent reduction in blood glucose was observed in GcgGut+/+ mice (Figure 5A,B). The various metformin doses did not impact insulin levels (Figure 5C); however, plasma GLP-1 levels were increased after metformin administration (Figure 5D). Notably, plasma glucose and insulin responses to metformin were similar in GcgGut+/+ and GcgGut−/- mice (Figure 5E–G), despite the lack of increase in plasma GLP-1 levels in GcgGut−/- mice (Figure 5H). Consistent with the oGTT results, metformin lowered glucose during an intraperitoneal glucose challenge independent of the changes in plasma GLP-1 (Figures 5I–5P). Taken together, these results suggest that the acute glucoregulatory actions of metformin do not require an increase in plasma levels of GLP-1.
Figure 5.
Gut Gcg expression and increases in plasma GLP-1 are not required for metformin-mediated glucoregulation. Oral glucose tolerance tests (oGTT, 2 g/kg BW) and intraperitoneal glucose tolerance tests (ipGTT, 1.5 g/kg BW) were conducted in adult GcgGut−/-, and littermate control mice. All mice were given water, metformin 50 mg/kg, or metformin 150 mg/kg by oral gavage 60 min prior to glucose bolus in a triple-crossover study design. (A, B) Glucose levels and AUC analysis from oGTT in GcgGut+/+ mice. (C) Insulin and (D) total GLP-1 (tGLP-1) levels during oGTT at 0, 15, and 60 min after glucose in GcgGut+/+ mice. (E, F) Glucose levels and AUC analysis from oGTT in GcgGut−/- mice. (G) Insulin and (H) tGLP-1 levels during oGTT at 0, 15, and 60 min after glucose in GcgGut−/- mice. (I, J) Glucose levels and AUC analysis from ipGTT in GcgGut+/+ mice. (K) Insulin and (L) Total GLP-1 (tGLP-1) levels during ipGTT at 0, 15, and 60 min after glucose in GcgGut+/+ mice. (M, N) Glucose levels and AUC analysis from ipGTT in GcgGut−/- mice. (O) Insulin and (P) tGLP-1 levels during ipGTT at 0, 15, and 60 min after glucose in GcgGut−/- mice. GcgGut+/+, n = 17–22 (males); GcgGut−/-, n = 6–9 (males). Statistical significance was assessed using one-way ANOVA with Tukey's multiple comparison test. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
4. Discussion
The findings that Gcg mRNA transcripts and corresponding levels of PGDPs, including GLP-1, are distributed throughout the small bowel and large bowel have engendered considerable debate toward understanding the relative importance of the proximal and the distal guts in the control of rapid meal-stimulated increases in circulating levels of GLP-1. Considerable evidence suggests that nutrient-stimulated hormonal mediators such as glucose-dependent insulinotropic polypeptide (GIP) liberated from the proximal gut or signals conveyed via neural transmission involving acetylcholine and gastrin-releasing peptide contribute to the amplification of L cell GLP-1 secretion from the distal gut in preclinical studies [10]. Furthermore, luminal perfusion of the proximal and the distal small bowel revealed a considerably greater increment in circulating GLP-1 following the stimulation of distal versus proximal gut L cells [28]. On the other hand, the levels of Gcg mRNA transcripts and GLP-1 content in the human proximal small bowel were not substantially different from those found more distally, highlighting potential contributions of the proximal small bowel to GLP-1 biosynthesis and secretion [9]. Moreover, the capacity of the isolated proximal rat gut to secrete GLP-1 in response to GRP or luminal peptone was not meaningfully different relative to the amounts of GLP-1 secreted by distal gut segments in acute short-term studies [12]. Hence, these contrasting findings illustrate the challenges in the interpretation of the relative importance of the proximal and the distal gut for the acute GLP-1 response to various secretagogues.
To better understand the contributions of the distal gut to the acute increase in circulating GLP-1 levels evident following the administration of nutrients and pharmacological agents, we employed mouse lines engineered to exhibit a substantial reduction of the Gcg expression throughout the small bowel and large bowel or predominantly in the terminal ileum and colon [13]. We previously determined that GLP-1 levels rose briskly following oral administration of glucose to GcgDistalGut−/- mice, indirectly affirming the functional competence of proximal gut L cells for maximal glucose-regulated GLP-1 secretion. The current studies using the administration of olive oil or arginine further substantiate the redundancy of distal gut L cells for nutrient-stimulated GLP-1 secretion, as plasma levels of GLP-1 were not substantially different after these nutrient challenges in GcgDistalGut+/+ versus GcgDistalGut−/- mice.
In marked contrast, plasma GLP-1 responses to the GPR119 agonist AR231453, or the MC4R agonist LY2112688, were substantially attenuated in GcgDistalGut−/- mice. A functional GPR119 recognized by several lipid-related ligands, including cannabinoids, monoacylglycerols such as 2-oleoyl glycerol (2-OG), and oleoylethanolamide, is expressed within murine enteroendocrine L cells in the small bowel and large bowel [29,30]. However, the relative contributions of regional L cell populations to the rise in circulating GLP-1 levels following acute GPR119 activation have not previously been determined. To answer this question, we used the validated pharmacological GPR119 agonist AR231435, an agent we and others have previously shown to be highly selective for the GPR119 receptor [[19], [20], [21]]. Remarkably, although AR231453 was previously shown to stimulate the secretion of GIP, a hormone predominantly secreted from the proximal intestine [19,20], plasma levels of GLP-1 did not rise in response to oral AR231453 in GcgDistalGut−/- mice. Intriguingly, the stimulation of GLP-1 secretion by enteral olive oil, while appearing to be mediated through the activation of proximal gut L cells in the current experiments, might theoretically have required contributions to the GLP-1 secretion derived from lipid metabolites acting through GPR119 in the distal gut. Collectively, our findings are consistent with established actions of GPR119 agonists acting predominantly through mouse and human colonic L cells [30,31] and further highlight the importance of the distal gut L cell as an important target for GPR119-dependent GLP-1 secretion.
MC4R is an extensively studied receptor mediating the regulation of food intake and body weight through central and peripheral mechanisms including control of food intake and energy expenditure. Studies in mice and rats demonstrated that GLP-1 reduces food intake through MC4R-independent pathways [32,33], providing a rationale for the successful use of GLP-1R agonists in the treatment of human subjects with obesity arising from genetic mutations in the MC4R pathway [34]. MC4R has also been localized to enteroendocrine L cells, most notably in the colon, functionally linked to the secretion of L cell peptides and increased circulating levels of peptide YY and GLP-1 [22]. Consistent with these findings, the MC4R agonist LY2112688 rapidly increased the circulating levels of GLP-1 in GcgDistalGut+/+ but not in GcgDistalGut−/- mice. Hence, although MC4R is expressed within cell populations of the stomach, small bowel, and large bowel [22], the distal gut is required for the acute GLP-1 response to the MC4R agonism.
Beyond roles in transduction of nutrient-related signals, substantial evidence supports a simultaneous role for L cells as pathogen sensors [35], linking inflammatory signals, including bacterial metabolites and cell wall products exemplified by LPS, to GLP-1 secretion in animals and humans [23,24,36]. Notably, transient vascular ischemia and mesenteric injury in the proximal small bowel of mice and human subjects produce a rapid rise in plasma GLP-1 levels [23]. Furthermore, LPS increased GLP-1 secretion from small bowel-derived STC-1 L cells [23], raising the possibility that both the small and large bowel L cells are capable of sensing tissue injury and inflammation linked to the enhanced GLP-1 secretion. Our findings reveal that the LPS-induced increment in circulating GLP-1 levels was attenuated, but not abolished, in GcgDistalGut−/- mice. Hence, it seems likely that LPS engages L cells in both the proximal and distal guts to enhance GLP-1 secretion.
The pleiotropic actions of metformin have also been linked to the acute GLP-1 secretion in preclinical studies [37] and in humans with type 2 diabetes [25]. Consistent with the importance of the gastrointestinal tract as a site of metformin action, gut-targeted metformin produces a rapid rise in plasma GLP-1 levels detectable within 60 min of metformin administration [38]. Moreover, intraduodenal metformin administration rapidly lowered glycemia and hepatic glucose production in rats through GLP-1R-dependent mechanisms [39], further highlighting the potential importance of the small bowel as a target for metformin-GLP-1 interactions. Nevertheless, our current findings reveal that low doses of enteral metformin are still capable of lowering glucose independent of any detectable changes in plasma GLP-1 levels in GcgGut−/- mice. These observations are consistent with previous results demonstrating the preservation of the glucoregulatory actions of a range of metformin concentrations in Glp1r−/- mice [27]. Although enteral metformin rapidly augments glucose-stimulated GLP-1 levels following perfusion of both the proximal and distal small bowels in humans [40], the GLP-1-stimulatory actions of metformin were markedly reduced in the absence of concomitant glucose challenge in GcgDistalGut−/- mice, implicating distal gut L cells as key intestinal targets of metformin action in mice. It is possible that the method and formulation of metformin administration in our studies preferentially enable metformin targeting to the distal gut. Nevertheless, our findings linking metformin action to the distal gut are also consistent with recent observations demonstrating that oral metformin induces GDF-15 expression predominantly through targeting cells within the distal small bowel and colon [41].
5. Conclusions and limitations
In summary, the current findings highlight the importance of the gastrointestinal tract, and particularly the distal gut, as key sites of the Gcg expression linked to the acute stimulation of L cell secretion and increased levels of circulating GLP-1 in mice. Nevertheless, these studies have limitations that restrict conclusions to the experimental models employed herein. It must be noted that rapid pharmacological parenteral administration of secretagogues would reach the distal gut and its relatively greater mass of L cells much faster than oral gavage of nutrient-related L cell secretagogues preferentially targeting the smaller number of L cells in the jejunum. Hence, the interpretation of the data should be tempered by these differences in routes of administration and rates of access of various secretagogues to L cells distributed within the proximal and distal guts.
Notably, we studied lean, nonobese mice without high-fat feeding, obesity, or diabetes, conditions that might modify the nature and responsivity of L cells along the gastrointestinal tract [42]. Importantly, GIP is known to increase GLP-1 secretion in mice [10], and some of the secretagogues employed here, such as olive oil and AR231453, are also known to enhance GIP secretion. Moreover, plasma GIP levels are increased in mice following the reduction of gut Gcg expression [13]. Hence, it remains possible that, for some secretagogues, the extent of change in plasma GLP-1 levels might also be influenced by simultaneous enhancement of the GIP secretion.
Our analyses of plasma GLP-1 levels were limited to measurements at baseline and only 1 or 2 time points, precluding definitive assessment of any pattern in GLP-1 secretory responses that might become apparent with more frequent blood sampling. Furthermore, experimental obesity and diabetes are frequently associated with pancreatic and islet inflammation and enhanced α-cell GLP-1 production [35], conditions absent in the lean healthy mice examined in our current studies. Moreover, our studies employed mice with germline elimination of Gcg expression within the small and large bowel; hence, it is possible that adaptive compensatory mechanisms arising during growth and development of the gut may have contributed to the pharmacological responses observed herein. Nevertheless, our results, together with recent studies linking selective colonic L cell activation to increased plasma levels of GLP-1 [43], clearly demonstrate that the distal gut represents an important site for robust L cell secretion, capable of impacting circulating levels of GLP-1. These findings have potential relevance for informing strategies targeting L cells for the treatment of metabolic disorders.
Authors’ Contributions
BP, BY, DM, JK, YS, and DD designed the experiments and, together with DS, reviewed and analyzed the data and wrote and/or reviewed the manuscript. BP, BY, DM, JK, and YS carried out the experiments. DJD secured funding for the studies and is the guarantor of the data.
Acknowledgments
DJD is supported in part by a Novo Nordisk, Banting and Best Diabetes Centre, University of Toronto Chair in incretin biology and CIHR Foundation (grant 154321). Mt. Sinai Hospital receives support for studies of incretin biology in the Drucker lab from Novo Nordisk. YS was supported by a fellowship from the BBDC and the Kangbuk Samsung Hospital.
Conflicts of interest
DJD receives consulting honoraria from Intarcia, Merck, Novo Nordisk, Pfizer, and Sanofi within the past 12 months for advisory boards and lectures related to incretin biology. None of the other authors have conflicts of interest. Following the completion of these studies, BP became a full-time employee of Roche Canada Inc.
References
- 1.Gribble F.M., Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nature Reviews Endocrinology. 2019;15(4):226–237. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 2.Drucker D.J. Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab. 2016;101(3):778–786. doi: 10.1210/jc.2015-3449. [DOI] [PubMed] [Google Scholar]
- 3.Mojsov S., Heinrich G., Wilson I.B., Ravazzola M., Orci L., Habener J.F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Journal of Biological Chemistry. 1986:26111880–26111889. [PubMed] [Google Scholar]
- 4.Orskov C., Holst J.J., Poulsen S.S., Kirkegaard P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia. 1987:30874–30881. doi: 10.1007/BF00274797. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval D.A., D'Alessio D.A. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiological Reviews. 2015;95(2):513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 6.Drucker D.J., Habener J.F., Holst J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. Journal of Clinical Investigation. 2017;127(12):4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Wielen N., Ten Klooster J.P., Muckenschnabl S., Pieters R., Hendriks H.F., Witkamp R.F. The noncaloric sweetener rebaudioside A stimulates glucagon-like peptide 1 release and increases enteroendocrine cell numbers in 2-dimensional mouse organoids derived from different locations of the intestine. Journal of Nutrition. 2016;146(12):2429–2435. doi: 10.3945/jn.116.232678. [DOI] [PubMed] [Google Scholar]
- 9.Jorsal T., Rhee N.A., Pedersen J., Wahlgren C.D., Mortensen B., Jepsen S.L. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61(2):284–294. doi: 10.1007/s00125-017-4450-9. [DOI] [PubMed] [Google Scholar]
- 10.Lim G.E., Brubaker P.L. Glucagon-like peptide 1 secretion by the L-cell: the view from within. Diabetes. 2006;55(Supplement_2):S70–S77. [Google Scholar]
- 11.Glass L.L., Calero-Nieto F.J., Jawaid W., Larraufie P., Kay R.G., Gottgens B. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab. 2017;6(10):1296–1303. doi: 10.1016/j.molmet.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svendsen B., Pedersen J., Albrechtsen N.J., Hartmann B., Torang S., Rehfeld J.F. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156(3):847–857. doi: 10.1210/en.2014-1710. [DOI] [PubMed] [Google Scholar]
- 13.Song Y., Koehler J.A., Baggio L.L., Powers A.C., Sandoval D.A., Drucker D.J. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 2019;30(5):976–986. doi: 10.1016/j.cmet.2019.08.009. e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchetti P., Lupi R., Bugliani M., Kirkpatrick C.L., Sebastiani G., Grieco F.A. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55(12):3262–3272. doi: 10.1007/s00125-012-2716-9. [DOI] [PubMed] [Google Scholar]
- 15.O'Malley T.J., Fava G.E., Zhang Y., Fonseca V.A., Wu H. Progressive change of intra-islet GLP-1 production during diabetes development. Diabetes Metab Res Rev. 2014;30(8):661–668. doi: 10.1002/dmrr.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers A.P., Sorrell J., Haller A., Roelofs K., Hutch C., Kim K.-S. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 2017;25(4):927–934. doi: 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019:3072–3130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemmensen C., Smajilovic S., Smith E.P., Woods S.C., Brauner-Osborne H., Seeley R.J. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology. 2013;154(11):3978–3983. doi: 10.1210/en.2013-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Z.L., Carroll C., Alfonso J., Gutierrez V., He H., Lucman A. A role for intestinal endocrine cell-expressed GPR119 in glycemic control by enhancing GLP-1 and GIP release. Endocrinology. 2008;149(5):2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 20.Flock G., Holland D., Seino Y., Drucker D.J. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011;152(2):374–383. doi: 10.1210/en.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panaro B.L., Flock G.B., Campbell J.E., Beaudry J.L., Cao X., Drucker D.J. Beta-cell inactivation of Gpr119 unmasks incretin dependence of GPR119-mediated glucoregulation. Diabetes. 2017;66(6):1626–1635. doi: 10.2337/db17-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panaro B.L., Tough I.R., Engelstoft M.S., Matthews R.T., Digby G.J., Moller C.L. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014;20(6):1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebrun L.J., Lenaerts K., Kiers D., Pais de Barros J.P., Le Guern N., Plesnik J. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Reports. 2017;21(5):1160–1168. doi: 10.1016/j.celrep.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen A.T., Mandard S., Dray C., Deckert V., Valet P., Besnard P. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes. 2014;63(2):471–482. doi: 10.2337/db13-0903. [DOI] [PubMed] [Google Scholar]
- 25.Migoya E.M., Bergeron R., Miller J.L., Snyder R.N., Tanen M., Hilliard D. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clinical Pharmacology & Therapeutics. 2010;88(6):801–808. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- 26.Mulherin A.J., Oh A.H., Kim H., Grieco A., Lauffer L.M., Brubaker P.L. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152(12):4610–4619. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
- 27.Maida A., Lamont B.J., Cao X., Drucker D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia. 2011;54(2):339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Young R.L., Bound M., Hu S., Jones K.L., Horowitz M. Comparative effects of proximal and distal small intestinal glucose exposure on glycemia, incretin hormone secretion, and the incretin effect in health and type 2 diabetes. Diabetes Care. 2019;42(4):520–528. doi: 10.2337/dc18-2156. [DOI] [PubMed] [Google Scholar]
- 29.Ekberg J.H., Hauge M., Kristensen L.V., Madsen A.N., Engelstoft M.S., Husted A.S. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40) Endocrinology. 2016;157(12):4561–4569. doi: 10.1210/en.2016-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss C.E., Glass L.L., Diakogiannaki E., Pais R., Lenaghan C., Smith D.M. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides. 2016:7716–7720. doi: 10.1016/j.peptides.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox H.M., Tough I.R., Woolston A.M., Zhang L., Nguyen A.D., Sainsbury A. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11(6):532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards C.M., Abbott C.R., Sunter D., Kim M., Dakin C.L., Murphy K.G. Cocaine- and amphetamine-regulated transcript, glucagon-like peptide-1 and corticotrophin releasing factor inhibit feeding via agouti-related protein independent pathways in the rat. Brain Research. 2000;866(1–2):128–134. doi: 10.1016/s0006-8993(00)02257-5. [DOI] [PubMed] [Google Scholar]
- 33.Nonogaki K., Suzuki M., Sanuki M., Wakameda M., Tamari T. The contribution of serotonin 5-HT2C and melanocortin-4 receptors to the satiety signaling of glucagon-like peptide 1 and liraglutide, a glucagon-like peptide 1 receptor agonist, in mice. Biochemical and Biophysical Research Communications. 2011;411(2):445–448. doi: 10.1016/j.bbrc.2011.06.175. [DOI] [PubMed] [Google Scholar]
- 34.Iepsen E.W., Zhang J., Thomsen H.S., Hansen E.L., Hollensted M., Madsbad S. Patients with obesity caused by melanocortin-4 receptor mutations can Be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 2018;28(1):23–32. doi: 10.1016/j.cmet.2018.05.008. e23. [DOI] [PubMed] [Google Scholar]
- 35.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Chimerel C., Emery E., Summers David K., Keyser U., Gribble Fiona M., Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Reports. 2014;9(4):1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuda N., Inoue T., Nagakura T., Yamazaki K., Kira K., Saeki T. Enhanced secretion of glucagon-like peptide 1 by biguanide compounds. Biochemical and Biophysical Research Communications. 2002;298(5):779–784. doi: 10.1016/s0006-291x(02)02565-2. [DOI] [PubMed] [Google Scholar]
- 38.DeFronzo R.A., Buse J.B., Kim T., Burns C., Skare S., Baron A. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: results from two randomised trials. Diabetologia. 2016;59(8):1645–1654. doi: 10.1007/s00125-016-3992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duca F.A., Cote C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21(5):506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borg M.J., Bound M., Grivell J., Sun Z., Jones K.L., Horowitz M. Comparative effects of proximal and distal small intestinal administration of metformin on plasma glucose and glucagon-like peptide-1, and gastric emptying after oral glucose, in type 2 diabetes. Diabetes Obes Metab. 2019;21(3):640–647. doi: 10.1111/dom.13567. [DOI] [PubMed] [Google Scholar]
- 41.Coll A.P., Chen M., Taskar P., Rimmington D., Patel S., Tadross J.A. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578(7795):444–448. doi: 10.1038/s41586-019-1911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dusaulcy R., Handgraaf S., Skarupelova S., Visentin F., Vesin C., Heddad-Masson M. Functional and molecular adaptations of enteroendocrine L-cells in male obese mice are associated with preservation of pancreatic alpha-cell function and prevention of hyperglycemia. Endocrinology. 2016;157(10):3832–3843. doi: 10.1210/en.2016-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reimann F., Lewis J.E., Miedzybrodzka E.L., Foreman R.E., Woodward O.R.M., Kay R.G. Selective stimulation of colonic L-cells improves metabolic outcomes in mice. Diabetologia. 2020 doi: 10.1007/s00125-020-05149-w. in press. [DOI] [Google Scholar]