Abstract
Feed as a cause of poisoning in horses can occur on small or large scales. It is challenging to work up cases of suspected feed contamination, but there are resources available to veterinarians and owners. Feed contamination can be chemical or biological. This article focuses on and provides examples of chemical feed contamination including misformulation, adulteration, and natural contaminants. Additionally, recommendations for feed sampling and diagnostic submission, including legal documentation, are included.
Keywords: equine, feed toxicology
INTRODUCTION
Feed contaminants can be broken into chemical and biological. Biological contamination usually refers to bacterial contamination. Because this is a toxicology lecture, we are most concerned about chemical contaminants although Clostridium botulinum will also be mentioned.
Although some individuals will reduce consumption when unfamiliar feed is introduced, sometimes feed refusal is an early sign of contamination, especially when a new batch of a familiar feed is introduced. Feed contamination is often suspected when multiple horses at the same facilities are affected with similar feed histories and clinical signs. Since local feed mills are likely to supply multiple facilities in the same geographic area, horses from more than one stable can be affected. Incidents of contamination of products from large national feed companies occur rarely and are usually due to misformulation, for example, excessive copper in sheep feed or sodium in poultry feed. Since many horse owners have a single or only a few horses and purchase national brands of feed in only small quantities, linking the feed to the clinical signs is likely to be challenging. It is very important that suspicions of feed contamination be reported so that an investigation can be initiated and further morbidity can be prevented. Feed contamination can be reported to the manufacturer, state officials, and the U.S. Food and Drug Administration’s Center for Veterinary Medicine. State officials can include the state veterinarian’s office or the state agricultural department. The U.S. FDA CVM has an online portal (https://www.safetyreporting.hhs.gov) that can be used to report suspected contamination. Regardless of who is contacted, critical information required will include manufacturer, product name, product details, and label information such as lot number, expiration date, and weight information.
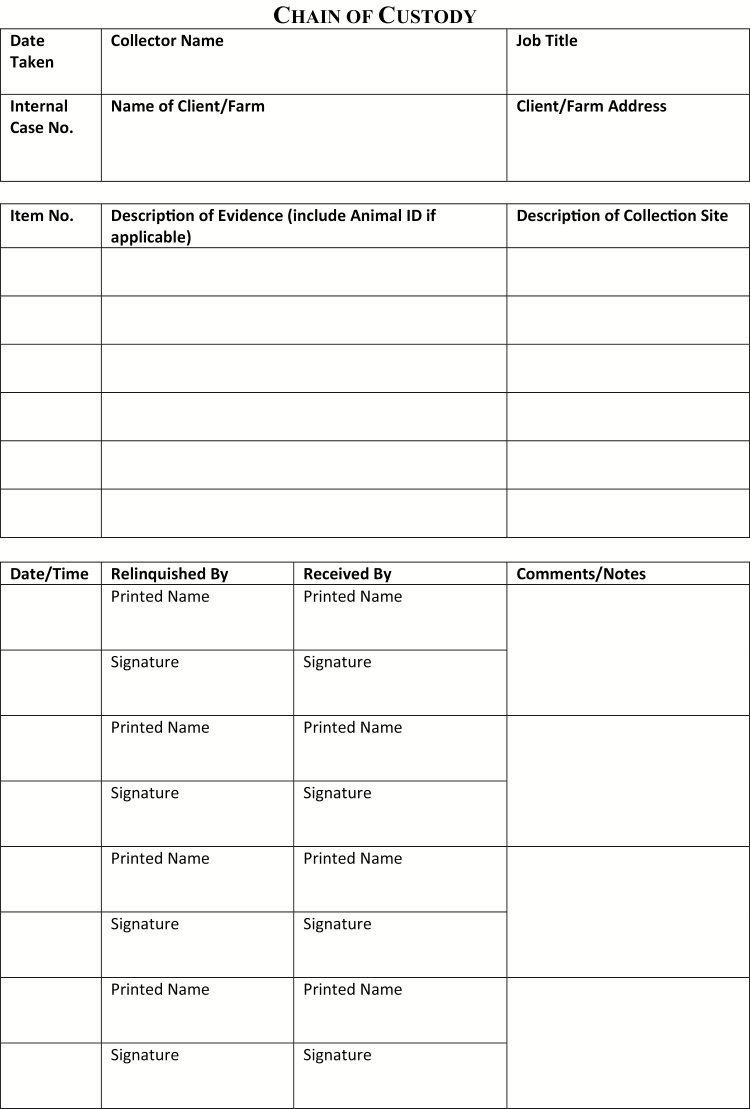
When feed contamination is suspected after a thorough clinical work-up, there are several important factors to consider. First, if the feed is a commercial product, then contamination confirmation may result in a recall. As noted above, the manufacturer, state veterinarian, and the FDA should be alerted to the suspected problem. Many times, though not always, manufacturers or regulatory agencies take financial responsibility for feed analyses, which can be cost prohibitive to horse owners. Second, if legal consequences could result from a diagnosis of contaminant-related morbidity and mortality, it is important to thoroughly document the case, including client communications, clinical findings, postmortem findings, and communications with manufacturers, laboratories, and officials at the state and national levels. Samples for analysis should be collected and placed in tamper-evident containers, individually labeled as to what they are, where and when they were collected, and who collected them, then sent to the laboratory with chain-of-custody. Tamper-evident containers can be purchased, or containers can be completely sealed with tamper-evident tape, which is dated and initialed by the collector (Gwaltney-Brandt, 2016). Chain-of-custody tags and forms can be purchased or printed. A typical chain-of-custody form has a header that includes the name, address, and contact information for the collector, date and time of collection and other sample identification information, and source information including the farm or animal owner. Below the header is a table to be filled out by anyone handling the sample. Each row includes a signature line and print name line for the person who has custody of the sample, the date, time, space for a description of the sample and container, another line for the signature of the person receiving the sample, and print name line for the recipient. Rows are filled out as the sample passes from a custodian to a recipient (Figure 1).
Figure 1.
A sample chain-of-custody form.
Sample Collection
Sampling feeds for analysis takes considerable planning and thought. The optimal feed sample is the one that made the animal sick, but that sample is in either the gastrointestinal tract or the compost heap. Often, contaminants are not homogenously dispersed within a lot of feed—for example, if the mill failed to completely clean their equipment between a batch of medicated feed for ruminants and a lot of horse feed, the first few bags of horse feed are likely to be more contaminated but the rest could be clean (Rumbeiha and Morrison, 2011). It is easy to see how spotty contaminants can be dispersed in hay, since all the weeds or insects can be located in just a few bales or just a few flakes of a bale. Some feed contaminants cause chronic toxicosis that may not present until long after the feed batch is gone. The best example of these is the pyrrolizidine alkaloids. Hepatic failure due to pyrrolizidine alkaloid-containing plants may not occur for weeks to months after exposure, by which time often the season has changed, the batch of feed is gone, and frequently the horse has been moved one or more times to a new location, all of which can make it impossible to pinpoint the source of the poisoning.
When sampling a mixed feed, like sweet feed or pellets, it is important to visually inspect the feed and look for possible hot spots, like areas of mold growth, discoloration, moisture, or differences in texture, and such areas should be collected. When submitting to the laboratory, include pertinent history, clinical signs, postmortem lesions, and a list of differential diagnosis. There is no “screen for all toxins”; therefore, specific analyses must be requested. If you do not know what to request, contact the veterinary professionals at the laboratory before submitting the sample. It is best to collect at least a gallon of feed from different areas of the lot (with emphasis on possible hot spots) to get the most representative sample possible. Feed can be stored in a cool, dry place or frozen for analysis.
When sampling hay, it is important to open several bales and inspect for weeds, insects, mold growth, dust, or other signs of contamination or poor quality. If weeds are detected, they can be submitted to an extension botanist for identification. Chemical analysis is available at veterinary diagnostic labs, and, as with the mixed feeds above, test requests must be specific. Representative sampling of hay for analysis can require submitting flakes from different areas of several bales, with emphasis on suspicious looking or weedy areas. Samples of hay for analysis should be kept in a cool and dry environment.
It is critical to collect samples from the affected animals for analysis if food contamination is suspected. Antemortem (before death) samples to collect are urine, feces, whole blood, and serum. Blood should be kept chilled, and other samples can be frozen. All samples must be placed in separate containers and individually labeled with the sample type, animal information, owner information, collector information, date, and time of collection.
Postmortem (after death), ideally, the carcass should be submitted to a veterinary pathology laboratory. However, this is not always possible; thus, a veterinarian or prosector will need to collect a full set of tissue samples to be saved in 10% neutral buffered formalin for histologic analysis by a pathologist. A full set of tissues includes liver, kidney, glandular and nonglandular stomach, including the margo plicatus, duodenum, jejunum, ileum, cecum, right ventral colon, left ventral colon, left dorsal colon, right dorsal colon, transverse colon, urinary bladder, lung, spleen, brain, and bone marrow, which can be submitted in a fragment of rib. The brain can be split through the mid-sagittal plane and half placed in formalin. Additionally, sample any tissue that looks unusual or contains an obvious lesion. Subsample from the margins of the lesion and include normal-appearing tissue from the organ. Tissue samples collected for histopathology should be less than a centimeter (4/10ths of an inch) thick, and there should be 10 times as much formalin as there is tissue in the collection container. Brain is the exception, since the tissue is too delicate for sectioning, but fixation time for brain is prolonged. Tissues can be stored in formalin indefinitely. If the samples are stored for more than a day or two before submission to the laboratory, fixation of properly collected samples will be complete and the formalin can be drained, allowing samples to be placed in a smaller container. It is important to closely examine the gastrointestinal tract grossly from the oral cavity to the anal sphincter, including the constituents and consistency of the ingesta and the integrity of the mucosa. Pathology is necessary to rule in or out feed contamination as a cause of illness and death. Toxicologic analyses are often based on the pathologist’s diagnosis. Large samples (100+ g) of liver, kidney, urine, half of the brain, and ingesta should be saved at the time of postmortem examination; placed in individual, labeled, dated, and initialed containers as noted for antemortem samples; and frozen in case analytical toxicology is needed (Gwaltney-Brandt, 2016). Be mindful that samples are easier to collect at the time of the postmortem examination than later, after the carcass has been buried or disposed of otherwise. Additionally, samples collected near the time of death and frozen are more useful to the analytic chemist, and more pleasant to work with, than tissues that have become severely autolyzed and putrefied.
Misformulation
Misformulation occurs when there is deviation from the “recipe,” or formulation, for a mixed feed, leading to partial or complete absence or else excess of one or more ingredients. Nutrient misformulations can lead to nutrient deficiencies or excesses, but in most cases do not cause significant short-term consequences. However, very high doses of certain nutrients can cause significant morbidity or mortality. Excessive vitamin D in a horse feed would be an example of a misformulation that could have clinical consequences, such as hypercalcemia and metastatic mineralization affecting the kidneys and other tissues. Misformulation of medicated feeds can also have consequences.
Adulteration
Adulteration occurs when something is present in the feed that is not part of the formulation. Adulteration can be intentional or accidental (Kosal and Anderson, 2004). An example of intentional adulteration was melamine contamination in pet foods in 2007 (Rumbeiha and Morrison, 2011), but in horse feed, documented cases have been accidental. Common adulterants of horse feeds are ionophores and antibiotics intended for ruminants. This can occur through mislabeling of feed ingredients or feeds, or through failure to properly clean equipment between batches. When the adulterant is an antibiotic, the horse can have loss of normal flora allowing colonization of the colon by Clostridium difficile, leading to severe and often fatal diarrhea. More commonly, the adulteration involves ionophores.
“Ionophores” are used as antibiotics, coccidiostats, and growth promotants in ruminants and poultry. These compounds were originally isolated from Streptomyces spp. Ionophore toxicosis has been reported in a large number of mammals and birds, and horses are particularly susceptible to adverse effects (Novilla, 2012).
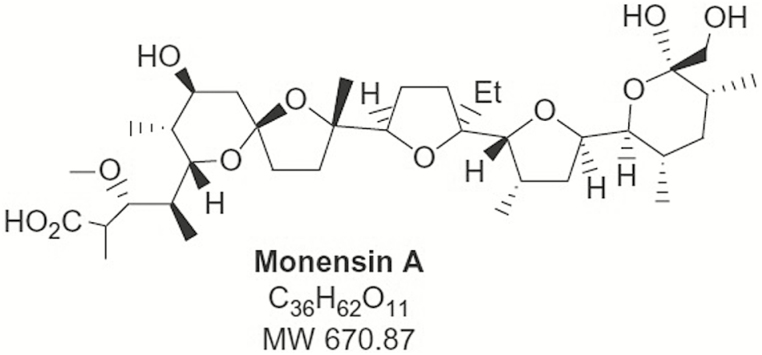
Common ionophores, based on generic names, include monensin, lasalocid, salinomycin, maduramicin, narasin, laidlomycin, and semduramicin (Figure 2). These tend to be long organic molecules with a combination of polar and nonpolar side chains. The side of the molecule with more polar groups wraps around a single ion so that the relatively nonpolar side is along the surface of the complex, which allows the complex to pass through lipid membranes that would be impermeable to the ion itself. Enhanced ion movement across cell plasma membranes disrupts ion balance and leads to functional changes, such as inability of mitochondria to produce ATP, changes in cellular pH, neurotransmitter release, and changes in polarization (Novilla, 2012).
Figure 2.
The chemical structure of monensin, a common ionophore.
The first observed clinical sign in horses is likely to be feed refusal. Later clinical signs include ataxia, jugular pulses, tachycardia, inability to stand, and sweating, but some horses are found dead before signs are noted. Survivor can have permanent myocardial damage (Decloedt et al., 2012a). Horses with ionophore toxicosis can have markedly elevated creatinine kinase and elevations in liver enzymes, serum urea nitrogen, creatinine, and phosphorus. Elevated cardiac troponin I has been used as a biomarker for ionophore exposure in horses (Decloedt et al., 2012b).
Postmortem lesions are sometimes mild or absent when animals die early in the progression of the toxicosis (Bautista et al., 2014). Cardiac myonecrosis, often with secondary pulmonary edema, ascites, and hepatic congestion, is the most common lesions. Skeletal myonecrosis is sometimes seen, and delayed axonal degeneration has been reported (Decloedt et al., 2012a). Diagnosis is based on clinical findings, postmortem lesions, exclusion of other causes, and feed analysis. Some laboratories offer tissue analysis, which can only be used to confirm exposure, since tissue concentrations of ionophores associated with toxicosis are not currently known (Bautista et al., 2014).
Treatment of ionophore toxicosis is supportive and symptomatic. As with other feed-related problems, animals must be placed on a new clean, high-quality feed. If the animal has recently ingested cattle feed or another more concentrated source of ionophores, gastrointestinal decontamination might be indicated. Under most circumstances, the only appropriate treatment is maintenance of hydration and electrolytes, quiet, and stall rest. Myocardial damage is permanent and can, in the long-term, be associated with exercise intolerance and congestive heart failure (Decloedt et al., 2012a).
Natural Contaminants
The most commonly isolated natural contaminants in livestock feeds are mycotoxins. Mycotoxins can include fumonisin, slaframine, ergot alkaloids in tall fescue, dicoumarol, tremorgenic mycotoxins, and aflatoxin (Riet-Correa et al., 2013). Weeds and weed seeds can also become incorporated into sweet feeds and pellets on occasion. Natural feed contaminants associated with hay include insects, such as the blister beetles, Epicauta spp. (Figure 3), which can become incorporated into alfalfa hay and alfalfa-based pellets, and toxic plants. A plague of caterpillars of the genus Malacosoma occurred in the Ohio Valley in 2001 and 2002, covering extensive areas of forage pastures and causing a disease termed Mare Reproductive Loss Syndrome, though it was associated with uveitis and fibrinous pericarditis as well as funisitis and placentitis (Sebastian et al., 2008; McDowell et al., 2010). Lastly, though this article is not intended to consider microbial contaminants, botulism will be mentioned as an important feed-associated toxic disease of horses.
Figure 3.
Epicauta spp. found in bales of alfalfa hay in the Midwestern US.
“Fumonisin” is relatively common in the United States and, of the mycotoxins, has been associated with dramatic and life-threatening clinical signs when fed to horses. The colloquial term for fumonisin toxicosis is “moldy corn poisoning,” and it has an almost pathognomonic lesion: leukoencephalomalacia. Fumonisin is possible a human carcinogen (Ostry et al., 2017).
Fumonisin is produced by Fumonisin verticillioides and other species. This fungus was once called “Fusarium moniliforme,” and the first letters were taken from the genus and species name to come up with the name of the mycotoxin. Like other mycotoxin-related feed problems, the presence of the fungus does not insure the production of fumonisin, and various climatic conditions and environmental factors are required for fumonisin production to occur (Riet-Correa et al., 2013). Since fumonisin does not occur in corn (or other crops) in the absence of Fusarium spp. growth, feeding good quality grain is the most important step in preventing moldy corn disease. Nevertheless, feed containing fumonisin may not be visibly moldy (Riet-Correa et al., 2013). Corn screenings and corn byproducts are risky since damaged kernels are more likely to grow mold. Currently, the FDA recommends that horse feed contains less than 5 ppm fumonisin (USFDA, 2001). However, lower feed fumonisin concentrations have been associated with equine leukoencephalomalacia. Fumonisin poisoning has occasionally been associated with hay (Vendruscolo et al., 2016).
Fumonisin B1, the most common form, is composed of a long carbon chain with several functional groups. This structure is similar to that of sphinganine and sphingosine. Ceramide synthase is the enzyme that uses sphinganine and sphingosine to produce sphingomyelin and other sphingolipids, and fumonisin acts on this process (Riet-Correa et al., 2013). Sphingolipids are important for cell membrane function, act as mediators for other cellular functions, and are integral to the formation of insulating myelin surrounding axons. Aside from loss of these functions, dysfunction of ceramide synthase is also associated with precursor buildup within cells, causing vacuolation and interfering with normal cell function (Caloni and Cortinovis, 2010).
Clinical signs of fumonisin toxicosis can occur within 12 h of exposure or much later. Unexpected death is occasionally the presenting complaint, but more commonly, horses present with tongue paralysis, ataxia, and proprioceptive defects followed later by head pressing, circling, blindness, agitation, belligerence, and other behavioral changes. Occasionally, a hepatotoxic syndrome occurs with or without the neurotoxic effects (Rissi and Sustra, 2013). Prognosis for affected horses is poor to grave, depending on severity, since the axonal damage in the central nervous system is not reversible. Clean feed, thiamin supplementation, and supportive care can be attempted, but caution is needed as these horses can be quite dangerous.
As noted, leukoencephalomalacia, literally softening of the white matter of the brain, is the lesion most associated with fumonisin in horses. Subcortical liquefactive necrosis of the white matter, which may or may not be bilateral, is caused by myelin damage (Rissi and Susta, 2013). Hepatomegaly and centrilobular necrosis can be evident in the hepatotoxic form of fumonisin poisoning. The diagnosis is supported by the detection of fumonisin in the feed.
Cantharidin
Cantharidin is a small terpenoid molecule produced by beetles of the family Meloidae. The genus Epicauta is found commonly in the United States and occurs worldwide, though Lytta is also a common genus in Europe and parts of Asia. Neither genus occur in Australia. Epicauta spp. are commonly referred to as “blister beetles” (Figure 2) and include Epicauta pensylvanica, the black blister beetle, E. vittata, the striped blister beetle, E. obesa, and others. They are approximately the size of fireflies. The mature beetles consume plant material, but larvae feed on grasshopper eggs. The adult beetles are most common from June through September and can congregate in large numbers in hay fields. Beetles will fly away when these fields are cut for hay, but if the hay is crimped, as typically occurs with alfalfa, the beetles become crushed and trapped in the hay bales. Thus, cantharidin toxicosis is associated with crimped alfalfa hay (Gwaltney-Brandt et al., 2012).
Cantharidin is produced by the male blister beetle and transferred to the female at mating. Cantharidin compose 1% to 5% of the tissue fluid of the beetle (Gwaltney-Brandt et al., 2012). This toxin acts to damage desmosomes, the adhesive structures that hold epithelial cells together, causing acantholysis and blisters (Al-Dawsari and Masterpol, 2016). When ingested, cantharidin blisters the mucosa throughout the gastrointestinal tract. It is eliminated via the kidneys, where it further damages the mucosa of the urinary tract. Cantharidin also causes acute myocardial necrosis (Helman and Edwards, 1997). Clinically, horses present with signs of colic and pain. Blistering and mucosal ulceration are sometimes evident in the oral cavity. Classic clinical pathology findings in horses with cantharidin poisoning are hypocalcemia, hypomagnesemia, increased creatinine kinase, and increased packed cell volume (Schmitz, 1989).
Diagnosis of cantharidin toxicosis is often based on appropriate clinical signs and lesions in addition to history of feeding alfalfa hay with visible beetles present. Many toxicology laboratories can analyze urine or stomach contents for cantharidin. Mineral oil or activated charcoal have been used as detox measures when recent large ingestions were suspected (Gwaltney-Brandt et al., 2012). Further treatment involves clean feed, intensive supportive care, and pain relief. Supportive care includes diuresis, correction of dehydration, and electrolyte imbalances. Due to the extreme pain associated with cantharidin, xylazine has been recommended for pain control.
Botulism is caused by botulinum neurotoxins (BoNTs) produced by C. botulinum, an environmentally ubiquitous spore-forming, anaerobic gram-positive rod. Each of several serotypes produces their own specific BoNT: serotype A (BoNT A) and B (BoNT B) are found in soil and are the most common forms that affect horses (Johnson et al., 2015, 2016). Serotype C (BoNT C) has been proposed as a cause for dysautonomia or mal seco in horses in Europe and South America, respectively, but convincing evidence is lacking (Böhnel et al., 2003). Serotype D (BoNT D) can also affect herbivores. Clostridium botulinum thrives in anerobic conditions and devitalized tissues, and poisoning incidents in horses and other livestock are usually associated with carrion in the feed, though hot spots for botulism due to high C. botulinum spore loads can occur in specific locations, for example, stalls within a barn (Johnson et al., 2016). Botulism in horses occurs most commonly when haylage is fed, though small animal carcasses can inadvertently be incorporated in to any kind of feed. The organism can also colonize wounded tissue or intestines in neonatal foals. BoNT is resistant to acid but is denatured by heat processing.
BoNT does not cross the placenta or blood–brain barrier, so the effects are peripheral and do not affect the fetus in a pregnant mare. BoNTs act by preventing the release of acetylcholine (ACh) at the synapse (Coffield and Welchel, 2012). The heavy chain of BoNT induces endocytosis of the molecule at the axon terminal, and the light chain cleaves proteins involved in neurotransmitter exocytosis, thus preventing vesicles of ACh from fusing at the membrane to release their contents into the synapse, resulting in paralysis. Early clinical signs are associated with dysphagia and reduced tongue tone. Head and neck edema is often reported; this is due to gravity because the horse can no longer hold up its head. Eventually, the horse becomes recumbent. Neonate acquire a toxicoinfectious form of botulism colloquially known as “shaker foal” because generalized muscle weakness causes them to tremble (Coffield and Welchel, 2012).
Because horses are exceptionally sensitive to BoNT, getting a definitive diagnosis of botulism is extremely challenging. Bioassays were once common, but could probably only detect about 30% of horses with botulism (Johnson et al., 2016). ELISA serology has also been used in horses. Currently, New Bolton Center has had good success with a PCR test, which they estimate can detect about 60% of positive horses (Johnson et al., 2015). Another method that has been used in foals is termed Repetitive Nerve Stimulation, in which, as the name implies, a nerve is repetitively stimulated and the response is measured to determine whether it fits the pattern associated with botulism (Prutton et al., 2016).
The prognosis for botulism in mature horses is guarded to grave and requires between 2 and 9 wk of hospitalization (Johnson et al., 2015). The expected mortality for horses that remain standing is only about 5%, but that rises to 80% for recumbent horses. Foals, being easier to manage, are reported have 4% to 12% mortality. Survival improves when antitoxin is given, but this can be cost prohibitive. Antitoxin is available through New Bolton Center.
No lesions are expected in horses that die from botulism, with the exception of secondary lesions such as subcutaneous edema of the head or pressure necrosis of muscle on the downside of the recumbent animal.
A vaccine is available to prevent type B botulism in horses. Only two cases of type B botulism were reported in fully vaccinated horses, and both had relatively mild clinical signs (Johnson et al., 2015). Use of an earlier vaccine was associated with tissue swelling, but the current product causes minimal tissue response (Whitlock and Buckley, 1997). The vaccine does not offer cross-protection for type A or C botulism. Still, because of the grave prognosis, preventative vaccination can be warranted even in horses that are at low risk for type B botulism.
CONCLUSION
With a myriad of possible contaminants, ranging from microbes to fungal metabolites like aflatoxin and vomitoxin, to misformulations producing nutritional excesses and deficiencies, and including even adulteration with ionophores, antibiotics, or other drugs and chemicals, it is challenging to diagnose feed-related toxicoses, but when contamination incidents occur, vigilance on the part of horse owners, veterinarians, and in some cases feed manufacturers and regulators will help minimize the consequences.
Conflict of interest statement. The authors have no conflicts of interest to report.
LITERATURE CITED
- Al-Dawsari N.A., and Masterpol K.S.. 2016. Cantharidin in dermatology. Skin Med. 14:111–114. [PubMed] [Google Scholar]
- Bautista A.C., Tahara J., Meta A., Gaskill C.L., Bryant U.K., and Puschner B.. 2014. Diagnostic value of tissue monensin concentrations in horses following toxicosis. J. Vet. Diag. Invest. 26:423–427. doi:10.1177/1040638714523774 [DOI] [PubMed] [Google Scholar]
- Böhnel H., Wernery U., and Gessler F.. 2003. Two cases of equine grass sickness with evidence for soil-borne origin involving botulinum neurotoxin. J. Vet. Med. B. 50:178–182. doi:10.1046/j.1439-0450.2003.00655.x [DOI] [PubMed] [Google Scholar]
- Caloni F., and Cortinovis C.. 2010. Effects of fusariotoxins in the equine species. Vet. J. 186: 157–161. doi:10.1016/j.tvjl.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Coffield J.A., and Welchel D.D.. 2012. Botulinum neurotoxins. In: Gupta R.C, editor. Veterinary toxicology basic and clinical principles. 2nd ed San Diego (CA): Academic Press; p. 937–949. [Google Scholar]
- Decloedt A., Verheyen T., De Clercq T., Sys S., Vercauteren G., Ducatelle R., Delahuat P., and Van Loon G.. 2012a. Acute and long-term cardiomyopathy and delayed neurotoxicity after accidental lasalocid poisoning in horses. J. Vet. Intern. Med. 26:1005–1011. doi:10.1111/j.1939-1676.2012.00933.x [DOI] [PubMed] [Google Scholar]
- Decloedt A., Verheyen T., Sys S., De Clercq D., and Van Loon G.. 2012b. Tissue Doppler imaging and 2-dimensional speckle tracking of left ventricular function in horses exposed to lasalocid. J. Vet. Intern. Med. 26:1209–1216. doi:10.1111/j.1939-1676.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- Gwaltney-Brandt S.M. 2016. Veterinary forensic toxicology. Vet. Pathol. 53:1067–1077. doi:10.1177/0300985816641994 [DOI] [PubMed] [Google Scholar]
- Gwaltney-Brandt S.M., Dunayer E., and Youssef H.. 2012. Terrestrial zootoxins. In: Gupta R.C, editor. Veterinary toxicology basic and clinical principles. 2nd ed San Diego (CA): Academic Press; p. 969–992. [Google Scholar]
- Helman R.G., and Edwards W.C.. 1997. Clinical features of blister beetle poisoning in equids: 70 cases (1983–1996). J. Am. Vet. Med. Assoc. 211:1018–1021. [PubMed] [Google Scholar]
- Johnson A. L., McAdams-Gallagher S.C., and Aceto H.. 2015. Outcome of adult horses with botulism treated at a veterinary hospital: 92 cases (1989–2013). J. Vet. Intern. Med. 29:311–319. doi:10.1111/jvim.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L., McAdams-Gallagher S.C., and Aceto H.. 2016. Accuracy of a mouse bioassay for the diagnosis of botulism in horses. J. Vet. Intern. Med. 30:1293–1299. doi:10.1111/jvim.13950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosal M.E., and Anderson D.E. 2004. An unaddressed issue of agricultural terrorism: a case study on feed security. J. Anim. Sci. 82:3394–3400. doi:10.2527/2004.82113394x [DOI] [PubMed] [Google Scholar]
- McDowell K.J., Webb B.A., Williams N.M., Donahue J.M., Newman K.E., Lindemann M.D., and Horohov D.W.. 2010. Invited review: the role of caterpillars in mare reproductive loss syndrome: a model for environmental cases of abortion. J. Anim. Sci. 88:1379–1387. doi:10/2527/jas.2009–2584 [DOI] [PubMed] [Google Scholar]
- Novilla M.N. 2012. Ionophores. In: Gupta R.C, editor. Veterinary toxicology basic and clinical principles. 2nd ed San Diego (CA): Academic Press; p. 1281–1299. [Google Scholar]
- Ostry V., Malir M.F., Toman J., and Grosse Y.. 2017. Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 33:65–73. doi:10.1007/s12550-016-0265-7 [DOI] [PubMed] [Google Scholar]
- Prutton J.S., Magdesian K.G., Plummer A., Williams D.C., and Aleman M.. 2016. Survival of a foal with type a botulism. J. Vet. Intern. Med. 30:675–678. doi:10.1111/jvim.13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riet-Correa F., Rivero R., Odriozola E., de Lourdes M., Medeiros R.M., and Schild A.L.. 2013. Mycotoxicoses of ruminants and horses. J. Vet. Diag. Invest. 25:692–708. doi:10.1177/10406387.13504572 [DOI] [PubMed] [Google Scholar]
- Rissi D.R., and Susta L.. 2013. Pathology in practice. J. Am. Vet. Med. Assoc. 243:57–59. doi:10.2460/javma.243.1.57 [DOI] [PubMed] [Google Scholar]
- Rumbeiha W., and Morrison J.. 2011. A review of class I and class II pet food recalls involving chemical contaminants from 1996 to 2008. J. Med. Toxicol. 7:60–66. doi:10.3390/nu5041024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D.G. 1989. Cantharidin toxicosis in horses. J. Vet. Intern. Med. 3:208–215. [DOI] [PubMed] [Google Scholar]
- Sebastian M.M., Bernard W.V., Riddle T.W., Latimer C.R., Fitgerald T.D., and Harrison L.R.. 2008. Review paper: mare reproductive loss syndrome. Vet. Pathol. 45:710–722. doi:10.1354/vp.45-5-710 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Adminstration 2001. Guidance for industry: fumonisin levels in human food and animal feeds; final guidance. Available from https://www.fda.gov/RegulatoryInformation/Guidances/ucm109231.htm [Google Scholar]
- Vendruscolo C.P., Frias N.C., de Carvalho C.B., de Sá L.R., Belli C.B., and Baccarin R.Y.. 2016. Leukoencephalomalacia outbreak in horses due to consumption of contaminated hay. J. Vet. Intern. Med. 30:1879–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock R.H., and Buckley C.. 1997. Botulism. Vet. Clin. North Am. Equine Pract. 13:107–128. [DOI] [PubMed] [Google Scholar]