Abstract
Bacillus velezensis JT3-1 was isolated from feces of the domestic yak (Bos grunniens) in Gansu province of China. Aim to know whether B. velezensis JT3-1 has the potency to be developed as a probiotic bacterium, works on the complete genome sequence, antimicrobial activity, growth performance in calves, and treatment effect on calf diarrhea of B. velezensis JT3-1 were carried out. The results showed that the complete genome of B. velezensis JT3-1 contains one gapless circular chromosome which is 3,929,799 bp, and has 3761 protein-encoding genes with an average GC content of 46.50%. From the antimicrobial activity results, B. velezensis JT3-1 has shown strong antagonistic activities against Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, Salmonella Typhimurium, Mannheimia haemolytica, Staphylococcus hominis, Clostridium perfringens, and Mycoplasma bovis. Compared with the control group, the average weight of the experiment animals from Bv1 group and Bv2 group which were supplemented with B. velezensis JT3-1 was significantly increased (P < 0.05). Meanwhile, the Bv1 and Bv2 supplement significantly improved the level of IgA, IgG, IgM, and IFN-γ in calves as compared with the controls (P < 0.05), but the IL-2 level was not obviously changed between the three groups. In addition, B. velezensis JT3-1 showed a good effect against diarrhea, as the cure rate reached 95.0% (171/180) in newborn calves (Angus cattle) in Xinjiang, and 100.0% (149/149) in yak calves in Qinghai, respectively. Our study will lay a good foundation for the elucidation of the molecular mechanisms of its antimicrobial activity, and supports the hypothesis that JT3-1 has the potential to be developed as a probiotic bacterium in cattle.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02235-z) contains supplementary material, which is available to authorized users.
Keywords: Bacillus velezensis, Genome, Antimicrobial activity, Growth performance, Diarrhea
Introduction
Antibiotics utilized in animal husbandry introduce various problems while improving animal productivity (Sharma et al. 2017). In many countries, the current tendency in animal production is towards a reduction or prohibition of the use of antibiotics, with an increase in the application of non-antibiotic methods (Lillehoj and Lee 2012). Of the non-antibiotic methods, probiotics are a safe alternative to antibiotics, and the dietary supplementation of probiotics can improve the productivity and immunological status of livestock (Abd El-Tawab et al. 2016; Khan et al. 2016; Reuter 2001). Probiotics are dietary supplements that are by definition “live microorganisms, which, upon ingestion in sufficient numbers, exert health benefits” (Schrezenmeir and de Vrese 2001). In ruminants, probiotics can change the rumen microbial ecosystem, and promote nutrient digestibility and feed efficiency. The most commonly used probiotics are Lactobacillus, Bifidobacterium, Bacillus, and yeast. Supplemental B. subtilis increases growth performance, disease resistance and immune response in animals (Guo et al. 2016, 2017). Previous reports have demonstrated that Bacillus velezenis QST713, Bacillus velezenis M75, and Bacillus velezenis S3-1 showed a strong ability to form biofilm and inhibit pathogens (Jin et al. 2017; Kim et al. 2017; Pandin et al. 2018).
B. velezensis JT3-1 was isolated from the feces of domestic yak (Bos grunniens) in Gansu Province, China. Our study showed that the B. velezensis JT3-1 strain had strong antagonistic activity against various intestinal pathogenic flora, and stimulation of animals (such as sheep, goat, mouse, and cattle) growth (not published). However, there are no published reports on the B. velezensis JT3-1 strain and its genome sequence is not found in Genebank. In this study, aim to know whether B. velezensis JT3-1 has the potency to be developed as a probiotic bacterium, works on the complete genome sequence, antimicrobial activity, growth performance in calves, and treatment effect on calf diarrhea of B. velezensis JT3-1 were carried out.
Materials and methods
Genome sequencing of B. velezensis JT3-1
Genomic DNA was extracted using Gentra Puregene Yeast/Bact (Qiagen, Germany), and the whole-genome sequencing of B. velezensis JT3-1 was performed using a PacBio Sequel sequencing platform at Beijing Genewiz Bioinformatics Technology Co., Ltd. A library with a 10 kb insert size was constructed for the PacBio Sequel platform, and the library was sequenced in a PacBio SMRT (Chin et al. 2013) instrument. Subsequently, the PacBio reads were assembled using SmrtLink, and a total of 199,380 reads, about 550 Mb, were obtained. Finally, we obtained a single circular gapless chromosome. Prodigal software (Hyatt et al. 2010) was utilized to find coding genes in the bacterium. Transfer RNAs (tRNAs) were detected in the genome using the program blast based on Rfam (Eric et al. 2014). The coding genes were annotated with the National Center for Biotechnology Information (NCBI) nr database by Diamond (Buchfink et al. 2015). Afterwards, the functions of genes were annotated using the Gene Ontology database, and the pathways were annotated using the Kyoto Encyclopedia of Genes and Genomes database. The proteins encoded by the genes were phylogenetically classified by the Clusters of Orthologous Groups database. The potential genes involved with the biosynthesis of bacteriocins were identified with antiSMASH4.0 (Blin et al. 2017) (https://antismash.secondarymetabolites.org/). Genes involved in promoting animal growth were analyzed.
Antimicrobial activity of B. velezensis JT3-1
An agar well diffusion assay was utilized to test the antibacterial properties of the JT3-1 culture supernatant, as previously described (Liu et al. 2017). Nutrient agar (NA) medium was utilized to culture the remaining bacteria, and PPLOB medium was utilized to culture Mycoplasma bovis. The diameter of the inhibition zones was calculated in millimeters, and the plus sign shows the range of the inhibition zone. The three-plus sign (+ + +) means that the diameter of the inhibition zone is more than 15 mm; the two-plus sign (+ +) means that the diameter of the inhibition zone is between 10 and 15 mm; and the one-plus sign ( +) means that the diameter of the inhibition zone is less than 10 mm (the diameter of the wells was 5 mm). Each test was repeated three times, and the mean values were adopted.
Calves and diets
A total of 45 six-month-old calves (Angus cattle) were randomly separated into three treatment groups (control, Bv1, and Bv2 diets). There were three replicates for each treatment with 5 calves per replicate; all calves were housed in isolators with iron fences, on the same farm. The ratio of concentrate to roughage was 40:60, and the concentrate was composed mainly of corn, soybean meal, wheat bran, rapeseed cake, salt and premix; the roughage comprised alfalfa, distiller’s grains and wheat straw, and the amount fed accounted for 19%, 15% and 26% of the feed, respectively. The ingredient composition and nutrient levels of the concentrates are shown in Table 1, according to the method described by Shao et al. (2019) with some modification. B. velezensis JT3-1 were mixed with concentrates and fed directly to the calves. In the experimental treatment, the calves received a daily dose of 2 × 109 cfu (for the Bv1 group) and 3 × 109 cfu (for the Bv2 group) of B. velezensis JT3-1 in 1 mL of culture during the morning feed, whereas no additives were fed to the control group. All calves had free access to feed and water. The body weight was measured on days 1 and 30, prior to the morning feed. Blood samples were collected in 9-mL Vacutainer tubes (Greiner Bio-one) from all calves on days 1 and 30. The serum was prepared immediately and stored at − 80 °C.
Table 1.
Composition and nutrient levels of concentrates (air-dry basis) (%)
| Item | Content | Nutrient levels | Content | ||
|---|---|---|---|---|---|
| Control | Bvl | Bv2 | |||
| Ingredients | |||||
| Corn | 51.00 | 51.00 | 51.00 | NE g /( MJ /kg)2 | 5.73 |
| Soybean meal | 16.5 | 16.5 | 16.5 | CP | 16.74 |
| Rapeseed cake | 8.00 | 8.00 | 8.00 | CF | 3.93 |
| Wheat bran | 18.00 | 17.99 | 17.99 | ADF | 7.13 |
| CaHPO4 | 1.50 | 1.50 | 1.50 | NDF | 16.37 |
| CaCO3 | 1.00 | 1.00 | 1.00 | Ca | 0.93 |
| NaHCO3 | 1.50 | 1.50 | 1.50 | p | 0.76 |
| NaCl | 1.50 | 1.50 | 1.50 | ||
| Bv1 or Bv2 | Bv1 | Bv2 | |||
| Premix1 | 1.00 | 1.00 | 1.00 | ||
| Total | 100 | 100 | 100 | ||
1The premix provided the following per kg of the diet: VA 8 000 IU, VD 1 200 IU, VE 50 IU, Cu 10 mg, Fe100 mg, Mn 40 mg, Zn 60 mg, I 0.50 mg, Se 0.3 mg, Co 0.1 mg
2NEg was calculated according to the NRC ( 2001), while the others were measured values
Measurement of immunoglobulin and cytokine concentrations
The concentrations of IgA, IgG, IgM, IL-2, and IFN-γ in bovine serum were detected by a bovine-specific ELISA quantitation kit (Cusabio Biotech Co., Ltd, Wuhan, China). The ELISA procedure was carried out according to the manufacturer’s guidelines, and the optical density (OD) values of each sample were measured at 450 nm using a microplate autoreader (iMark; Bio-Rad, USA).
Treatment of diarrhea in newborn calves
In 2018, diarrhea broke out in newborn Angus cattle on a farm in Xinjiang and on a yak (Bos mutus) farm in Qinghai, respectively. There was a total of 329 cases of diarrhea, including 180 newborn Angus calves in Xinjiang Province and 149 yak calves in Qinghai Province. The three-strain probiotic preparation, temporarily named CBBLE, contains B. velezensis JT3-1, Lactobacillus plantarum FBL and Enterococcus faecium DT in appropriate proportions, and was utilized to treat calf diarrhea in the two provinces. The calves received a daily dose of no less than 2 × 109 cfu CBBLE by oral administration. The cure rate of diarrhea cases in the calves was calculated.
Statistical analysis
All experimental data were subjected to one-way analysis of variance (ANOVA) to determine whether significant differences occurred in calves fed the different diets. Values were expressed as mean ± SD. The SPSS 20.0 software was utilized to determine the effects of dietary treatment. Significance was defined as P < 0.05.
Results and discussion
General genomic features of B. velezensis JT3-1
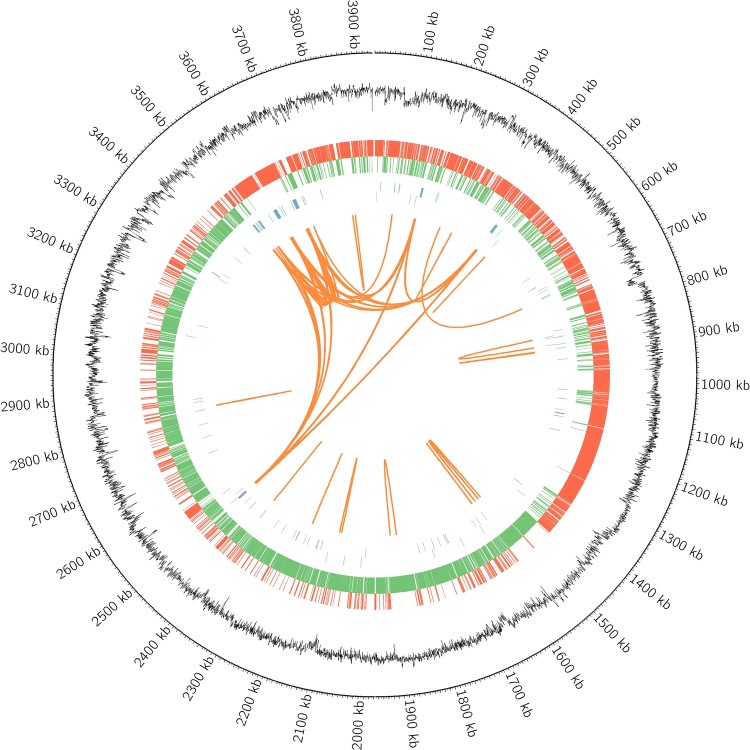
The complete genome of B. velezensis JT3-1 contains one gapless circular chromosome of 3,929,799 bp, 3761 protein-encoding genes, 86 tRNA genes, 27 rRNA genes and 83 other ncRNA; it shows an average G + C content of 46.50%, and no plasmids were found. The circular genome map of the complete genome of B. velezensis JT3-1 is displayed in Fig. 1. This strain was deposited at the China General Microbiological Culture Collection Center under the accession number CGMCC No.15545, and the MixS information of B. velezensis JT3-1 was presented in Table 1S. In addition, the data from the comparative genomic analysis of B. velezensis JT3-1 with other Bacillus genomes are presented in Table 2.
Fig. 1.
The whole genome of Bacillus velezensis JT3-1. The circular genome map consists of 7 circles. From the outer circle inward, each circle displays information about (1) scale, (2) complete genome, (3) forward CDS, (4) reverse CDS, (5) ncRNA, (6) G + C content, and (7) repetitive sequence
Table 2.
Comparative genomic analysis of B. velezensis JT3-1 with other Bacillus genomes
| Strain/genbank accession number | Genome size (bp) | GC content% | Protein-CDS | rRNA | tRNA | Plasmid | Surfactin% | Fengycin% | Rhizocticin | Source | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B. velezensis JT3-1 / CP032506 | 3,929,799 | 46.50 | 3761 | 27 | 86 | 0 | 82 | 100 | – | Faeces (yak) | In this study |
| B. velezensis FZB42 / NC_009725.1 | 3,918,589 | 46.49 | 3693 | 30 | 86 | 0 | 95 | 100 | – | Sugar beet field | Chen et al. (2009) |
| B. velezensis 157 / CP022341 | 4,013,317 | 46.41 | 3789 | 27 | 86 | 2 | 86 | 100 | – | Bark (Eucommia ulmoides) | Chen et al. (2018) |
| B. velezensis ZY-1–1 / CP027061 | 3,899,251 | 46.57 | 3688 | 27 | 87 | 0 | 82 | 100 | – | Larval gut (scarab beetle) | Zhang et al. (2018) |
| B. velezensis 9912D / NZ_CP017775.1 | 4,241,576 | 45.99 | 4436 | 27 | 86 | 1 | 86 | 100 | – | Sediment (Bohai Sea) | Pan et al. (2017) |
| B. velezensis QST713/NZ_CP025079.1 | 4,233,757 | 45.90 | 4159 | 25 | 79 | 0 | 82 | 100 | 22% | Not available | Pandin et al. (2018) |
| B.velezensis LS69 / CP015911 | 3,917,761 | 46.40 | 3643 | 21 | 72 | 0 | 86 | 100 | – | Rice field | Liu et al. (2017) |
| B. velezensis M75 / CP016395 | 4,007,450 | 46.60 | 3803 | 27 | 86 | 0 | 82 | 100 | – | Cotton waste | Kim et al. (2017) |
The symbol “–” means the potential genes involved with the biosynthesis of rhizocticin were not found with antiSMASH4.0
Secondary metabolite clusters
Through the antiSMASH 4.0 genome analysis tool (Blin et al. 2017), 30 clusters of secondary metabolites were identified in the genome of the JT3-1 strain: two transATPKS-NRPS, two transATPKS (trans-acyl transferase polyketide synthetase), one lantipeptide, one encoding NRPS (non-ribosomal peptide synthetase), one T3PKS, one bacteriocin-Nrps, two terpenes, one other KS, three Cf_fatty_acid, five Cf_saccharide, and 11 Cf_putative (Table 3). Eight clusters were clearly involved in the synthesis of difficidin, surfactin, fengycin, butirosin, macrolactin, bacilysin, bacillaene and bacillibactin, and they were reported to be closely involved with antimicrobial activity (Chen et al. 2009, 2017; Koumoutsi et al. 2004; Niazi et al. 2014; Ongena et al. 2005; Liu et al. 2017; Kim et al. 2017; Stein 2005; Pandin et al. 2018; Wu 2017; Adeniji et al. 2019). This analysis showed that at least 17% of the JT3-1 genome contributes to the molecular mechanism of antibiotic biosynthesis. These antimicrobial compounds include mainly peptides that are either non-ribosomally produced, or post-translationally modified (lantibiotics) and ribosomally synthesized. Of the metabolites, bacillibactin is a microbial competitor metabolite; bacilysin is an antibacterial and anti-Candida albicans metabolite; butirosin, bacilysin, difficidin, bacillaene, and macrolactin are antibacterial metabolites; fengycin and surfactin are antifungal metabolites. The cyclic lipopeptide fengycin is reported to have strong antifungal activity, specifically against filamentous fungi, and it has a distinct inhibitory effect on tumor tissues and tumor cells (Ntushelo et al. 2019). The lipopeptide surfactin has also demonstrated potential as an antibacterial, -viral, -hypocholesterolemic, and -tumor agent, and colonizes roots and the gastrointestinal tract (Wu 2017; Chen et al. 2009; Adeniji et al. 2019). Bacillus-derived peptides show antibacterial, -fungal, -viral, -tumor, -amebocytic, and -mycoplasmic activities (Yilmaz et al. 2006; Rabbee et al. 2019). The operons in B. velezensis JT3-1 encoding the biosynthetic gene cluster for the metabolites fengycin (BGC0001095_c1), dificidin (BGC0000176_c1), bacilysin (BGC0001184_c1), bacillibactin (BGC0000309_c1), macrolactin (BGC0000181_c1), and bacillaene (BGC0001089_c1) were found to be 100% homologous with B. velezensis FZB42, and the surfactin (BGC0000433_c1) exhibits 82% identity with that of B. velezensis FZB42 at the nucleotide level.
Table 3.
Secondary metabolite clusters identified in the genome of B. velezensis JT3-1
| Cluster Number | Sequence size (bp) | Synthetase | Metabolites | MIBiG BGC-ID (Simility) | Bioactivity |
|---|---|---|---|---|---|
| 1 | 25,038 | Cf_fatty_acid | – | – | Not determined |
| 2 | 29,590 | Cf_saccharide | – | – | Not determined |
| 3 | 22,551 | Cf_putative | – | - | Not determined |
| 4 | 41,244 | Otherks | Butirosin | BGC0000693_c1(7%) | Antimicrobial |
| 5 | 20,977 | Cf_fatty_acid | – | – | Not determined |
| 6 | 7135 | Cf_putative | – | – | Not determined |
| 7 | 20,740 | Terpene | – | – | Not determined |
| 8 | 24,825 | Cf_fatty_acid | Citrulline | BGC0000895_c1(18%) | Not determined |
| 9 | 8911 | Cf_putative | – | – | Not determined |
| 10 | 17,703 | Cf_putative | Molybdenum | BGC0000916_c1(11%) | Not determined |
| 11 | 45,150 | Lantipeptide | – | – | Not determined |
| 12 | 85,905 | Transatpks | Macrolactin | BGC0000181_c1(100%) | Antimicrobial |
| 13 | 102,674 | Transatpks-Nrps | Bacillaene | BGC0001089_c1(100%) | Antimicrobial |
| 14 | 137,801 | Transatpks-Nrps | Fengycin | BGC0001095_c1(100%) | Filamentous fungi |
| 15 | 21,883 | Terpene | – | – | Not determined |
| 16 | 4727 | Cf_putative | – | – | Not determined |
| 17 | 41,100 | T3pks | – | – | Not determined |
| 18 | 100,453 | Transatpks | Difficidin | BGC0000176_c1(100%) | Antimicrobial |
| 19 | 8466 | Cf_putative | – | – | Not determined |
| 20 | 12,930 | Cf_putative | – | - | Not determined |
| 21 | 66,791 | Bacteriocin-Nrps | Bacillibactin | BGC0000309_c1(100%) | Microbial competitors |
| 22 | 11,114 | Cf_putative | – | – | Not determined |
| 23 | 25,411 | Cf_saccharide | – | – | Not determined |
| 24 | 54,812 | Cf_saccharide | Teichuronic_acid | BGC0000868_c1(100%) | Not determined |
| 25 | 14,022 | Cf_putative | – | – | Not determined |
| 26 | 72,378 | Cf_saccharide | Bacilysin | BGC0001184_c1(100%) | Bacteria, Candida albicans |
| 27 | 24,874 | Cf_saccharide | – | – | Not determined |
| 28 | 6391 | Cf_putative | – | – | Not determined |
| 29 | 65,407 | Nrps | Surfactin | BGC0000433_c1(82%) | Virus, mycoplasma, tumour |
| 30 | 25,038 | Cf_putative | – | – | Not determined |
Genes involved in promoting animal growth
Bacillus can produce some metabolites that are bioactive against pathogens, provide various enzymes and nutrients that enhance growth, maintain beneficial gut microflora, and improve the immune system of the host (Koumoutsi et al. 2004; Abd El-Tawab et al. 2016; Guo et al. 2016, 2017; Yi et al. 2018). Biofilm is defined as a complex structure of bacteria adhering to surfaces, and embedded in an extracellular matrix. Spo0A is a master regulator for sporulation in B. subtilis, and influences the expression of over 500 genes, but most may be mediated indirectly by regulatory genes (Molle et al. 2003). Spo0A promotes the transcription of sinI and represses abrB transcription; SinR is a transcriptional regulator of postexponential phase response genes, and binds to multiple sites in the regulatory region of the operon; SinI is an antagonist of SinR. In the biofilms of B. subtilis, the exopolysaccharide is produced by enzymes encoded by the epsA-O operon and the encoding gene is located in the yqxM-sipW-tasA operon. TasA and epsA-O are controlled by the repressor SinR (Chai et al. 2008). The operons of yqxM-sipW-tasA and epsA–O are controlled by SinR and AbrB (Chu et al. 2008). Biofilm formation and swimming motility in B. thuringiensis are controlled by the transition phase regulators Spo0A, AbrB and SinR, and the findings are similar in B. subtilis (Annette et al. 2014). The interaction between TasA and dextran-associated extracellular polysaccharides (EP) plays an important role in inter-species interactions during initial stages of multispecies biofilm development, and amyloid-like fibers (ALF) contribute to the structural complexity of dual-species biofilms (Duanis-Assaf et al. 2018). The analysis of the genome of the B. velezensis JT3-1 showed that JT3-1 also contains many genes involved in biofilm formation, including the spo0A, sinI, sinR, tasA and epsA-0 genes. B. velezensis JT3-1 contains xynD and xynA genes, which encode xylanase and are related to the use of hemicelluloses and celluloses present in the animal intestinal tract. At present, phytase is widely used as an animal feed supplement. JT3-1 also contains the gene encoding phytase, and it is reported to be able to degrade phytate and improve the efficacy of phosphorus metabolism and the performance of animals and plants (Frias et al. 2003). In addition, B. velezensis JT3-1 also harbors the genes that encode ATP-dependent Clp protease (clpE), α-glucosidase (glyA), β-glucosidase (gmuD), catalase (katE), and thiol peroxidase (tpx), among others, demonstrating that B. velezensis JT3-1 is able to use many organic materials as nutritional supplies to promote its survivability.
Antimicrobial activity of B. velezensis JT3-1
The results of the antimicrobial activity of B. velezensis JT3-1 against pathogens are presented in Table 4 and Fig. 2. Our study identified that B. velezensis JT3-1 showed strong antimicrobial activities against some Gram-negative human or animal pathogens (such as E. coli ATCC43888, E. coli ATCC25922, E. coli LQ1-2, Yersinia enterocolitis 23,715, Proteus vulgaris ATCC29905, Shigella bogdii ATCC9207, Salmonella typhimurium ATCC13311, and Mannheimia haemolytica ATCC29696), several Gram-positive animal and human pathogenic bacteria (such as Streptococcus pyogenes ATCC19615, Listeria monocytogenes ATCC19115, Staphylococcus hominis ZSY2, Staphylococcus aureus ATCC6538, and Clostridium perfringens MQF5), and the Mycoplasma bovis CYF. In this study, the B. velezensis JT3-1 strain demonstrated broad-spectrum antibacterial properties against both Gram-positive and Gram-negative bacteria. Similarly, other Bacillus spp., such as the B. cereus M15 strain, also have an inhibitory effect against both Gram-positive and Gram-negative bacteria (Yilmaz et al. 2006).
Table 4.
Antimicrobial activity of the B. velezensis JT3-1 culture supernatant
| Pathogens | Broth medium | Antimicrobial activity |
|---|---|---|
| Gram-negative bacteria | ||
| E.coli ATCC43888 | NAa | + + + |
| E.coli ATCC25922 | NA | + + + |
| E.coli LQ1-2 | NA | + + + |
| Yersinia enterocolitis 23,715 | NA | + + |
| Proteus vulgaris ATCC29905 | NA | + |
| Shigella bogdii ATCC9207 | NA | + |
| Salmonella typhimurium ATCC13311 | NA | + + |
| Mannheimia haemolytica ATCC29696 | NA | + + |
| Gram-positive bacteria | ||
| Listeria monocytogenes ATCC19115 | NA | + + + |
| Staphylococcus aureus ATCC6538 | NA | + + + |
| Streptococcus pyogenes ATCC19615 | NA | + + + |
| Staphylococcus hominis ZSY2 | NA | + + + |
| Clostridium perfringens MQF5 | NA | + + + |
| Mycoplasma bovis CYF | PPLOBb | + + + |
aNA Nutrient agar
bPPLOB PPLOB medium was utilized to culture Mycoplasma bovis
Fig. 2.
The inhibition zones of B. velezensis JT3-1 culture supernatant against different pathogens
Growth performance
The effects of B. velezensis JT3-1 on the growth performance of weaning calves are presented in Table 5. Compared with the control group, supplemental B. velezensis JT3-1 significantly increased average body weight (ABW) in the Bv1 group and Bv2 group (P < 0.05), along with the average daily gain (ADG) of calves over the entire experiment (from day 1 to day 30). In addition, the average body weight of calves receiving Bv2 supplementation was significantly higher than that of those given Bv1 during the overall period (P < 0.05); the average body weight of calves receiving Bv2 supplementation was very significantly higher (P < 0.01) than that of the control group during the overall period.
Table 5.
Effects of B. velenensis JT3-1 on growth performance of weaning calves1
| Item | Treating | SEM | P-value | ||
|---|---|---|---|---|---|
| Control | Bv1 | Bv2 | |||
| IBW2 /kg | 82.69 ± 15.99 | 85.81 ± 5.91 | 99.58 ± 11.22 | / | / |
| FBW3 /kg | 104.00 ± 17.01 | 110.88 ± 7.01 | 128.83 ± 9.45 | / | / |
| ADG/kg | 0.73 ± 0.08c | 0.86 ± 0.11b | 1.0 ± 0.14a | 0.44 | 0.001 |
a,b,cMean values within a row with different superscripts differ (P < 0.05)
1Each value is the mean of data from 15 calves per group; IBW2 initial body weight, FBW3 Final body weight
Immunoglobulin and cytokine concentrations
The immunoglobulin and cytokine concentrations in plasma are presented in Table 6. The concentrations of bovine IgA, IgG, IgM, and IFN-γ changed to different degrees after supplementation with Bv1 and Bv2. The Bv1 and Bv2 supplement significantly improved serum IgA, IgG, IgM, and IFN-γ levels in calves when compared with control (P < 0.05). However, no significant difference (P > 0.05) was observed between the Bv1 and Bv2 groups. In addition, the IL-2 level was not obviously different among the three groups (control, Bv1 and Bv2).
Table 6.
Effects of B. velezensis JT3-1 supplementation on plasma immunoglobulin and cytokine concentrations of weaning calves1
| Item | Time | Control | Bv1 | Bv2 |
|---|---|---|---|---|
| IgA (µg/ml) | Day 1 | 1184.71 ± 96.37 | 1157.07 ± 100.93 | 1187.03 ± 70.27 |
| Day 30 | 1241.92 ± 107.75b | 2858.40 ± 153.08a | 2897.82 ± 292.28a | |
| IgG (mg/ml) | Day 1 | 7.53 ± 0.54 | 7.98 ± 0.81 | 7.84 ± 0.64 |
| Day 30 | 7.99 ± 0.83b | 17.76 ± 1.45a | 19.38 ± 2.29a | |
| IgM (µg/ml) | Day 1 | 1310.50 ± 102.04 | 1362.03 ± 101.22 | 1378.64 ± 119.27 |
| Day 30 | 1314.89 ± 128.12b | 1970.95 ± 297.57a | 1984.36 ± 206.20a | |
| IL-2 (pg/ml) | Day 1 | 82.12 ± 9.80 | 80.58 ± 2.86 | 81.68 ± 6.12 |
| Day 30 | 82.74 ± 2.79 | 81.93 ± 5.84 | 82.04 ± 7.13 | |
| IFN-γ (pg/ml) | Day 1 | 196.01 ± 36.45 | 199.41 ± 65.43 | 205.63 ± 61.11 |
| Day 30 | 193.01 ± 50.53b | 409.08 ± 74.04a | 411.24 ± 131.52a |
a,bMean values within a row with different superscripts differ (P < 0.05)
1IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, IL-2 interleukin-2, and INF-γ interferon-gamma
Treatment of calf diarrhea
The results of treatment with CBBLE in Xinjiang Province showed that, after three days of oral feeding, 80% (144/180) of diarrhea cases recovered, and a further 15% (27/180) of diarrhea cases recovered after 4–6 days of oral administration, but 5% (9/180) died. In Qinghai Province, after three days of oral feeding, 89.94% (134/149) of diarrhea cases recovered; the rest recovered after 4–5 days of oral administration, and no deaths occurred. The cure rate of diarrhea cases in newborn Angus calves reached 95.0% (171/180) in Xinjiang Province, and it was 100.0% (149/149) in yak calves in Qinghai province. Recently, the applications of B. velezensis in biomedicine have been gradually reported. Li et al. (2020) reported that B. velezensis CPA1-1 isolated from Macrobrachium nipponense had a potential application in prevention and control of a variety of aquatic animal diseases, owing to its good antibacterial ability. Gao et al. (2017) reported that B. velezensis V4 caused an obvious reduction in the mortality of fish and also improved their growth. B. velezensis JW has the potential to be developed as a probiotic bacterium, and B. velezensis C-11 was also utilized as a potential gut microbiota-targeted therapy to prevent fish from pathogen invasion in aquaculture (Yi et al. 2018; Zhang et al. 2019). B. velezensis K68 isolated from traditional Korean fermented foods has functionality in the prevention of dental caries with Streptococcus mutans (Yoo et al. 2018). Our study has identified that B. velezensis JT3-1 plays an important role in the prevention and cure of calf diarrhea caused by bacteria, and improving growth performance of calves by the weaning period.
Conclusion
Until now, 168 B. velezensis strains have been sequenced and released in the NCBI genome database. Among the B. velezensis reported, most are isolated from plants; only one B. velezensis was reported to be isolated from animal (yak). This study also confirmed that JT3-1, when given to calves, produced improvements in growth, immune responses, and disease resistance against Escherichia coli and other bacteria. Our study will not only enrich the genome database but also provide some valuable information regarding the molecular mechanism of antimicrobial action and build the foundation for the utilization of JT3-1 as a probiotic agent in animal husbandry.
Genome sequence accession number
The complete genome sequence of B. velezensis JT3-1 was deposited at GenBank under the accession number CP032506.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFD0501804, 2018YFD0502305 and 2017YFD0501200), MOST; NBCITS (CARS-37). We thank Guodong Sun and Zhengying Zhang for collecting yak fecal samples, and thank Edanz and Dr. Junlong Liu for revising the manuscript.
Author contributions
YL and XL designed the experiments; DJ and JL extracted the genomic DNA. JW, AL, and GG conducted the genome sequencing and annotation. ZL and JX conducted the genome assembly and comparisons. HY conducted the phylogenetic analysis and the manual inspection of the annotation. YL and HY conceived the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Lanzhou Veterinary Research Institute, CAAS (No. LVRIAEC2013-010). The use of these field samples was approved by the Animal Ethics Procedures and Guideline of China.
Contributor Information
Youquan Li, Email: youquan-li@163.com.
Hong Yin, Email: yinhong@caas.cn.
References
- Abd El-Tawab MM, Youssef IM, Bakr HA, Fthenakis GC, Giadinis ND. Role of probiotics in nutrition and health of small ruminants. Pol J Vet Sci. 2016;19(4):893–906. doi: 10.1515/pjvs-2016-0114. [DOI] [PubMed] [Google Scholar]
- Adeniji AA, Babalola OO. Bacillus velezensis: phylogeny, useful applications, and avenues for exploitation. App Microbiol Biot. 2019;103(9):3669–3682. doi: 10.1007/s00253-019-09710-5. [DOI] [PubMed] [Google Scholar]
- Annette F, Thomas D, Ole-Andreas Ø, Emilie V, Nathalie G, Imène B, Stéphane P, Myriam G, Stéphane A, Anne-Brit K, Didier L, Michel G. SinR Controls Enterotoxin Expression in Bacillus thuringiensis Biofilms. PLoS ONE. 2014;9(1):e87532. doi: 10.1371/journal.pone.0087532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, Suarez Duran HG, de Los Santos ELC, Kim HU, Nave M, Dickschat JS, Mitchell DA, Shelest E, Breitling R, Takano E, Lee SY, Weber T, Medema MH. antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017;45(W1):W36–W41. doi: 10.1093/nar/gkx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Complete genome sequence of Bacillus velezensis LM2303, a biocontrol strain isolated from the dung of wild yak inhabited Qinghai-Tibet plateau. J Biotechnol. 2017;251:124–127. doi: 10.1016/j.jbiotec.2017.04.034. [DOI] [PubMed] [Google Scholar]
- Chen L, Gu W, Xu H, Yang GL, Shan XF, Chen G, Wang CF, Qian AD. Complete genome sequence of Bacillus velezensis 157 isolated from Eucommia ulmoides with pathogenic bacteria inhibiting and lignocellulolytic enzymes production by SSF. 3 Biotech. 2018;8:114. doi: 10.1007/s13205-018-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R, Piel J, Borriss R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10(6):563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- Chu F, Kearns DB, McLoon A, Chai Y, Kolter R, Richard L. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duanis-Assaf D, Duanis-Assaf T, Zeng G, Meyer RL, Reches M, Steinberg D, Shemesh M. Cell Wall Associated Protein TasA Provides an Initial Binding Component to Extracellular Polysaccharides in Dual-Species Biofilm. Sci Rep. 2018;8(1):9350. doi: 10.1038/s41598-018-27548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eric PN, Sarah WB, Alex B, Jennifer D, Ruth YE, Sean RE, Evan WF, Paul PG, Thomas AJ, John T, Robert DF. Rfam 12.0: updates to the RNA families database. Nucleic Acids Res. 2014;37:D136–D140. [Google Scholar]
- Frias J, Doblado R, Antezana JR, Vidal-Valverde CN. Inositol phosphate degradation by the action of phytase enzyme in legume seeds. Food Chem. 2003;81(2):233. doi: 10.1016/S0308-8146(02)00417-X. [DOI] [Google Scholar]
- Gao XY, Liu Y, Miao LL, Li EW, Sun GX, Liu Y, Liu ZP. Characterization and mechanism of anti-Aeromonas salmonicida activity of a marine probiotic strain, Bacillus velezensis V4. Appl Microbiol Biotechnol. 2017;101:3759–3768. doi: 10.1007/s00253-017-8095-x. [DOI] [PubMed] [Google Scholar]
- Guo M, Hao G, Wang B, Li N, Li R, Wei L, Chai T. Dietary Administration of Bacillus subtilis Enhances Growth Performance, Immune Response and Disease Resistance in Cherry Valley Ducks. Front Microbiol. 2016;7:1975. doi: 10.3389/fmicb.2016.01975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wu F, Hao G, Qi Q, Li R, Li N, Wei L, Chai T. Bacillus subtilis Improves Immunity and Disease Resistance in Rabbits. Front Immunol. 2017;8:354. doi: 10.3389/fimmu.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt D, Chen GL, LoCascio PF, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC bioinformatics 11 [DOI] [PMC free article] [PubMed]
- Jin Q, Jiang Q, Zhao L, Su C, Li S, Si F, Li S, Zhou C, Mu Y, Xiao M. Complete genome sequence of Bacillus velezensis S3–1, a potential biological pesticide with plant pathogen inhibiting and plant promoting capabilities. J Biotechnol. 2017;259:199–203. doi: 10.1016/j.jbiotec.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Khan RU, Shabana N, Kuldeep D, Karthik K, Ruchi T, Mutassim MA, Alhidary IA, Zahoor A. Direct-fed microbial: beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. Int J Pharm. 2016;12:220–231. doi: 10.3923/ijp.2016.220.231. [DOI] [Google Scholar]
- Kim SY, Lee SY, Weon HY, Sang MK, Song J. Complete genome sequence of Bacillus velezensis M75, a biocontrol agent against fungal plant pathogens, isolated from cotton waste. J Biotechnol. 2017;241:112–115. doi: 10.1016/j.jbiotec.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cylic lipopeptides in Bacillus amyloliquefaciens FZB42. J Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao X, Zhang S, Jiang Z, Yang H, Liu X, Jiang Q, Zhang X. Characterization of a Bacillus velezensis with antibacterial activity and inhibitory effect on common aquatic pathogens. Aquaculture. 2020 doi: 10.1016/j.aquaculture.2020.735165. [DOI] [Google Scholar]
- Lillehoj HS, Lee KW. Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Poult Sci. 2012;91:1286–1291. doi: 10.3382/ps.2012-02374. [DOI] [PubMed] [Google Scholar]
- Liu G, Kong Y, Fan Y, Geng C, Sun M. Whole-genome sequencing of Bacillus velezensis LS69, a strain with a broad inhibitory spectrum against pathogenic bacteria. J Biotechnol. 2017;249:20–24. doi: 10.1016/j.jbiotec.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Molle V, Fujita M, Jensen ST, Eichenberger P, González-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50(5):1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- Niazi A, Manzoor S, Asari S, Bejai S, Meijer J, Bongcam-Rudloff E. Genome analysis of Bacillus amyloliquefaciens subsp plantarum UCMB5113: a rhizobacterium that improves plant growth and stress management. PLoS One. 2014;9:e104651. doi: 10.1371/journal.pone.0104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntushelo K, Ledwaba LK, Rauwane ME, Adebo OA, Njobeh PB (2019) The Mode of Action of Bacillus Species against Fusarium graminearum, Tools for Investigation, and Future Prospects. Toxins 11(10) [DOI] [PMC free article] [PubMed]
- Ongena M, Jacques P, Touré Y, Destain J, Jabrane A, Thonart P. Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl Microbiol Biotechnol. 2005;69:29–38. doi: 10.1007/s00253-005-1940-3. [DOI] [PubMed] [Google Scholar]
- Pan HQ, Li QL, Hu JC. The complete genome sequence of Bacillus velezensis 9912D reveals its biocontrol mechanism as a novel commercial biological fungicide agent. J Biotechnol. 2017;247:25–28. doi: 10.1016/j.jbiotec.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Pandin C, Le Coq D, Deschamps J, Védie R, Rousseau T, Aymerich S, Briandet R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J Biotechnol. 2018;278:10–19. doi: 10.1016/j.jbiotec.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Rabbee MF, Ali M, Choi J, Hwang BS, Jeong SC, Baek KH. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. doi: 10.3390/molecules24061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G. Probiotics–possibilities and limitations of their application in food, animal feed, and in pharmaceutical preparations for men and animals. Berl Münch Tierärztl Wochenschr. 2001;114:410–419. [PubMed] [Google Scholar]
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr. 2001;73(2 Suppl):361S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Shao Y, Wang Z, Wang H, Peng Q, Xue B, Wang L, Hu R, Zou H, Kang K, Hu J. Effects of Yeast Cell Wall on Growth Performance, Nutrient Apparent Digestibility and Serum Immune Indices of Calves. Chin J An N. 2019;31(1):378–387. [Google Scholar]
- Sharma C, Rokana N, Chandra M, Pal SB, Gulhane RD, Gill JPS, Ray P, Puniya AK, Panwar H. Antimicrobial Resistance: Its Surveillance, Impact, and Alternative Management Strategies in Dairy Animals. Front Vet Sci. 2017;4:237. doi: 10.3389/fvets.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Wu YS. Anticancer Activities of Surfactin and Potential Application of Nanotechnology Assisted Surfactin Delivery. Front Pharmacol. 2017;8:761. doi: 10.3389/fphar.2017.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Zhang Z, Zhao F, Liu H, Yu L, Zha J, Wang G. Probiotic potential of Bacillus velezensis JW: Antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immun. 2018;78:322–330. doi: 10.1016/j.fsi.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Soran H, Beyatli Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol Res. 2006;161(2):127–131. doi: 10.1016/j.micres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yoo Y, Seo D-H, Lee H, Nam Y-D, Seo M-J. Inhibitory effect of Bacillus velezensis on biofilm formation by Streptococcus mutans. J Biotechnol. 2018;298:57–63. doi: 10.1016/j.jbiotec.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Kang YH, Zhan S, Zhao ZL, Jin SN, Chen C, Zhang L, Shen JY, Wang CF, Wang GQ, Shan XF, Qian AD. Effect of Bacillus velezensis on Aeromonas veronii-Induced Intestinal Mucosal Barrier Function Damage and Inflammation in Crucian Carp (Carassius auratus) Front Microbiol. 2019;10:2663. doi: 10.3389/fmicb.2019.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Raza MF, Zheng Z, Zhang X, Dong X, Zhang H. Complete genome sequence of Bacillus velezensis ZY-1-1 reveals the genetic basis for its hemicellulosic/cellulosic substrate-inducible xylanase and cellulase activities. 3 Biotech. 2018;8(11):465. doi: 10.1007/s13205-018-1490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.